Abstract
Background: Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to reduce cardiovascular mortality at a dose of ≈1 g/d. Studies using higher doses have shown evidence of reduced inflammation and improved endothelial function. Few studies have compared these doses.
Objective: The objective of this study was to compare the effects of a nutritional dose of EPA+DHA (0.85 g/d) with those of a pharmaceutical dose (3.4 g/d) on serum triglycerides, inflammatory markers, and endothelial function in healthy subjects with moderately elevated triglycerides.
Design: This was a placebo-controlled, double-blind, randomized, 3-period crossover trial (8 wk of treatment, 6 wk of washout) that compared the effects of 0.85 and 3.4 g EPA+DHA/d in 23 men and 3 postmenopausal women with moderate hypertriglyceridemia (150–500 mg/dL).
Results: The higher dose of EPA+DHA lowered triglycerides by 27% compared with placebo (mean ± SEM: 173 ± 17.5 compared with 237 ± 17.5 mg/dL; P = 0.002), whereas no effect of the lower dose was observed on lipids. No effects on cholesterol (total, LDL, and HDL), endothelial function [as assessed by flow-mediated dilation, peripheral arterial tonometry/EndoPAT (Itamar Medical Ltd, Caesarea, Israel), or Doppler measures of hyperemia], inflammatory markers (interleukin-1β, interleukin-6, tumor necrosis factor-α, and high-sensitivity C-reactive protein), or the expression of inflammatory cytokine genes in isolated lymphocytes were observed.
Conclusion: The higher dose (3.4 g/d) of EPA+DHA significantly lowered triglycerides, but neither dose improved endothelial function or inflammatory status over 8 wk in healthy adults with moderate hypertriglyceridemia. The trial was registered at clinicaltrials.gov as NCT00504309.
INTRODUCTION
Elevated triglycerides are a risk marker for cardiovascular disease (CVD), with an estimated 30% of the adult US population being affected (1–10). The omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) reduce triglyceride concentrations when administered at higher doses (>3 g/d combined) (11–13). However, lower doses (≈1 g EPA+DHA/d) are recommended for CVD risk reduction on the basis of evidence from large secondary prevention trials, which showed that this intake can reduce cardiovascular mortality by 20–30% without significant reductions in triglycerides (14, 15). Therefore, the cardioprotective action of omega-3 (n−3) fatty acids is thought to occur via multiple benefits, such as antiarrhythmic (16, 17) and antiinflammatory (18, 19) effects secondary to changes in cell membrane properties that affect cell signaling and gene expression (18–21). These benefits may also manifest in improved vascular endothelial function when omega-3 fatty acids are given acutely (22–24) or chronically (24–31).
Despite the significant reductions in triglycerides achieved with higher pharmacologic doses of omega-3 fatty acids, establishing recommended intakes to reduce CVD risk in persons with moderate hypertriglyceridemia has been complicated. Moderate hypertriglyceridemia often coexists with high LDL cholesterol (combined hyperlipidemia), and pharmacologic doses of omega-3 fatty acids can increase LDL cholesterol when large reductions in triglycerides are achieved (12). Because of the lack of morbidity and mortality data with high doses of omega-3 fatty acids, the current guidelines only recommend the higher dose for those who have substantial elevations in triglycerides (>500 mg/dL), not for minimizing general CVD risk (6).
A greater understanding of the effects of different doses of EPA+DHA on intermediate CVD risk markers, such as inflammation and vascular endothelial function, would help to establish evidence-based recommendations, particularly for adults with moderate hypertriglyceridemia.
The objective of this study was to compare the effects of a lower dose (ie, 0.85 g EPA+DHA/d, recommended for secondary prevention of CHD) and a higher dose (3.4 g EPA+DHA/d, indicated to lower very high triglycerides) on endothelial function, lipids, and inflammatory markers in healthy persons with moderately elevated triglycerides. We hypothesized that the higher dose would significantly decrease fasting triglycerides and that improvements in endothelial function would be dose-dependent and accompanied by reductions in inflammation.
