Abstract
Background
Infection with human herpesvirus 8 (HHV-8) is common among men who have sex with men (MSM) in North America and Europe, and is also found to be endemic in some regions of South America. Little is known about HHV-8 prevalence and its correlates among MSM in the Andean region.
Methods
We assessed HHV-8 seroprevalence among 497 MSM recruited for the 2002 Peruvian HIV sentinel surveillance program using a combined HHV-8 enzyme immunoassay and immunofluorescence assay algoritm. Logistic regression was used to estimate Odds Ratios (OR) and their 95% confidence intervals (CI) to determine the association between selected covariates and HHV-8 seropositivity.
Results
483 (97%) of 497 men had stored sera and demographic data available for analysis. 131 (66.5%, 95% CI 63.1%-69.9%) of 197 HIV-infected and 80 (26.7%, 95% CI 24.4%-29.0%) of 300 HIV-uninfected MSM had serologic evidence of HHV-8 infection. Factors independently associated with HHV-8 infection were education <12 years (OR 1.7, 95% CI 1.1-2.7), anal receptive sex with the last partner (OR 2.0, 95% CI 1.2-3.3), self-reported STI symptoms during the last year (OR: 1.9, 95% CI 1.2-3.0), and co-infection with HIV (OR 4.2, 95% CI 2.8-6.4) and Chronic Hepatitis B (OR 4.9, 95% CI 1.5-15.8). MSM with long-standing HIV infection were more likely to have serologic evidence of HHV-8 infection when compared to men with recently-acquired HIV (OR: 3.8, 95% CI 1.7-9.1).
Conclusions
HHV-8 infection is common among both HIV-infected and negative MSM in Lima, Peru. HHV-8 seropositivity is correlated with anal receptive sex, self-reported STI symptoms, and HIV infection among these MSM, and thus appears to be sexually transmitted. HHV-8 infection appears to be acquired after HIV infection, suggesting that future studies should evaluate the mode of HHV-8 transmission and prevention strategies among HIV-infected MSM.
Keywords: Human Herpesvirus 8, Homosexual Men, Human Immunodeficiency Virus, Peru, Sexually Transmitted Infections
Introduction
Human Herpesvirus 8 (HHV-8), the etiologic agent of Kaposi Sarcoma (KS), has a wide distribution and variable prevalence across populations and geographical regions. Infection is most frequent among the general population in Africa, with intermediate prevalence among some ethnic groups in the Mediterranean region. In North America and Europe, HHV-8 seroprevalence is higher among men who have sex with men (MSM), especially those with HIV-1 infection, when compared with the general population, such as blood donors. Additionally, a high prevalence of infection has been observed among indigenous populations living in remote tribes of Amazonia(1).
In North America and Europe, HHV-8 appears to be sexually transmitted, particularly among MSM(1-3). Prevalent and incident infections have been found to be associated with HIV-1 infection, age, number of sexual partners, oroanal sex, orogenital sex, hepatitis B infection, and syphilis(2, 3). Non-sexual routes of transmission have also been postulated in regions where classic and endemic KS occur(1).
High prevalence of HIV-1 and sexually transmitted infections (STI) and high-risk sexual behaviors have been reported among MSM of major cities in Peru(4, 5). More comprehensive understanding of the magnitude and extension of the epidemiology of HHV-8 in this setting would identify appropriate populations for further intervention strategies to reduce HHV-8 transmission. To better understand the epidemiology of HHV-8 among MSM, we assessed the prevalence of and associated risk factors for HHV-8 infection among MSM participating in a HIV and STI survey in Lima, Peru.
