Abstract
Purpose
To investigate the effects of tibial nerve stimulation on bladder overactivity induced by acetic acid (AA) irritation.
Material and Methods
Cystometry was performed in 10 α-chloralose anesthetized female cats by infusing saline or AA through a urethral catheter that was secured by a ligature around the urethra. Intravesical infusion of 0.25% AA was used to irritate the bladder and induce bladder overactivity. Multiple cystometrograms (CMGs) were performed before, during, and after tibial nerve stimulation to determine the inhibitory effect on the micturition reflex.
Results
Infusion of 0.25% AA irritated the bladder, induced bladder overactivity, and significantly reduced the bladder capacity to about 20% of the control capacity measured during saline infusion. Tibial nerve stimulation at either low (5 Hz) or high (30 Hz) frequency significantly increased bladder capacity to about 40% of the saline control capacity when it was applied during AA infusion CMG. The amplitude of bladder contractions was smaller during AA irritation than during saline distention due to a significantly smaller bladder capacity. Tibial nerve stimulation at 5 Hz not only increased bladder capacity but also increased the amplitude of bladder contractions.
Conclusion
Activation of somatic afferents in the tibial nerve of cats can partially reverse the bladder overactivity induced by intravesical administration of a chemical irritant that activates C-fiber afferent nerves. These data are consistent with clinical studies showing that tibial nerve neuromodulation is effective in treating overactive bladder symptoms.
Keywords: Bladder, Tibial nerve, Electrical stimulation, Cat
INTRODUCTION
Many clinical reports 1–17 have shown that percutaneous tibial nerve stimulation is effective in treating patients with overactive bladder symptoms. However, only a few animal studies 18,19 have investigated the inhibitory effect of tibial/peroneal nerve stimulation on reflex bladder activity. These studies showed that stimulation frequencies of 0.5–10 Hz were effective 18,19, while most clinical studies used 20 Hz 1–17. The animal studies used saline to fill the bladder and induce normal reflex micturition mediated by Aδ afferent fibers 20. However, in pathological conditions C-fiber afferents contribute to overactive bladder symptoms 21; and currently there is no animal study demonstrating that tibial nerve stimulation can modulate bladder overactivity induced by C-fiber afferents. Due to the lack of basic science evidence supporting the clinical treatment of bladder overactivity by tibial nerve stimulation, the efficacy of this treatment is often questioned by many clinicians.
In this animal study using cats we aimed at answering the following two questions: 1) Is the tibial nerve stimulation effective in suppressing pelvic C-fiber afferent mediated bladder overactivity and 2) Which stimulation frequencies are effective? The results of our study showed the effectiveness of low and high frequency tibial nerve stimulation in suppressing chemically-induced bladder overactivity. These data provide support for the clinical use of tibial nerve neuromodulation in the treatment of overactive bladder symptoms.
MATERIAL AND METHODS
All protocols involving the use of animals in this study were approved by the Animal Care and Use Committee at the University of Pittsburgh.
Experimental setup
Experiments were conducted in 10 female cats (2.2 kg to 3.3 kg) under α-chloralose anesthesia (65 mg/kg, I.V. supplemented as necessary) after induction with isoflurane (2–3% in O2). Systemic blood pressure was monitored throughout the experiment via a catheter inserted in the right carotid artery. A tracheotomy was performed and a tube was inserted to keep the airway patent. A catheter for I.V. infusion was introduced into the right ulnar vein. The ureters were cut and drained externally. A double lumen catheter was inserted through the urethra into the bladder and secured by a ligature around the urethra. One lumen of the catheter was connected to a pump to infuse the bladder with either saline or 0.25% acetic acid (AA) at a rate of 0.5–2 ml/min, and the other lumen was connected to a pressure transducer to measure the pressure change in the bladder. The tibial nerve was exposed on the medial side of left hindlimb above the ankle. After a tripolar cuff electrode (NC223pt, MicroProbe Inc., Gaithersburgh, MD) was implanted on the tibial nerve for stimulation, the skin was closed by sutures.
