Abstract
PR-924 is an LMP-7-selective tripeptide epoxyketone proteasome inhibitor that covalently modifies proteasomal N-terminal threonine active sites. In the present study, we show that PR-924 inhibits growth and triggers apoptosis in multiple myeloma (MM) cell lines and primary patient MM cells, without significantly affecting normal peripheral blood mononuclear cells. PR-924-induced apoptosis in MM cells is associated with activation of caspase-3, caspase8, caspase-9, BID, and PARP, translocation of cleaved-BID to mitochondria, and cytochrome-c release. In vivo administration of PR-924 inhibits tumor growth in human plasmacytoma xenografts. Results from SCID-hu model show a significant reduction in the shIL-6R levels in mice treated with PR-924 versus vehicle-control. PR-924 treatment was well tolerated as evidenced by the lack of weight loss. Importantly, treatment of tumor-bearing mice with PR-924, but not vehicle alone, prolonged survival. Our preclinical findings therefore validate immunoproteasome LMP-7 subunit as a novel therapeutic target in MM.
Introduction
Therapeutic efficacy of targeting the ubiquitinproteasome pathway led to the FDA approval of dipeptidyl boronic acid bortezomib, first-in-class proteasome inhibitor, for the treatment of multiple myeloma (MM) (Adams 2004) (Hideshima, et al 2001). However, bortezomib therapy can be associated with off-target activities and development of drug resistance (Orlowski, et al 2002) (Richardson, et al 2003) (Richardson and Mitsiades 2005). Recent studies have therefore led to the development of other proteasome inhibitors containing novel scaffolds: NPI-0052 (Chauhan, et al 2005), a beta lactone inhibitor, and carfilzomib (Kuhn, et al 2007), a tetrapeptide epoxyketone inhibitor. In contrast to bortezomib, these two new agents are irreversible in nature and exhibit potent anti-MM activity in preclinical models; both agents are under clinical investigation for the treatment of MM.
The 26S proteasome mediates chymotrypsin-like (CT-L, β5), caspase-like (C-L, β5) and trypsin-like activities (TL, β2), respectively (Ciechanover 2005) (Orlowski and Wilk 2000) These catalytic subunits have corresponding inducible immunoproteasome components LMP7 (β5i), LMP2 (β1i) and MECL1 (β2i), which are thought to play a role in presentation of antigens on major histocompatability complex 1 (Rock, et al 2002) (Rivett and Hearn 2004). Recent reports link immunoproteasome expression to several diseases including Huntington disease (Diaz-Hernandez, et al 2003), Alzheimer’s disease (Mishto, et al 2006), macular degeneration (Ethen, et al 2007), inflammatory bowel disease (Fitzpatrick, et al 2007), (Mishto, et al 2006), and rheumatoid arthritis (Egerer, et al 2006). Interestingly, increased expression of the immunoproteasome has also been observed in MM (Altun, et al 2005). In this context, a recent study showed that immunoproteasome-specific inhibitor IPSI-001 preferentially targets the β1i-subunit of the immunoproteasome; inhibits MM cell growth; and overcomes bortezomib-resistance (Kuhn, et al 2009). These findings suggest that targeting the immunoproteasome may achieve potent anti-MM activity.
In the present study, we have utilized PR-924, a tripeptide epoxyketone related to carfilzomib. (Parlati, et al 2009) PR-924 is 100-fold selective for β5i and less selective for CT-L activity compare to bortezomib and carfilzomib, which can target other activities as well.
Our data show that PR-924 inhibits growth of MM cell lines and primary patient tumor cells, including those resistant to conventional and novel prior therapies. Animal studies show that PR-924 inhibits tumor growth in both SCID-hu and plasmacytoma xenograft mouse models, without significant toxicity. Our preclinical findings therefore provide the basis for phase-1 clinical trials of PR-924 to improve patient outcome in MM.
Material and Methods
Cell Culture
Human MM cell lines MM.1S, MM.1R, RPMI8226, Doxorubicin resistant (Dox-40), LR5 (derivative of RPMI-8226), OPM-1, OPM-2, KMS12PE and INA-6 were cultured in complete medium (RPMI-1640 media supplemented with 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine). MM patient tumor cells were purified by CD138 positive selection using the Auto MACS magnetic cell sorter (Miltenyi Biotec Inc., Auburn, CA). Informed consent was obtained from all patients in accordance with the Helsinki protocol. PBMCs from normal healthy donors were maintained in culture medium, as above. Drug source: PR-924 was obtained from Onyx Pharmaceuticals, Inc., South San Francisco, CA.
