Abstract
Prenatal cocaine exposure has been linked to increased child behavior difficulties in some studies but not others.
Objective
The primary aim was to estimate the relationship between in utero cocaine exposure and child behavioral functioning at age 7 years with ratings made by blinded examiners during a structured testing session. A second aim was to examine whether caregiver drug use and psychological problems might mediate suspected relationships between prenatal cocaine exposure and aspects of examiner-rated behavior.
Methods
407 children (212 cocaine-exposed, 195 non-exposed) participating in the longitudinal Miami Prenatal Cocaine Study (MPCS) were rated with regard to their behavior during a neuropsychological assessment conducted at age 7 years. Raters were trained research psychometricians blinded to drug exposure. Individual behavioral items were summarized and the cocaine-behavior relationship was estimated within the context of latent variable modeling, using Mplus software.
Results
Two latent variables, Behavioral Regulation and Sociability, were derived via exploratory latent structure analysis with promax rotation. Prenatal cocaine exposure, statistically controlling for child sex, test age, and prenatal exposure to alcohol, tobacco, and marijuana, was associated with Behavioral Regulation (estimated slope ß = -0.25; 95% CI = -0.48, -0.02; p = 0.04) but not Sociability (estimated slope ß = -0.03; 95% CI = -0.26, 0.20; p = 0.79). Neither postnatal drug use by caregivers nor the severity of their psychological problems at age 5 follow-up predicted levels of child Behavioral Regulation or Sociability at age 7 years (p>0.10).
Conclusions
Examiner ratings of child behavior at age 7 revealed less optimal behavioral regulation for prenatally cocaine-exposed compared to non-exposed children, in contrast with what had been previously found from parent-report data. This evidence highlights the potential value of trained observers in assessing behavioral outcomes of children exposed in utero to drugs and other toxicants.
Keywords: prenatal cocaine exposure, behavior, examiner ratings, caregiver drug use, caregiver psychological functioning, behavioral regulation
1. INTRODUCTION
Evidence from neuropsychopharmacological and neurodevelopmental studies suggests that in utero cocaine exposure alters arousal, attention regulation, and stress responses in offspring [Mayes, 1999; Mayes, 2002]. In preclinical models, for example, gestational cocaine exposure has been shown to result in structural and functional modifications to monoaminergic pathways, including pathways thought to be integral to regulatory abilities. Additionally, in utero cocaine exposure has been associated in several animal models with behavioral responses indicative of regulatory difficulties [Brunzell et al., 2002; Gendle et al., 2004; He et al., 2004; Morgan et al., 2002; Spear et al., 1998]. In some clinical studies, prenatally cocaine-exposed infants and toddlers have shown more negative affect, poorer impulse control, greater arousal in response to stress, and decreased ability to physiologically regulate during a stressful laboratory task [Bard et al., 2000; Bendersky and Lewis, 1998a; Bendersky and Lewis, 1998b; Chaplin et al., 2009; Schuetze et al., 2007], findings which collectively suggest a profile of cocaine-associated regulatory difficulties. It is unclear, however, the extent to which these early regulatory deficits might manifest as children with prenatal cocaine exposure (PCE) mature. In theory, cocaine-associated impairments in behavioral regulation may appear at a later age in the form of learning and attentional difficulties, or emotional and behavioral problems, especially in the context of stressful environmental conditions [Bendersky et al., 2006; Bennett et al., 2002; Lester et al., 2009; Lester and Padbury, 2009; Mayes, 2002].
Published reports on the long-term behavioral outcomes of prenatally cocaine-exposed children are limited, and results to date are mixed. Some studies have linked PCE to increased childhood behavior problems [Bada et al., 2007; Chasnoff et al., 1998; Delaney-Black et al., 2000; Linares et al., 2006], while others have failed to support this association [Bennett et al., 2002; Phelps et al., 1997; Richardson et al., 1996; Warner et al., 2006]. Discrepant findings may be attributed partly to methodological variations across studies, including differences in the measurement of behavior. In prior research, parent-report measures, such as the Achenbach Child Behavior Checklist (CBCL), have often been used to measure behavioral outcomes. These measures are efficient and can yield valuable information regarding child behavioral functioning from the parent’s perspective. Nonetheless, they have limitations. Global measures such as the CBCL may not be sufficiently sensitive to detect subtle and/or specific behavioral and emotional difficulties. Additionally, parents offer only one viewpoint of the child’s behavior, and ratings can be influenced by other factors such as parental psychological functioning [Chilcoat and Breslau, 1997]. It is important, therefore, that additional perspectives on child behavior be considered, particularly when caregiver functioning may be compromised (e.g., by the parent’s continued drug use or depression). Several studies that have found an association between PCE and child behavior problems have utilized teacher-report measures [Delaney-Black et al., 1998; Delaney-Black et al., 2000]. Teachers have the advantage of observing the child’s behavior in the structured classroom setting under conditions of academic stress or demand and have a basis of comparison of a child’s behavior to that of his/her same-aged peers. However, teachers may be aware of the child’s history of PCE and thus also have the potential for bias in their behavioral ratings.
In previous reports from the Miami Prenatal Cocaine Study (MPCS) using the parent-report CBCL, we found no evidence of prenatal cocaine-associated increases in behavior problems at either age 5 or 7 years [Accornero et al., 2002; Accornero et al., 2006]. In the current study, we sought to extend this line of work by examining the behavior of prenatally cocaine-exposed and non-cocaine-exposed children from the perspective of a different type of informant—namely, an independently trained and blinded observer (i.e., one who is blinded to the cocaine exposure status of the child). Specifically, the primary aim of this study was to estimate the relationship between PCE and aspects of behavior at age 7 years using behavioral ratings made in a structured testing session by trained examiners. Secondly, we sought to examine whether the suspected cocaine-behavior relationship might be mediated by aspects of primary caregiver behavioral health (i.e., drug use and psychological distress). This research question was motivated by prior MPCS results that linked maternal behavioral health (recent drug use and psychological distress) to higher levels of externalizing behaviors at 5 years of age, as well as a substantial literature linking parental drug abuse and psychological syndromes to negative child developmental and emotional/behavioral outcomes [Ashman et al., 2008; Clark et al., 2004; Glasheen et al., 2010; Johnson and Leff, 1999; Luthar and Sexton, 2007; Stanger et al., 1999].
2. METHODS
2.1 Study Design
As part of a larger epidemiological study, 476 mother-infant dyads were enrolled prospectively into a follow-up cohort to evaluate effects of PCE on long-term developmental outcomes. Participants were recruited from the delivery service of the University of Miami Miller School of Medicine-Jackson Memorial Medical Center (UM-JMMC). The overall study design and recruitment of the follow-up cohort for the longitudinal MPCS, briefly summarized below, has been detailed in a separate report [Bandstra et al., 2001a]. The study was approved by the Institutional Review Board and conducted under a federal Department of Health and Human Services Certificate of Confidentiality.
2.2 Study Participants
The MPCS longitudinal follow-up sample of 476 infants was recruited from November 1990 through July 1993. The study sample was homogeneous with regard to full-term gestational age (≥37 completed weeks), low socioeconomic status, inner-city residence, and African-American race/ethnicity. Study exclusion criteria were as follows: maternal HIV/AIDS; prenatal exposure to opiates, methadone, amphetamines, barbiturates, benzodiazepines or phencyclidine; major congenital malformation; chromosomal abnormality; or disseminated congenital infection. PCE was determined by maternal self-report of cocaine use during pregnancy and/or by positive assay on one or more biologic markers, including maternal urine, infant urine, and meconium.
