Abstract
Gene therapy approaches delivering fibroblast growth factor-2 (FGF-2) have shown promise as a potential treatment for increasing blood flow to ischemic limbs. Currently, effective non-invasive techniques to deliver plasmids encoding genes of therapeutic interest, such as FGF-2, are limited. We sought to determine if intradermal injection of plasmid DNA encoding FGF-2 (pFGF) followed by non-invasive cutaneous electroporation (pFGFE+) could increase blood flow and angiogenesis in a rat model of hindlimb ischemia. pFGFE+ or control treatments were administered on postoperative day 0. Compared to injection of pFGF alone (pFGFE-), delivery of pFGFE+ significantly increased FGF-2 expression for 10 days. Further, the increase in FGF-2 expression with pFGFE+ was sufficient to significantly increase ischemic limb blood flow, measured by laser Doppler perfusion imaging, beginning on postoperative day 3. Ischemic limb blood flow in the pFGFE+ treatment group remained significantly higher than all control groups through the end point of the study, postoperative day 14. Immunohistochemical staining of gastrocnemius cross-sections determined there was a two-fold increase in capillary density in the pFGFE+ treatment group. Our results suggest that pFGFE+ is a potential non-invasive, non-viral therapeutic approach to increase perfusion and angiogenesis for the treatment of limb ischemia.
Keywords: peripheral artery disease, hindlimb ischemia, FGF-2, electroporation
Introduction
Peripheral artery disease (PAD), resulting from atherosclerosis, is one of the leading causes of morbidity and mortality in the western world.1 The primary pathophysiology of PAD is reduction of blood flow to the lower extremities that commonly results in one of two clinical presentations, intermittent claudication (IC) or critical limb ischemia (CLI). IC is manifested by insufficient blood flow during exercise. The more severe manifestation, CLI, results from insufficient blood flow to the limb even when the effected limb is at rest. Current treatment options for PAD include risk factor reduction, physical therapy and training, and pharmacological treatment of the underlying atherosclerosis. Also, in patients with severe IC or CLI, interventional (catheter based) or open surgical procedures may be used to revascularize the ischemic limb. However, direct revascularization is often impossible due to the anatomic extent of PAD and is also limited by associated co-morbidities, such as diabetes, coronary artery disease or stroke, requiring a majority of patients to undergo limb amputation.2-5 Currently, an effective pharmacological treatment to increase blood flow to ischemic limbs is not available.6 The lack of an effective pharmacological treatment and the limits of interventional and surgical procedures have spurred an intense investigation of alternative approaches, such as therapeutic angiogenesis, for the treatment of PAD.
Angiogenesis plays a major role in both health and disease. The formation of new blood vessels occurs during normal physiological processes, such as embryonic development and wound healing.7 Insufficient neovascularization is characteristic of several diseases including ischemic heart and limb diseases, neurodegeneration and osteoporosis. 8 The initiation and progression of angiogenesis is mediated through the production of growth factors and cytokines in response to disease or injury. Therapeutic approaches delivering angiogenic growth factors, such as members of the vascular endothelial growth factor and FGF families, are emerging as promising treatment options for IC and CLI in patients when direct revascularization is not possible.9 FGFs play an integral role in angiogenesis by modulating multiple steps including degradation of the extracellular matrix and promotion of vascular endothelial cell proliferation, migration and morphogenesis.10 There are currently greater than 20 known FGFs that share approximately 30–70% homology in their amino-acid sequences. Of the FGF family members FGF-1 and FGF-2 have been the most intensely studied and most frequently utilized in emerging therapeutic angiogenesis approaches.
The overall goal of this study was to elucidate a non-invasive therapeutic approach for delivering FGF-2 as a potential treatment for IC and CLI. In previous studies, intramuscular injection of plasmid FGF-2 11, alone or followed by intramuscular electroporation (EP), 12,13 increased angiogenesis and blood flow to ischemic limbs. In this study, we demonstrate that delivery of a plasmid encoding FGF-2 (pFGF) to the skin by intradermal injection and non-invasive EP (pFGFE+) significantly increases FGF-2 expression compared to injection of the plasmid without EP (pFGFE-). The increase in FGF-2 expression with pFGFE+ was sufficient to significantly increase blood flow and angiogenesis in a rat model of limb ischemia. The results presented here suggest that pFGFE+ is a novel potential non-invasive therapeutic angiogenesis approach for the treatment of PAD related limb ischemia.