Quantification of the LMP-7 subunits in hematopoetic cells MM patient tumor cells and normal cells from healthy donor were subjected to immunoproteasome expression analysis using methodology reported previous study (Parlati, et al 2009). In brief the level of LMP-7 in MM patients as well as cell lines were quantitated and total amount of LMP-7 per total protein were measured, as described previously (Findlay, et al 2000) (Parlati, et al 2009)
Cell Viability and Apoptosis Assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5diphenyltetrazolium bromide (MTT; Chemicon International Inc., Temecula, CA), as previously described. (Hideshima, et al 2000) Percent cell death in control vs. untreated cells was obtained by trypan blue exclusion assay. Apoptosis was assessed by Annexin V/Propidium iodide (PI) staining assay kit, as per manufacturer’s instructions (R&D Systems, Inc. Minneapolis, MN), and analysis on a FACSCalibur (Becton Dickinson, San Jose, CA). Cell proliferation assay was performed by using WST (4-[3(4Iodophenyl)-2-(nitrophenyl)2H-5-tetrazolio, 1-3benezene dissulfonate) proliferation kit (BioVision, Mountain View, CA).
Western Blot Analysis
Protein lysates from control and drug-treated cells were subjected to immunoblotting using antibodies to PARP, caspase-8, caspase-9, caspase-3, Bcl-2, MCl-1, Bax, Cleaved BID or GAPDH as a loading control (Cell Signaling, Beverly, MA, 1:1000 dilution). Blots were then developed by enhanced chemiluminesence (ECL; Amersham, Arlington Heights, IL).
Mitochondrial Membrane Potential and Human Cytochrome-c (cyto-c) Immunoassays
Mitochondrial membrane potential was measured by using JC-1 cationic dye (Invitrogen, Eugene, Oregon), as previously described (Chauhan, et al 2003b) Briefly, MM.1S and RPMI cells were treated with PR-924 (3 μM, 24hr and carbonyl cyanide 4(trifluromethoxy)phenylhydrazone (cccp; 25μM), was used as a positive control), washed with PBS, and incubated with 1ug/ml of JC-1 dye for 20min at Room Temperature (RT) in the dark. After incubation, cells were washed twice in PBS, and FACS analysis was done as suggested by the manufacturer. Cytochrome-c release from mitochondria to cytosol was measured in cytosolic vs. mitochondrial fraction by using ELISA, as previously described (Chauhan, et al 2003a). Briefly, MM.1S were treated with PR-924 (3μM for 48 hr) and cytosolic/mitochondrial fractions were prepared by using mitochondrial isolation kit (Pierce, Rockford, IL) as per manufacturer’s instructions. After separation, 20μg of cytosolic or mitochondrial protein were used for cytochrome-c measurement using human cytochrome c immunoassay kit (R & D, Quantikine, Minneapolis, MN).
Human Plasmacytoma Xenograft and SCID Hu Model
All animal studies were approved by DFCI Institutional Animal Care and Use Committee. The in vivo anti-MM activity of PR-924 was assessed using the xenograft tumor model, as previously described (Chauhan, et al 2005),(Chauhan, et al 2008) (LeBlanc, et al 2002). Briefly, CB-17 SCID-mice (n = 12) (Charles River Labs, MA) were subcutaneously injected with MM.1S cells (5.0 × 106) in 100 μl of serum free RPMI1640 medium. When tumors were measurable (~350-400 mm3) approximately two weeks after MM.1S cell injection, mice were treated intravenously with PR-924 (6 mg/kg) or vehicle alone for 21 days on a twice-weekly schedule (Day1/Day2). Dosing solution for PR-924 contains 20% PEG 400 (polyethylene glycol 400, Fluka, Sigma), 0.05% Tween 80, 0.07% DMSO, and 79.88% of double distilled water.
We also utilized a SCID-hu mouse model, as described previously(Chauhan, et al 2009), to assess PR-924 activity. Briefly, 2.5 × 106 INA-6 MM cells were injected directly into human bone chips implanted subcutaneously in SCID mice. Tumor growth was assessed once weekly by measuring circulating levels of soluble human shIL-6R in mouse sera using ELISA (R & D Systems, MN, USA). Animals were treated with PR-924 at doses and schedule described above. Statistical significance of differences observed in vehicle vs. PR-924 treated mice was determined using a Student’s t test.
In situ detection of apoptosis, immunohistochemical (IHC) determination of proliferation, and assessment of microvessel density (MVD) Tumors from untreated and PR-924treated mice were excised and analyzed for proliferation, apoptosis, and angiogenesis by IHC staining using antibodies against Ki67, caspase-3, Factor VIII, and VEGFR1/FLT-1, as previously described(Chauhan, et al 2008), (Chatterjee, et al 2008)
Statistical Analysis
Statistical significance of differences observed in drug-treated vs. control cultures was determined using the Student’s t test. The minimal level of significance was P < 0.05. Tumor volume was determined using a Student’s t test. Survival of mice was measured using Kaplan-Meier curves (GraphPad Software, SanDiego, CA).
