SUMMARY
Gene therapy is an attractive method for the treatment of cardiovascular disease. However, using current strategies, induction of gene expression at therapeutic levels is often inefficient. In this study, we demonstrate a novel electroporation method to enhance delivery of a plasmid expressing an angiogenic growth factor (vascular endothelial growth factor, i.e. VEGF), which previously documented to stimulate revascularization in coronary artery disease. DNA expression plasmids were delivered in vivo to the porcine heart with or without co-administered electroporation in order to determine the potential effect of electrically mediated delivery. The results demonstrated that plasmid delivery through electroporation significantly increased cardiac expression of VEGF compared to injection of plasmid alone. This is the first report demonstrating successful intracardiac delivery, through in vivo electroporation, of a protein expressing plasmid in a large animal.
Keywords: Electroporation, Cardio-vascular, VEGF, Heart, Non-viral
Advances in the treatment of coronary artery disease (CAD) have been attained through interventions such as angioplasty and coronary artery bypass surgery1. However, some CADs are not amenable to these interventions, indicating that development of other therapies is needed. One such therapeutic strategy is the induction of revascularization through targeted gene therapy2-6. Vascular endothelial growth factor (VEGF) is an angiogenic protein used to stimulate angiogenesis in models of CAD and, as such, has been evaluated as a therapeutic target. Genes encoding VEGF isoforms can potentially circumvent many of the obstacles presented by restenosis and other cardiovascular pathologies through the stimulation of potentially therapeutic collateral vessel formation.
Direct naked/non-viral DNA plasmid injection and adenoviral-mediated gene transfer6, 7 have shown promise for treating CAD. In addition to providing symptomatic relief during ischemia, studies indicated that VEGF stimulates collateral vessel formation even in non-ischemic hearts8. However, some drawbacks exist for adenoviral-mediated and non-viral naked DNA direct injection gene therapy methods. For example, non-viral DNA delivery has been demonstrated to often mediate low and short-term gene expression while adenoviral vectors have some toxicity concerns. The toxicity issues for the adenoviral-based vectors include the generation of “memory” immune responses, after the delivery of the vector, against the adenoviral vector backbone. This is likely due to the previous exposure to a number of “natural” adenoviruses during life.
The non-viral naked DNA approach, although somewhat inefficient, deserves further scrutiny due to it’s, to date, more favorable patient safety profile. In fact, numerous subjects have been “vaccinated” with antigens expressed from naked DNA plasmids without the development of significant adverse events. Several non-viral methods have been developed in an attempt to enhance delivery of DNA and have been the focus of several reviews9-12. Both chemical and physical techniques including liposomes13-15, particle bombardment16-18 and hydrodynamic delivery19, 20 have been described and used to increase the efficiency of tissue DNA uptake.
Another physical delivery method to circumvent the problems of low protein expression associated with naked non-viral based DNA plasmids is delivery of the genes through in vivo electroporation (EP). The demonstration that electric fields can be safely and effectively applied in vivo to deliver small molecules21 and the widespread use of EP to deliver plasmid DNA to cells in vitro provided a foundation for the use of EP to deliver plasmid DNA in vivo. EP is a simple and direct, in vivo method to deliver genes for therapy. The use of electric pulses for the in vivo delivery of plasmid DNA has been steadily increasing. Preliminary results indicate that therapeutic plasmid DNA delivery could potentially achieve similar success to conventional drug delivery. Multiple studies have demonstrated the feasibility of this approach, primarily in skin, skeletal muscle, and tumors22-25.
Importantly, the EP delivery method has recently been successfully evaluated in a clinical trial in which an IL-12 expressing plasmid was delivered to patients with metastatic melanoma26. A second clinical trial delivered a cancer vaccine to muscle27. In terms of the application of EP mediated delivery of therapies against CAD, two studies have reported using electric pulses to transfer genes to the heart. Both of these studies utilized explanted / ex vivo hearts either from mice or embryonic chicks18, 28.
Therefore, it was hypothesized that EP could deliver plasmid DNA to the heart for the potential treatment of CAD. The goal of the study presented here was to establish a safe and potentially therapeutic EP based plasmid DNA delivery to the heart in a large animal model, which could potentially be translated to human medicine. The pig is a useful model for these studies because of similarities between pigs and humans in terms of cardiac and coronary artery anatomy and physiology. This proof-of-concept brief communication describes the successful novel delivery of expression plasmids to the porcine heart.