Methods
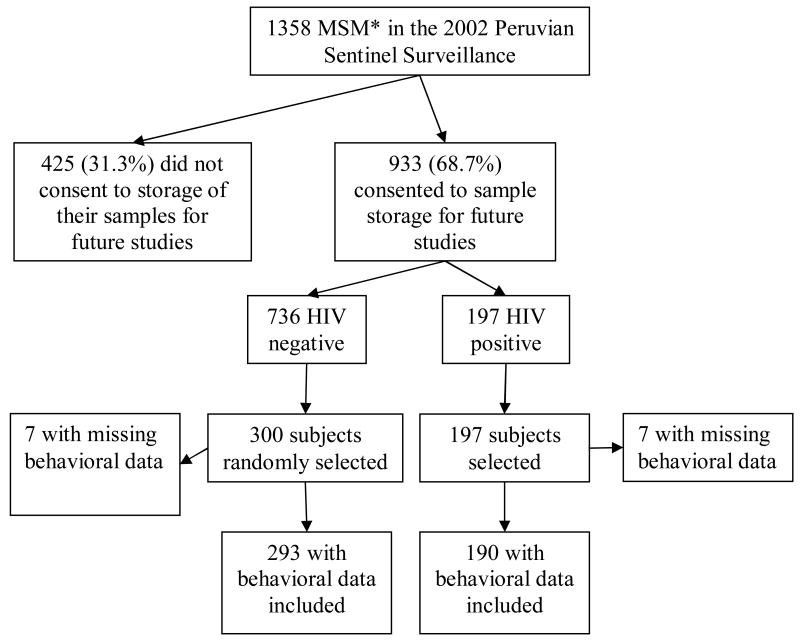
Among 1358 men participating in the HIV and STI Sentinel Surveillance for MSM between 2002-2003 in Lima, Peru, which it methodology has been described elsewhere (5, 6), 933 (68.7%) consented to blood sample storage for further STI testing Of these 933, all 197 HIV-infected, and 300 randomly selected HIV-uninfected, participants were tested for HHV-8 (Figure 1). The random sample selection of HIV-negative participants was weighted by the total number screened at each of the sentinel sites.
Figure 1. Selection of study population.
*Men who have Sex with Men (MSM)
Demographic and behavioral information, including sexual risk behaviors, and partner-specific information about sexual practices with the last 3 sexual partners during the last 3 months was obtained by a computer-assisted self-administrated interview. Detection of serum antibodies to HIV-1 (EIA, Vironostika, Organon Tecnica; and Western blot, Biorad Laboratories); Treponema pallidum (RPR, Organon Tecnica; and MHA-TP, Organon Tecnica) and Herpes Simplex Virus 2 (HerpeSelect-2 EIA, Focus Technology) were conducted in all survey participants. Among men consenting for further storage, sera were additionally tested for Hepatitis B surface antigen ([HBsAg] EIA, Hepanostika HBsAg Ultra, bioMerieux, Inc). HHV-8 infection was determined by a whole virus lysate EIA combined with a confirmatory immunofluoresence assay as described elsewhere (88% sensitivity and 97% specificity)(7). Syphilis seroreactivity was defined by a RPR titer ≥1:1 and a positive MHA-TP. A presumptive diagnosis of early syphilis was made for RPR titer >1:8 and a positive MHA-TP. HSV-2 seropositivity was defined using a cut-off of 3.4 to improve specificity(5). Presumptive recent HIV-1 infection was categorized among HIV-infected participants who had an optical density signal-to-cutoff ratio <0.75 in a sensitive/less sensitive EIA testing (Vironostika, Organon Tecnica)(8).
This study was approved by the Asociacion Civil Impacta Salud y Educacion and University of Washington Institutional Review Boards, and all participants provided written informed consent.
Statistical analysis was performed with Intercooled Stata 8.0 (Stata Corporation). HHV-8 prevalence and 95% confidence intervals (CI) were computed using the normal approximation to a binomial distribution. In order to obtain the most conservative CI's, the larger standard error for either the HIV-infected or uninfected group was used to calculate the CI's for HHV-8 prevalences at each group. Bivariate comparisons of selected variables were performed using non-parametric tests and Odds Ratios (OR) were computed. Variables (p<0.10) associated with HHV-8 infection in the bivariate analysis were entered in a forward stepwise logistic regression analysis to estimate independently associated variables.
Results
Comparison of HIV infected and non-infected participants revealed that HIV-infected MSM were older and a higher proportion identified themselves as homosexual. They also reported both receptive and insertive anal sex (‘versatile’) and an STI symptom during the last year. They described themselves as transvestites, and had higher HSV-2, syphilis and HBsAg prevalence than HIV-non-infected men (Table 1).