The originating birth cohort consisted of 253 prenatally cocaine-exposed (with or without concomitant exposure to alcohol, tobacco, or marijuana) and 223 comparison infants, of whom 147 were drug-free and 76 were exposed to varying combinations of alcohol, tobacco, or marijuana. The sample for the current report is a subsample of the original follow-up cohort, including only the children seen for the 7-year follow-up exam and who had a complete neuropsychological assessment and corresponding behavior rating form (n=407; 212 cocaine-exposed; 195 non-cocaine-exposed). Participants were excluded for the following reasons: 2 infants were deceased; 32 moved out of the area, too far to return to the study clinic for assessments; 11 declined participation in the 7-year exam; and 16 were lost to follow-up. There was no differential attrition between cocaine-exposed and non-cocaine-exposed groups, and nor were there differences in enrollment characteristics between those evaluated at the 7-year visit and those who were not. Table 1 presents a description of selected maternal and child characteristics measured at birth for this study subsample.
Table 1.
Maternal and Infant Characteristics at Birth Enrollment (n=407)
| Non-Cocaine-Exposed (n=195) | Cocaine-Exposed (n=212) | |
|---|---|---|
| Maternal Characteristics | ||
| Maternal age (years)* | 23.8 (5.4) | 28.9 (4.9) |
| Education (years) | 11.3 (1.4) | 11.1 (1.4) |
| Unemployed (%)* | 83 | 95 |
| Never married (%) | 88 | 91 |
| Primigravida (%)* | 24 | 7 |
| Prenatal care ≤ 3 visits (%)* | 15 | 31 |
| Infant Characteristics | ||
| Birth weight (gm)* | 3296 (510) | 2962 (467) |
| Birth length (cm)* | 50.7 (2.3) | 48.9 (2.5) |
| Birth head circumference (cm)* | 33.8 (1.5) | 33.0 (1.5) |
| Gestational age (wks)* | 39.7 (1.4) | 39.3 (1.4) |
| Male (%) | 51 | 48 |
Numbers represent means (S.D.) or percentages where indicated.
p <0.01
2.3 Data Collection Procedures
During the immediate postpartum period, experienced research staff performed a standardized research interview, and biological specimens were collected for analysis. Pertinent medical and demographic information were collected from the medical record at birth. Primary caregiver and child data relevant to the present study were drawn from the serial follow-up visits at ages 5 and 7 years. Examiners blinded to in utero drug exposure status performed all child assessments.
2.4 Drug Exposure Measures at Birth
A structured standardized interview was conducted by trained research staff within the first 36 hours postpartum to ascertain maternal substance use and additional demographic information. To enhance timeline recall, targeted recall periods were outlined and anchored to important calendar dates. Drug use questions during pregnancy were asked by trimester and included number of weeks used, most days/week, fewest days/week, usual days/week, and usual dose/day. Dosage was measured in number of cigarettes smoked, number of marijuana joints smoked, number of drinks of beer, wine or hard liquor, and number of cocaine lines/rocks. Standardized definitions were used for determining 1-drink units for each type of alcohol (beer 12 oz., wine 5 oz., and liquor 1.5 oz) [Schneiderman, 1990]. Pregnancy exposure composites were calculated for each drug by multiplying the number of weeks used by the usual days/week and the usual dose/day. Table 2 (columns 1 and 2) shows the percentage of mothers in each group self-reporting use of alcohol, tobacco, marijuana and cocaine, respectively. The total pregnancy self-report composites, based on mothers admitting use of each specific drug, are presented descriptively in Table 2 to reflect the median cohort exposure levels (columns 2 and 4).
Table 2.
Maternal Self-Reported Alcohol, Tobacco, Marijuana and Cocaine Use during Pregnancy
| Substance | Non-Cocaine-Exposed (n = 195) | Cocaine-Exposed (n = 212) | ||
|---|---|---|---|---|
| % (n) a | Median (Min, Max)b | % (n) a | Median (Min, Max) b | |
| Alcohol (# drinks) c | 30 (58) | 57 (2, 1680) | 67 (141) | 105 (1, 5226) |
| Tobacco (# cigarettes)c, d | 15 (30) | 1043 (21, 5880) | 77 (163) | 2184 (1, 8820) |
| Marijuana (# joints) c | 12 (23) | 32 (1, 807) | 45 (95) | 28 (1, 1229) |
| Cocaine/crack (# lines/rocks) | 68 (145) | 144 (1, 19320) | ||
Percentage (number) of mothers in each study group self-reporting drug use.
Median values based only on mothers reporting usage, calculated using total exposure composites: (number of weeks used) x (usual number of days per week) x (usual dose per day).
p<0.01, between group comparisons of percentage of mothers reporting drug use (columns 1 and 3).
p<0.05, between group comparisons of median amount of maternal drug use (columns 2 and 4).
Screening of urine and meconium for cocaine metabolite (benzoylecgonine) was performed by EMIT® (Syva D.A.U.), at a cut-off of 150 ng/ml for urine and 150 ng/gm for meconium, respectively, and cocaine-positive specimens were confirmed by gas chromatography/mass spectrometry (GC/MS) [Mulé and Casella, 1988]. Urine specimens were also assayed by EMIT® for marijuana (cannabinoids), opiates, amphetamines, barbiturates, benzodiazepines, and phencyclidine. Meconium specimens were assayed by EMIT® for marijuana and opiates. Infant urine and maternal urine was available for 98% and 79% of the total sample, respectively. Infant meconium was available for 86% of the original cohort. Of the original MPCS cohort, 100% had at least 1 biological marker, 96% had at least 2 biological markers, and 68% had all 3 biological markers.
Based on maternal self-report data and biological markers, infants were classified into two groups: non-cocaine-exposed and cocaine-exposed. Non-cocaine-exposed infants had a negative maternal self-report of cocaine during pregnancy and three months prior to pregnancy, as well as cocaine-negative results on all available specimens. Cocaine-exposed infants had documented maternal self-report of cocaine use during pregnancy and/or at least 1 GC/MS-confirmed cocaine-positive biological marker.
In the current study, PCE is expressed in all analyses as a dichotomous variable (no/yes) based on maternal self-report of cocaine use during pregnancy and/or by positive assay on one or more biologic markers. Self-reported pregnancy composites of alcohol, tobacco and marijuana use during pregnancy were used as covariates.
2.5 Child Measures
2.5.1 Behavior Observation Record (BOR)
The MPCS Behavior Observation Record (BOR) is an investigator-developed measure capturing the examiner’s perception of the child’s physical appearance, use/understanding of language, quality of social interaction, regulatory abilities, and test-taking skills as observed during standardized testing conducted at the 7-year follow-up visit. Using a Likert-type scale (1 through 5), examiners blinded to drug exposure status rated items following the administration of each of the primary measures comprising the comprehensive 7-year neuropsychological exam; i.e., the Wechsler Intelligence Scale for Children-III (WISC-III) Short Form [Wechsler, 1991], the NEPSY [Korkman et al., 1997], and the Wechsler Individual Achievement Test (WIAT) [Wechsler, 1993]. These measures were typically administered on the same clinic day (with corresponding behavioral ratings made on the same day by the same psychometrician), with the total testing/observation time averaging approximately 4 hours. All examiners were trained in coding by the same doctoral-level supervisor. Training included didactic components as well as extensive piloting of the measure (as part of the comprehensive neuropsychological battery) with repeated observation of each examiner and feedback of coding accuracy by the supervisor. A total of 27 items from the social interaction, regulatory abilities, and test-taking sections of the BOR were included in analyses. BOR items assessing physical appearance were not included in these analyses, as described below. Pairwise Spearman correlations between similar items from the BOR and the well-established CBCL (parent-report obtained at the 7-year visit) Attention Subscale score were statistically robust, providing preliminary evidence of construct validity. For example, the CBCL Attention Subscale score was correlated with the BOR items of ‘attention’, ‘persistence’ and ‘difficult to test’ (all with p<0.001).