The initial experiment involved injection of a plasmid encoding luciferase, pLuc (gWizLuc, Gene Therapy Systems, San Diego, CA) followed by the administration of EP. This was carried out in four pigs with injection of the plasmid made in two sites marked with polypropylene sutures in the anterior left ventricular wall in a line 1.5 cm lateral to the left anterior descending coronary artery approximately 2 cm apart. A second set of injection sites was made 2 cm lateral to the initial line with this line of sites now in the lateral ventricular wall supplied by the left circumflex artery. The sites were again 2 cm apart. A total of four injection sites were made in each heart. Three sites received EP delivered to individual sites following each injection. One site received plasmid injection without EP and a biopsy site was taken in the far lateral ventricular wall without plasmid injection or EP. This same delivery technique was used in each group of pigs described below.
These pulses were administered at a rate of 8 per second and were not synchronized with the heart rhythm (ASYNC). The pLuc delivery experiments were performed to determine which of the EP parameters resulted in the highest levels of gene expression. The rationale for use of ASYNC pulses was to assess whether such a delivery could be applied safely and was effective in enhancing gene expression. Forty-eight hours after pLuc and EP administration myocardial expression of luciferase was quantified in cardiac biopsy samples (collected with a 6 mm biopsy punch) using commercial firefly luciferase (Sigma-Aldrich Chemical Co., St. Louis, MO). These data are shown in Figure 1a. There was a significant increase of expression when pLUC was delivered with electroporation compared to injection alone. The highest expression was obtained using an applied field strength of 100 V/cm and a pulse width of 250 ms. These optimal EP parameters (that is, 250ms pulse width and 100V/cm of applied field strength) were then subsequently used to deliver pVEGF. Two or seven days after delivery of pVEGF to pig hearts (two injection and EP sites, one injection only site and one no-treatment site), a myocardial tissue punch (6 mm) of the treated sites was excised and VEGF expression in the samples was measured (expressed as picogram VEGF per gram tissue) using a commercial Quantikine human VEGF quantitative sandwich ELISA kit (R and D Systems, Minneapolis, MN 55413). In these experiments, VEGF expression was significantly higher at 2 but not at 7 days post-treatment compared to the pVEGF treatment without EP (Figure 1b). In summary, for these initial electroporation delivery experiments, EP increased expression by 25 fold and 5 fold for luciferase and VEGF, respectively. Although both proteins were expressed from vectors containing the CMV promoter, differences in protein fold increase levels and duration of expression were observed. Several possible variables may have produced this difference. Different CMV promoter/enhancers do not necessarily produce identical expression. Other plasmid structural elements, for example the polyadenylation signal, may affect expression. Luciferase was detected using a functional assay, whereas VEGF was detected using an antibody assay. Finally, the proteins expressed may differ in half-life. While the luciferase protein half-life in mammalian cell culture is three hours 29, the half-life of VEGF in vivo has been measured at 6 minutes 30 and 50 minutes 30, 31.
Figure 1. Cardiac expression of luciferase or VEGF following ASYNC EP mediated delivery of pLuc or pVEGF.
