Abstract
In 7 of 8 children with idiopathic pulmonary arterial hypertension treated with intravenous epoprostenol for > 1 year, concomitant use of bosentan allowed a reduction of epoprostenol and decreased its associated side effects without deterioration of clinical and hemodynamic parameters. In 3 children with normal or near-normal pulmonary artery pressure on epoprostenol, the addition of bosentan allowed discontinuation of epoprostenol and stabilization of hemodynamics for up to 1 year.
Long-term therapy with epoprostenol, a potent prostacyclin and short-acting vasodilator, has been shown to improve hemodynamics, exercise capacity, and survival in adults and children with pulmonary hypertension.1–6 However, epoprostenol has several inherent drawbacks. Epoprostenol is administered through continuous intravenous infusion, and its dosing and regulation is complex. Endothelin-1, a potent vasoconstrictor peptide, is increased in the pulmonary arteries of patients with pulmonary hypertension7; endothelin levels are also increased and correlated with the severity of disease in adults with idiopathic primary pulmonary arterial hypertension (IPAH).8 Bosentan, a dual endothelin receptor antagonist, lowers pulmonary artery pressure and resistance and improves exercise tolerance in adults with pulmonary arterial hypertension.9 In children with pulmonary arterial hypertension related to congenital heart disease or IPAH, bosentan lowered pulmonary pressure and resistance and was well tolerated.10 A recent case series has suggested that epoprostenol may be withdrawn from a select group of adult patients with normal pulmonary pressure.11 Our study was undertaken to determine whether the addition of bosentan concomitant with stepwise epoprostenol dose reductions could decrease the severity of side effects experienced with epoprostenol while maintaining hemodynamic stability in children with IPAH.
After institutional review board approval, informed consent, and assent where appropriate, patients were enrolled in a longitudinal follow-up study. Pediatric patients with IPAH in World Health Organization (WHO) functional class II or III, who received continuous epoprostenol therapy for > 1 year at the Pediatric Pulmonary Hypertension Program of the Children's Hospital in Denver, Colorado, were recruited (Table 1). All known causes of pulmonary hypertension were excluded, including congenital heart disease and lung disease. All patients had severe, life-threatening pulmonary hypertension before initiation of epoprostenol (Table 2).
TABLE 1. Baseline Characteristics Before and After the Addition of Bosentan to Epoprostenol.
| Patient | Age (yrs)/Sex | Diagnosis | Weight (kg) | WHO | EPO | Bosentan Duration (yrs) | Walk Before | Walk After | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Duration (yrs) | |||||||
| 1 | 8.5 M | IPAH | 25 | 2 | 2 | 37 | 62 | 3.2 | 2.2 | 427 | 581 |
| 2 | 8.7 F | IPAH | 24 | 2 | 2 | 62 | 0 | 8.2 | 1.6 | 369 | 427 |
| 3 | 9.1 M | IPAH | 18 | 2 | 2 | 147 | 60 | 8.0 | 2.3 | 556 | 579 |
| 4 | 10.5 M | IPAH | 25 | 2 | 2 | 130 | 4 | 10.3 | 1.4 | 593 | 579 |
| 5 | 14.4 F | IPAH | 48 | 2 | 1 | 72 | 0 | 8.0 | 2.5 | 518 | 546 |
| 6 | 16.3 M | IPAH | 40 | 2 | 1 | 55 | 0 | 9.9 | 1.8 | 704 | 616 |
| 7 | 16.7 F | IPAH | 49 | 3 | 3 | 97 | 67 | 7.5 | 2.5 | 412 | 470 |
| 8 | 17.8 F | IPAH | 48 | 3 | 3 | 88 | 38 | 5.6 | 1.8 | 402 | 346 |
| Mean | 2.3 | 2.0 | 86 | 29* | 7.6 | 2.0 | 498 | 518 | |||
| SD | 0.5 | 0.8 | 37 | 31 | 2.3 | 0.4 | 116 | 94 | |||
p <0.05 EPO before versus EPO after.
EPO = epoprostenol dose nanogram per kilogram per minute; walk = 6-minute walk.