Stimulation protocol
Uniphasic rectangular pulses (0.2 ms pulse width) at low (5 Hz) or high (30 Hz) frequency were delivered to the tibial nerve via the cuff electrode. The intensity threshold (T) for inducing toe movement was determined at 5 Hz by gradually increasing the stimulation intensity. Since our preliminary test indicated that stimulation intensity of 2–4 T was required to inhibit isovolumetric bladder contractions induced by saline distention, this intensity range was used for tibial nerve stimulation to suppress bladder overactivity induced by infusion of 0.25% AA.
The inhibitory effect of tibial nerve stimulation was tested by performing repeated cystometrograms (CMGs) which consisted of a slow infusion of saline or AA starting with an empty bladder to determine the bladder capacity. Bladder infusion was stopped immediately after the initiation of the first large amplitude bladder contraction. First, two or three saline CMGs were performed without stimulation to obtain the control bladder capacity and evaluate reproducibility. Then, another 3–5 CMGs were performed during a 30–50 minute period with infusion of 0.25% AA to irritate the bladder and induce bladder overactivity that markedly reduced bladder capacity. After the capacity of the irritated bladder stabilized, tibial nerve stimulation (frequency: 5 or 30 Hz; intensity: 2–4 times threshold for inducing toe movement) was applied during two sequential CMGs. The order of application of the two stimulation frequencies (5 or 30 Hz) was randomized in different animals. At the end of the experiment, another CMG with AA infusion was performed without stimulation. The bladder was emptied after each CMG and a 5–10 min rest period was inserted between CMGs to allow the micturition reflex to recover. Repeated CMGs during 0.25% AA infusion were performed over a period of 1.5–2 hours.
Data analysis