2.6 Postnatal Caregiver Measures
2.6.1 Psychosocial Interview
A structured psychosocial interview was conducted with the mother/primary caregiver at each assessment visit. The primary caregiver was defined as the family member or custodial guardian responsible for the physical, emotional, and financial well-being of the child. Biological mothers residing with and parenting the child were prioritized for interview purposes as the primary caregiver. The psychosocial interview assessed key caregiver and family characteristics including: whether the biological mother was currently a primary caregiver; current caregiver age, educational level, employment and marital status; number of primary caregiver changes since birth; and child’s receipt of developmental services. Terms for these characteristics were included in the models to assess the relationship between PCE and examiner-rated behavior controlling for these factors.
2.6.2 Addiction Severity Index (ASI),[McLellan et al., 1992]Modified
An adapted ASI was administered to the child’s current primary caregiver during a follow-up assessment visit when the child was approximately 5 years of age. The alcohol and drug section of the ASI was modified to include frequency of substance use in the previous 12 months. Total number of days of substance use in the past year was calculated separately for alcohol, tobacco, marijuana, and cocaine and then recoded into categorical variables based on the frequency distribution for each of the variables. The Composite Score from the Psychiatric Status section of the ASI was included as a measure of caregiver psychological functioning. The weighted summary score, a continuous variable ranging from 0 (no problem) to 1 (most severe), is based on self-report of psychological symptom occurrence and duration as well as perceived impact of symptoms in the past 30 days. For descriptive purposes, the percentage of caregivers reporting recent drug use and the mean ASI Psychiatric Composite Score are presented by study group in Table 3.
Table 3.
Caregiver Characteristics at 5-year Follow-Up
| Non-Cocaine-Exposed (n=195) | Cocaine-Exposed (n=212) | |
|---|---|---|
| Biological mother as caregiver* | 96% | 65% |
| Age* | 29.1±6.8 | 38.6±9.8 |
| Never married | 66% | 59% |
| Employed | 40% | 36% |
| Highest grade completed | 11.5±1.5 | 11.2±1.8 |
| Past year alcohol use | 64% | 60% |
| Past year tobacco use* | 22% | 56% |
| Past year marijuana use* | 16% | 27% |
| Past year cocaine use* | 3% | 22% |
| ASI Psychiatric Composite Score | 0.08±0.15 | 0.08±0.14 |
Numbers represent means (standard deviation) or percentages where indicated.
p <0.01
2.7 Statistical Analyses
First, the three ratings for each BOR item (i.e., rated after the administration of each of the WISC-III, Short Form, NEPSY, and WIAT measures) were standardized (mean=0, variance=1) and summarized into a single scaled score via the Stata ‘alpha’command [Statacorp, 2010a; Statacorp, 2010b]. This approach yields a summary score that is highly correlated with a ‘principal factor’ solution such that ratings with greater discrimination are given slightly greater weight than ratings with lower discrimination. This approach is superior to the standard principal factor solution because there is no casewise deletion if a rating is missing; an imputation approach is used to derive the score for all cases using values for the non-missing ratings [Statacorp, 2010a; Statacorp, 2010b]. Cronbach’s alpha for these item scaled scores were generally high (≥ .90). Due to significant skewness, item scaled scores were then transformed into ordered categorical variables; the exception was the item ‘smiles’ where the distribution approached normal and therefore was treated as a continuous variable. The direction and amount of skew varied across items and thus so did the number of categories created in the transformation. As ‘impulsivity’ showed considerable variability, four ordered categorical indicators were created based on quartiles of the distribution. Items such as ‘anger/temper outbursts’, ‘aggression’, and ‘tearful/crying’ were relatively low frequency items and were thus recoded with only two categories (none/any).
Next, latent variable modeling via Mplus software, version 6.0. [Muthén and Muthén, 2004], was used to derive latent variables of examiner-rated behavior (i.e., latent dimensions observed via manifest indicators) from the 27 BOR items. The resulting behavior observation constructs were used to test the hypothesized PCE-behavior relationship, with PCE expressed dichotomously (no/yes) in all analyses. The hypothesized models, based on theory and extant research findings, specified a direct negative effect of PCE on dimensions of examiner-rated behavior at age 7 years, as well as an indirect negative effect through caregiver behavioral health measured by caregiver drug use (latent variable of self-reported alcohol, tobacco, marijuana and cocaine use in the past year) and psychological functioning assessed at the 5 year follow-up visit (Figures 1 and 2 depict the hypothesized models). The hypothesized models also included terms for child sex and age as well as prenatal alcohol, tobacco and marijuana exposure for statistical control of their possible impact on behavioral outcomes. Within this framework, primary caregiver drug use and psychological problem variables are hypothesized endogenous with respect to PCE, and the child behavior latent constructs are endogenous with respect to PCE, caregiver drug use, caregiver psychological problems, and the other hypothesized explanatory covariates listed above in the description of the specified models. These models represent only a subset of all possible models that might be fit to the observed study data. They are not necessarily expected to be the ‘correct’ models. Rather, they are specified as alternatives to the null models in which (a) there is no tangible PCE relationship with examiner-rated behavior at age 7 years; and (b) there is no mediation (indirect paths) through caregiver drug use or caregiver psychological problems. In analyses, each estimated regression coefficient from the structural equations model (herein referred to as ‘estimated slope’) and model fit statistics were used to evaluate the hypothesized model.
Figure 1.
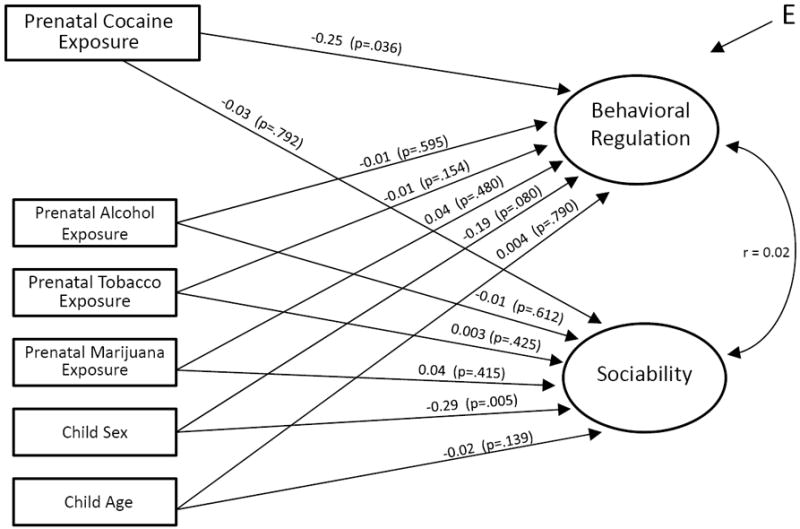
Estimated effects of prenatal cocaine exposure on examiner-rated behavior at age 7 years. n=407. Unstandardized regression coefficients and p-values shown along path arrows. Comparative Fit Index (CFI) = 0.97; Tucker-Lewis index (TLI) = 0.97; Root Mean Square Error of Approximation (RMSEA) = 0.04.
Figure 2.