SUBJECTS AND METHODS
Study population
Healthy persons with moderate hypertriglyceridemia (fasting triglycerides: 150–500 mg/dL) were recruited for this study. Estrogen fluctuations alter endothelial function, so all women were postmenopausal (no menses for > 12 mo). Other inclusion criteria were an age of 21 to 65 y, a body mass index (BMI; in kg/m2) of 20–39, and generally good health. Exclusion criteria were tobacco use; acute or chronic inflammatory conditions; hypertension (systolic blood pressure: ≥150 mm Hg; diastolic blood pressure: ≥95 mm Hg); liver or kidney dysfunction (self-reported or abnormal screening blood results); an unwillingness to discontinue nutritional supplements (except for calcium, which was allowed at a stable dose); intakes of fish, flaxseed, or walnuts ≥2 servings/wk; use of oral contraceptives or hormone replacement therapy; use of lipid-lowering, antiinflammatory, antidepressant, or blood pressure medication; abnormal screening electrocardiogram; or a history of heart disease. Potential participants were advised that they would be expected to maintain a low consumption of omega-3 fatty acids during the study, refrain from the use of all supplements, and maintain their body weight. A complete blood count and standard chemistry profile were obtained at screening to rule out the presence of serious illness (eg, autoimmune disease, cancer, and immunodeficiency). Seated blood pressure was measured by nurses in a controlled environment using a calibrated mercury sphygmomanometer and appropriately sized cuffs after a 5-min quiet rest according to JNC 7 guidelines (32). Three readings were taken, and the average of the last 2 readings was used to determine the eligibility for study participation and baseline characteristics. The blood pressure criterion (<150 mm Hg SBP and <95 mm Hg DBP) was established to avoid the exclusion of persons with unmedicated stage 1 hypertension.
Recruitment and ethical aspects
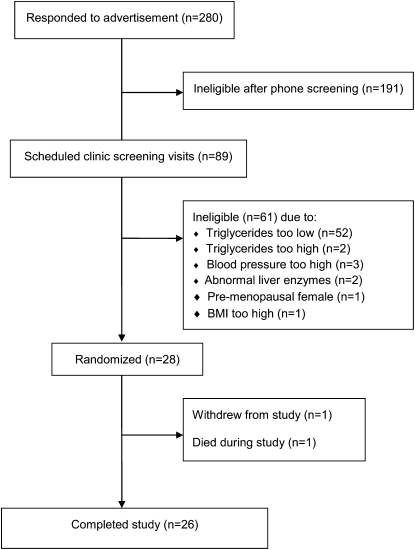
Subjects were recruited through advertisements in the local newspaper, fliers in the community, and campus E-mail lists. Two hundred eighty potential subjects called to indicate interest in participating in the study. They were given information about the study and, if interested, were asked a series of medical and lifestyle questions. Of the 280 respondents, 89 met the study criteria and were scheduled for a clinic screening at the Penn State General Clinical Research Center. After written informed consent was provided, a screening blood sample was drawn and a 12-lead electrocardiogram was obtained. Body weight and height were recorded to calculate BMI. From the 89 persons who were screened, 28 were eligible to participate in the study (Figure 1). Subjects with a glucose concentration >100 mg/dL, blood pressure ≥140/90 mm Hg, or high cholesterol were required to obtain a release form from their physicians because of the length of the trial and medication restrictions. A randomization scheme was developed in advance, and subjects were assigned to a treatment sequence at enrollment. The study protocol was approved by the Institutional Review Board of the Pennsylvania State University.
FIGURE 1.
Schematic of subject flow and reasons for exclusion.