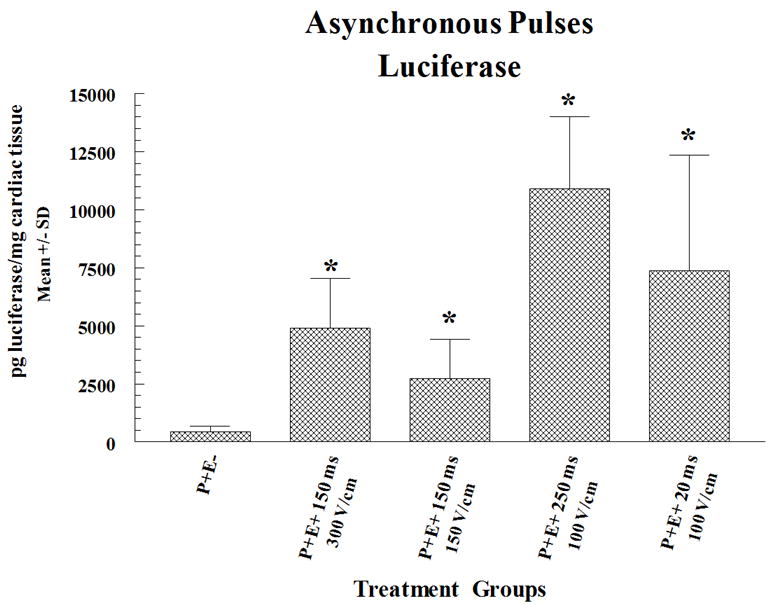
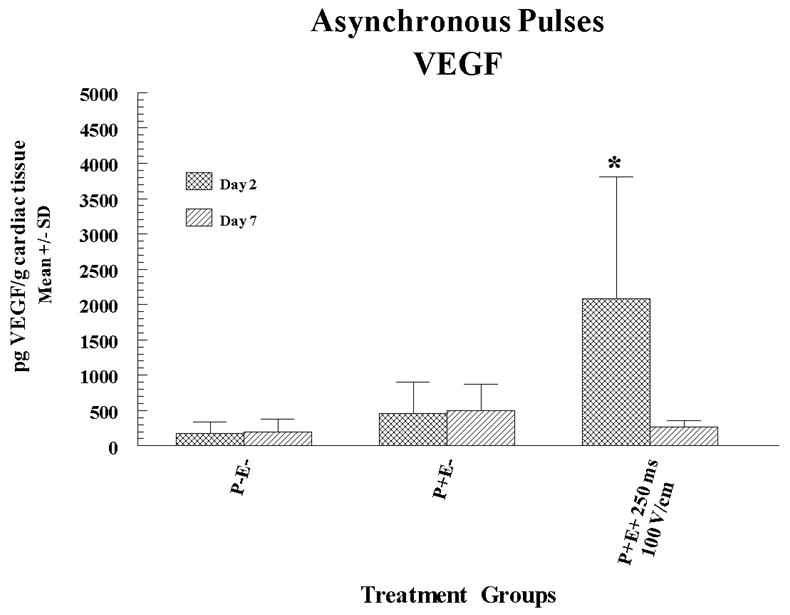
pLuc (a) or pVEGF (b) were administered by intracardiac injection followed by ASYNC pulses. Expression for luciferase and VEGF are given as mean pg/mg and pg/g cardiac tissue sample ± standard deviation (SD) respectively. In the figures P+ and P- designates with or without plasmid injection while E+ and E- designates with or without delivery via EP. Results for luciferase expression represent a mean and SD of 3 sites (4 sites for injection only) and for VEGF the results are a mean and SD of 6 sites. A total of 10 pigs (30-35 kg) were utilized for these sets of experiments (4 for luciferase and 6 for VEGF). Multiple sites were utilized on the heart of each animal.
Statistical Analysis: Statistical comparisons for protein expression were determined between the groups receiving electroporation and P+E- (plasmid injection alone) by Student’s unpaired T test. For statistical comparisons which included greater than two groups (comparison between experimental groups) the analysis was done by nonparametric ANOVA. The asterisk symbol (*) indicates significantly elevated expression (p<0.05) compared to control.
Plasmids: To construct pVax1-hVEGF165, a fragment containing hVEGF165 was subcloned from pBLAST49-hVEGFv2.0 (HYPERLINK “https://webmail.odu.edu/owa/www.invivogen.com” InvivoGen, San Diego, CA) into pVax1 (Invitrogen Corp., Carlsbad, CA), which contains the CMV promoter and the BGH polyadenylation signal. The plasmid was commercially prepared (Aldevron, Fargo, ND) and suspended in sterile injectable saline. Endotoxin levels were <0.1 EU/μg plasmid. The pLuc used in the studies was a gWizLuc plasmid (Aldevron, Fargo, ND).
Anesthesia: Animals were anesthetized during DNA injection and electric pulse administration. Pre-anesthetic Agents: Induction: Ketamine 10-20 mg/kg IM for immobilization, Atropine 0.02 mg/kg IV, Sufentanil 0.015 mg/kg/h IV followed by 0.007 mg/kg Sufentanil bolus after 5 min. Maintenance: Sufentanil 0.015-0.030 mg/kg/h IV infusion supplemented by 0.5 -1.0% isoflurane in oxygen via inhalation (i.e. intubation). Monitoring: Animals were monitored using EKG monitoring, pulse oximetry (O2 sat), capnography (end-tidal CO2), and rectal temperature. Medications: Antibiotics: Cephalexin 10 mg/kg IV once for antibiotic prophylaxis, Anti-arrhythmic: Amiodarone 5-10 mg/kg was injected intravenously just prior to cardiac manipulation and every 30 min as required with use of Lidocaine intravenously prn.