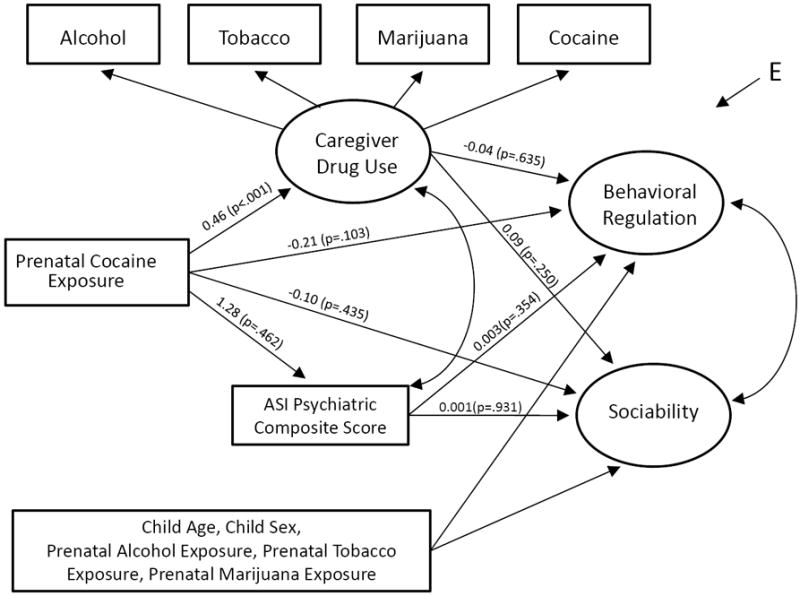
Impact of caregiver behavioral health on the relationship between prenatal cocaine exposure and facets of examiner-rated behavior at age 7 years. Caregiver drug use and psychological functioning were measured at the 5-year follow-up exam. n=372. Unstandardized regression coefficients and p-values shown along path arrows. Comparative Fit Index (CFI) = 0.97; Tucker-Lewis index (TLI) = 0.97; Root Mean Square Error of Approximation (RMSEA) = 0.04. In the n=372 sample, due to its item-level limited dispersion, it was necessary to drop the BOR aggression item from the Figure 2 model.
3. RESULTS
3.1 Sample Demographics
Maternal and infant characteristics of the study subsample (n=407) at delivery enrollment are presented in Table 1. Cocaine-using mothers were older, less often employed, had higher gravidity, and utilized less prenatal care than non-cocaine-using mothers. Prenatally cocaine-exposed infants had somewhat shorter gestation and were smaller in birth weight, length and head circumference than non-cocaine-exposed infants. Table 2 shows the amounts of prenatal alcohol, tobacco, marijuana, and cocaine use reported by the mothers admitting use of each drug within each study group. A higher percentage of cocaine-using mothers reported prenatal use of alcohol, tobacco, and marijuana. Among cigarette smokers, cocaine-using mothers also smoked a higher mean number of cigarettes than non-cocaine-using mothers. Of the mothers in the cocaine group (group status determined by positive self-report and/or bioassay), 68% self-reported cocaine powder or crack use during pregnancy.
There were no differences between the cocaine-exposed and non-cocaine-exposed groups in terms of child assessment age in months (88.6± 3.9; 88.8±4.2) or the child’s estimated Full Scale IQ (FSIQ) from the WISC-III Short Form (81.9±13.2; 83.1±13.7) at the 7-year follow-up visit when behavioral data were obtained (data not shown in table). In order to test mediation via caregiver behavioral health, caregiver data on drug use and psychological functioning were drawn from the preceding 5-year follow-up assessment. Accordingly, key primary caregiver characteristics of the study subsample at 5-year follow-up are summarized in Table 3. Cocaine-exposed children were less likely to be in the primary care of the biological mother at the time of the 5-year assessment. Also, caregivers in the prenatally cocaine-exposed group were older and reported more tobacco, marijuana, and cocaine use in the past year compared to those in the control group.
3.2 Multivariate Modeling
Using Mplus software, a total of 27 items from the behavior rating form were subjected to an exploratory latent structure analysis with categorical indicators and promax rotation. Examination of the scree plot and model fit statistics revealed a best-fitting two-trait solution. The first trait labeled “Behavioral Regulation”, was tapped by strongly loaded rating items such as “sustained attention” and “degree of cooperativeness”. The second trait, “Sociability”, was tapped by strongly loaded rating items such as “social relatedness” and “offers spontaneous information”. Five items (i.e., quality of expressive speech, fearful/anxious, fatigue, response to success, and response to failure) loaded on neither trait and were not included in this analysis. Discrimination parameter estimates (‘factor loadings’) for the items are summarized in Table 4. The two traits were allowed to be inter-correlated but the actual inter-correlation estimate was modest (r=0.02, 0.03).
Table 4.
MPCS Behavior Observation Record: Results of Latent Structure Analyses
| Item # | Item Description | Factor Loadings | |
|---|---|---|---|
| Behavioral Regulation | Sociability | ||
| 1 | Difficult to test | - .88 | - .10 |
| 2 | Degree of cooperativeness | .86 | .004 |
| 3 | Degree of structuring needed | - .86 | .08 |
| 4 | Overall sustained attention | .82 | .11 |
| 5 | Aggressive towards self or others | - .75 | - .08 |
| 6 | Anger/temper outbursts | - .75 | .11 |
| 7 | Persistence on difficult tasks | .74 | .18 |
| 8 | Work habits | .70 | .07 |
| 9 | Complains | - .69 | .33 |
| 10 | Motor restlessness/overactivity | - .65 | .32 |
| 11 | Tearful/crying | - .65 | - .21 |
| 12 | Impulsivity | - .58 | .31 |
| 13 | Benefits from intervention | - .50 | .15 |
| 14 | Understands verbal directions | .47 | .28 |
| 15 | Ability to maintain rapport | .46 | .71 |
| 16 | Amount of eye contact | .45 | .45 |
| 17 | Asks questions | - .30 | .75 |
| 18 | Laughter | - .14 | .66 |
| 19 | Overall social relatedness/interactions | .13 | .86 |
| 20 | Offers spontaneous information | - .10 | .84 |
| 21 | Smiles | .07 | .77 |
| 22 | Amount of expressive speech | .003 | .83 |
| 23* | Quality of expressive speech | 0.27 | 0.37 |
| 24* | Fearful/anxious | -0.14 | -0.36 |
| 25* | Fatigue | 0.29 | 0.22 |
| 26* | Response to success | 0.09 | 0.38 |
| 27* | Response to failure | -0.30 | 0.30 |
Note:
Items 23-27 were eliminated in initial exploratory latent structure models due to insufficient loadings on both factors (<0.40). Loadings as presented for Items 1-22 are from the final latent structure model. Model fit for the two factor solution is indicated by an estimated Root Mean Square Residual (RMR) = .07.
Next, within the framework of the structural equations model (SEM), the level of Behavioral Regulation and the level of Sociability were regressed simultaneously on a covariate term for PCE with ancillary covariate terms used to hold constant child sex, test age, and prenatal exposure to alcohol, tobacco, and marijuana. In this base model, the level of Behavioral Regulation was found to depend upon PCE (estimated slope ß = -0.25; 95% CI = -0.48, -0.02; p = 0.04), but this was not the case for level of Sociability (estimated slope ß = -0.03; 95% CI = -0.26, 0.20; p = 0.79; see Figure 1). Child sex was associated with Sociability, with males evidencing lower scores on the Sociability trait (p=0.005), but child sex did not predict Behavioral Regulation (p=0.08). The prenatal cocaine estimate of interest remained stable when the covariate vector was extended to include a term for each additional covariate including mother’s age, educational level, marital status and employment status at time of delivery, and primigravida (entered into the model one at a time). The size and sign of the estimated male-female difference in Behavioral Regulation and Sociability provide insight into the meaning of the observed PCE relationships. For example, the estimated Behavioral Regulation trait value for males was 0.19 units lower than the estimate for females, and the estimated trait value for PCE-exposed was 0.25 units lower than the estimate for non-exposed. Accordingly, it follows that the size of the effect of PCE on Behavioral Regulation is not much larger in size to the male-female difference in Behavioral Regulation. As for Sociability, the estimated trait value for males was -0.29 standard units lower than the estimate for females (corresponding to a one unit difference in the male-female variable, coded 0 for female and 1 for male). As compared to this understandable male-female difference of -0.29 units, the PCE relationship to Sociability was much smaller at -0.03, with the PCE variable also dummy-coded with 0 for non-exposed and 1 for exposed.