Design and interventions
This was a randomized, double-blind, 3-period crossover, placebo-controlled study with 8-wk treatment periods and 6-wk washout periods. Treatment was provided as 4 identical capsules per day during all periods. All capsules were provided by GlaxoSmithKline (Research Triangle Park, NC) (Lovaza and identical corn-oil placebo). Each 1-g capsule of omega-3 fatty acid ethyl ester contained ≈465 mg EPA and 375 mg DHA (ratio of 1.2:1). Most fish-oil supplements contain EPA and DHA at a ratio of 1.5:1. We performed an independent analysis of one sample of active and placebo (methods previously described; 33). The corn-oil placebo contained 56% linoleic acid (18:2n−6), 28% oleic acid (18:1n−9), 12% palmitic acid (16:0), and small amounts of other fatty acids. The fatty acid profile of these treatments, as determined by our independent analysis, is summarized in Table 1. During the 3 treatment periods, subjects received in random order 0 g EPA+DHA/d (corn oil placebo), 0.85 g EPA+DHA/d, and 3.4 g EPA+DHA/d. The lower dose was provided as 4 capsules: one capsule of Lovaza and 3 placebo capsules. Treatments were matched to a coded numeric identifier so that the researchers and participants were blinded to treatment assignment. The subjects were instructed to maintain their weight and activity level during the course of the study, and they were counseled to exclude fatty fish meals (including salmon, tuna, mackerel, and herring), fish-oil supplements, flax products, walnuts, and omega-3–enriched eggs during the study. The subjects were contacted 2 wk into each phase to determine compliance and discuss any difficulties with taking the capsules. At the midpoint of each treatment period (4 wk), the subjects reported to the General Clinical Research Center to have their bottles weighed and to receive new supplies. Sample size was based on a power calculation, with flow-mediated dilation (FMD) as the primary outcome. Twenty-two subjects were estimated to provide 90% power to detect a 30% change in FMD values (27) with α of 0.05, based on the variability of fasting FMD values in our previous work (34).
TABLE 1.
Fatty acid composition (mg/4-g daily dose) of the 3 study interventions1
| EPA+DHA |
|||
| Fatty acid | 0 g/d | 0.85 g/d | 3.4 g/d |
| Palmitic, 16:0 | 464 | 348 | 1 |
| Stearic, 18:0 | 73 | 55 | 2 |
| Oleic, 18:1n−9 | 1121 | 843 | 10 |
| Linoleic, 18:2n−6 | 2240 | 1681 | 2 |
| Eicosadienoic, 20:2n−6 | 1 | 18 | 70 |
| Arachidonic, 20:4n−6 | 0 | 15 | 62 |
| EPA, 20:5n−3 | 0 | 486 | 1944 |
| Docosapentaenoic acid (n−3), 22:5n−3 | 0 | 35 | 141 |
| Docosapentaenoic acid (n−6), 22:5n−6 | 0 | 12 | 48 |
| DHA, 22:6n−3 | 0 | 421 | 1686 |
Values were calculated from independent analysis of fatty acid composition of a sample of active and placebo capsules (only fatty acids detected ≥1% of total fatty acids for either active treatment or placebo capsules are shown). EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Blood sample collection and assays
At the beginning of the study and at the end of each treatment period, blood samples were collected in the fasting state (12 h with nothing but water, 48 h without alcohol, and 2 h without vigorous exercise). A general health profile was obtained with fresh serum samples to monitor liver enzyme concentrations (Chem 24 panel; Quest Diagnostics, Pittsburgh, PA). The fasting lipid profile was measured on 2 separate days at the end of each period. Except for endpoints that required unfrozen samples, samples were portioned and stored at −80°C for batch analysis.