Surgical Procedure: Animals were placed in supine position and on a warming blanket. Steri-drape was used to cover from neck to mid-abdomen. Incision was made in skin from sterno-manubrial junction to xyphoid using a #10 Blade. The subcutaneous tissue was divided to sternum using electrocautery and then the sternum divided with a stryker oscillating saw. Arrhythmias were treated as indicated above and the sternum closed with 0 wire and soft tissues closed in layers with absorbable suture.
Plasmid Injection and Electroporation: Plasmid DNA (pLuc or pVEGF) was injected (200μg in 100 μl of sterile saline) in multiple sites in the left ventricle followed by EP at specific delivery parameters that varied by field strength and pulse width. EP was administered via an epicardial probe at the designated parameters. The electric fields were applied using an applicator containing an array of 16 electrodes and a firing sequence of a series of 2 × 2 mm squares. Four pulses were fired in each direction (total of 72 pulses for entire array). Internal defibrillation was administered as needed (10 – 50 joules) in all animals receiving asynchronous EP and the heart allowed to recover for 10 – 15 minutes following restoration of normal sinus rhythm. Location of treatment site(s) were marked with 4-0 prolene suture. Two controls were performed; plasmid delivery without EP and an untreated control in which there was neither plasmid injection nor administration of EP.
Evaluation: At 2 or 7 days post-operatively, the sternotomy was re-opened. The injection sites were excised with a 6 mm punch and sent for luciferase or VEGF assays. Luciferase expression was quantified using commercial firefly luciferase (Sigma, St. Louis, MO). A sandwich ELISA kit was used for detection of VEGF. The animals were euthanized with intracardiac-administered potassium chloride. This experimental animal surgery and treatment protocol was approved by the University of South Florida Institutional Animal Care and Use Committee and all experiments were performed in accordance with all relevant guidelines and regulations.
Although the primary objective of increasing protein expression was obtained, it was observed that ASYNC pulses resulted in ventricular fibrillation in all of the pigs. Although most of the pigs were successfully defibrillated, it was reasoned that the development of a synchronous (SYNC) EP pulse delivery method, in which the pulses could be delivered in synchrony with the normal heart rhythm, would circumvent this problem. Therefore, in a subsequent set of plasmid injection experiments, electrical pulses were delivered to the heart synchronously with the QRS complex determined with the use of a surface electrocardiogram. Importantly, the initial experiments indicated that delivering SYNC pulses eliminated ventricular fibrillation.
Synchronous electrical pulses, delivered during the absolute refractory period of the cardiac electrical cycle, have long been known to have minimal risk of causing electrical fibrillation; for example, as used to treat atrial arrhythmias. Hojman, et al. 32, have shown that EP induces changes in Na+ and K+ fluxes, and in Ca2+ content in skeletal muscle with larger changes when DNA is present with EP. These effects may also be present in cardiac muscle and in treatment of the heart, but have not been definitely demonstrated. The larger and more determinant effect is the timing of the pulse delivery within the absolute refractory period of the cardiac cycle.
To determine the SYNC EP conditions that result in maximal expression, experiments were again performed in six pigs with pLuc injection plus EP utilizing six injection and electroporation sites, two injection only sites and two no-treatment sites in each heart (half were evaluated for expression and the other half underwent histological evaluation). In these experiments, it was determined that the pulse length was required to be shortened in order to be synchronized with the QRS wave. In the experiment summarized in Figure 2a, expression of luciferase was measured in the porcine hearts either 2 or 7 days post-treatment. In this experiment, the highest level of expression was 10,000 and 15 fold higher at day 2 or 7 respectively compared to pLuc treatment without EP. Interestingly, utilizing different pulsing conditions resulted in not only different peak levels of luciferase expression but also different expression kinetics. Peak short-term expression (2 days) that was significantly higher than injection alone was obtained using an applied field strength of 80 V/cm and a pulse width of 20 ms. Expression was no longer significantly higher than injection alone by seven days. In contrast, when pLUC was delivered with an applied field strength of 120 V/cm and pulse width of 20 ms, peak expression was delayed to 7 days and was significantly higher. This suggests the versatility of delivery with EP as certain expression patterns can be obtained utilizing different EP delivery parameters. In experiments in which pVEGF was delivered (in six pigs utilizing five injection sites and one no-treatment site in each heart) with SYNC pulses, maximal VEGF expression was 5 fold higher than pVEGF injection alone, when measured at a 2 day post-treatment time point (Figure 2b). No episodes of ventricular fibrillation were experienced using the synchronous EP delivery technique. VEGF expression was evaluated at peak expression (Day 2) based on the results presented in Figure 1b which showed a drop in VEGF expression to background by Day 7.
