Abstract
We describe a 15-year-old boy who presented with a stroke. Brain MRI imaging showed thalamic and multiple cerebral infarcts. An echocardiogram revealed multiple atrial masses, which were resected. Histological examination confirmed multiple atrial myxomas. Further clinical examination of the patient revealed subtle buccal and peri-oral lentigenes. The diagnosis of Carney complex was made clinically. The patient was subsequently diagnosed with testicular seminomas and a cutaneous angiomyxoma. Genetic investigation revealed a pathological mutation in the PRKAR1A gene. We review the reported manifestations and presentations of Carney complex, along with current diagnostic guidelines. We emphasise the importance of recognising the cutaneous manifestations of this rare autosomal dominantly inherited neoplasia syndrome.
Introduction
Carney complex (CNC, OMIM 160980) is a rare neoplasia syndrome characterised by atrial and cutaneous myxomas, endocrine abnormalities and testicular tumours [2]. This condition has previously been described as ‘Carney myxoma-endocrine complex’, ‘nevi, atrial myxomas and ephelides’ and ‘lentigenes, atrial myxomas and blue nevi’ syndromes [14].
The inheritance pattern of CNC is autosomal dominant. Linkage to two loci at chromosomes 2p16 (CNC2, OMIM 605244) and 17q22–24 (CNC1, OMIM 160980) was reported [7]. One hundred fifty patients with a clinical diagnosis of CNC have been found to carry various inactivating mutations in the protein kinase A type I-α regulatory subunit gene (PRKAR1A, OMIM 18830 at CNC1). There were no significant differences observed between patients with clinical features of CNC who carry a PRKAR1A mutation and those who do not [14]. There are two mutation hotspots observed, but no clear genotype–phenotype correlation. Haploinsufficiency for PRKAR1A with nonsense-mediated decay is reported but the precise mechanism of tumourigenesis remains controversial [5, 17]. A single family with distal arthrogryposis and some clinical features of CNC (CNC-TPC) was reported with mutations in the MYH8 gene (17p13.1, OMIM 160741) [16].
A variety of cutaneous and visceral manifestations of the condition have now been reported, along with consensus diagnostic criteria [12, 14]. CNC has similarities to the McCune–Albright syndrome (polyostotic fibrous dysplasia, OMIM 174800) [14]. Mucosal lentiginosis is a feature of other neoplasia syndromes including Peutz–Jeghers and multiple endocrine neoplasia type 2B [11, 13]. We report a 15-year-old male patient with no family history, who presented unusually with an acute stroke and subtle cutaneous lentigines.
Case report
A previously healthy 15-year-old boy presented with a collapse whilst playing football. He described a headache for the previous 24 h. Clinical examination revealed a right hemiplegia (mild weakness and upper motor neurone signs) and a non-localising unsteady gait. Examination of the cardiac system revealed no abnormalities.
MRI imaging of the brain showed acute left posterior thalami (Figure 1a, arrow), with involvement of the posterior limb of the internal capsule and multiple established infarcts (Figure 1b, arrows). Trans-thoracic echocardiography revealed a 4-cm mass arising from the interatrial septum and prolapsing through the mitral valve (Figure 2a). Through a median sternotomy and utilising cardiopulmonary bypass, four myxomata were removed from both right and left atria. Histology revealed characteristic stellate cells. The patient made an uneventful recovery from surgery with almost complete resolution of neurological symptoms.
Figure 1.
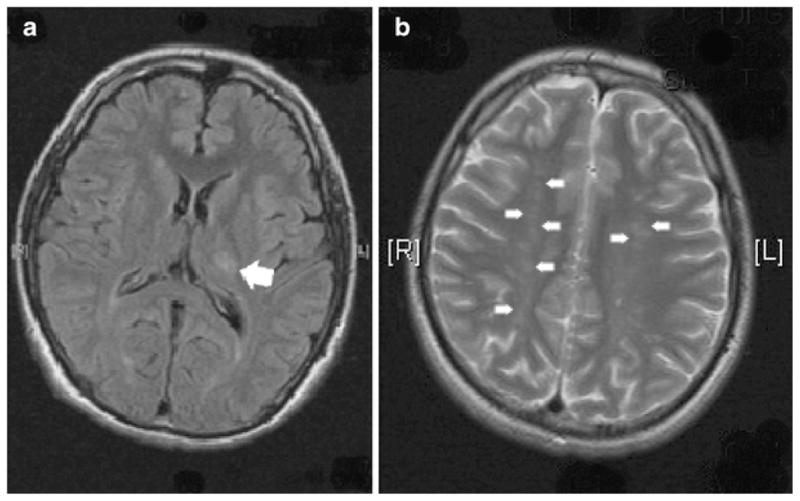
MRI imaging of the brain showed acute left posterior thalamic infarct (a, arrow) and multiple established watershed infarcts (b, arrows)
Figure 2.