Table 1. Behavioral and demographic characteristics of the study participants.
| Variables* | Total N=497 |
HIV + N=197 |
HIV - N=300 |
|---|---|---|---|
| Median age (range)££ | 26 (18-59) | 28 (18-55) | 24 (18-59) |
| Education level | |||
| Less than 12 years | 306 (63.4) | 125 (65.8) | 181 (61.8) |
| 12 or more years | 177 (36.6) | 65 (34.2) | 112 (38.2) |
| Sexual Identity | |||
| Bisexual | 173 (35.9) | 41 (21.6) | 132 (45.2) |
| Homosexual££ | 309 (64.1) | 149 (78.4) | 166 (54.8) |
| Sexual Role during anal sex | |||
| Insertive (top) | 165 (34.2) | 40 (21.1) | 125 (42.7) |
| Receptive (bottom)££ | 179 (37.1) | 83 (43.7) | 96 (32.8) |
| Versatile (insertive and receptive)££ | 139 (28.8) | 67 (35.2) | 72 (24.6) |
| Transvestite£ | 118 (24.5) | 62 (32.6) | 56 (19.2) |
| Any self-reported STI symptoms during the last year£ | 138 (28.7) | 71 (37.6) | 67 (23.0) |
| >5 sexual partners in the last 3 months. | |||
| Male | 82 (16.5) | 36 (18.3) | 46 (15.3) |
| Female | 8 (1.6) | 1 (0.5) | 7 (2.3) |
| Total | 97 (19.5) | 37 (18.8) | 60 (20.0) |
| Prevalence of STIs | |||
| HSV-2££ | 270 (54.3) | 159 (80.7) | 111 (37.0) |
| Syphilis**££ | 68 (13.7) | 43 (21.8) | 25 (8.3) |
| Early Syphilis*** | 16 (3.2) | 9 (4.6) | 7 (3.2) |
| Chronic Hepatitis B$££ | 25 (5.0) | 18 (9.1) | 7 (2.3) |
Demographic and Behavioral information not available for 14 persons
MHA-TP+ & RPR+
MHA-TP+ & RPR> 1:8 dils
HBsAg +
Chi2 test for independence, p-value=0.01
Chi2 test for independence, p-value<0.001
Abbreviations used in table: Human Immunodeficiency virus (HIV), Sexually transmitted infection (STI), Herpes simplex virus type 2 (HSV-2)
Serologic evidence of HHV-8 infection was found among 131 (66.5%, 95% CI 63.1%-69.9%) of 197 HIV-infected MSM and 80 (26.7%, 95% CI 24.4%-29.0%) of 300 HIV-uninfected MSM. In bivariate analysis, men with HHV-8 infection were slightly older and reported a lower level of education than HHV-8 negative men. Behavioral risk factors associated with HHV-8 infection were self-identification as a homosexual or transvestite, reporting their main sexual role with men as receptive or versatile and self-reported STI symptoms during the past year (Table 2). HHV-8 infection was associated in the bivariate analyses with HSV-2 seropositivity, syphilis reactivity, HBV chronic infection and HIV infection. Among HIV-infected men (n=197), those men chronically HIV-infected were more likely than those recently HIV-infected to be HHV-8 seropositive (70.8% vs. 38.5%, p<0.001).
Table 2. Bivariate and multivariate predictors of HHV-8 seropositivity.