Figure 2. Cardiac expression of luciferase or VEGF following SYNC EP mediated delivery of pLuc or pVEGF.
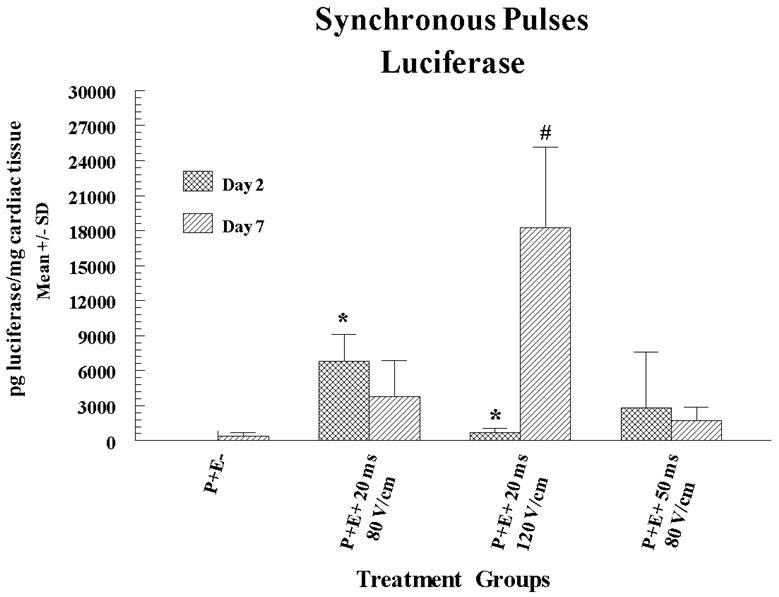
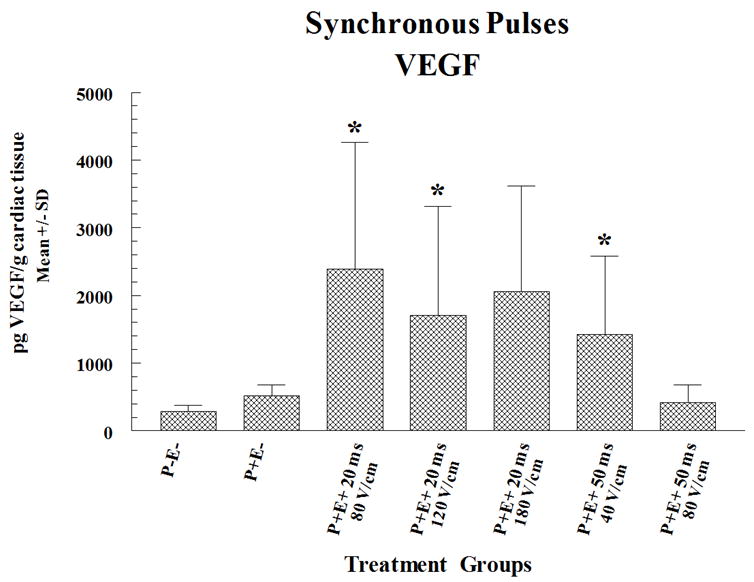
pLuc (a) or pVEGF (b) were administered by intracardiac injection followed by SYNC pulses. The electric fields were applied using an applicator containing 4 needle electrodes that were 6 mm long and arranged to form a 5 × 5 mm square. Four pulses were fired in two perpendicular directions (total of 8 pulses for entire array). Expression for luciferase and VEGF are given as mean pg/mg and pg/g cardiac tissue sample ± standard deviation (SD) respectively. In the figures P+ and P- designates with or without plasmid injection while E+ and E- designates with or without delivery via EP. Methods are as described in the legend for Figure 1 except that EP pulses were synchronized with the heart rhythm. Electroporation pulses were administered individually just before the peak of the r wave (within the qrs complex) and completed prior to the initiation of the t wave. Results for luciferase expression represent a mean and SD of 3 sites and for VEGF the results are a mean and SD of 4 sites except for injection only (5 sites) and injection and electroporation with 20 ms and 120 V/cm (5 sites). A total of 12 pigs (30-35 kg) were utilized for these experiments (6 for luciferase and 6 for VEGF). Multiple sites were utilized on the heart of each animal. For histological evaluation, four sections were prepared from each biopsy and stained with hematoxylin and eosin and then assessed by pathologic examination to determine any evidence of damage as well as immune or inflammatory reactions. Electroporation sites were treated prior to the non electroporated sites.