Results
Immunoproteasome expression in MM cells
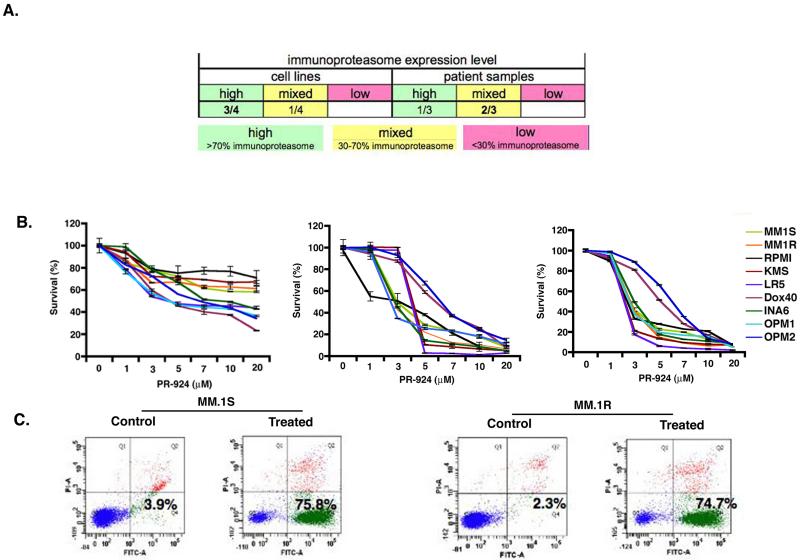
Analysis of immunoproteasome expression in MM cells showed high expression of immunoproteasomes in both MM cell lines and primary patient (CD138+) tumor cells, suggesting a role of immunoproteasomes in MM (Figure 1A). Previous study measured the potency of PR-924 against all six proteasome catalytic subunits using ProCISE, an activity based ELISA designed to differentiate between catalytic subunits with overlapping activities such as b5 and β5i; and showed that PR-924 is more selective inhibitor of β5i (LMP-7) than carfilzomib (Parlati, et al 2009).
Figure 1.
Immunoprotesome expression level in MM patient tumor cells and MM cell lines and PR-924 inhibits growth and triggers apoptosis in MM. (A) Differential expression of immunoproteasome content in MM cell lines and MM patients purified CD138+ cells. (B) Human MM cell lines (MM.1S, MM.1R, RPMI-8226, KMS12, LR-5, DOX40, INA-6, OPM1 and OPM2) were treated with PR-924 (dose range: 1-20 μM) for 24h, 48h, and 72h; cell viability was measured using MTT assays. Data presented are mean plus or minus SD of three independent experiments (P <. 05 for all cell lines at different time points). (C) MM.1S and MM.1R cell lines were treated with PR-924 (3μM) for 48h, and apoptosis was measured using Annexin V/PI binding assay and FACS analysis (P < 0.05; n=3,). A representative graph from three independent experiments is shown.
PR-924 inhibits growth and triggers apoptosis in MM cells
We next examined whether inhibiting β5i immunoproteasome activity using PR-924 affects MM cell viability. Human MM cell lines (MM.1S, MM.1R, RPMI-8226, KMS12PE, LR-5, DOX40, INA-6, OPM1 and OPM2) were treated with PR-924 (dose range: 1-20 μM) for 24h, 48h, and 72h, and cell viability was then measured using MTT assays. As shown in Fig 1B, PR-924 significantly decreases the viability of all the MM cell lines in a time-and dose-dependent manner (IC50 range for cell lines: 3-7 μM for 48 h). Since the MM cell lines examined include those with adverse cytogenetics and resistance to conventional therapies, our data suggests that PR-924 may overcome drug resistance. To confirm whether cell death induced by PR924 is due to apoptosis, we treated MM.1S and MM.1R cells with PR-924 (IC50 dose: 3μM at 48h) and then analyzed for apoptosis. As shown in Fig 1C, PR-924 triggered a significant increase in the Annexin V+/PI-apoptotic cell population versus untreated controls. These data suggest that PR-924 triggers apoptosis in MM cells.