For the repeated CMG recordings, the bladder capacities were measured and normalized to the measurement of the first control CMG during saline infusion. Repeated measurements in the same animal under the same conditions were averaged. The normalized data from different animals are presented as means ± SE. Student’s t-test was used to detect statistical significance (P<0.05).
RESULTS
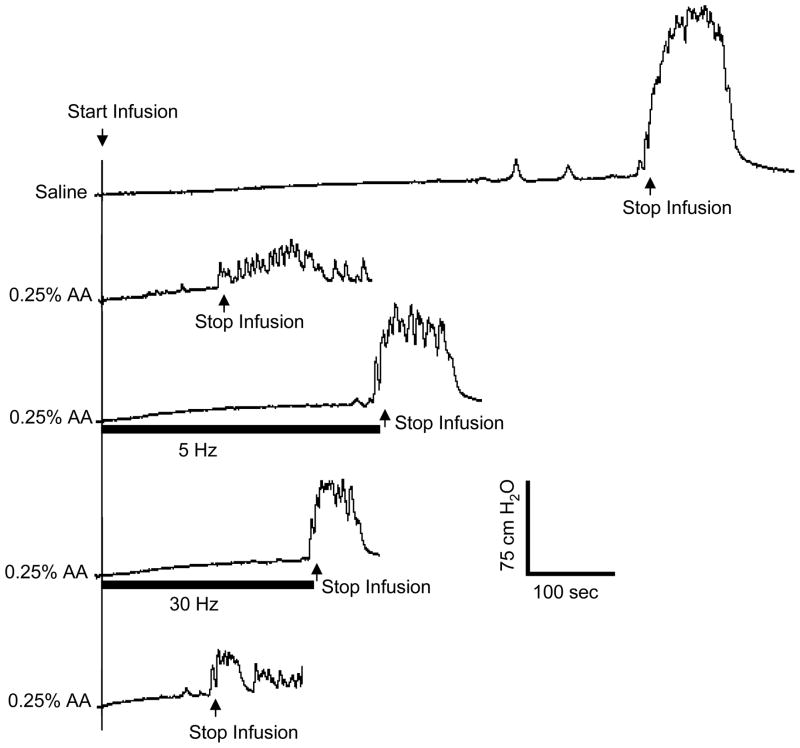
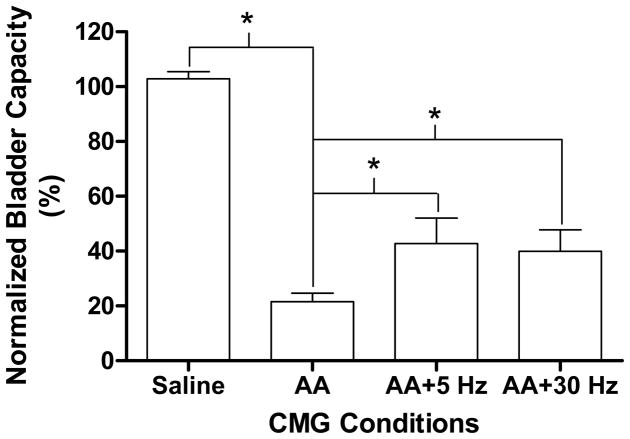
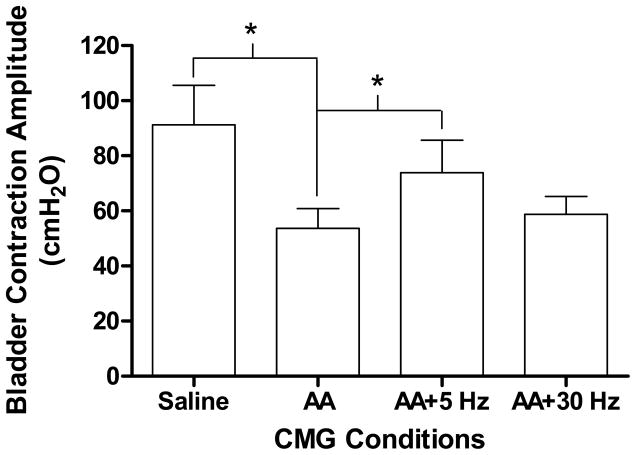
During saline infusion, large amplitude (>30 cmH2O) long duration (>30 seconds) bladder contractions were induced when the bladder volume reached the micturition threshold volume (i.e. bladder capacity, 10.7±1.8 ml) (see the first trace in Fig. 1). Irritation of the bladder by infusion with 0.25% AA markedly reduced (P<0.05) bladder capacity to about 20% of the capacity during saline infusion (see Figs.1 and 2). The amplitude of bladder contractions was also reduced (Figs.1 and 3) presumably due to the smaller bladder volume at the time of reflex micturition. Tibial nerve stimulation at either 5 or 30 Hz applied during the CMGs following the AA induced irritation significantly (P<0.05) increased bladder capacity to about 40–50% of the saline control (see the third and fourth traces in Fig. 1), but did not completely reverse the effect of AA irritation and return bladder capacity to control levels (Fig. 2). There was no significant difference between 5 Hz and 30 Hz in increasing bladder capacity (Fig. 2). However 5 Hz but not 30 Hz stimulation also significantly (P<0.05) increased the amplitude of bladder contractions (Fig. 3). Bladder capacity during control CMGs at the end of the experiments following recovery from tibial nerve stimulation were not significantly different from the bladder capacities measured during AA irritation prior to tibial nerve stimulation indicating that the CMGs were stable over the course of the experiment.
Fig. 1.
Tibial nerve stimulation suppresses bladder overactivity induced by infusion of 0.25% acetic acid (AA) and significantly increases bladder capacity. Stimulation: pulse width 0.2 ms, intensity 6 V (4 T). T- intensity threshold for inducing toe movement. Infusion rate: 1 ml/min.
Fig. 2.