Materials and Methods
Experimental animals
All procedures were approved by the Animal Use and Care Committee of the University of South Florida College of Medicine. A total of 34 male Sprague Dawley rats (275-300 g) were used in this study; 10 rats were use to evaluate FGF-2 expression kinetics and 24 rats were used for analysis of perfusion and capillary density. Operations were performed using standard aseptic techniques. Prior to all procedures animals were anesthetized with 2.5-3.0% isoflurane in oxygen.
Hindlimb ischemia model
Hindlimb ischemia was induced in the right hindlimb of the Sprague Dawley rats. Through an approximately 2-cm long incision parallel to the inguinal ligament the saphenous artery was ligated distally of the bifurcation of the femoral artery into the saphenous and popliteal arteries. The most distal end of the external iliac was ligated immediately adjacent to the inguinal ligament and the femoral artery was ligated proximal and distal to the bifurcation of the superficial epigastric artery and vein. The superficial epigastric artery and vein were also ligated at 2 sites adjacent to the femoral bifurcation and cut between these sites. The femoral artery was then cut between the ligations adjacent to the inguinal ligament and distally of the superficial epigastric artery and vein. The femoral artery was then dissected free from the point of the distal ligation of the saphenous artery to the ligation of the femoral placed distally to the bifurcation of the superficial artery and vein.
FGF-2 plasmid construction
The CMV promoter was removed from the pVAX1 plasmid (Invitrogen) to create a promoterless pVAX1. The human FGF-2 plasmid (pFGF) was cloned by sub-cloning the hEF1-IF4g-hbFGF sequence from pBLAST45-hbFGF2 (Invivogen) into the promoterless backbone of pVAX1. Where indicated vector backbone controls are the pVAX1 backbone containing the hEF1-IF4g promoter.
Electroporation
After the operation to induce hindlimb ischemia animals were randomized into 1 of 4 treatment groups: injection of pFGF with electroporation (pFGFE+), injection of pFGF without electroporation (pFGFE-), injection of vector backbone with electroporation (pVAXE+) and no treatment (P-E-). All injections were performed using a 25 gauge, 5/8-inch length needle. Animals received two 50 μL intradermal injections of plasmid DNA (2 mg/mL) in sterile injectable saline. The multielectrode array (MEA)11 was then placed over the injection bubble and 150 ms pulses applied at a field strength of 300 V/cm (delivery to skin of rat flank for initial expression kinetics) or 250 V/cm (delivery to the hindlimb). FGF-2 expression levels resulting from pFGFE+ and pFGFE- treatments were equivalent at 300 V/cm when delivered to skin on flank of a rat and 250 V/cm for delivery to the skin of the hindlimb and did not differ between ischemic and non-ischemic limbs (data not shown).
FGF-2 Protein Expression
FGF-2 protein expression was determined by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN)). At the indicated time points, the delivery sites were excised and snap frozen on dry ice. Tissue samples were homogenized in 1-2 mLs of lysis buffer (150 mmol/l NaCl, 20 mmol/l sodium phosphate pH 7.4, 10% glycerol, 1% NP-40 and protease inhibitors ((Roche, Indianapolis, IN)). Homogenates were then centrifuged at 4°C for 10 minutes at 10,000 rpm. The supernatants were removed and assayed for FGF-2 expression according to manufacturer’s instructions.
Measurement of blood flow by laser Doppler perfusion imaging
A Laser Doppler Perfusion Imager (Moor Instruments Ltd., Devon, UK) was used to measure hindlimb blood flow. Before measuring perfusion, animals were anesthetized and placed on a warming pad to ensure constant body temperature. A low intensity (2mW) laser light beam (λ= 632.8 nm) scanned the surface of the skin without contact at a standardized working distance of 25 cm. Scan modus was set at 10 ms/pixel and resolution at 256 × 256 pixels. Three scans were completed per time point for each animal for both the ischemic and non-ischemic limbs and average perfusion in arbitrary units (flux) was determined separately for each limb. Perfusion in the ischemic limb was normalized to the contra-lateral non-ischemic limb (I/NI) to minimize variation due to ambient light and temperature. Baseline perfusion was assessed immediately preoperatively and postoperative perfusion assessed immediately after surgery but before treatment. The I/NI was used to calculate percentage of baseline perfusion at the postoperative time points.