| HHV-8 Serostatus | ||||
|---|---|---|---|---|
| Variable | Positive N=211 N (%) |
Negative N=286 N (%) |
Bivariate OR (95% CI) |
Multivaiate AOR (95%CI) |
| Median age (range,y) | 27(18-59) | 25 (18-58) | 1.0 (1.0-1.1)§ | 1.0 (1.0-1.0) |
| Education Level | ||||
| Less than 12 years | 144 (70.2) | 162 (58.3) | 1.7 (1.2-2.5)§ | 1.7 (1.1-2.7)§ |
| 12 or more years | 61 (29.8) | 116 (41.7) | 1 | 1 |
| Sexual Identity | ||||
| Bisexual | 52 (25.5) | 121 (43.5) | 1 | - |
| Homosexual | 152 (74.5) | 157 (56.5) | 2.3 (1.5-3.3)§ | - |
| Sexual Role | ||||
| Insertive (top) | 49 (23.9) | 116 (40.6) | 1 | 1 |
| Receptive (bottom) | 93 (45.4) | 86 (30.1) | 2.6 (1.6-4.0)§ | 2.0 (1.2-3.3)§ |
| Versatile (insertive and receptive) | 63 (30.7) | 76 (26.6) | 2.0 (1.2-3.2)§ | 1.4 (0.8-2.4) |
| Transvestite | 62 (29.4) | 56 (19.6) | 1.7 (1.1-2.6)§ | |
| Self-reported STI symptoms during the last year | 76 (37.4) | 62 (22.3) | 2.1 (1.4-3.1)§ | 1.9 (1.2-3.0)§ |
| >5 sexual partners in the last 3 months. | ||||
| Male | 39 (19.0) | 43 (15.5) | 1.3 (0.8-2.1) | - |
| Female | 2 (1.0) | 6 (2.2) | 0.5 (0.1-2.2) | - |
| Total | 43 (21.0) | 54 (19.4) | 1.1 (0.7-1.7) | - |
| Last partner in the last 3 months | ||||
| Receptive oral sex without condom | 87 (56.9) | 102 (52.6) | 1.2 (0.8-1.8) | - |
| Insertive oral sex without condom | 36 (23.5) | 55 (28.3) | 0.8 (0.5-1.3) | - |
| Receptive anal sex without condom | 51 (33.3) | 60 (30.9) | 1.1 (0.7-1.8) | - |
| Insertive anal sex without condom | 20 (13.1) | 43 (22.2) | 0.5 (0.3-0.9)§ | - |
| Prevalence of STIs | ||||
| HSV-2* | 150 (71.0) | 120(42.0) | 3.4 (2.3-5.0)§ | - |
| Syphilis** | 39 (18.5) | 29 (10.1) | 2.0 (1.2-3.4)§ | - |
| Early Syphilis*** | 10 (4.7) | 6 (2.1) | 2.3 (0.8-6.5) | |
| Chronic Hepatitis B$ | 21 (10.0) | 4 (1.4) | 7.8 (2.6-23.1)§ | 4.9 (1.5-15.8)§ |
| HIV infection | ||||
| HIV positive | 131 (62.1) | 66 (23.1) | 5.5 (3.7-8.1)§ | 4.2 (2.8-6.4)§ |
| Chronic HIV Infection$$ | 121/171 (70.8) | 50/171 (29.2) | 3.8 (1.7-9.1)§ | - |
| Early HIV infection$$ | 10/26 (38.5) | 16/26 (61.5) | 1 | - |
Focus Select-2 EIAIndex Value ≥3.5
MHA-TP+ & RPR+
MHA-TP+ & RPR> 1:8 dils
HBsAg +
By Detuned ELISA
Statistically significant difference (p-value<0.05)
Abbreviations used in table: Human Immunodeficiency virus (HIV), Sexually transmitted infection (STI), Herpes simplex virus type 2 (HSV-2)
In the multivariate analysis, lower education level (OR 1.7, 95% CI [1.1-2.7]), being primarily receptive in sex with men (OR 2.0, 95% CI [1.2-3.3]), reporting STI symptoms during the last year (OR 1.9, 95% CI [1.2-3.0]), having chronic hepatitis B infection (OR 4.9, 95% CI [1.5-15.8]) and HIV infection (OR 4.2, 95% CI [2.8-6.4)]were independently associated with HHV-8 seropositivity (Table 2).
Discussion
In this study of HHV-8 seroprevalence from the 2002 Peruvian Sentinel Surveillance, we found a similar proportion of Peruvian MSM had detectable antibodies to HHV-8 when compared with MSM in the Americas and Europe(9, 10). Again consistent with what has been reported in MSM populations elsewhere around the world (including Latin America(11, 12)), we found that HIV-infected MSM had substantially higher rates of HHV-8 infection when compared with those who are HIV-negative.
The epidemiology of HHV-8 infection, with specific geographic and demographic pockets of endemicity, has long been puzzling(1). In Latin America, HHV-8 prevalence in the general population of Latin America has been described as low(12, 13), but rural populations with a high prevalence of antibodies to HHV-8 have been described in Brazil(14) and Peru(15). The moderate seroprevalence of HHV-8 among HIV-negative MSM in our study suggests that the previous reports of high HHV-8 seroprevalence and KS incidence(16) among the persons in Peru suggests that there may be substantial heterogeneity in the prevalence of HHV-8 infection among residents of Peru, and bears further study.