TABLE 2. Cardiac Catheterization Data.
| Patient | Age (yrs) | Status | PAP (mm Hg) | AOP (mm Hg) | CI (l/min/m2) | PVRI (U · m2) | PVR/SVR |
|---|---|---|---|---|---|---|---|
| 1 | 4.9 | Baseline | 70 | 74 | 4.2 | 15.0 | 0.95 |
| 6.3 | EPO | 56 | 73 | 4.3 | 11.3 | 0.69 | |
| 6.6 | EPO + BOS | 27 | 68 | 4.5 | 4.3 | 0.29 | |
| 2 | 0.1 | Baseline | 55 | 55 | 2.5 | 22.0 | 1.00 |
| 6.6 | EPO | 25 | 64 | 4.9 | 3.1 | 0.27 | |
| 8.2 | BOS | 24 | 62 | 2.6 | 5.6 | 0.24 | |
| 3 | 0.4 | Baseline | 67 | 60 | 2.2 | 27.3 | 1.20 |
| 6.7 | EPO | 39 | 80 | 4.5 | 6.6 | 0.40 | |
| 7.0 | EPO + BOS | 35 | 69 | 4.6 | 6.5 | 0.46 | |
| 4 | 0.1 | Baseline | 63 | 59 | 2.1 | 28.6 | 1.50 |
| 4.7 | EPO | 55 | 68 | 5.3 | 7.0 | 0.75 | |
| 10.2 | EPO + BOS | 31 | 71 | 3.4 | 7.1 | 0.40 | |
| 5 | 5.5 | Baseline | 65 | 68 | 3.2 | 17.0 | 0.90 |
| 11.9 | EPO | 25 | 70 | 4.3 | 4.9 | 0.31 | |
| 14.5 | BOS | 30 | 84 | 3.1 | 7.2 | 0.28 | |
| 15.5 | BOS | 30 | 80 | 3.8 | 6.0 | 0.34 | |
| 6 | 5.4 | Baseline | 98 | 75 | 2.6 | 32.0 | 1.39 |
| 14.5 | EPO | 24 | 78 | 4.1 | 3.4 | 0.20 | |
| 15.3 | BOS | 24 | 78 | 4.9 | 3.5 | 0.23 | |
| 7 | 9.1 | Baseline | 75 | 102 | 5.0 | 13.2 | 0.68 |
| 14.2 | EPO | 66 | 84 | 6.0 | 9.9 | 0.75 | |
| 14.4 | EPO + BOS | 58 | 79 | 6.4 | 8.0 | 0.66 | |
| 8 | 10.3 | Baseline | 120 | 85 | 2.9 | 46.0 | 1.30 |
| 15.1 | EPO | 103 | 84 | 3.8 | 23.4 | 1.18 | |
| 16.2 | EPO + BOS | 115 | 70 | 5.5 | 19.5 | 0.79 | |
| Baseline | 77 ± 22 | 73 ± 16 | 3.1 ± 1.0 | 25.1 ± 10.8 | 1.1 ± 0.3 | ||
| EPO | 49 ± 27* | 74 ± 7 | 4.5 ± 0.5* | 8.6 ± 7.2* | 0.5 ± 0.4* | ||
| BOS ± EPO | 43 ± 33 | 72 ±7 | 4.1 ±1.1 | 7.7 ± 5.4 | 0.4 ± 0.2 |
p <0.05 baseline versus EPO.
AOP = mean arterial pressure; BOS = bosentan; CI = cardiac index; PAP = mean pulmonary artery pressure; PVRI = pulmonary vascular resistance index; SVR = systemic vascular resistance; other abbreviation in Table 1.
From April 2001 to July 2002, patients were enrolled and their treatment regimen was modified to include bosentan, with dosing according to published guidelines.10 The target dose for children 10 to 20 kg was 31.25 mg twice daily, for children 20 to 40 kg, the dose was 62.5 mg twice daily, and for children >40 kg, the dose was 125 mg twice daily. Epoprostenol doses were decreased 1 to 2 ng/kg/min weekly, depending on stable or decreasing right ventricular peak systolic pressure as determined by echocardiography and/or cardiac catheterization. Patients remained on concomitant medications, including warfarin (8 of 8 patients), digoxin (2 of 8), diuretics (2 of 8), dipyridamole (6 of 8), calcium channel blockers (1 of 8), and nighttime oxygen (7 of 8). Cardiopulmonary stress testing was conducted in all patients >7 years of age at start of the study. Maximal symptom-limited exercise testing was performed on an electronically braked cycle ergometer using the James protocol. After familiarization with the exercise testing procedure, patients underwent testing before and periodically during weaning of their epoprostenol dosage. Also, submaximal exercise capacity was tested periodically using the 6-minute walk test. WHO functional class was assessed at start of the study and on the last assessment date. Hemodynamic values were determined by cardiac catheterization before the beginning of the study and at 3 months to 2.0 years after the addition of bosentan.