Immunohistochemistry
Two weeks after treatment, animals were humanely euthanized and tissue samples from the gastrocnemius muscle were excised and fixed in 10% neutral buffered formalin for at least 12 hours at room temperature before embedding in paraffin and sectioning (4 μm). Capillaries in the cross-sections were visualized using the Blood Vessel Staining Kit (Millipore, Billerica, MA) according to manufactures instructions for Factor VIII with Hematoxylin counterstaining. The number of Factor VIII positive vessels was manually counted in a blinded fashion in 5 randomly selected fields (400X) for 5 animals from each treatment group. The stained sections were analyzed using a standard light microscope (Olympus BX51; Olympus, Center Valley, PA). The average capillary density per high power field (400X) for each treatment group is reported. Representative fields from each treatment group were photographed at 400X with a Spot Insight 2 digital camera (Diagnostic Instruments, Sterling Heights, MI).
Statistical Analysis
All values are reported as the mean ± SEM unless otherwise noted. Analysis of FGF-2 expression and capillary density was completed using a two-tailed Student’s t-test. Analysis of blood flow was completed by ANOVA with post-hoc Fisher’s Least Significant Difference to adjust for multiple group comparisons. A post-hoc Bonferroni correction was used to adjust for multiple comparisons to pFGFE+ for analysis of capillary density. All statistical analysis was completed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL).
Results
FGF-2 expression kinetics
After determining pFGF transfection increased FGF-2 protein expression in vitro (data not shown) we then determined if pFGFE+ could increase FGF-2 protein expression in vivo (Figure 1). At the time points indicated in Figure 1, skin from the treated areas was excised and assayed for FGF-2 expression by enzyme-linked immunosorbent assay. For 10 days after treatment, pFGFE+ significantly increased FGF-2 protein levels, compared to pFGFE-, (p<0.05) before decreasing to background levels at days 14 and 17. The application of an electric field to tissues alone can transiently induce expression of some genes, including angiogenic growth factors14,15, but injection of the vector backbone, lacking the FGF-2 cDNA insert, followed by EP (pVAXE+) resulted in similar levels of FGF-2 expression as untreated controls (P-E-) (n=4, 1416 ± 326 total pg / sample).
Figure 1. FGF-2 expression kinetics.
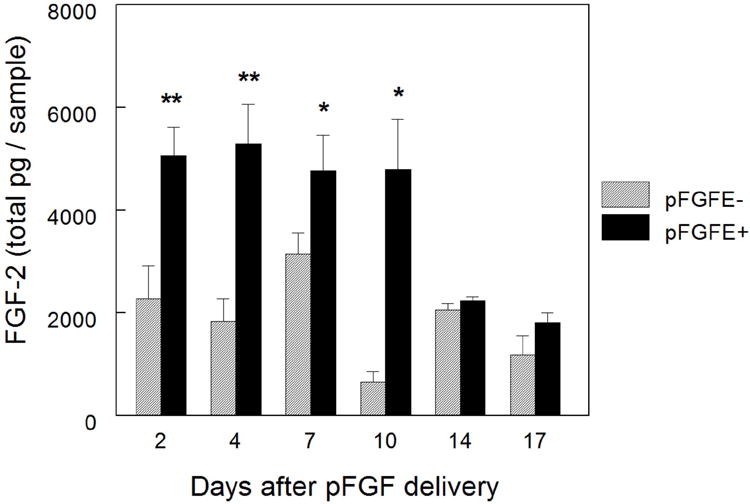
At the indicated time points skin samples were harvested from the delivery site and assayed for FGF-2 protein expression by ELISA. To determine FGF-2 expression resulting from pFGFE+ and pFGFE- the average FGF-2 expression in untreated skin (n=4, 1416 ± 326 total pg / sample) was subtracted from the total pg/sample for each treatment site. Day 2 n=10, days 4, 7, and 10 n=6 and days 14 and 17 n=8 per group per time point. pFGFE+= 300 V/cm, 150 ms. ** p< 0.01, * p< 0.05. pFGFE-: intradermal injection of plasmid FGF-2; pFGFE+: intradermal injection of plasmid FGF-2 followed by electroporation.