In this population of MSM in Peru, HHV-8 seropositivity is associated with high risk sexual behavior similar to that associated with HIV or other STIs, similar to risk factors found among MSM in Europe or the US. In the Amsterdam cohort, the number of sexual partners in the prior 5 years was associated with prevalent HHV-8 infection, and in a nested case-control study of seroconverters in that cohort, incident HHV-8 infection correlated with insertive and receptive orogenital sex as well as HIV infection and age(10). One study found that HIV serodiscordant partners and oroanal sex with an HIV-infected partner was associated with HHV-8 positivity among MSM(2). Several other studies of correlates of HHV-8 infection among MSM showed that contact with body fluids such as saliva or semen were associated with HHV-8 seropositivity(3, 9), which is biologically plausible, given that the oral cavity is the most frequent anatomic site of HHV-8 shedding, with higher shedding rates than in semen, and is the only site where infectious virions have been identified(1, 17).
We found that HHV-8 infection is strongly associated with HIV infection. A possible explanation is that HIV infection increases HHV-8 shedding, frequency and titer(18), thus increasing the chances of HHV-8 transmission from an HIV-infected partner. Some reports indicate that HIV-infected persons tend to have sex with other HIV-infected persons (i.e., ‘serosorting’)(19), which would increase the chances of HHV-8 infection among HIV-infected MSM, if serosorting were occurring in the MSM studied in Peru, as reported in one study(20). Interestingly, our results based on the ‘detuned’ HIV-1 ELISA(8), which has reasonable specificity for identifying recently infected persons in clade B settings, suggest that HHV-8 may be acquired after HIV infection among Peruvian MSM, the lowest prevalence among the HIV-uninfected and the highest among the HIV-chronic infected, being those HIV-recently infected in between, which could be relevant to the natural history of KS since prior reports indicate a higher risk for developing KS if HHV-8 is acquired after HIV infection(1). Alternatively, the detection of HHV-8 antibody may be higher among HIV-infected persons. Seronegative HHV-8 infection has been reported(17), and the hypergammaglobulinemia observed in HIV infection may result in higher antibody titers that allow for laboratory detection of antibody that prior to HIV infection was too low for detection by current antibody assays. This may also reflect the biology of HHV-8 infection, as viral replication may occur more briskly in HIV-infected persons(21), and thus HHV-8 specific immune response may be more marked. Careful cohort studies evaluating viral activity and immune response over time may help elucidate further the host response to HHV-8.
This study had a number of limitations. First, this sample was chosen from a convenience-based sample of MSM which used a “snow-ball technique” and outreach workers to recruit high risk MSM from different venues and representing diverse subgroups of MSM, which may lead to recruitment of participants with similar characteristics as the outreach workers, and possibly a higher proportion of transvestites than is representative of MSM in Lima(4). Second, the behavioral questionnaire used in this surveillance activity was not designed to comprehensively capture information about exposure to saliva, so information about this potential mode of HHV8 acquisition could not be ascertained. Third, the sexual behavior questions focused on the prior 3 months and the timing of HHV-8 could not be determined in this cross-sectional study, limiting analyses of behavioral correlates of HHV-8 infection. Lastly, HHV-8 infection status may be misclassified, given the sensitivity and specificity of this serologic algorithm(7).
Since this is one of the first epidemiological studies of HHV-8 infection among MSM conducted in Peru, it highlights opportunities and challenges in this field of study. First, future studies of HHV-8 in Peru should define the seroprevalence in other populations, including HIV-infected women, many of whom might have acquired HIV from bisexual partners, characteristic reported by a third of MSM at Lima (4). More importantly, little is directly known about factors linked to transmission and acquisition of HHV-8 in Peru. Only well-designed cohort studies will be able to elucidate such factors and describe their association with HHV-8 associated malignancies. Most of the currently available information on KS in Peru is based on case reports or cases series describing location, number of lesions and stage of HIV disease(22). Given the high seroprevalence of HHV-8 among MSM in Peru, additional information about the natural history of HHV-8 and KS epidemiology will be important in Peru and other Latin American countries in order to identify effective prevention strategies and to provide treatment for KS.