Additionally, the estimate did not change appreciably when the multivariate response vector was extended to include other possibly endogenous consequences of maternal cocaine use such as prenatal care and fetal growth, entered one at a time (i.e., the introduction of the term in the model did not alter the base model estimate to any great extent; by ‘appreciably’ we mean a shift in estimate so large that the resulting PCE effect estimate falls outside an interval defined by the base estimate +/- one standard error of that estimate). Nor did the estimate change appreciably with the inclusion of terms for the following postnatal characteristics: biological mother as the primary caregiver; number of primary caregiver changes; current primary caregiver’s age, level of education, employment status, and marital status; caregiver’s recent use of cocaine; and child’s estimated WISC-III FSIQ and receipt of developmental services. In a post-estimation exploration step, the ‘multiple indicators, multiple causes’ (MIMIC) approach described by Rabe-Hesketh and Skrondahl [2005] was used to check whether individual facets of child behavior might have been directly influenced by PCE, over and above the indirect paths leading from PCE through the latent constructs and onward to the manifest variables. Because the evidence indicated no direct path at p<0.05 for any of the individual facets of child behavior, the resulting estimates are not presented here.
In the next series of analyses, latent variable modeling was used to examine whether caregiver behavioral health, defined here by caregiver drug use and psychological problems as assessed at the 5-year follow-up visit, might mediate the observed relationship between PCE and Behavioral Regulation. Recent caregiver drug use was expressed as a latent variable measured by four categorical indicators of alcohol, tobacco, marijuana, and cocaine use in the year preceding the 5-year assessment. The ASI Psychiatric Composite Score was entered in the model as an estimate of recent caregiver psychological problems. Due to missing values on these age 5 variables for some participants, the sample size for this analysis was slightly smaller (n=372). When caregiver drug use and psychological problems were entered separately and then together (shown here in Figure 2), as an extension of the more basic model (Figure 1), neither was associated with Behavioral Regulation or Sociability (p>0.10). As expected, there was a statistically robust slope linking cocaine use during pregnancy to caregiver drug use measured at the 5-year follow-up (ß= 0.46; 95% CI= 0.22, 0.67; p <0.001). The inclusion of these caregiver behavioral health indicators (as measured at the 5-year follow-up exam) did not appreciably change the estimate linking PCE and Behavioral Regulation at age 7 years (e.g., ß= -0.21, well within the prior 95% CI of -0.48, -0.02 as shown in Figure 1).
4. DISCUSSION
Prior research on the behavior of prenatally cocaine-exposed school-aged children is largely based on reports of parents or teachers (e.g., CBCL ratings). In contrast, the novel findings of this longitudinal research, with evidence of a suspected PCE effect on behavioral regulation, are based upon ratings made by independent examiners, blind to the cocaine exposure status of the children. Two behavioral traits, derived from exploratory latent structure analysis, were examined: Behavioral Regulation and Sociability. Behavioral Regulation at 7 years, but not Sociability, was found to be linked to PCE. Estimates remained fairly stable with consideration of multiple key covariates, including factors such as prenatal exposures to other drugs, maternal characteristics measured at birth, and caregiver characteristics measured during follow-up assessments. Moreover, levels of caregiver drug use and psychological distress at the age 5 study visit did not seem to mediate the relationship between PCE and Behavioral Regulation observed at the age 7 study visit.
Behavioral regulation, a component of the more general construct of self-regulation, refers to the ability to control and modify behavior in response to environmental stimuli and situational demands. While the emphasis is on overt strategies and behaviors, behavioral regulation is supported by cognitive processes such as executive functioning and is likely influenced by internal emotional and arousal states. The Behavioral Regulation trait here, derived through latent structure analysis, was tapped by behaviors such as degree of cooperativeness, structure needed to maintain testing, sustained attention, anger/temper outbursts, persistence on difficult tasks, overactivity, and impulsivity, which are similar to indicators used by others in the assessment of behavioral regulation during childhood [Calkins and Dedmon, 2000; Clark et al., 2008; Dawes et al., 1997; Martin et al., 1994]. Behavioral regulation has been inversely linked to greater externalizing behavior problems in children [Eisenberg et al., 2000] and has been shown to impact functioning in multiple domains of adaptation including social and academic competence [McClelland et al., 2007; Schonfeld et al., 2009; von Suchodoletz et al., 2009]. Moreover, high-risk children and adolescents have been shown to exhibit poorer self-regulation compared to low-risk controls [Calkins and Dedmon, 2000; Dawes et al., 1997; Martin et al., 1994], and manifestations of behavioral dysregulation have been linked to drug use and high-risk sexual behavior in adolescence [Mezzich et al., 1997; Tarter et al., 2003].
Study findings lend support to the theory of a hypothesized disturbance in regulatory abilities secondary to in utero cocaine exposure and are consistent with other observational data indicating less optimal behavioral regulation among cocaine-exposed children. For example, Espy et al.[1999] found that cocaine-exposed toddlers were rated by examiners as less regulated and less engaged during the administration of the Bayley Scales of Infant Development (BSID), although raters in this cited study were not blinded to the children’s prenatal drug exposure status. Similarly, Heffelfinger and colleagues [Heffelfinger et al., 2002], with ratings from blinded observers, reported cocaine-associated differences on the BSID Emotional Regulation factor in a small group of children aged 14 to 60 months. Richardson [1998] also reported poorer engagement at 1 year of age and shorter attention span and greater restlessness at 3 years as observed and rated by masked examiners during developmental testing. In another study examining reactivity and regulation during the preschool years, PCE was associated with shorter latency to frustration during a problem-solving task, particularly among boys [Dennis et al., 2006]. Cocaine-exposed children have also displayed more inattention and impulsivity in the laboratory setting as measured by the Continuous Performance Test (CPT)[Bandstra et al., 2001b; Dow-Edwards et al., 1999; Richardson et al., 1996; Savage et al., 2005] and the delay of gratification task [Bendersky and Lewis, 1998b]. Moreover, greater emotional and behavioral problems, a possible manifestation of dysregulation, have been reported in preschool- and school-aged cocaine-exposed children compared to controls based on teacher-report data [Delaney-Black et al., 1998; Delaney-Black et al., 2000] and child responses [Bendersky et al., 2006; Linares et al., 2006]. Cocaine-associated increases in parent-reported behavioral difficulties have also been shown in some studies [Bada et al., 2007; Chasnoff et al., 1998; Hawley et al., 1995] but not others [Bennett et al., 2002; Phelps et al., 1997].