Plasmid FGF-2 with electroporation increases blood flow in the ischemic hindlimb
We next evaluated if pFGFE+ treatment could increase blood flow in a rat model of hindlimb ischemia. Immediately postoperatively (Day 0) pFGFE+ or control treatments were administered at two sites on the medial aspect of the ischemic limb. A laser Doppler perfusion Imager was used to measure perfusion in the distal area of both the ischemic and non-ischemic limbs preoperatively (baseline), postoperatively but prior to treatment (Day 0) and on postoperative days (PODs) 1, 3, 7, and 14 (Figure 2a). Immediately postoperatively, blood flow decreased to approximately 40% of baseline indicating the hindlimb was effectively rendered ischemic. In Figure 2b blood flow is reported for each treatment group as the ratio of blood flow in the ischemic hindlimb to the non-ischemic hindlimb (I/NI) (Figure 2b, top panel) and as a percent of the perfusion recorded at baseline (Figure 2b, bottom panel). There was a significant difference in limb blood flow beginning on POD 3 between all treatment groups for I/NI (p<0.02) and as a percentage of baseline levels (p<0.001). Also on POD 3, blood flow in the pFGFE+ treatment group was significantly greater than all of the control groups (p < 0.05). Blood flow in the ischemic limb continued to be higher in the pFGFE+ treatment group, compared to control treatment groups, at all subsequent time points in the study (p<0.05, all time points for percent of baseline blood flow and I/NI). The pFGFE- treatment group showed a slight, but not significant, increase in blood flow compared to the pVAXE+ and P-E- treatment groups on PODs 7 and 14.
Figure 2. Effect of pFGFE+ on ischemic limb blood flow.

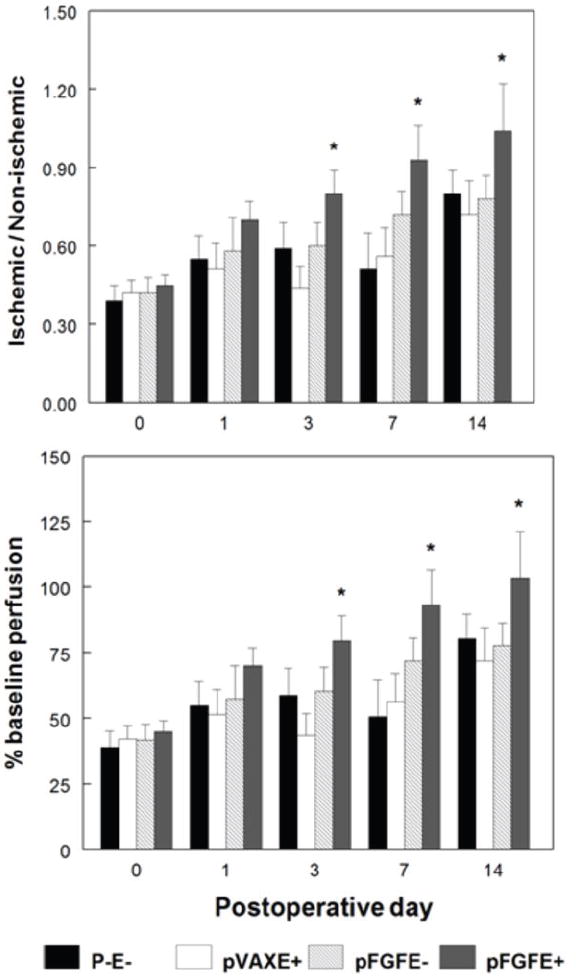
A, Representative laser Doppler perfusion images for the pFGFE+ and pFGFE- treatment groups at day 14 postoperatively. The white box indicates the approximate area where blood flow was assessed. The arrows indicate the absence of perfusion in the area of the femoral artery after the operation to induce hindlimb ischemia. B, Quantification of blood flow as determined by laser Doppler perfusion imaging. (Top panel) Ratio of blood flow in the ischemic limb to the non-ischemic limb (I/NI). (bottom) Postoperative blood flow as a percentage of baseline line blood flow. n=6 per group per time point. * p<0.05 compared to all controls. pFGFE+: intradermal injection of plasmid FGF-2 followed by electroporation; pFGFE-: Intradermal injection of plasmid FGF-2; pVAXE+: intradermal injection of vector backbone followed by electroporation; P-E-: no treatment.
Plasmid FGF-2 with electroporation increases angiogenesis in the ischemic hindlimb
Next, we determined if the increase in limb perfusion in the pFGFE+ treatment group resulted from an increase in angiogenesis. In rodent models of hindlimb ischemia, as well as in patients with PAD, angiogenesis typically occurs in the gastrocnemius muscle, or distal to the arterial occlusion.16 Thus, on POD 14 samples were harvested from the gastrocnemius muscle of the ischemic limb and capillary density visualized by immunohistochemical staining for the endothelial cell marker factor-VIII associated antigen. Representative fields (400X) from each of the 4 treatment groups are shown in Figure 3a. In Figure 3b, the average number of vessels in 5 randomly selected high power fields (400 X) for 5 animals in each treatment group is reported. There was an approximately two-fold increase in the number of capillaries in the pFGFE+ treatment group, compared to all control groups (p<0.001), indicating the increase in blood flow to the ischemic limb with pFGFE+ was in fact due to an increase in angiogenesis.