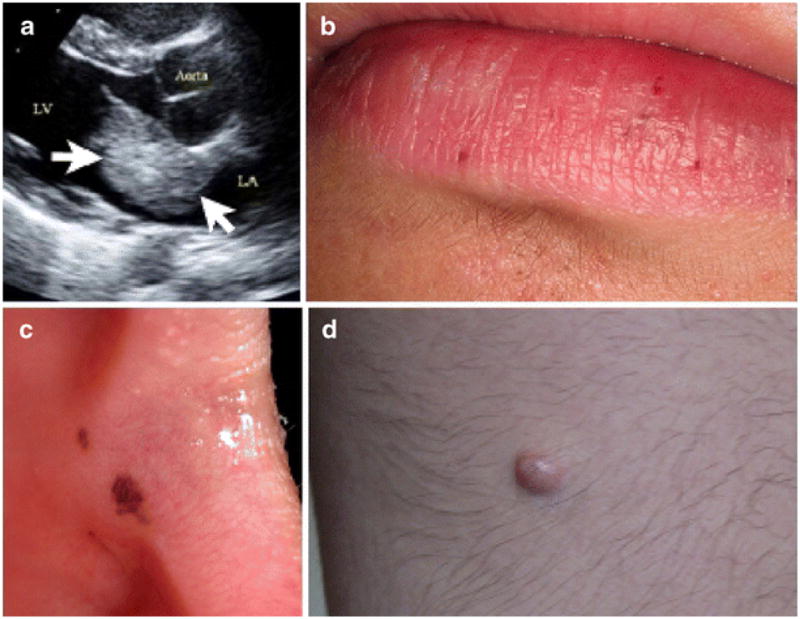
Trans-thoracic echocardiogram showing a large atrial myxoma (a, arrows). Cutaneous findings: small peri-oral (b) and buccal (c) lentigines and leg angiomyxoma (d)
The intra-operative findings of multiple myxomata prompted a detailed clinical examination which revealed extremely small lip (Fig. 2b) and buccal lentigenes (Fig. 2c). The association of multiple myxomas with the cutaneous findings led to the clinical diagnosis of CNC. Subsequent endocrine investigation of our patient showed normal ACTH, cortisol, TSH and GH. He had a polypoid mass on his posterior right upper leg (Fig. 2d), which was resected and shown to be an angiomyxoma.
A testicular ultrasound revealed a calcified right testicle, which was resected, and biopsy revealed a calcified Sertoli tumour. The patient’s DNA was sequenced and found to carry an R288X mutation in the PRKAR1A gene encoding protein kinase A regulatory subunit on chromosome 17q.
Discussion
CNC is a rare disease with a total of 500 cases reported worldwide by 2007. Patients from many ethnicities have been reported, and 70% of cases were from 67 known families. Clinical criteria for the diagnosis reflect the diversity of clinical presentations and include typical cutaneous pigmentary abnormalities, myxomata, endocrine abnormalities and a definite diagnosis in a first-degree relative [12, 14].
Patients with CNC present at a younger age and with multiple left and right side cardiac lesions [12]. Cardiac myxoma may present with symptoms of left heart outflow obstruction (heart failure, dyspnoea, syncope and sudden death), constitutional symptoms (autoimmune/vasculitic symptoms, weight loss, fatigue or fever) or embolic disease. Clinical findings on auscultation are only found in a minority of cases whilst transthoracic echocardiography has excellent sensitivity [8]. Cardiac myxoma in CNC patients usually presents in the second or third decade. Screening of at-risk family members has highlighted the variability in age-related penetrance of cardiac myxoma, from age 4 to 61 years [9, 18]. Atrial myxoma is a benign neoplasm of multipotential mesenchymal origin with two gross anatomical subtypes: solid and gelatinous [15]. Cardiac myxoma may recur post-resection and has been reported in one individual with CNC in conjunction with multiple fusiform cerebral aneurysms [10]. Non-syndromic bilateral atrial myxoma is rare [6].
The endocrine manifestations of Carney complex include adrenocorticotropic hormone-independent Cushing’s syndrome secondary to a characteristic primary pigmented nodular adrenocortical disease [2]. Patients with Carney complex infrequently present with florid clinical features of acromegaly secondary to growth hormone secreting pituitary macroadenoma. A larger proportion may have a milder presentation with subtle baseline and dynamic abnormalities in their GH/IGF1 levels [4].
Carney complex predisposes to the development of various tumours. Large cell calcifying Sertoli cell tumours may be bilateral and multifocal and are a complex management issue given a potential risk of malignancy and reduced fertility observed in men with CNC [1, 17]. Psammatomatous melanotic schwanomma in the gastrointestinal system and paraspinal sympathetic chain may be metastatic and have been reported in 33 patients with CNC. Papillary and follicular thyroid cancers are found in 10% of CNC patients. Osteochondromyxoma has also been reported [12]. A single case of a woman with a known diagnosis of CNC who presented with hepatocellular adenoma at age 19 was recently reported [3].