The patients' family members or primary caregivers graded epoprostenol-related side effect severity. Symptoms were evaluated every 3 months on a 4-point scale. Liver enzyme levels were monitored throughout the study; elevations were managed according to published guidelines for bosentan.10 Parents and female patients were warned of possible teratogenic effects and male patients were advised of the possibility of male infertility during treatment with bosentan.
Baseline patient demographics are shown in Table 1. Eight patients, 4 of whom had participated in the Bosentan Randomized Trial of Endothelin Antagonist Therapy 3,10 were enrolled in the study. Epoprostenol doses were reduced steadily in 7 patients and increased in 1. Mean epoprostenol dosage was downtitrated from study start to study end in November 2003 (p <0.05). Epoprostenol therapy was discontinued in 3 patients who had normal or near-normal pulmonary artery pressure on epoprostenol (Table 1). In 1 patient (patient 1), the epoprostenol dose was increased to treat progressive pulmonary hypertension. Results from cardiac catheterizations performed before epoprostenol as well as before and after treatment with bosentan are listed in Table 2. Exercise testing results are given in Table 3. Despite the reduction in epoprostenol dosage, no patient demonstrated a decrease in aerobic capacity. Peak workload and peak oxygen pulse increased (p <0.05), suggesting an improvement in cardiac output on the subsequent exercise studies.
TABLE 3. Results of Exercise Testing Before and After the Addition of Bosentan to Epoprostenol (n = 5).
| Variable | Before | After | p Value |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 32 ± 15 | 43 ±11 | 0.22 |
| Work (W) | 73 ± 26 | 102 ± 21 | 0.02 |
| Peak VO2 (ml/kg/min) | 26 ± 4 | 29 ± 8 | 0.25 |
| Predicted VO2 (%) | 58 ± 6 | 66 ± 15 | 0.25 |
| ΔVE/ΔVCO2 | 44 ± 9 | 45 ± 10 | 0.16 |
| Predicted ΔVE/ΔVCO2 (%) | 138 ± 28 | 140 ± 28 | 0.39 |
| Peak oxygen pulse (ml/beat) | 4.9 ± 1.3 | 5.4 ± 1.4 | 0.03 |
VO2 = oxygen consumption; ΔVE/ΔVCO2 = change in ventilation per amount of expired CO2.
Epoprostenol side effects were monitored after the initiation of bosentan (Figure 1). Many children experienced a transient worsening of epoprostenol symptoms after the initiation of bosentan. All but 1 patient reported overall improvement in symptom severity soon after epoprostenol doses were reduced. Before the addition of bosentan and reduction in epoprostenol dosage, most of the patients experienced ≥3 of the 7 side effects monitored. Headache and rash were the most frequent symptoms reported; fatigue and nausea/vomiting/stomachaches were the least frequent symptoms noted. By study end, all patients reported improvement or elimination of rashes. At the start of the study, 5 patients had reported jaw pain, which was eliminated completely in the 4 patients who had epoprostenol reduced. By study end, no side effect was rated severe by any patient, and ≥1 side effects were eliminated in all patients.
FIGURE 1.