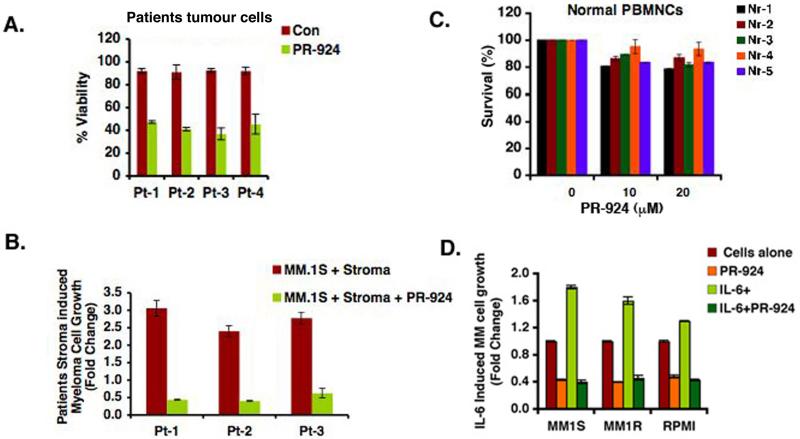
PR-924 induces cell death in patient tumor (CD138+) cells and overcomes bone marrow stromal cells (BMSCs)mediated drug resistance MM patient (CD138+) tumor cells were next treated with 3 μM of PR-924 for 48hr, and cell viability was measured by using trypan blue exclusion assays. As in MM cell lines, PR-924 induced significant increases in cell death in the four MM patient samples tested (Fig 2A; P < 0.05; n = 4). In contrast, examination of normal PBMCs showed a minimal decrease in viability even after treatment with high doses of PR-924 (20 μM) (Fig 2B). Of note, Pt#2 and Pt#3 had newly diagnosed MM, Pt#1 had MM, which relapsed after Vorinostat/Dex/lenalidomide therapy; and Pt#3 relapsed after bortezomib treatment. These findings demonstrate specific anti-MM activity of PR-924 and suggest a favorable therapeutic index.
Figure 2.
PR-924 induces cell death in patient tumor (CD138+) cells and inhibits BMSC-induced MM cell growth (A) Purified patient MM cells were treated with PR-924 (3μM) for 48h, and cell death was measured using trypan blue exclusion assays. Data presented are mean plus or minus SD of triplicate samples (P < 0.05 for all patients) (B) PBMCs from 5 normal healthy donors were treated with indicated concentrations of PR-924 for 48h, and then analyzed for viability using MTT assay. Data presented are mean plus or minus SD of triplicate samples (P < 0.05; n=3). (C) MM.1S cells were treated with PR-924 (3μM, 48hr) in the presence or absence of three different patient BMSCs, and cell growth was measured using WST cell proliferation assay. Data presented are mean plus or minus SD of triplicate samples (P < 0.05; n=3,). (D) MM.1S, MM.1R, and RPMI-8226 cells were treated with PR-924 (3μM, 48h) in the presence or absence of rhIL-6 (10ng/ml), and then cell growth was measured using WST cell proliferation assay. Data presented are mean plus or minus SD of triplicate samples (P < 0.05, for all cell lines).
Recently a study showed selective blockade of immunoproteasome subunits-β5i inhibit cytokine production and associated chronic inflammatory diseases [Muchamuel T, 2009]. Since BM microenvironment also confers growth advantage to MM cells (Chauhan, et al 1996), (Anderson 2007) we examined whether immunoproteasome subunits using PR-924 affects BMSCs-induced MM cell growth. MM.1S and MM.1R MM cells were cultured in the presence or absence of BMSCs and treated with PR-924 (3 μM) for 48h; cell proliferation was measured using WST colorimetric assay. As shown in Fig 3C, PR-924 significantly inhibits MM cell growth even in the presence of BMSCs (P < 0.05; n = 3). Our prior studies showed that adhesion of MM cells to BMSCs triggers transcription and secretion of MM cell growth and survival factor such as interleukin-6 (IL-6) (Chauhan, et al 1996) and we therefore also examined whether PR-924 retains its anti-MM activity in the presence of IL-6. MM.1S, MM.1R, and RPMI-8226 MM cells were treated with PR-924 (3 μM) for 48h in the presence or absence of IL-6 (10 ng/ml), and then analyzed for growth using the WST proliferation assay. Results show that PR-924 abrogates IL-6-induced proliferation of MM cells (Fig 2D).
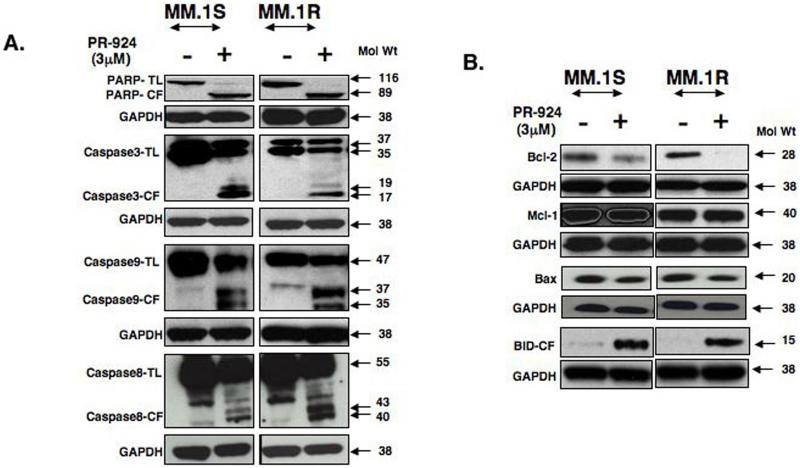
Figure 3.