Figure 3. Effect of pFGFE+ on neovascularization.

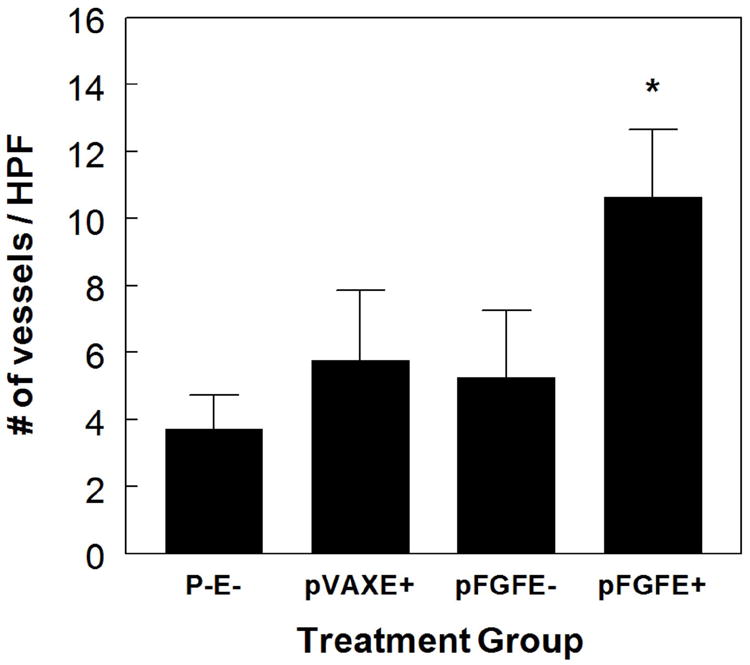
A, Representative cross-sections (400X) of factor VIII-asscociated antigen immunohistological staining of cross-sections excised from the gastrocnemius on POD 14. Arrows indicate examples of stained vessels. B, Quantification of capillary density. The average number of capillaries in 5 fields, for 5 animals in each treatment group. * p<0.01 compared to all controls. HPF: High power field (400 X). pFGFE+: intradermal injection of plasmid FGF-2 followed by electroporation; pFGFE-: Intradermal injection of plasmid FGF-2; pVAXE+: intradermal injection of vector backbone followed by electroporation; P-E-: no treatment.
Discussion
Direct injection of naked plasmid DNA has been intensively investigated as a gene delivery approach for the treatment of a variety of diseases. One drawback to this approach is inefficient uptake of the plasmid by cells resulting in low levels of gene expression.17 In vivo EP has been successfully used to increase the uptake and expression of naked plasmid DNA in the skin and other target tissues. The skin is an attractive target for the delivery of plasmid DNA because it allows for enhanced control over expression levels and aids in targeting expression to specific tissue areas. Further, if higher expression levels are needed, the area treated or number of treatments can be increased. Plasmid DNA has been successfully delivered to the skin with in vivo EP for both systemic and tissue-specific expression 18-22. This approach has shown promise in several fields including vaccines 22-26 and wound healing 27-30.
The design of the electrode used for plasmid delivery in this study, multielectrode array (MEA) 11, allows for this potential treatment for limb ischemia to be readily translated to a clinical setting. One drawback of other electrode designs is delivery of plasmid DNA to a large surface area requires the distance between the electrode pairs to be increased thereby the applied voltage must also be increased for cell permeabilization to be effective. The design of the MEA allows for its dimensions to be expanded by incorporating a larger number of electrode pairs thus enabling a larger surface area to be treated without the need to increase the distance between electrodes.
In addition to direct injection of naked plasmid DNA, the most prevalent methods of therapeutic administration of FGF-2 in the literature are viral-mediated gene transfer and recombinant protein delivery. These methods are promising for treatment of PAD but have drawbacks. In human clinical trials viral vectors have caused inflammatory responses, formation of antibodies to the viruses, transient fever 31 and hepatotoxicity 32. Also, relative to non-viral gene transfer approaches, viral-mediated delivery results in long-term and high levels of gene expression increasing the potential for the occurrence of the adverse side effects associated with high levels of FGF-2 expression, such as toxicity and proteinuria.6,33 In both animal models 34-36 and clinical trials 37-39 intramuscular injection or intra-arterial administration of recombinant FGF-2 protein improved PAD related symptoms and or blood flow to the ischemia limb, but the use of recombinant protein in a clinical setting is often not practical due to its short half live37,40, poor bioavailability and consequently frequent administration is often required to sustain lasting effects. The strategy developed here to deliver FGF-2 to an ischemic limb is an attractive alternative to viral and recombinant protein approaches because it allows for control of localized expression level and duration. Also, pFGFE+ circumvents the possibility of adverse side-effects and practicality issues associated with viral and recombinant protein delivery approaches.