Lipids and lipoproteins
Whole blood was drawn into serum separator tubes, allowed to clot, and centrifuged. Total cholesterol and triglycerides were measured by enzymatic procedures (Quest Diagnostics, Pittsburgh, PA; CV < 2% for both). HDL cholesterol was estimated according to the modified heparin-manganese procedure (CV < 2%). LDL cholesterol was calculated by using the Friedewald equation [LDL cholesterol = TC – (HDL cholesterol + TG/5)] except in cases where triglyceride values exceeded recommended ranges. In these cases a direct LDL-cholesterol test was ordered that measures LDL-cholesterol concentrations using a chromogenic reaction after removal of all non-LDL cholesterol (N-geneous LDL-ST-C; Quest Diagnostics). The between-run CV of this assay is < 3%.
Inflammatory markers
Plasma concentrations of interleukin-1β ( IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were measured with high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits from R&D Systems (Minneapolis, MN) in duplicate (assay CV < 11% for all). Serum high-sensitivity C-reactive protein was measured by latex-enhanced immunonephelometry (Quest Diagnostics; assay CV < 8%).
Mononuclear cell gene expression
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient separation from EDTA-anticoagulated blood. After isolation and washing of the buffy coat with saline, the cells were counted on a hemocytometer. At least 8 × 106 cells were saved for RNA isolation. Cells for RNA isolation were suspended in RNALater solution before being stored at −80°C. After RNA isolation (RNEasy mini kit; Qiagen; Valencia, CA), a high-capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) was used for reverse transcription. cDNA (500 ng) was amplified with SYBR Green PCR Master Mix (Applied Biosystems) and detected with an ABI 7300 Sequence Detection System (Applied Biosystems). The expression of IL-1β, IL-6, and TNF-α was measured by using real-time polymerase chain reaction with the following primers: 1173F CACGGCCACATTTGGTTCTAA (IL-1β), 1224R CAGAATGTGGGAGCGAATGAC (IL-1β), 803F ATCAATCGGCCCGACTATCTC (TNF-α), 887R TGGATGTTCGTCCTCCTCACA (TNF-α), 197F GCCACTCACCTCTTCAGAACG (IL-6), and 250R CCGTCGAGGATGTACCGAATT (IL-6). The expression of these genes was normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The GAPDH forward and reverse primers were TGGGTGTGAACCATGAGAAG and GCTAAGCAGTTGGTGGTGC, respectively.
Insulin and glucose
Insulin was measured by radioimmunoassay with 125I-labeled human insulin and a human insulin antiserum (Linco Research, St Charles, MO; cross-reactivity with proinsulin < 0.2%). Glucose was measured with an immobilized enzyme biosensor by using the YSI 2300 STAT Plus Glucose & Lactate Analyzer (Yellow Springs Instruments, Yellow Springs, OH). The quantitative insulin-sensitivity check index (QUICKI) was calculated as 1/(log[fasting glucose] + log[fasting insulin]) (35). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as glucose × insulin/405 (36).
Liver enzymes
Liver enzymes were measured as part of a general health profile battery of blood tests (Chem 24 panel; Quest Diagnostics).
Erythrocyte fatty acids
Blood samples were drawn into EDTA-containing tubes at each visit. Red blood cells (RBCs) were separated from plasma by centrifugation, and a 0.5-mL aliquot was collected from the RBC pack. RBCs were frozen at –80°C until analyzed. Fatty acid analysis was performed as previously described (33). Briefly, lipids were extracted, methylated to form fatty acid methyl esters (FAMEs), and analyzed by gas chromatography on a GC2010 (Shimadzu Corporation, Columbia, MD) equipped with a 100-m SP-2560 column (Supelco, Bellefonte, PA). FAME composition is reported as the percentage by weight of total identified FAMEs. The omega-3 index is the sum of EPA and DHA.