In our previous work [Accornero et al., 2002; Accornero et al., 2006], we did not find PCE-associated elevations in behavior problems at ages 5 or 7 years as reported by the caregivers, but as noted in the introduction, this variation might be attributed to the source of information. That is, the difference between those results and the current evidence from blinded behavioral raters are not entirely surprising given the differences in the assessment methods: a) the rater (i.e., parent vs. examiner), b) the context in which behavior was measured (i.e., home vs. clinic setting), and c) the specificity of the measures. Other prenatal cocaine researchers have also observed discrepancies across behavioral outcome measures. For example, Linares and colleagues [2006] reported that PCE was related to child self-reported symptoms of oppositional defiant disorder (ODD) and attention deficit hyperactivity disorder (ADHD), but not parent-reported behavior on the CBCL at age 6 years. Similarly, Bendersky et al.[2006] found that the strength of the relationship between PCE and aggression at age 5 varied depending on the measure considered. When parent ratings, teacher ratings, child report and direct observation data were examined individually, only the child’s report of aggressive behavior in response to hypothetical situations was associated with PCE. There was also a relationship between PCE and a composite aggression indicator that combined high aggression values from all the measures. In a review of the literature, Dixon and colleagues [2008] noted that the majority of studies showing a prenatal cocaine impact on behavior used narrow-band or direct observation assessments as opposed to the broad-band CBCL, suggesting that more specific and sensitive measures may be needed to capture subtle cocaine-associated behavioral difficulties.
In reference to our own data, we cannot assume that the examiner ratings of child behavior generalize to other settings, although it is possible that such ratings may reveal behavioral difficulties not found with parent-report. While parent-report measures such as the Achenbach CBCL are an important part of a behavioral assessment, there is concern about bias. Much of the literature on the possible bias in parental ratings focuses on the potential for parents to exaggerate behavioral problems in certain circumstances such as when the parent is depressed or has other psychological difficulties [Chilcoat and Breslau, 1997]. Nevertheless, it is also possible that parents may in some cases minimize child behavior problems. One study investigated the relationship between measures of social desirability and the parent-report CBCL and found an inverse association between measures; that is, parents with higher scores on social desirability instruments (adapted to reflect social desirability with regard to their child’s behavior) reported fewer behavior problems on the CBCL, particularly for externalizing behavior [Merydith et al., 2003]. Laboratory measures, including tester ratings, may circumvent some of the potential biases associated with parent-report and teacher report, although it is critical that psychometricians remain blinded to prenatal drug exposure status and other key characteristics that might influence their ratings or interpretation of tests/tasks [Rose-Jacobs et al., 2002]. Moreover, well-trained examiners knowledgeable about child development may be able to detect subtle behavioral difficulties not perceived by the parent, and behavioral regulation deficits may be more apparent in the clinic setting in the context of the highly structured, demanding nature of neuropsychological testing.
In this study, we did not find evidence that an intermediate measure of caregiver behavioral health functioned as a mediator in the relationship between PCE and Behavioral Regulation at age 7 as measured in this study. As might be expected, mother’s use of cocaine during pregnancy predicted presence of caregiver drug use at the 5-year follow-up; nonetheless, neither caregiver drug use nor psychological distress as measured at the 5-year follow-up predicted child Behavioral Regulation or Sociability at age 7. Additionally, the estimated relationship between PCE and child behavioral regulation was not appreciably attenuated when terms for recent caregiver behavioral health were introduced into the model.
These results are somewhat unexpected in light of our previously reported findings of an association between maternal behavioral health and child behavior problems on the parent-report CBCL at age 5 [Accornero et al., 2002]. In addition, a number of studies have linked parental drug use and psychological problems with greater childhood behavioral problems [Clark et al., 2004; Luthar and Sexton, 2007; Stanger et al., 1999]. There is also mounting evidence pointing to the role of environmental risks, including caregiver characteristics and caregiving quality, and contextual factors in influencing a variety of outcomes among cocaine-exposed children [Ackerman et al., 2008; Arendt et al., 2004; Bada et al., 2008; Bendersky et al., 2006; Bennett et al., 2002; Singer et al., 2008; Warner et al., 2006]. The absence of a link between caregiver behavioral health and child behavior in the current study may be attributed in part to the timing of the measurements. In an effort to establish mediation, we included measures of caregiver behavioral health that were taken approximately 2 years prior to the assessment of behavior. Fluctuations in caregiver drug use and psychological symptoms may have occurred during this period. It is possible that mediation via caregiver behavioral health may have been observed if indicators of more current caregiver functioning were included in the model. However, the research protocol for this study did not allow assessments of caregiver functioning just prior to the 7-year exam, which would be required to accurately ascertain if caregiver functioning has a more proximal effect on child behavioral regulation.
The MPCS has a number of strengths including prospective enrollment of a large cohort of prenatally cocaine-exposed infants and demographically-similar non-cocaine-exposed controls, verification of prenatal substance exposure through bioassays, substantial retention through the 7-year assessment, and well-trained, blinded examiners for all child assessments. However, there are limitations to this study that deserve mention. Because the sample was restricted to African-American, full-term infants from low-socioeconomic neighborhoods, findings may not generalize to other populations. Also, given some evidence suggesting a possible prenatal cocaine effect on gestational age, the exclusion of premature infants may have eliminated one pathway through which cocaine exposure might impact later developmental outcomes; if so, the effect of PCE on Behavioral Regulation observed in this study might actually be an underestimate. Caregiver drug use and psychological symptoms are based solely on self-report and thus are likely an underestimate, which may have affected findings. Specific to the current report, the behavioral outcome measure used, the MPCS Behavior Observation Record (BOR), is an investigator-developed rating scale not yet normed and without more rigorous psychometric evaluation beyond what is described in this initial report. The latent structure analysis of the BOR items, as well as statistically robust correlations between similar items on the BOR and the CBCL Attention Subscale score, provide initial evidence of construct validity for the measure. However, further assessment of the BOR’s reliability and validity is warranted. Definitive conclusions regarding the impact of PCE on behavioral regulation, as measured by the BOR in this sample, should await further evidence of the BOR’s psychometric properties. Also, while results indicate lower Behavioral Regulation scores in prenatally cocaine-exposed children compared to controls, the clinical significance of these findings is not yet clear. Additional research is needed to ascertain the impact, if any, of cocaine-associated differences on the BOR on ‘real-world’ outcomes in the domains of academic performance and more tangible behavioral outcomes (e.g., school suspensions). Finally, there are postnatal environmental factors other than caregiver behavioral health that may influence child behavioral functioning (e.g., parenting; exposure to violence). It will be important in future work to investigate the influence of PCE on behavior within the context of a full array of environmental risks.
In summary, prenatally cocaine-exposed children were rated as less behaviorally regulated during a structured testing session at age 7 when compared to non-exposed children of the same age. These findings are congruent with the well-documented hypothesis that in utero cocaine exposure alters regulatory abilities in offspring [Mayes, 1999] and are in line with other studies reporting evidence of cocaine-associated emotional and behavioral dysregulation in preschool and school-aged children [Dennis et al., 2006; Heffelfinger et al., 2002; Linares et al., 2006; Richardson, 1998]. Although it cannot be asserted that observer ratings are accurate and caregiver ratings inaccurate, these new findings raise concern that there might be prenatal cocaine-behavior associations not ordinarily identifiable in studies that base their conclusions solely upon maternal or caregiver report or through broad-band measures alone (e.g., Achenbach Child Behavior Checklist scales). Replication studies are needed, with multiple measures from multiple informants to assess emotional and behavioral functioning and with continued followup through the school-age and adolescent years to determine whether the observed behavioral dysregulation confers risk for poorer academic performance and adverse psychological outcomes, including high risk behaviors such as drug use and risky sexual behavior.
Acknowledgments
This research was supported by grants from the National Institutes of Health (NIH) National Institute on Drug Abuse (NIDA; K01 DA 016720, Principal Investigator [PI]: V.H. Accornero; RO1 DA 006556, PI: E.S. Bandstra; K05 DA 015799, PI: J.C. Anthony), NIH Office of Research on Women’s Health and NIDA (P50 DA 024584, PI: E.S. Bandstra), the NIH Center for Research Resources, University of Miami General Clinical Research Center (MO1-RR 16587), the Health Foundation of South Florida, and the Kenneth A. Lattman Foundation.