Tibial nerve stimulation at both 5 Hz and 30 Hz significantly suppressed bladder overactivity induced by infusion of 0.25% acetic acid (AA). Stimulation: pulse width 0.2 ms, intensity 2–4 T. T - intensity threshold for inducing toe movement. * indicate a statistical significance. N = 10.
Fig. 3.
The amplitude of bladder contractions was significantly reduced by infusion of 0.25% acetic acid (AA). Tibial nerve stimulation significantly increased the contractionamplitude. Stimulation parameters were same as Fig. 2. * indicate a statistical significance. N = 10.
DISCUSSION
This study revealed that tibial nerve stimulation in cats at both low (5 Hz) and high (30 Hz) frequencies is effective in suppressing bladder overactivity induced by intravesical administration of 0.25% AA (Figs.1 and 2). The results provide basic science support for the clinical use of tibial nerve stimulation in treating overactive bladder symptoms.
Previous studies in non-human primates 18 and cats 19 showed that tibial/peroneal nerve stimulation is effective in inhibiting reflex bladder activity induced by saline infusion into the bladder. Under these conditions reflex bladder activity is induced primarily by activation of bladder Aδ-fiber afferents. However following AA irritation bladder C-fiber afferents are sensitized and induce bladder overactivity including a marked reduction in bladder capacity 20,21 (Figs.1 and 2). This is the first animal study showing that the bladder overactivity can be suppressed by activating the somatic afferent fibers in the tibial nerve. Because reflex micturition after bladder irritation very likely depends on a normal reflex pathway activated by Aδ-fiber afferents that is in turn facilitated by C-fiber afferent input, it is not possible to determine if tibial nerve stimulation specifically reverses the C-fiber afferent induced facilitation or acts by suppressing the Aδ-fiber activated pathway. Nevertheless the present data show that irritated bladders are still sensitive to the inhibitory effects of tibial nerve stimulation.
However tibial nerve stimulation only partially reversed (about 40%, Fig. 2) the effect of AA irritation on bladder capacity. This could be due to the intense afferent activation in this AA irritation model. The magnitude of the bladder irritation induced by 0.25% AA was very large (average 80% reduction in bladder capacity) and therefore represents a very robust pathological condition that probably produces extreme changes in voiding function that exceed those in OAB patients being treated with tibial neuromodulation. Thus a more moderate level of irritation using a lower AA concentration such as 0.1% instead of 0.25% might yield a more appropriate and sensitive model for future exploration of the mechanisms underlying the effects of tibial nerve stimulation. The study of different pathological models is important because it could provide insight into how the therapeutic effect of tibial nerve stimulation might vary according to the severity of overactive bladder symptoms.
Tibial nerve stimulation did not decrease the amplitude of the bladder contractions as might be expected to occur during an inhibitory response but rather increased contraction amplitude (Figs.1 and 3). This is attributable to an indirect effect resulting from the reversal of the effect of AA on bladder capacity. After administering AA bladder capacity was markedly reduced and micturition contractions occurred at a very small volume that decreased the amplitude of the contractions (Figs.1 and 3). During tibial nerve stimulation the contractions occurred at a larger bladder volume and this in turn presumably enhanced the amplitude of the contractions.
The magnitude of the effect of tibial nerve stimulation on bladder capacity in the AA irritation model was comparable to the effect of pudendal nerve stimulation on bladder capacity in a model of neurogenic bladder dysfunction induced by chronic spinal cord injury in the cat 22. However the frequency dependence of the effects of tibial and pudendal nerve stimulation are different. Both 5 Hz and 30 Hz tibial nerve stimulation inhibits bladder activity, in contrast to the effect of pudendal nerve stimulation 22,23 that induces an inhibitory effect at a low (5 Hz) frequency but an excitatory effect at a high (20–30 Hz) frequency. This difference indicates that the underlying mechanisms for pudendal nerve and tibial nerve neuromodulation might be significantly different. The current study also indicated that the 20 Hz frequency currently used in clinical applications of tibial nerve stimulation might not be a critical parameter and that lower frequencies such as 5 Hz might be able to achieve the same therapeutic effect as the 20 Hz. Our recent study in cats 24 showed that electrical stimulation applied to the surface of the foot could also significantly suppress bladder overactivity. Similar to the results in the present study, foot stimulation at both low (5 Hz) and high (20 Hz) frequencies was effective. However, the foot stimulation is not specific to the tibial nerve since the peroneal nerve which innervates the foot 25 could also be activated.