Flow-mediated dilation, Doppler assessment of reactive hyperemia, and peripheral arterial tonometry
After a 12-h fast, endothelial function was assessed by FMD with high-frequency ultrasound, as described previously (including test-retest reliability) in a quiet, dimly lit room at 71–75°F (22, 34). The brachial artery above the elbow of the right arm was scanned in a longitudinal section after a 15 min rest, and continuous cross-sectional images were recorded at rest (1 min), during cuff inflation (5 min), and during increased blood flow after cuff release (2 min). An automated rapid cuff inflator set to 250 mm Hg (Hokanson, Bellevue, WA) was placed on the forearm distal to the ultrasound probe to induce ischemia. Changes in arterial diameter were measured by external B mode ultrasound imaging (Acuson Aspen 128XP equipped with a 10-mHz linear array transducer; Acuson, Mountain View, CA) by a single well-trained sonographer (P Wagner). The images were gated by using R-wave detection so that scans were assessed at end diastole. Automated edge detection software (Brachial Analyzer; MIA, Iowa City, IA) was used to quantify artery diameter continuously throughout the test. Peak artery diameter was determined as the largest diameter recorded in the 2-min postdeflation segment. Resting diameters were the average of all images collected over a 1-min period. FMD was calculated by 2 independent scorers as the percentage change in artery diameter at peak dilation compared with baseline and is reported as a percentage. If FMD values differed by >2%, a third technician reviewed the scan. FMD is reported as the average of the 2 readings.
Average flow velocity (m/s) across the cardiac cycle, maximum flow velocity, and velocity time integral across the cardiac cycle (m) were measured by using duplex pulsed Doppler at 2 time points: resting baseline and immediately after cuff release. Flow (mL/min) was calculated as described previously (34).
During the FMD test, the EndoPAT 2000 (Itamar Medical Ltd, Caesarea, Israel) was used to measure relative changes in pulse wave amplitude before and after occlusion (37). The EndoPAT technique has been validated (37) and was used in the Framingham Heart Study, which found significant, inverse associations between EndoPAT scores and multiple CVD risk factors (38). Two flexible probes were placed on the index fingers of the right (ischemic) and left (control) hands. Measurements were made during baseline (5 min), occlusion (5 min), and reactive hyperemia (5 min). The Reactive Hyperemia Index (RHI) was calculated as the ratio of the average pulse wave amplitude during hyperemia (60 to 120 s of the postocclusion period) to the average pulse wave amplitude during baseline in the occluded hand divided by the same values in the control hand and then multiplied by a baseline correction factor. The Framingham RHI (F-RHI) is an alternative calculation derived from the same raw data and differs in that it uses the period from 90 to 120 s of postocclusion hyperemia, does not incorporate a baseline correction factor, and has a natural log transformation applied to the resulting ratio. F-RHI has been shown to correlate with other CVD risk markers (38, 39). The EndoPAT device also generates the Augmentation Index (AI)—a measure of vascular stiffness (pulse wave reflection) that is calculated from the shape of the pulse wave recorded by the probes during baseline. AI can be adjusted to a heart rate of 75 beats/min to correct for the independent effect of heart rate on this measure (40). Both unadjusted and adjusted AIs are reported here.
Statistical analyses
Statistical analyses were performed by using SAS (version 9.2; SAS Institute, Cary, NC). The natural logarithmic transformation was used for positively skewed outcome variables. The mixed-models procedure (PROC MIXED) in SAS was used to test the effects of treatment, period, and treatment by period interactions on each outcome. Subject was treated as a random effect, and the remaining factors were fixed effects. When period and treatment by period interactions were nonsignificant, they were removed from the model. For all outcomes, no treatment by period effect was observed. When period effects were significant, they were retained in the final model of treatment effects. Tukey-Kramer–adjusted P values were used for post hoc comparisons between the 3 groups. Values that were measured in duplicate [lipids (separate days), plasma cytokines (assay duplicate), and FMD (2 independent scorers)] were averaged before analysis. Means are reported as least-squares means ± SEM.
Regression modeling was performed with Minitab (version 16.1; Minitab, State College, PA). Predictor by dose interactions were tested in SAS to assess whether the slope of the regression line for the predictor were equal across both treatments. Regression lines for the 2 treatment groups were also visually examined for equal slope. When that requirement was met, regressions were reported as pooled values, collapsing across the treatments. Graphic representations were generated in Minitab as scatter plots for outcome vs predictor with regression lines. Residual vs fit plots were examined to ensure homoscedasticity.