Statistical Analysis: Statistical comparisons were performed as described in Figure 1 legend. The asterisk symbol (*) on a column indicates that the expression for that treatment on Day 2 was significantly elevated (p<0.05) compared to Day 2 control. The hash (#) symbol on a column indicates that the expression for that treatment on Day 7 was significantly elevated (p<0.05) compared to Day 7 control.
An ability to tailor the EP parameters for differential expression may be useful for optimal results and to minimize complications in a clinical situation. It is unclear at this time when the most optimal delivery periods would be and how long they would persist. Further study is necessary. The VEGF levels in this study are higher than in a clinical study by Kastrup, et al., but the levels in this study were myocardial tissue levels while the clinical study measured serum levels 33, 34. No significant elevation of serum levels of VEGF were seen in this study. The regional localization and lack of systemic increases in VEGF may have significant advantages in mitigation of complications due to increased VEGF levels with neovascularization in the retina in diabetes and neovascularity to support tumor formation/growth. More study is necessary to define the optimal target levels of VEGF both in the myocardium and in the serum, both in an experimental and in clinical situations.
In addition to the assessment of expression, biopsies of the injection/EP sites were also examined histologically. There was evidence of pericarditis in all samples. This was an expected sequela of the cardiac manipulations. Some mild to moderate inflammatory responses were also noted in several samples which could not be directly associated with treatment conditions.
To our knowledge, this is the first report of the successful delivery of a plasmid expressing a vascularization modifying protein such as VEGF to the porcine heart through EP. Expression of VEGF was increased significantly in tissue surrounding the cardiac injection site that had received pVEGF plus EP. These studies indicate a proof of concept validation of this method and provide the impetus for further investigation. These studies will include safety analysis as an assessment of potential treatment-associated biological and clinical effects. Ultimately, these studies will indicate the potential for this technique and strategy for the treatment of CAD in humans.
Acknowledgments
Supported by a research grant from the National Institutes of Health R21 HL089017 and by the University of South Florida College of Medicine, Florida Center of Excellence for Biomolecular Identification and Targeted Therapeutics and Cook Medical, Inc.
Footnotes
Declaration of conflict of interest
Richard Heller has been funded by the National Institutes of Health. Drs. Heller, Marshall and Jaroszeski are co-inventors on a patent application which covers the technology that was used in the work reported in this manuscript. Drs. R. Heller and Jaroszeski are also inventors on other patents that were used in this work. Drs. Heller and Jaroszeski have ownership interest in RMR Technologies and own stock and stock options of Inovio Biomedical Corporation. Brian Boone, Jose D. Burgos, Sylvia I. Gografe, Margaret K. Baldwin, Michele L. Danielson, Mary J. Larson, Denise R. Caretto, Yolmari Cruz, Bernadette Ferraro and Kenneth E. Ugen declare no potential conflict of interest.