Cutaneous lentiginosis in a typical distribution is a major criterion for the diagnosis of CNC [12]. Flat, poorly circumscribed brown to black macules are found in an unusual distribution along the vermillion border, eyelids, ears and genitalia. They typically present pre-pubertally and fade in the 40th decade. There is variability with lentiginosis observed at birth until the 80th decade [13]. Individuals of African-American descent with lentiginosis may manifest with slightly raised dark papules. Blue nevi, café-au-lait spots and depigmented lesions are also observed in individuals with CNC. It may be difficult to distinguish CNC lentiginosis from benign freckling (benign, patterned centrofacial lentiginosis) on clinical or histological grounds [13].
Acknowledgments
This work was supported in part by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health & Human Development intramural project Z01-HD-000642-04 to Dr. C.A. Stratakis. We thank the medical photographers at Guy’s and St. Thomas’ NHS Foundation Trust.
References
- 1.Brown B, Ram A, Clayton P, Humprey G. Conservative management of bilateral Sertoli cell tumours of the testicle in association with the Carney complex: a case report. J Pediatr Surg. 2007;42:E13–E15. doi: 10.1016/j.jpedsurg.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Carney JA, Gordon H, Carpenter PC, et al. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore. 1985 Jul;64(4):270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Gennari M, Stratakis CA, Hovarth A, et al. A novel PRKAR1A mutation with hepatocellular carcinoma in a young patient and a variable Carney complex phenotype in affected subjects in older generations. Clin Endocrinol (Oxf. 2008;69:751–755. doi: 10.1111/j.1365-2265.2008.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giralsi FP, Fatti LM, Berlota, et al. Carney’s complex with acromegaly as the leading clinical condition. Clin Endocrinol (Oxf. 2007;68:322. doi: 10.1111/j.1365-2265.2007.03024.x. [DOI] [PubMed] [Google Scholar]
- 5.Horvath A, Bossis I, Giatzakis C, et al. Large deletions of the PRKAR1A gene in Carney complex. Clin Cancer Res. 2008;14:388–395. doi: 10.1158/1078-0432.CCR-07-1155. [DOI] [PubMed] [Google Scholar]
- 6.Irani AD, Estrera AL, Buja LM, Safi H. Biatrial myxoma: a case report and review of the literature. J Card Surg. 2008;23:385–390. doi: 10.1111/j.1540-8191.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 7.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 8.O’Rourke F, Dean N, Mouradian MS, et al. Atrial myxoma as a cause of stroke: case report and discussion. Can Med Assoc J. 2003;169(10):1049–1051. [PMC free article] [PubMed] [Google Scholar]
- 9.Puntila J, Hakala T, Salminen J, Pihkala J. Positive genetic test led to an early diagnosis of myxoma in a 4-year-old boy. Interact CardioVasc Thorac Surg. 2006;5:662–663. doi: 10.1510/icvts.2006.134817. [DOI] [PubMed] [Google Scholar]
- 10.Ryou KS, Lee SH, Park SH. Multiple fusiform myxomatous cerebral aneurysms in a patient with Carney complex. J Neurosurg. 2008;109:318–320. doi: 10.3171/JNS/2008/109/8/0318. [DOI] [PubMed] [Google Scholar]
- 11.Sallai A, Hosszu E, Gergics P, et al. Orolabial signs are important clues for the diagnosis of the rare endocrine syndrome MEN 2B. Presentation of two unrelated cases. Eur J Pediatr. 2008;167:441–446. doi: 10.1007/s00431-007-0532-x. [DOI] [PubMed] [Google Scholar]
- 12.Sosibatros B, Stratakis CA. Carney complex, the first 20 years. Curr Opin Oncol. 2007;19:24–29. doi: 10.1097/CCO.0b013e32801195eb. [DOI] [PubMed] [Google Scholar]
- 13.Stratakis CA. Genetics of Peutz–Jeghers syndrome, Carney complex and other familial lentiginoses. Horm Res. 2000;54:334–343. doi: 10.1159/000053283. [DOI] [PubMed] [Google Scholar]
- 14.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrin Metab. 2001;86(9):4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 15.Swartz MF, Lutz CJ, Chandan VS, et al. Atrial myxomas: pathological types, tumor location, and presenting symptoms. J Card Surg. 2006;21:435–440. doi: 10.1111/j.1540-8191.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- 16.Veugelers M, Bressan M, McDermott DA, et al. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. New Engl J Med. 2004;351:460–469. doi: 10.1056/NEJMoa040584. [DOI] [PubMed] [Google Scholar]
- 17.Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol. 2005;6:501–508. doi: 10.1016/S1470-2045(05)70244-8. [DOI] [PubMed] [Google Scholar]
- 18.Zahedi RG, Wald DS, Ohri S. Carney complex. Ann Thorac Surg. 2006;82:320–322. doi: 10.1016/j.athoracsur.2005.07.099. [DOI] [PubMed] [Google Scholar]