PR-924-induced apoptosis in MM cells is associated with caspase activation and alteration in the expression of Bcl-2 family proteins. (A) MM.1S and MM.1R MM cells were treated with PR-924 (3μM) for 48h; harvested; and total protein lysates were subjected to western blot analysis using antibodies against PARP, caspase-3, caspase-9, caspase-8, or GAPDH. TL indicates total length; CL indicates cleaved fragment. Blots shown are representative of two independent experiments. (B) MM.1S and MM.1R MM cells were treated with PR-924 (3 μM) for 48h; harvested; and total protein lysate was subjected to western blot analysis using antibodies against Bcl-2, Mcl-1, BAX, cleaved BID, or GAPDH. Blots shown are representative of two independent experiments.
PR-924-induced apoptosis in MM cells correlates with activation of caspases and down-regulation of pro-survival proteins
Caspases are aspartate specific cysteine proteases and mediate apoptosis(Thornberry and Lazebnik 1998),(Wolf and Green 1999). Upon activation, caspases inactivate several key cellular proteins, such as DNA repair enzyme PARP [wolf BB 1999]. Our data shows that PR-924-induced apoptosis is associated with PARP cleavage in MM cells (Fig 3A). Furthermore, PR-924 triggers activation of caspase-3, caspase-8 and caspase-9 as can be determined by the appearance of the processed forms by western blotting (Fig 3A). These findings indicate the involvement of caspases in PR-924-induced apoptosis in MM cells. Bcl-2 confers survival in cancer cells (Chen, et al 2005), and we therefore next examined whether Bcl-2 affects PR-924induced MM cell death. Our data show that PR-924 significantly down-regulates the expression of Bcl-2 protein, without altering Bax or MCL-1 protein levels (Fig 3B).
PR-924 induces BID cleavage and its translocation to mitochondria, as well as cyto-c release BID, a proapoptotic BH-3 family protein, is linked to mitochondria-mediated apoptotic signaling pathways via cyto-c release (Yin 2006),(Wang, et al 1996). Importantly, PR-924 induces activation of BID, evidenced by a robust cleavage of BID (Fig 3B). We next asked whether PR-924 alters mitochondrial membrane potential by using JC-1 dye incorporation assay and measuring shift of red fluroscence to green fluroscence as an indicator of changes. Our results using two different cell lines MM.1S and RPMI-8226 show that after PR-924 triggered a marked shift from red to green fluroscence, suggesting a disruption of mitochondrial membrane potential (55-60% cells showed loss of mitochondrial membrane potential upon treatment (Fig 4A).
Figure 4.
PR-924 causes disruption of mitochondrial membrane potential and release of cytochrome-c (A) MM.1S and RPMI8226 cells were treated with PR-924 (3 μM) for 24h, and flow cytometric assay for JC-1 were performed as described in material and methods. The shift of fluroscence from red to green indicates loss of mitochondrial membrane potential. CCCP was used as a positive control. Data is plus or minus SD of two independent experiments (P < 0.05 for both cell lines). (B) MM.1S and RPMI cells were treated with PR-924 (3μM for 48h, and cyto-c release was measured in mitochondrial vs. cytosolic fractions using ELISA. Data is plus or minus SD of two independent experiments (P < 0.05).
(C) MM.1S and RPMI-8226 cells were treated with PR-924 (3μM) for 48h, and cytosolic fractions were subjected to immunoblot analysis using antibodies against cyto-c or GAPDH. Bortezomib (Velcade) (24nM, 24h) was used as a positive control in experiment using RPMI-8226 cells. Blots shown are representative of two independent experiments.
As shown in Fig 4B and 4C (left panel), treatment of MM.1S cells with PR-924 increases levels of cytosolic cytochrome-c, with a concurrent decrease in mitochondrial cytochrome-c fraction. Taken together, our data therefore suggest that PR-924-induced apoptosis is mediated, at least in part, via mitochondria.
PR-924 inhibits tumor growth in both human plasmacytoma xenograft and SCID-hu mouse models
Having shown the in vitro anti-MM activity of PR-924, we next examined in vivo efficacy of PR-924 using two distinct human MM xenograft murine models. MM tumor-bearing mice were treated intravenously with PR-924 (6mg/kg) or vehicle alone twice a week for 3 weeks. Results from the SCID-hu model show a significant reduction in tumor growth, evidenced by decreased levels of shIL-6R in PR-924-treated mice vs. vehicle controls (Fig 5A; P < 0.05). Similarly, PR-924 treatment inhibited tumor growth and prolonged survival in mice xenografted with MM.1S cells subcutaneously (Fig 5B and 5C; P < 0.05). Importantly, PR-924 treatment was well tolerated, without significant weight loss or neurological changes (Fig 5D).
Figure 5.