The neural pathways involved in tibial nerve inhibition of the bladder have only been partially elucidated. Stimulation at intensities 2–4 times threshold for inducing toe movement were used in the present experiments to suppress bladder overactivity (Figs.1 and 2) and are used clinically to control overactive bladder symptoms. At this intensity the stimulation probably only activates large afferent nerve fibers rather than the small Aδ- and C-fiber afferents that induce painful sensations. Tibial afferent nerves project to the segments of the lumbosacral spinal cord that also receive inputs from bladder afferents travelling in the pelvic nerves 25. Because somatic afferents are known to synapse on populations of spinal interneurons that also receive visceral inputs from the pelvic organs 26,27, it is possible that synaptic interactions between the tibial and bladder sensory pathways occur at the spinal level and mediate the inhibitory effect of tibial nerve stimulation. However a previous study in cats 28 showed that the inhibitory effect on bladder activity elicited by electrical stimulation of the nerves from hindlimb muscles was lost after chronic spinal cord transection at the thoracic level. This indicates that supraspinal rather than spinal mechanisms are essential for somato-vesical inhibition. In this regard it is well known that extensive viscerosomatic convergence occurs at thalamic synapses 29, raising the possibility that circuitry in the thalamus is important for tibial nerve inhibition of bladder activity.
In summary our current study focused on the inhibitory effect of tibial nerve stimulation in a model of bladder overactivity induced by irritation of chemosensitive C-fiber afferent nerves in the bladder. Although many questions about tibial nerve neuromodulation still need to be answered, this animal study and a recent multicenter, double-blind, randomized, controlled clinical trial 1 indicate that tibial nerve neuromodulation could be a very promising clinical treatment for overactive bladder symptoms.
Acknowledgments
This study is supported by NIH under grants DK-068566, DK090006, and DK-077783, and by the Christopher and Dana Reeve Foundation.
References
- 1.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 2.MacDiarmid SA, Peters KM, Shobeiri SA, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183:234. doi: 10.1016/j.juro.2009.08.160. [DOI] [PubMed] [Google Scholar]
- 3.Peters KM, MacDiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: results from the overactive bladder innovative therapy trial. J Urol. 2009;182:1055. doi: 10.1016/j.juro.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 4.McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 5.Kabay SC, Yucel M, Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urol. 2008;71:641. doi: 10.1016/j.urology.2007.11.135. [DOI] [PubMed] [Google Scholar]
- 6.Vandoninck V, van Balken MR, Agro EF, et al. Percutaneous tibial nerve stimulation in the treatment of overactive bladder: urodynamic data. Neurourol Urodynam. 2003;22:227. doi: 10.1002/nau.10111. [DOI] [PubMed] [Google Scholar]
- 7.Klingler HC, Pycha A, Schmidbauer J, et al. Use of peripheral neuromodulation of the S3 region for treatment of detrusor overactivity: a urodynamic-based study. Urol. 2000;56:766. doi: 10.1016/s0090-4295(00)00727-5. [DOI] [PubMed] [Google Scholar]
- 8.Govier FE, Litwiller S, Nitti V, et al. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol. 2001;165:1193. [PubMed] [Google Scholar]
- 9.van Balken MR, Vandoninck V, Gisolf KWH, et al. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol. 2001;166:914. doi: 10.1097/00005392-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Vandoninck V, van Balken MR, Agro EF, et al. Posterior tibial nerve stimulation in the treatment of urge incontinence. Neurourol Urodynam. 2003;22:17. doi: 10.1002/nau.10036. [DOI] [PubMed] [Google Scholar]