Other studies have shown that intramuscular injection of plasmid FGF-2 alone 11 or followed by intramuscular EP 12,13 can improve ischemic hindlimb blood flow and increase angiogenesis. Here, we have presented a simple, non-invasive approach for delivery of pFGF with EP that effectively increases FGF-2 protein expression, ischemic limb blood flow and angiogenesis. The increased blood flow and angiogenesis with pFGFE+ treatment may be occurring through several known in vivo mechanisms. FGF-2 is normally confined to the cell compartment but is exported from the cell during active angiogenesis to exert downstream effects. 41 Thus, although pFGFE+ treatment is administered to the skin, increased levels of FGF-2 protein after treatment and subsequent export could activate downstream angiogenic pathways. For example, a recent study by Fujii, et al.11 demonstrated that in a model of hindlimb ischemia intramuscular injection of a plasmid encoding FGF-2 increased the expression of placental growth factor via the upregulation of vascular endothelial growth factor expression. Placental growth factor alone is known to increase angiogenesis in models of hindlimb ischemia, and it is possible a similar mechanism may be occurring with pFGFE+. 42,43 Another possible mechanism for the increase in angiogenesis and hindlimb blood flow with pFGFE+ treatment is the upregulation of HGF. FGF-2 stimulates hepatocyte growth factor expression, which itself has also been successful in increasing perfusion in hindlimb ischemia models.44,45 Further studies to characterize the exact mechanism of pFGFE+ mediated angiogenesis are currently underway.
In summary, pFGFE+ treatment significantly increased FGF-2 expression for 10 days after delivery and this increase was sufficient to significantly increase blood flow and angiogenesis in a rat model of hindlimb ischemia. Translation of this therapeutic approach to an appropriate large animal model, and eventually to the clinic, will be the focus of future studies. Overall, EP-mediated intradermal delivery of plasmid FGF-2 is a potential non-invasive, non-viral therapeutic approach to increase perfusion and angiogenesis in ischemic limbs and warrants further investigation as a possible treatment for PAD.
Acknowledgments
The authors would like to thank William Marshall, M.D. (University of South Florida, College of Medicine) for his insight into vascular biology and valuable review of the manuscript and Mark Jaroszeski (University of South Florida, College of Engineering) for construction of the MultiElectrode Array. This work was supported by the Defense Threat Reduction Agency and the Florida Center of Excellence for Biomolecular Identification and Targeted Therapeutics.
Footnotes
Declaration of conflict of interest Richard Heller is an inventor on patents used in this work. These patents have been licensed to RMR Technologies, LLC and Inovio Biomedical Corporation. Richard Heller has ownership interest in RMR Technologies and owns stock and stock options of Inovio Biomedical Corporation.