In vivo anti-MM activity of PR-924. (A) INA-6 MM cells (2.5 × 106) were injected directly into the human fetal bone chip implanted in SCID-hu mice, and mouse sera samples were analyzed for shIL-6R by ELISA. Upon detection of measurable shIL-6R, mice (5 mice/group) were treated intravenously with PR-924 (6 mg/kg) or vehicle alone twice weekly for 3 weeks (Bars indicate mean ± SD; P < 0.05; n=2).
(B) MM.1S cells (5 × 106 in 100 μl of serum free RPMI-1640 medium) were implanted subcutaneously in mice (6 mice/group); average and standard deviation of tumor volume (mm3) was monitored every third day. Mice were treated intravenously with PR-924 (6 mg/kg) or vehicle alone twice weekly for 3 weeks. Bars indicate mean ± SD; P < 0.05). (C) Kaplan-Meier plot showing survival of mice treated with PR924 compared to vehicle-treated controls. PR-924-treated mice survived significantly longer than controls (P=0.016; median survival in control was 23.5 days vs. 42.5 days in PR-924 treatment group). (D) Body weight of PR-924-treated vs. control mice was monitored once a week. Data shows plus or minus SD of 6 different mice/group.
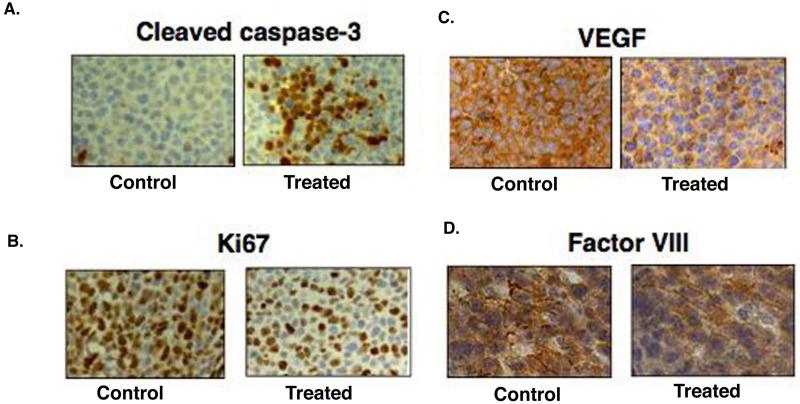
Tumor growth inhibition by PR-924 treatment is due to increased apoptosis and inhibition of angiogeneis We next harvested tumors from PR-924-treated vs. control mice, and performed immunostaining for cleaved caspase-3, Ki67, and angiogenesis related markers including VEGF and Factor VIII. As shown in Fig 6A, a robust increase in cleaved-caspase-3, together with a decrease in proliferation marker Ki-67 (6B), was observed in tumor sections harvested from PR-924-treated group versus controls. Moreover, the anti-angiogenic activity of PR-924 was evidenced by a significant reduction in VEGF and Factor VIII expression (Fig 6C and 6D). These results demonstrate that in vivo anti-MM activity of PR-924 may, at least in part, be due to its anti-angiogenic activity.
Figure 6.
Immunohstiochemistry analysis of tumor tissue samples from PR-924 treated vs control mice Tumors from control and PR-924-treated mice were excised and subjected to immunostaining using antibodies against (A) cleaved caspase-3, (B) Ki67, (C) VEGF, or (D) Factor-VIII. Photographs (A, B, C and D shown are representative of similar observations in 2 different mice receiving same treatment.
Discussion
Preclincal and clinical studies have established proteasome inhibition with bortezomib as a potent anti-MM therapy; however, it can be associated with toxicity and drug resistance.(Orlowski, et al 2002)(Richardson, et al 2003),(Richardson and Mitsiades 2005),(Kuhn, et al 2007) The off-target activity of bortezomib (Richardson, et al 2005) may contribute to associated observed adverse effects. In this context, prior studies have shown that bortezomib predominantly inhibits proteasome CT-L, and to a lesser extent C-L, activities(Chauhan, et al 2005). Importantly, carfilzomib, a novel proteasome inhibitor under clinical investigation, is a more selective inhibitor of CT-L activity than bortezomib (Kuhn, et al 2007). Nonetheless, both agents at their pharmacological concentrations affect constitutive and immunoproteasome activities (Orlowski, et al 2007), (Richardson, et al 2005), (Kuhn, et al 2007).