Acknowledgments
The authors would like to thank Jorge Vergara for local study leadership; to Mrs. Rosa Galvan for laboratory study coordination; to all recruiters and peer educators; and specially, to all study participants. Special thanks to US NMRCD support in the implementation of the Second Generation Sentinel Surveillance.
This study was supported by funds of The Fogarty International Center thru the UW International AIDS Research and Training Program, US National Institute of Health Research Grant #s D43 TW000007 and work unit number 62787A S17 H B0002,
Footnotes
Disclaimers:
The findings of this study were presented in part at the IX International Conference in Malignancies on AIDS and Other Acquired Immunodeficiencies, Bethesda, Maryland, September 26th – 27th, 2005. Abstract 74.
Study protocols were approved by the Asociacion Civil Impacta Salud y Educacion, US Naval Medical Research Center and University of Washington Institutional Review Boards in compliance with all applicable federal regulations governing the protection of human subjects.
The opinions and assertions made by the authors do not reflect the official position or opinion of the government of the Republic of Peru, the Ministry of Health of Peru, the US Department of the Navy or Army, or the Fogarty International Center, nor Department of the Navy, Department of Defense, nor the U.S. Government.
Dr. Silvia Montano is an employee of the U.S. Government. This work was prepared as part of her official duties. Title 17 U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Conflicts: There are no conflicts to report.
PERUVIAN HIV SENTINEL SURVEILLANCE WORKING GROUP
Members of the Peruvian HIV Sentinel Surveillance Working Group include: Luis Suarez, Monica Pun (General Directorate of Epidemiology, Ministry of Health, Lima, Peru); César Cabezas, Patricia Caballero (National Institute of Health, Ministry of Health, Lima, Peru); Jorge Sanchez, Javier Lama, Juan Guanira, Aldo Lucchetti, Pedro Goicochea, Jorge Vergara (Investigaciones Medicas en Salud, Lima, Peru); Martin Casapia, Juan Carlos Hinojosa (Asociacion Civil Selva Amazonica, Iquitos, Peru); Victoria Zamalloa (Instituto Sur Peruano de Infectologia, Arequipa, Peru); Abner Ortiz (Centro Medico Cayetano Heredia, Pucallpa, Peru); Nora Ojeda (Asociacion de Servicios Generales de Salud y Educacion, Sullana, Peru); Anabeli Tataje (Policlinico Daniel Alcides Carrion, Ica, Peru); Pablo Campos, Patricia Garcia, Cesar Carcamo (Universidad Peruana Cayetano Heredia, Lima, Peru); Connie L. Celum, King K. Holmes, Joseph Zunt, William L. H. Whittington, James P. Hughes (University of Washington, Seattle, WA); Jose L. Sánchez (past member), Silvia Montano, Victor A. Laguna-Torres and Tadeusz Kochel (US Naval Medical Research Center Detachment, Lima, Peru).
References
- 1.Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and do not know. AIDS. 2003;17:1717–1730. doi: 10.1097/01.aids.0000076337.42412.86. [DOI] [PubMed] [Google Scholar]
- 2.Casper C, Carrell D, Miller KG, et al. HIV serodiscordant sex partners and the prevalence of human herpesvirus 8 infection among HIV negative men who have sex with men: baseline data from the EXPLORE Study. Sex Transm Infect. 2006;82:229–235. doi: 10.1136/sti.2005.016568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martro E, Esteve A, Schulz TF, et al. Risk factors for human Herpesvirus 8 infection and AIDS-associated Kaposi's sarcoma among men who have sex with men in a European multicentre study. Int J Cancer. 2007;120:1129–1135. doi: 10.1002/ijc.22281. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez J, Lama JR, Kusunoki L, et al. HIV-1, sexually transmitted infections, and sexual behavior trends among men who have sex with men in Lima, Peru. J Acquir Immune Defic Syndr. 2007;44:578–585. doi: 10.1097/QAI.0b013e318033ff82. [DOI] [PubMed] [Google Scholar]
- 5.Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006;194:1459–1466. doi: 10.1086/508548. [DOI] [PubMed] [Google Scholar]