References
- 1.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 2.Robeer G, Brandsma J, van den Heuvel S, Smit B, Oostendorp R, Wittens C. Exercise therapy for intermittent claudication: a review of the quality of randomized clinical trials and evaluation of predictive factors. Eur J Vasc Endovasc. 1998;15:36–43. doi: 10.1016/s1078-5884(98)80070-1. [DOI] [PubMed] [Google Scholar]
- 3.Dormandy J, Rutherford R. Management of Peripheral Arterial Disease (PAD) J Vasc Surg. 2000;31:s3. [PubMed] [Google Scholar]
- 4.Rissanen TT, Vajanto I, Yla-Herttuala S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb - on the way to the clinic. European Journal of Clinical Investigation. 2001;31:651–666. doi: 10.1046/j.1365-2362.2001.00864.x. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) European Journal of Vascular and Endovascular Surgery. 2007;33:S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Aviles RJ, Annex BH, Lederman RJ. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor (FGF-2) Br J Pharmacol. 2003;140:637–646. doi: 10.1038/sj.bjp.0705493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 9.Bobek V, Taltynov O, Pinterova D, Kolostova K. Gene therapy of the ischemic lower limb -- therapeutic angiogenesis. Vasc Pharmacol. 2006;44:395–405. doi: 10.1016/j.vph.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth F R. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Fujii T, Yonemitsu Y, Onimaru M, Inoue M, Hasegawa M, Kuwano H, et al. VEGF function for upregulation of endogenous PlGF expression during FGF-2-mediated therapeutic angiogenesis. Atherosclerosis. 2008;200:51–57. doi: 10.1016/j.atherosclerosis.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Lee J-S, Kim J-M, Kim KL, Jang H-S, Shin I-S, Jeon E-S, et al. Combined administration of naked DNA vectors encoding VEGF and bFGF enhances tissue perfusion and arteriogenesis in ischemic hindlimb. Biochem Bioph Res Co. 2007;360:752–758. doi: 10.1016/j.bbrc.2007.06.120. [DOI] [PubMed] [Google Scholar]
- 13.Nishikage S, Koyama H, Miyata T, Ishii S, Hamada H, Shigematsu H. In vivo electroporation enhances plasmid-based gene transfer of basic fibroblast growth factor for the treatment of ischemic limb. J Surg Res. 2004;120:37–46. doi: 10.1016/j.jss.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaka M, Kohzuki M, Fujii T, Kanno S, Kawamura T, Onodera H, et al. Effect of low-voltage electrical stimulation on angiogenic growth factors in ischaemic rat skeletal muscle. Clin Exp Pharmacol Physiol. 2006;33:623–627. doi: 10.1111/j.1440-1681.2006.04417.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanno S, Oda N, Abe M, Saito S, Hori K, Handa Y, et al. Establishment of a Simple and Practical Procedure Applicable to Therapeutic Angiogenesis. Circulation. 1999;99:2682–2687. doi: 10.1161/01.cir.99.20.2682. [DOI] [PubMed] [Google Scholar]
- 16.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, et al. Contribution of Arteriogenesis and Angiogenesis to Postocclusive Hindlimb Perfusion in Mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 17.Vogel J. Nonviral Skin Gene Therapy. Hum Gene Ther. 2000;11:2253–2259. doi: 10.1089/104303400750035780. [DOI] [PubMed] [Google Scholar]
- 18.Heller R, Schultz J, Lucas ML, Jaroszeski MJ, Heller LC, Gilbert RA, et al. Intradermal delivery of interleukin-12 plasmid DNA by in vivo electroporation. DNA Cell Biol. 2001;20:21–26. doi: 10.1089/10445490150504666. [DOI] [PubMed] [Google Scholar]
- 19.Glasspool-Malone J, Somiari S, Drabick J, Malone R. Efficient nonviral cutaneous transfection. Mol Ther. 2000;2:140–146. doi: 10.1006/mthe.2000.0107. [DOI] [PubMed] [Google Scholar]
- 20.Dujardin N, Van Der Smissen P, Preat V. Topical gene transfer into rat skin using electroporation. Pharm Res. 2001;18:61–66. doi: 10.1023/a:1011026726938. [DOI] [PubMed] [Google Scholar]
- 21.Maruyama H, Ataka K, Higuchi N, Sakamoto F, Gejyo F, Miyazaki J. Skin-targeted gene transfer using in vivo electroporation. Gene Ther. 2001;8:1808–1812. doi: 10.1038/sj.gt.3301604. [DOI] [PubMed] [Google Scholar]
- 22.Drabick JJ, Glasspool-Malone J, Somiari S, King A, Malone RW. Cutaneous transfection and immune responses to intradermal nucleic acid vaccination are significantly enhanced by in vivo electropermeabilization. Mol Ther. 2001;3:249–255. doi: 10.1006/mthe.2000.0257. [DOI] [PubMed] [Google Scholar]