References
- 1.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis--how many patients might be eligible? Am J Cardiol. 1999;84:598–600. A598. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 2.Brewster LP, Brey EM, Greisler HP. Cardiovascular gene delivery: The good road is awaiting. Adv Drug Deliv Rev. 2006;58:604–629. doi: 10.1016/j.addr.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortuin FD, Vale P, Losordo DW, Symes J, DeLaria GA, Tyner JJ, et al. One-year follow-up of direct myocardial gene transfer of vascular endothelial growth factor-2 using naked plasmid deoxyribonucleic acid by way of thoracotomy in no-option patients. Am J Cardiol. 2003;92:436–439. doi: 10.1016/s0002-9149(03)00661-1. [DOI] [PubMed] [Google Scholar]
- 4.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar N, Ruck A, Kallner G, Y-Hassan S, Blomberg P, Islam KB, et al. Effects of intramyocardial injection of phVEGF-A165 as sole therapy in patients with refractory coronary artery disease--12-month follow-up: angiogenic gene therapy. J Intern Med. 2001;250:373–381. doi: 10.1046/j.1365-2796.2001.00905.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 7.Dean DA. Nonviral gene transfer to skeletal, smooth, and cardiac muscle in living animals. Am J Physiol Cell Physiol. 2005;289:C233–245. doi: 10.1152/ajpcell.00613.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schalch P, Rahman GF, Patejunas G, Goldschmidt RA, Carbray J, Retuerto MA, et al. Adenoviral-mediated transfer of vascular endothelial growth factor 121 cDNA enhances myocardial perfusion and exercise performance in the nonischemic state. J Thorac Cardiovasc Surg. 2004;127:535–540. doi: 10.1016/j.jtcvs.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Herweijer H, Wolff JA. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther. 2003;10:453–458. doi: 10.1038/sj.gt.3301983. [DOI] [PubMed] [Google Scholar]
- 10.Niidome T, Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001;12:861–870. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 12.Wells DJ. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- 13.Caplen NJ, Alton EW, Middleton PG, Dorin JR, Stevenson BJ, Gao X, et al. Liposome-mediated CFTR gene transfer to the nasal epithelium of patients with cystic fibrosis. Nat Med. 1995;1:39–46. doi: 10.1038/nm0195-39. [DOI] [PubMed] [Google Scholar]
- 14.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolau C, Sene C. Liposome-mediated DNA transfer in eukaryotic cells. Dependence of the transfer efficiency upon the type of liposomes used and the host cell cycle stage. Biochim Biophys Acta. 1982;721:185–190. doi: 10.1016/0167-4889(82)90067-2. [DOI] [PubMed] [Google Scholar]
- 16.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc Natl Acad Sci U S A. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun WH, Burkholder JK, Sun J, Culp J, Turner J, Lu XG, et al. In vivo cytokine gene transfer by gene gun reduces tumor growth in mice. Proc Natl Acad Sci U S A. 1995;92:2889–2893. doi: 10.1073/pnas.92.7.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci U S A. 1990;87:9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D, Knapp JE. Hydrodynamics-based gene delivery. Curr Opin Mol Ther 2001. 2001;3:192–197. [PubMed] [Google Scholar]
- 20.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 21.Mir LM. Therapeutic perspectives of in vivo cell electropermeabilization. Bioelectrochemistry (Amsterdam, Netherlands) 2001;53:1–10. doi: 10.1016/s0302-4598(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 22.Aihara H, Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- 23.Glasspool-Malone J, Somiari S, Drabick JJ, Malone RW. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2:140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- 24.Heller LC, Heller R. In vivo electroporation for gene therapy. Hum Gene Therapy. 2006;17:890–897. doi: 10.1089/hum.2006.17.890. [DOI] [PubMed] [Google Scholar]
- 25.Lucas ML, Heller R. Immunomodulation by electrically enhanced delivery of plasmid DNA encoding IL-12 to murine skeletal muscle. Mol Ther. 2001;3:47–53. doi: 10.1006/mthe.2000.0233. [DOI] [PubMed] [Google Scholar]
- 26.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I Trial of Interleukin-12 Plasmid Electroporation in Patients With Metastatic Melanoma. J Clin Oncol. 2008;26(35):5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Low L, Mander A, McCann KJ, Dearnaley D, Tjelle TE, Mathiesen I, et al. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20(11):1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 28.Harrison RL, Byrne BJ, Tung L. Electroporation-mediated gene transfer in cardiac tissue. FEBS Lett. 1998;435:1–5. doi: 10.1016/s0014-5793(98)00987-9. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JF, Hayes LS, Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–177. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- 30.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu L-Q, Bunting S, et al. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou A, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 32.Hojman P, Gissel H, Andre FM, Cournil-Henrionnet C, Eriksen J, Gehl J, et al. Physiological effects of high and low voltage pulse combinations for gene electrotransfer in muscle. Hum Gene Ther. 2008;19:1249–1260. doi: 10.1089/hum.2008.059. [DOI] [PubMed] [Google Scholar]
- 33.Kastrup J, Jørgensen E, Rück A, Tägil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmid vascular endothelial growth factor-a165 gene therapy in patients with stable severe angina pectoris. J Am Coll Cardiol. 2005;45(7):982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 34.Gaffney MM, Hynes SO, Barry F, O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol. 2007;152:175–188. doi: 10.1038/sj.bjp.0707315. [DOI] [PMC free article] [PubMed] [Google Scholar]