In the present study, we show that a novel agent PR-924, which predominantly targets β5i, triggers MM cell death without affecting normal cell viability. In MM, adhesion of tumor cells to BMSCs trigger transcription and secretion of various cytokines mediating MM cell growth, survival, migration, and drug resistance (Chauhan, et al 1996), (Chauhan, et al 2004), (Hideshima, et al 2007). Thus, the BM microenvironment and associated cytokine secretion regulate the growth and survival of MM cells in a paracrine manner. A recent preclinical study suggest that selective targeting of immunoproteasome subunits-β5i can block cytokine production and therefore inhibits chronic inflammatory diseases (Muchamuel, et al 2009). These findings together suggested that inhibition of immunoproteasome in MM may inhibit the growth of MM cells mediated by adherence to BMSCs. Indeed, our in vitro study show that PR-924 block the growth of MM cells in the presence of BMSCs, and this effect is likely mediated via blockade of cytokine production. Our in vivo animal model study also demonstrates similar results: SCID-hu models reflects the human BM microenvoirnment; and importantly, PR-924 inhibit the growth of xenografted MM cells, as measured by a decrease in secretion of shIL-6R. These in vitro and in vivo findings suggest that MM cell growth inhibitory activity of PR-924 is, at least in part, mediated indirectly via affecting paracrine MM cell growth factors. Importantly, PR-924 overcomes the survival advantage to MM cells conferred by BMSCs.
Our mechanistic studies show that the anti-MM activity of PR-924 is primarily due to induction of apoptosis. PR924-induced MM cell apoptosis is associated with activation of caspase-3, caspase-8 and caspase-9, and PARP. Our results also suggest predominant involvement of mitochondria-mediated signaling cascade during PR-924triggered apoptosis in MM cells. Specifically, mitochondria are major organelles involved in signal transduction and execution of cell death (Lindsten, et al 2000),(Kroemer, et al 2007), and one of the mechanisms regulating induction of mitochondrial apoptotic signaling is BH3 only protein BID. (Landshamer, et al 2008),(Chauhan, et al 2007) In our study, treatment of MM cells with PR-924 decreases mitochondrial membrane potential and triggers BID cleavage and its translocation to mitochondria, thereby inducing caspase-9 activation and cyto-c release. These mechanistic data suggest that PR-924-induced MM cell apoptosis occurs in a mitochondria-dependent fashion via BID activation.
To confirm our in vitro finding, we also utilized two different xenograft mouse models of human MM (Chauhan, et al 2009). PR-924 significantly inhibits tumor growth and prolongs survival of mice in both plasmacytoma xenograft and SCID-hu models. The SCID-hu mouse model best reflects the interaction between the human BM microenvironment and human MM cells; importantly, our data in this animal model demonstrate the ability of PR-924 to overcome the cytoprotective effects of the BM microenvironment on MM cells. Moreover, no significant toxicity was observed in mice receiving PR-924 treatment, as evident by less than 10% weight loss. Analysis of tumor sections from PR-924treated mice show: decreased expression of proliferation marker Ki67; increased apoptosis assessed by cleavedcaspase-3 staining; and marked anti-angiogenic activity, evidenced by reduction in VEGF and Factor VIII expression. Overall, our in vitro and in vivo preclinical data therefore provide the framework for clinical trials of PR924 to improve patient outcome in MM.
Acknowledgement
Grant support: This investigation was supported by NIH grants SPORE-P50100707, PO1-CA078378, and RO1CA050947; and MRF funds.
Footnotes
The LeBow Institute for Myeloma Therapeutics and Jerome Lipper Myeloma Center, Department of Medical Oncology, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA 02115; and Onyx Pharmaceuticals, San Francisco, CA 92121
Authors’ contributions and disclosure of Conflicts of Interest: DC designed research, analyzed data, and wrote the manuscript; AVS performed experiments, analyzed data, and wrote the manuscript; MB performed the experiments; MA, CJK provided PR -924 and immunoproteasome expression/activity data; PR, provided bone marrow samples; and KCA wrote the manuscript. MA and CJK are employees of Onyx pharmaceuticals.