- 23.Medi BM, Hoselton S, Marepalli R, Singh J. Skin targeted DNA vaccine delivery using electroporation in rabbits: I: Efficacy. Int J Pharm. 2005;294:53–63. doi: 10.1016/j.ijpharm.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- 25.Hooper J, Golden J, Ferro A, King A. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirao LA, Wu L, Khan AS, Satishchandran A, Draghia-Akli R, Weiner DB. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine. 2008;26:440–448. doi: 10.1016/j.vaccine.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 27.Marti G, Ferguson M, Wang J, Byrnes C, Dieb R, Qaiser R, et al. Electroporative transfection with KGF-1 DNA improves wound healing in a diabetic mouse model. Gene Ther. 2004;11:1780–1785. doi: 10.1038/sj.gt.3302383. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P-Y, Chesnoy S, Huang L. Electroporatic delivery of TGF-B1 gene works synergistically with electric therapy to enhance diabetic wound healing in db/db mice. J Investig Dermatol. 2004;123:791–798. doi: 10.1111/j.0022-202X.2004.23309.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin MP, Marti GP, Dieb R, Wang J, Ferguson M, Qaiser R, et al. Delivery of plasmid DNA expression vector for keratinocyte growth factor-1 using electroporation to improve cutaneous wound healing in a septic rat model. Wound Repair Regen. 2006;14:618–624. doi: 10.1111/j.1743-6109.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 31.Ylä-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 32.Muruve D, Barnes M, Stillman I, Libermann T. Adenoviral Gene Therapy Leads to Rapid Induction of Multiple Chemokines and Acute Neutrophil-Dependent Hepatic Injury in Vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 33.Cooper LT, Jr, Hiatt WR, Creager MA, Regensteiner JG, Casscells W, Isner JM, et al. Proteinuria in a placebo-controlled study of basic fibroblast growth factor for intermittent claudication. Vascular Medicine. 2001;6:235–239. doi: 10.1177/1358836x0100600406. [DOI] [PubMed] [Google Scholar]
- 34.Wafai R, Tudor EM, Angus JA, Wright CE. Vascular Effects of FGF-2 and VEGF-B in Rabbits with Bilateral Hind Limb Ischemia. J Vasc Res. 2008;46:45–54. doi: 10.1159/000139132. [DOI] [PubMed] [Google Scholar]
- 35.Cao R, Brakenhielm E, Pawliuk R, Wariaro D, Post M, Wahlberg E, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–613. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 36.Masaki I, Yonemitsu Y, Yamashita A, Sata S, Tanii M, Komori K, et al. Angiogenic Gene Therapy for Experimental Critical Limb Ischemia: Acceleration of Limb Loss by Overexpression of Vascular Endothelial Growth Factor 165 but not of Fibroblast Growth Factor-2. Circ Res. 2002;90:966–973. doi: 10.1161/01.res.0000019540.41697.60. [DOI] [PubMed] [Google Scholar]
- 37.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, et al. Basic fibroblast growth factor in patients with intermittent claudication: results of a phase I trial. J Am Coll Cardiol. 2000;36:1239–1244. doi: 10.1016/s0735-1097(00)00882-2. [DOI] [PubMed] [Google Scholar]
- 38.Lederman R, Mendelsohn F, Anderson R, Saucedo J, Tenaglia A, Hermiller J, et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. The Lancet. 2002;359:2053–2058. doi: 10.1016/s0140-6736(02)08937-7. [DOI] [PubMed] [Google Scholar]
- 39.Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, et al. A Novel Approach to Therapeutic Angiogenesis for Patients With Critical Limb Ischemia by Sustained Release of Basic Fibroblast Growth Factor Using Biodegradable Gelatin Hydrogel: An Initial Report of the Phase I-IIa Study. Circ J. 2007;71:1181–1186. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- 40.Bush MA, Samara E, Whitehouse MJ, Yoshizawa C, Novicki DL, Pike M, et al. Pharmacokinetics and pharmacodynamics of recombinant FGF-2 in a phase I trial in coronary artery disease. J Clin Pharmacol. 2001;41:378–385. doi: 10.1177/00912700122010230. [DOI] [PubMed] [Google Scholar]
- 41.Kandel J, Bossy-Wetzel E, Radvanyi F, Klagsbrun M, Folkman J, Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991;66:1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- 42.Korpisalo P, Rissanen TT, Bengtsson T, Liimatainen T, Laidinen S, Karvinen H, et al. Therapeutic angiogenesis with placental growth factor improves exercise tolerance of ischaemic rabbit hindlimbs. Cardiovasc Res. 2008;80:263–270. doi: 10.1093/cvr/cvn195. [DOI] [PubMed] [Google Scholar]
- 43.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8:831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 44.Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, et al. Therapeutic Angiogenesis Induced by Human Hepatocyte Growth Factor Gene in Rat Diabetic Hind Limb Ischemia Model: Molecular Mechanisms of Delayed Angiogenesis in Diabetes. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- 45.Onimaru M, Yonemitsu Y, Tanii M, Nakagawa K, Masaki I, Okano S, et al. Fibroblast growth factor-2 gene transfer can stimulate hepatocyte growth factor expression irrespective of hypoxia-mediated downregulation in ischemic limbs. Circ Res. 2002;91:923–930. doi: 10.1161/01.res.0000043281.66969.32. [DOI] [PubMed] [Google Scholar]