Footnotes
Conflict of Interest The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Veronica H. Accornero, University of Miami Miller School of Medicine, Department of Pediatrics, P.O. Box 016960 (M-808), Miami, FL 33101, vaccornero@med.miami.edu
James C. Anthony, Michigan State University, Department of Epidemiology, B601 West Fee Hall, East Lansing, MI 48824, janthony@epi.msu.edu
Connie E. Morrow, University of Miami Miller School of Medicine, Department of Pediatrics, P.O. Box 016960 (M-808), Miami, FL 33101, cmorrow@med.miami.edu
Lihua Xue, University of Miami Miller School of Medicine, Department of Pediatrics, P.O. Box 016960 (M-808), Miami, FL 33101, lxue@med.miami.edu
Elana Mansoor, University of Miami Miller School of Medicine, Department of Pediatrics, P.O. Box 016960 (M-808), Miami, FL 33101, emansoor@med.miami.edu
Arnise L. Johnson, University of Miami Miller School of Medicine, Department of Pediatrics, P.O. Box 016960 (M-808), Miami, FL 33101, Arnisepsy@earthlink.net
Clyde B. McCoy, Department of Epidemiology and Public Health, University of Miami Miller School of Medicine, 1120 NW 14th Street, Room 906, Miami, Fl. 33136, cmccoy@med.miami.edu
Emmalee S. Bandstra, University of Miami Miller School of Medicine, Department of Pediatrics, Division of Neonatology, P.O. Box 016960 (R-131), Miami, FL 33101, ebandstr@med.miami.edu
Reference List
- Accornero VH, Anthony JC, Morrow CE, Xue L, Bandstra ES. Prenatal cocaine exposure: An examination of childhood internalizing and externalizing behavior problems at age 7 years. Epidemiol Psichiatr Soc. 2006;15:20–9. PMCID: PMC2641031. [PMC free article] [PubMed] [Google Scholar]
- Accornero VH, Morrow CE, Bandstra ES, Johnson AL, Anthony JC. Behavioral outcome of preschoolers exposed prenatally to cocaine: role of maternal behavioral health. J Pediatr Psychol. 2002;27:259–69. doi: 10.1093/jpepsy/27.3.259. PMCID: PMC2760334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman JP, Llorente AM, Black MM, Ackerman CS, Mayes LA, Nair P. The effect of prenatal drug exposure and caregiving context on children’s performance on a task of sustained visual attention. J Dev Behav Pediatr. 2008;29:467–74. doi: 10.1097/DBP.0b013e3181903168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt RE, Short EJ, Singer LT, Minnes S, Hewitt J, Flynn S, Carlson L, Min MO, Klein N, Flannery D. Children prenatally exposed to cocaine: developmental outcomes and environmental risks at seven years of age. J Dev Beh Ped. 2004;25:83–90. doi: 10.1097/00004703-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H. Trajectories of maternal depression over 7 years: relations with child psychophysiology and behavior and role of contextual risks. Dev Psychopathol. 2008;20:55–77. doi: 10.1017/S0954579408000035. [DOI] [PubMed] [Google Scholar]
- Bada HS, Das A, Bauer CR, Shankaran S, Lester B, LaGasse L, Hammond J, Wright LL, Higgins R. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007;119:e348–e359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- Bada HS, Langer J, Twomey J, Bursi C, LaGasse L, Bauer CR, Shankaran S, Lester BM, Higgins R, Maza PL. Importance of stability of early living arrangements on behavior outcomes of children with and without prenatal drug exposure. J Dev Behav Pediatr. 2008;29:173–82. doi: 10.1097/DBP.0b013e3181644a79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Churchill SS, Chitwood DD, Steele BM, Ofir AY, Xue L. Intrauterine growth of full-term infants: impact of prenatal cocaine exposure. Pediatrics. 2001a;108:1309–19. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicol Teratol. 2001b;23:545–59. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Dev Psychobiol. 2000;36:194–212. [PubMed] [Google Scholar]
- Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. J Pediatr Psychol. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Arousal modulation in cocaine-exposed infants. Dev Psychol. 1998a;34:555–64. [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Lewis M. Prenatal cocaine exposure and impulse control at two years. Ann N Y Acad Sci. 1998b;846:365–7. [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Dev Psychol. 2002;38:648–58. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Ayres JJB, Meyer JS. Effects of prenatal cocaine exposure on latent inhibition in 1-year-old female rats. Pharmacol Biochem Behav. 2002;72:795–802. doi: 10.1016/s0091-3057(02)00773-6. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. J Abnorm Child Psychol. 2000;28:103–18. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Fahy T, Sinha R, Mayes LC. Emotional arousal in cocaine exposed toddlers: prediction of behavior problems. Neurotoxicol Teratol. 2009;31:275–82. doi: 10.1016/j.ntt.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasnoff IJ, Anson A, Hatcher RP, Stenson H, Iaukea K, Randolph LA. Prenatal exposure to cocaine and other drugs: outcome at four to six years. Ann N Y Acad Sci. 1998;846:314–28. [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Does psychiatric history bias mothers’ reports? An application of a new analytic approach. J Am Acad Child Adolesc Psychiatry. 1997;36:971–9. doi: 10.1097/00004583-199707000-00020. [DOI] [PubMed] [Google Scholar]
- Clark CA, Woodward LJ, Horwood LJ, Moor S. Development of emotional and behavioral regulation in children born extremely preterm and very preterm: biological and social influences. Child Dev. 2008;79:1444–62. doi: 10.1111/j.1467-8624.2008.01198.x. [DOI] [PubMed] [Google Scholar]
- Clark DB, Cornelius J, Wood DS, Vanyukov M. Psychopathology risk transmission in children of parents with substance use disorders. Am J Psychiatry. 2004;161:685–91. doi: 10.1176/appi.ajp.161.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes MA, Tarter RE, Kirisci L. Behavioral self-regulation: Correlates and 2 year follow-ups for boys at risk for substance abuse. Drug Alcohol Depend. 1997;45:165–76. doi: 10.1016/s0376-8716(97)01359-8. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager J, Nordstrom-Klee B, Martier S, Leddick L, Czerwinski RH, Sokol RJ. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–91. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager JW, Martier SS, Sokol RJ. Prenatal cocaine exposure and child behavior. Pediatrics. 1998;102:945–50. doi: 10.1542/peds.102.4.945. [DOI] [PubMed] [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Dev Psychol. 2006;42:688–97. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DR, Kurtz PF, Chin MD. A systematic review of challenging behaviors in children exposed prenatally to substances of abuse. Res Dev Disabil. 2008;29:483–502. doi: 10.1016/j.ridd.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, Mayes L, Spear L, Hurd Y. Cocaine and development: clinical, behavioral, and neurobiological perspectives--a symposium report. Neurotoxicol Teratol. 1999;21:481–90. doi: 10.1016/s0892-0362(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Guthrie IK, Fabes RA, Shepard S, Losoya S, Murphy BC, Jones S, Poulin R, Reiser M. Prediction of elementary school children’s externalizing problem behaviors from attentional and behavioral regulation and negative emotionality. Child Dev. 2000;71:1367–82. doi: 10.1111/1467-8624.00233. [DOI] [PubMed] [Google Scholar]
- Espy KA, Kaufmann P, Glisky M. Neuropsychologic function in toddlers exposed to cocaine in utero: a preliminary study. Dev Neuropsychol. 1999;15:447–60. [Google Scholar]