- 6.Lama JR, Sanchez J, Suarez L, et al. Linking HIV and antiretroviral drug resistance surveillance in Peru: a model for a third-generation HIV sentinel surveillance. J Acquir Immune Defic Syndr. 2006;42:501–505. doi: 10.1097/01.qai.0000221677.29693.dd. [DOI] [PubMed] [Google Scholar]
- 7.Casper C, Krantz E, Taylor H, et al. Assessment of a combined testing strategy for detection of antibodies to human herpesvirus 8 (HHV-8) in persons with Kaposi's sarcoma, persons with asymptomatic HHV-8 infection, and persons at low risk for HHV-8 infection. J Clin Microbiol. 2002;40:3822–3825. doi: 10.1128/JCM.40.10.3822-3825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 9.Grulich AE, Cunningham P, Munier ML, et al. Sexual behaviour and human herpesvirus 8 infection in homosexual men in Australia. Sex Health. 2005;2:13–18. doi: 10.1071/sh04029. [DOI] [PubMed] [Google Scholar]
- 10.Dukers NH, Renwick N, Prins M, et al. Risk factors for human herpesvirus 8 seropositivity and seroconversion in a cohort of homosexual men. Am J Epidemiol. 2000;151:213–224. doi: 10.1093/oxfordjournals.aje.a010195. [DOI] [PubMed] [Google Scholar]
- 11.Perez C, Tous M, Benetucci J, et al. Correlations between synthetic peptide-based enzyme immunoassays and immunofluorescence assay for detection of human herpesvirus 8 antibodies in different Argentine populations. J Med Virol. 2006;78:806–813. doi: 10.1002/jmv.20627. [DOI] [PubMed] [Google Scholar]
- 12.Souza VA, Sumita LM, Freire W, et al. Prevalence of antibodies to human herpesvirus-8 in populations with and without risk for infection in Sao Paulo State. Braz J Med Biol Res. 2004;37:123–127. doi: 10.1590/s0100-879x2004000100017. [DOI] [PubMed] [Google Scholar]
- 13.Perez C, Tous M, Gallego S, et al. Seroprevalence of human herpesvirus-8 in blood donors from different geographical regions of Argentina, Brazil, and Chile. J Med Virol. 2004;72:661–667. doi: 10.1002/jmv.20029. [DOI] [PubMed] [Google Scholar]
- 14.Cunha AM, Caterino-de-Araujo A, Costa SC, et al. Increasing seroprevalence of human herpesvirus 8 (HHV-8) with age confirms HHV-8 endemicity in Amazon Amerindians from Brazil. J Gen Virol. 2005;86:2433–2437. doi: 10.1099/vir.0.81087-0. [DOI] [PubMed] [Google Scholar]
- 15.Mohanna S, Portillo JA, Carriquiry G, et al. Human herpesvirus-8 in Peruvian blood donors: a population with hyperendemic disease? Clin Infect Dis. 2007;44:558–561. doi: 10.1086/511044. [DOI] [PubMed] [Google Scholar]
- 16.Mohanna S, Ferrufino JC, Sanchez J, et al. Epidemiological and clinical characteristics of classic Kaposi's sarcoma in Peru. J Am Acad Dermatol. 2005;53:435–441. doi: 10.1016/j.jaad.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 17.Casper C, Redman M, Huang ML, et al. HIV infection and human herpesvirus-8 oral shedding among men who have sex with men. J Acquir Immune Defic Syndr. 2004;35:233–238. doi: 10.1097/00126334-200403010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med. 2000;343:1369–1377. doi: 10.1056/NEJM200011093431904. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JT, Schrimshaw EW, Wolitski RJ, et al. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. Aids. 2005;19:S13–S25. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 20.Lama JR, Goicochea P, Laguna A, et al. Sexual behavior and sexually transmitted infections among HIV-1-infected MSM in Peru. XVI International AIDS Conference; Toronto, Canada. 2006. [Google Scholar]
- 21.Zeng Y, Zhang X, Huang Z, et al. Intracellular Tat of human immunodeficiency virus type 1 activates lytic cycle replication of Kaposi's sarcoma-associated herpesvirus: role of JAK/STAT signaling. J Virol. 2007;81:2401–2417. doi: 10.1128/JVI.02024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velásquez J. Human Medicine. Lima: Universidad Peruana Cayetano Heredia; 2000. Dermatological manifestation of HIV/AIDS in patients older than 13 years seen at the Cayetano Heredia National Hospital. 1992-1999; p. 52. [Google Scholar]