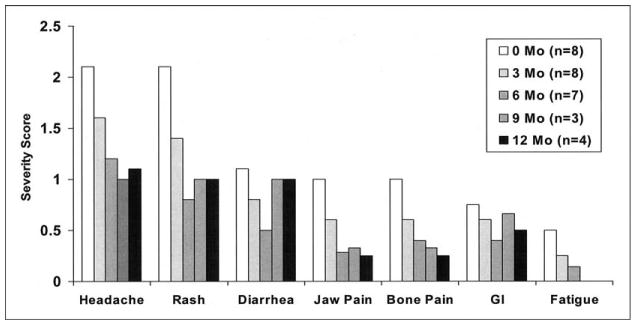
Epoprostenol side effect symptoms were evaluated at 3-month intervals after the initiation of bosentan. Symptoms were evaluated on a 4-point scale (0 = no discomfort or interference with daily activity; 1 = mild discomfort but no interference; 2 = moderate discomfort sufficient to reduce or affect daily activity; and 3 = severe, incapacitating discomfort with inability to perform normal daily activities).
Four patients experienced a liver enzyme elevation. One patient (patient 5) experienced elevations 5× the upper limit of normal after treatment with oxacillin >1 year after the study ended. Bosentan therapy for this patient was halted for 2 weeks and then reinstituted at the initial dose and later increased to full dose without incident. By study end, only 1 patient (patient 7) discontinued treatment with bosentan due to elevated liver function tests.
Children receiving long-term epoprostenol therapy experience severe side effects associated with high-dose treatment. Although reducing epoprostenol dosage can attenuate epoprostenol-related side effect severity, this comes at the risk of reducing the beneficial hemodynamic effects obtained at the higher dose. The addition of bosentan to the treatment regimen may resolve this dilemma. Recent successes of bosentan in improving cardiopulmonary hemodynamics in adults with IPAH9 and children with congenital heart disease or IPAH10 make it reasonable to consider the concomitant use of these 2 agents, which have different mechanisms of action.
In most children, as epoprostenol doses decreased dramatically, side effect severity also decreased significantly, without clinical or hemodynamic deterioration. Patients with longer term epoprostenol treatment withstood greater dose reductions, suggesting that the best candidates for dose reductions may be those with a stable, extended duration of drug use. We only attempted discontinuation of epoprostenol in children with normal or near-normal pulmonary artery pressure. One patient (patient 1), who had been on epoprostenol therapy the shortest time, was unable to reduce epoprostenol dose due to continued worsening of pulmonary hypertension. Epoprostenol therapy was discontinued in 3 patients with no clinical worsening or elevation of pulmonary pressure up to 1 year after epoprostenol discontinuation.
This study has several limitations. The patient population was small, and it is possible that these patients may have been able to wean off of epoprostenol without the addition of bosentan. This was not attempted because IPAH is a progressive disease without a cure. Most patients treated with bosentan in addition to epoprostenol did not show a marked improvement in hemodynamics or exercise capacity; rather they showed a stable clinical profile on weaning of epoprostenol. It is likely that the major beneficial effect was due to prolonged epoprostenol therapy.3
We demonstrated that bosentan facilitates the reduction of epoprostenol dosages and its associated side effect severity, without adversely affecting hemodynamic parameters in this select pediatric patient population. Further randomized studies are required to determine if use of bosentan concomitantly with epoprostenol improves hemodynamics and may allow safe weaning or discontinuation of epoprostenol.
Acknowledgments
This research was supported by grant MO1 RR00069 from the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health, Bethesda, Maryland. Dr. Ivy's address is: Pediatric Cardiology, The Children's Hospital, 1056 East 19th Avenue, Denver, Colorado 80218.
References
- 1.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann Intern Med. 1990;112:485–491. doi: 10.7326/0003-4819-112-7-485. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338:273–277. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–1208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 7.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 8.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest. 2001;120:1562–1569. doi: 10.1378/chest.120.5.1562. [DOI] [PubMed] [Google Scholar]
- 9.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 10.Barst RJ, Ivy D, Dingemanse J, Widlitz A, Schmitt K, Doran A, Bingaman D, Nguyen N, Gaitonde M, van Giersbergen PL. Pharmacokinetics, safety, and efficacy of bosentan in pediatric patients with pulmonary arterial hypertension. Clin Pharmacol Ther. 2003;73:372–382. doi: 10.1016/s0009-9236(03)00005-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim NH, Channick RN, Rubin LJ. Successful withdrawal of long-term epoprostenol therapy for pulmonary arterial hypertension. Chest. 2003;124:1612–1615. [PubMed] [Google Scholar]