- Gendle MH, White TL, Strawderman M, Mactutus CF, Booze RM, Levitsky DA, Strupp BJ. Enduring effects of prenatal cocaine exposure on selective attention and reactivity to errors: evidence from an animal model. Behav Neurosci. 2004;118:290–7. doi: 10.1037/0735-7044.118.2.290. [DOI] [PubMed] [Google Scholar]
- Glasheen C, Richardson GA, Fabio A. A systematic review of the effects of postnatal maternal anxiety on children. Arch Womens Ment Health. 2010;13:61–74. doi: 10.1007/s00737-009-0109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley TL, Halle TG, Drasin RE, Thomas NG. Children of addicted mothers: effects of the ‘crack epidemic’ on the caregiving environment and the development of preschoolers. Am J Orthopsychiatry. 1995;65:364–79. doi: 10.1037/h0079693. [DOI] [PubMed] [Google Scholar]
- He N, Bai J, Champoux M, Suomi SJ, Lidow MS. Neurobehavioral deficits in neonatal rhesus monkeys exposed to cocaine in utero. Neurotoxicol Teratol. 2004;26:13–21. doi: 10.1016/j.ntt.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: implications for behavioral regulation. J Int Neuropsychol Soc. 2002;8:12–21. [PubMed] [Google Scholar]
- Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103:1085–99. [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. A Developmental Neuropsychological Assessment Manual. San Antonio, TX: Psychological Corporation; 1997. NEPSY. [Google Scholar]
- Lester BM, Bagner DM, Liu J, LaGasse LL, Seifer R, Bauer CR, Shankaran S, Bada H, Higgins RD, Das A. Infant neurobehavioral dysregulation: behavior problems in children with prenatal substance exposure. Pediatrics. 2009;124:1355–62. doi: 10.1542/peds.2008-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Dev Neurosci. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Linares TJ, Singer LT, Kirchner HL, Short EJ, Min MYO, Hussey P, Minnes S. Mental health outcomes of cocaine-exposed children at 6 years of age. J Pediatr Psychol. 2006;31:85–97. doi: 10.1093/jpepsy/jsj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, Sexton CC. Maternal drug abuse versus maternal depression: vulnerability and resilience among school-age and adolescent offspring. Dev Psychopathol. 2007;19:205–25. doi: 10.1017/S0954579407070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Blackson TC, Vanyukov MM, Moss HB, Tarter RE. Aggressiveness, inattention, hyperactivity, and Impulsivity in boys at high and low-risk for substance-abuse. J Abnorm Child Psychol. 1994;22:177–203. doi: 10.1007/BF02167899. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicol Teratol. 2002;24:385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers’ literacy, vocabulary, and math skills. Dev Psychol. 2007;43:947–59. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The 5th edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Merydith SP, Prout HT, Blaha J. Social desirability and behavioral rating scales: An exploratory study with the Child Behavior Checklist/4-18. Psychol Schools. 2003;40:225–35. [Google Scholar]
- Mezzich AC, Tarter RE, Giancola PR, Lu S, Kirisci L, Parks S. Substance use and risky sexual behavior in female adolescents. Drug Alcohol Depend. 1997;44:157–66. doi: 10.1016/s0376-8716(96)01333-6. [DOI] [PubMed] [Google Scholar]
- Morgan RE, Garavan HP, Mactutus CF, Levitsky DA, Booze RM, Strupp BJ. Enduring effects of prenatal cocaine exposure on attention and reaction to errors. Behav Neurosci. 2002;116:624–33. doi: 10.1037//0735-7044.116.4.624. [DOI] [PubMed] [Google Scholar]
- Mulé S, Casella GA. Confirmation and quantitation of cocaine, benzoylecgonine, ecgonine methyl ester in human urine by GC/MS. J Anal Toxicol. 1988;12:153–5. doi: 10.1093/jat/12.3.153. [DOI] [PubMed] [Google Scholar]
- Muthén BO, Muthén L. Mplus software. Los Angeles, CA: Muthén, B.O. & Muthén, L; 2004. [Google Scholar]
- Phelps L, Wallace NV, Bontrager A. Risk factors in early child development: is prenatal cocaine/polydrug exposure a key variable? Psychol Schools. 1997;34:245–52. [Google Scholar]
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using STATA. College Station, TX: Stata Press; 2005. [Google Scholar]
- Richardson GA. Prenatal cocaine exposure: A longitudinal study of development. Ann N Y Acad Sci. 1998;846:144–52. [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: effects on the development of school-age children. Neurotoxicol Teratol. 1996;18:627–34. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Rose-Jacobs R, Cabral H, Posner MA, Epstein J, Frank DA. Do “we just know”? Masked assessors’ ability to accurately identify children with prenatal cocaine exposure. J Dev Behav Pediatr. 2002;23:340–6. doi: 10.1097/00004703-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Savage J, Brodsky NL, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. J Dev Beh Ped. 2005;26:42–7. [PubMed] [Google Scholar]
- Schneiderman JF. Nonmedical drug and chemical use in pregnancy. In: Koren G, editor. Maternal-fetal toxicology: a clinician’s guide. New York: Marcel Dekker, Inc.; 1990. pp. 301–20. [Google Scholar]
- Schonfeld AM, Paley B, Frankel F, O’Connor MJ. Behavioral regulation as a predictor of response to Children’s Friendship Training in children with fetal alcohol spectrum disorders. Clin Neuropsychol. 2009;23:428–45. doi: 10.1080/13854040802389177. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD, Coles CD. Prenatal cocaine and other substance exposure: effects on infant autonomic regulation at 7 months of age. Dev Psychobiol. 2007;49:276–89. doi: 10.1002/dev.20215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Nelson S, Short E, Min MO, Lewis B, Russ S, Minnes S. Prenatal cocaine exposure: drug and environmental effects at 9 years. J Pediatr. 2008;153:105–11. doi: 10.1016/j.jpeds.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Campbell J, Snyder K, Silveri M, Katovic N. Animal behavior models. Increased sensitivity to stressors and other environmental experiences after prenatal cocaine exposure. Ann N Y Acad Sci. 1998;846:76–88. [PubMed] [Google Scholar]
- Stanger C, Higgins ST, Bickel WK, Elk R, Grabowski J, Schmitz J, Amass L, Kirby KC, Seracini AM. Behavioral and emotional problems among children of cocaine- and opiate-dependent parents. J Am Acad Child Adolesc Psychiatry. 1999;38:421–8. doi: 10.1097/00004583-199904000-00015. [DOI] [PubMed] [Google Scholar]
- Statacorp [Internet] College Station, Texas: StataCorp LP; Factor analysis. ©1996-2010 [updated 2010 Nov 2010a; cited 2010 Nov 19a]. Available from: http://www.stata.com/capabilities/factor.html. [Google Scholar]
- Statacorp [Internet] College Station, Texas: StataCorp LP; ©1996-2010; Stata 11 help for alpha. [updated 2010 Nov 2010b; cited 2010 Nov 19b]. Available from: http://www.stata.com/help.cgi?alpha. [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–85. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- von Suchodoletz A, Trommsdorff G, Heikamp T, Wieber F, Gollwitzer PM. Transition to school: the role of kindergarten children’s behavior regulation. Learning and Individual Differences. 2009;19:561–6. [Google Scholar]
- Warner TD, Behnke M, Wei H, Garvan CW, Wobie K, Eyler FD. Predicting caregiver-reported behavior problems in cocaine-exposed children at 3 years. J Dev Beh Ped. 2006;27:83–92. doi: 10.1097/00004703-200604000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children--Third Edition (WISC-III) San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Test (WIAT) San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]