References
- Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- Altun M, Galardy PJ, Shringarpure R, Hideshima T, LeBlanc R, Anderson KC, Ploegh HL, Kessler BM. Effects of PS-341 on the activity and composition of proteasomes in multiple myeloma cells. Cancer Res. 2005;65:78967901. doi: 10.1158/0008-5472.CAN-05-0506. [DOI] [PubMed] [Google Scholar]
- Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–162. doi: 10.1016/j.exphem.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Rancso C, Stuhmer T, Eckstein N, Andrulis M, Gerecke C, Lorentz H, Royer HD, Bargou RC. The Y-box binding protein YB-1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood. 2008;111:3714–3722. doi: 10.1182/blood-2007-05-089151. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Catley L, Li G, Podar K, Hideshima T, Velankar M, Mitsiades C, Mitsiades N, Yasui H, Letai A, Ovaa H, Berkers C, Nicholson B, Chao TH, Neuteboom ST, Richardson P, Palladino MA, Anderson KC. A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from Bortezomib. Cancer Cell. 2005;8:407–419. doi: 10.1016/j.ccr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC. JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem. 2003a;278:17593–17596. doi: 10.1074/jbc.C300076200. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Podar K, Hideshima T, Mitsiades C, Schlossman R, Munshi N, Richardson P, Cotter FE, Anderson KC. Targeting mitochondria to overcome conventional and bortezomib/proteasome inhibitor PS-341 resistance in multiple myeloma (MM) cells. Blood. 2004;104:2458–2466. doi: 10.1182/blood-2004-02-0547. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Sattler M, Podar K, Mitsiades C, Mitsiades N, Munshi N, Hideshima T, Anderson KC. Superoxide-dependent and independent mitochondrial signaling during apoptosis in multiple myeloma cells. Oncogene. 2003b;22:6296–6300. doi: 10.1038/sj.onc.1206734. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109:1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2008;111:1654–1664. doi: 10.1182/blood-2007-08-105601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, Raje N, Jaye DL, Kumar SK, Richardson P, Munshi N, Anderson KC. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009;16:309–323. doi: 10.1016/j.ccr.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87:1104–1112. [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- Diaz-Hernandez M, Hernandez F, Martin-Aparicio E, Gomez-Ramos P, Moran MA, Castano JG, Ferrer I, Avila J, Lucas JJ. Neuronal induction of the immunoproteasome in Huntington’s disease. J Neurosci. 2003;23:11653–11661. doi: 10.1523/JNEUROSCI.23-37-11653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerer T, Martinez-Gamboa L, Dankof A, Stuhlmuller B, Dorner T, Krenn V, Egerer K, Rudolph PE, Burmester GR, Feist E. Tissue-specific up-regulation of the proteasome subunit beta5i (LMP7) in Sjogren’s syndrome. Arthritis Rheum. 2006;54:1501–1508. doi: 10.1002/art.21782. [DOI] [PubMed] [Google Scholar]
- Ethen CM, Hussong SA, Reilly C, Feng X, Olsen TW, Ferrington DA. Transformation of the proteasome with age-related macular degeneration. FEBS Lett. 2007;581:885–890. doi: 10.1016/j.febslet.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, Khan MN, Bowsher RR. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000;21:1249–1273. doi: 10.1016/s0731-7085(99)00244-7. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LR, Small JS, Poritz LS, McKenna KJ, Koltun WA. Enhanced intestinal expression of the proteasome subunit low molecular mass polypeptide 2 in patients with inflammatory bowel disease. Dis Colon Rectum. 2007;50:337–348. doi: 10.1007/s10350-006-0796-7. discussion 348-350. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, Demo SD, Bennett MK, van Leeuwen FW, Chanan-Khan AA, Orlowski RZ. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DJ, Hunsucker SA, Chen Q, Voorhees PM, Orlowski M, Orlowski RZ. Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood. 2009;113:4667–4676. doi: 10.1182/blood-2008-07-171637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landshamer S, Hoehn M, Barth N, Duvezin-Caubet S, Schwake G, Tobaben S, Kazhdan I, Becattini B, Zahler S, Vollmar A, Pellecchia M, Reichert A, Plesnila N, Wagner E, Culmsee C. Bid-induced release of AIF from mitochondria causes immediate neuronal cell death. Cell Death Differ. 2008;15:15531563. doi: 10.1038/cdd.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc R, Catley LP, Hideshima T, Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien CS, Adams J, Gupta D, Richardson PG, Munshi NC, Anderson KC. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, Shwonek P, Parlati F, Demo SD, Bennett MK, Kirk CJ, Groettrup M. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- Orlowski M, Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Arch Biochem Biophys. 2000;383:1–16. doi: 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, Guerciolini R, Anderson JK, Depcik-Smith ND, Bhagat R, Lehman MJ, Novick SC, O’Connor OA, Soignet SL. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol. 2002;20:4420–4427. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, Micklem DR, Ruurs P, Sylvain C, Lu Y, Shenk KD, Bennett MK. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–369. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Mitsiades C. Bortezomib: proteasome inhibition as an effective anticancer therapy. Future Oncol. 2005;1:161–171. doi: 10.1517/14796694.1.2.161. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Schlossman R, Mitsiades C, Hideshima T, Munshi N, Anderson K. Emerging trends in the clinical use of bortezomib in multiple myeloma. Clin Lymphoma Myeloma. 2005;6:84–88. doi: 10.3816/CLM.2005.n.033. [DOI] [PubMed] [Google Scholar]
- Rivett AJ, Hearn AR. Proteasome function in antigen presentation: immunoproteasome complexes, Peptide production, and interactions with viral proteins. Curr Protein Pept Sci. 2004;5:153–161. doi: 10.2174/1389203043379774. [DOI] [PubMed] [Google Scholar]
- Rock KL, York IA, Saric T, Goldberg AL. Protein degradation and the generation of MHC class I-presented peptides. Adv Immunol. 2002;80:1–70. doi: 10.1016/s0065-2776(02)80012-8. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:13121316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]