- 11.Gennaro MD, Capitanucci ML, Mastracci P, et al. Percutaneouss tibial nerve neuromodulation is well tolerated in children and effective for treating refractory vesical dysfunction. J Urol. 2004;171:1911. doi: 10.1097/01.ju.0000119961.58222.86. [DOI] [PubMed] [Google Scholar]
- 12.van Balken MR. Percutaneous tibial nerve stimulation: the Urgent PC® device. Expert Rev Med Dev. 2007;4:693. doi: 10.1586/17434440.4.5.693. [DOI] [PubMed] [Google Scholar]
- 13.Pal FVD, van Balken MR, Heesakkers JPFA, et al. Percutaneous tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: is maintenance treatment necessary? BJU International. 2006;97:547. doi: 10.1111/j.1464-410X.2006.06055.x. [DOI] [PubMed] [Google Scholar]
- 14.Agro EF, Campagna A, Sciobica F, et al. Posterior tibial nerve stimulation: is the once-a-week protocol the best option? Minerva Urol Nefrol. 2005;57:119. [PubMed] [Google Scholar]
- 15.Pal FVD, van Balken MR, Heesakkers JPFA, et al. Implant-drive tibial nerve stimulation in the treatment of refractory overactive bladder syndrome: 12-month follow-up. Neuromodulation. 2006;9:163. doi: 10.1111/j.1525-1403.2006.00056.x. [DOI] [PubMed] [Google Scholar]
- 16.Andrews BJ, Reynard JM. Transcutaneous posterior tibial nerve stimulation for treatment of detrusor hyperreflexia in spinal cord injury. J Urol. 2003;170:926. doi: 10.1097/01.ju.0000080377.71804.fd. [DOI] [PubMed] [Google Scholar]
- 17.Amarenco G, Ismael SS, Even-Schneider A, et al. Urodynamic effect of acute transcutaneous posterior tibial nerve stimulation in overactive bladder. J Urol. 2003;169:2210. doi: 10.1097/01.ju.0000067446.17576.bd. [DOI] [PubMed] [Google Scholar]
- 18.McGuire E, Morrissey S, Zhang S, et al. Control of reflex detrusor activity in normal and spinal injured non-human primates. J Urol. 1983;129:197. doi: 10.1016/s0022-5347(17)51982-5. [DOI] [PubMed] [Google Scholar]
- 19.Sato A, Sato Y, Schmidt RF. Reflex bladder activity induced by electrical stimulation of hind limb somatic afferents in the cat. J Auto Nerv Syst. 1980;1:229. doi: 10.1016/0165-1838(80)90019-3. [DOI] [PubMed] [Google Scholar]
- 20.Fowler CJ, Griffths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC, Araki I, Vizzard MA, et al. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioural Brain Res. 1998;92:127. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 22.Tai C, Smerin SE, de Groat WC, et al. Pudendal-to-bladder reflex in chronic spinal cord injured cat. Exp Neurol. 2006;197:225. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Boggs JW, Wenzel BJ, Gustafson KJ, et al. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577:115. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai C, Shen B, Chen M, et al. Suppression of bladder overactivity by activation of somatic afferent nerves in the foot. BJU Int. 2010 doi: 10.1111/j.1464-410X.2010.09358.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reighard J, Jennings HS. Anatomy of the cat. New York: Holt, Rinehart and Winston; 1935. [Google Scholar]
- 26.de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurons concerned with micturition in the cat. J Physiol (Lond) 1969;200:87. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. J Physiol (Lond) 2001;533:57. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McPherson A. The effects of somatic stimuli on the bladder in the cat. J Physiol. 1966;185:185. doi: 10.1113/jphysiol.1966.sp007980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci. 1994;14:6796. doi: 10.1523/JNEUROSCI.14-11-06796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]