RESULTS
Twenty-eight people began the study and 26 completed it (23 men and 3 postmenopausal women). One male subject withdrew because of gastrointestinal symptoms that began during the placebo period and intensified during the 3.4-g/d period. One male subject died during the washout phase after the placebo dose, and this event was judged to be unrelated to study procedures. The final study population was, on average, middle-aged, overweight, and normotensive (Table 2). The sample was predominantly white and non-Hispanic and included one subject of Southeast Asian descent. Compliance was excellent (>95% for all subjects during all periods) as determined by capsule logs and bottle weights. Erythrocyte EPA and DHA also increased in a dose-dependent manner (discussed further below). Body weight did not change during the study (data not shown).
TABLE 2.
Baseline characteristics of the subjects who completed the study (n = 26)1
| Value (n = 23 M, 3 F) | |
| Age (y) | 44.3 ± 9.8 (22–65) |
| Weight (kg) | 92.8 ± 18.2 (59.7–137.7) |
| BMI (kg/m2) | 29.0 ± 3.6 (23.7–36.5) |
| SBP (mm Hg) | 122.9 ± 9.5 (98–139) |
| DBP (mm Hg) | 81.9 ± 7.8 (65–96) |
| Lipids and lipoproteins | |
| TG (mg/dL) | 222.8 ± 56.3 (140.5–339) |
| TC (mg/dL) | 206.2 ± 42.3 (133–269) |
| LDL-C (mg/dL) | 121.2 ± 38.1 (57–188.5) |
| HDL-C (mg/dL) | 40.4 ± 8.1 (26–57) |
| Glucose metabolism | 103.5 ± 15.7 (85–163) |
| Glucose (mg/dL) | |
| Insulin (μIU/mL) | 18.0 ± 9.0 (5–40) |
| Markers of inflammation | |
| hs-CRP (mg/L) | 1.26 ± 0.83 (<0.2–2.8) |
| IL-1β (pg/mL) | 0.14 ± 0.09 (0–0.34) |
| IL-6 (pg/mL) | 0.77 ± 0.53 (0.15–2.48) |
| TNF-α (pg/mL) | 1.22 ± 0.30 (0.55–1.71) |
| Liver enzymes (IU/L) | |
| AST | 21.5 ± 5.9 (12–37) |
| ALT | 28.8 ± 12.2 (12–56) |
| Erythrocyte fatty acid content (% by weight) | |
| Eicosapentaenoic acid | 0.49 ± 0.21 (0.21–0.95) |
| Docosapentaenoic acid | 2.68 ± 0.36 (2.00–3.54) |
| Docosahexaenoic acid | 3.97 ± 0.96 (2.32–6.65) |
| Omega-3 index | 4.46 ± 1.13 (2.63–7.45) |
All values are means ± SDs; ranges in parentheses. Values were obtained by using the UNIVARIATE procedure (version 9.2; SAS Institute Inc, Cary, NC). Lipids and lipoproteins are the average of 2 samples taken on 2 separate days. LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Effects of lower and higher EPA+DHA doses on blood-derived measures
Compared with placebo and the low dose, triglycerides were significantly lower after the 3.4-g/d dose of EPA+DHA (Table 3). Relative to placebo, the reduction with the 3.4-g/d dose was 27% (P = 0.002). The 0.85-g/d dose did not alter triglyceride values. Total cholesterol, LDL-cholesterol, and HDL-cholesterol values did not differ significantly by treatment.
TABLE 3.
Effects of treatment on plasma and serum measures (n = 26)1
| EPA+DHA |
P value for treatment effect2 | |||
| 0 g/d | 0.85 g/d | 3.4 g/d | ||
| Lipids and lipoproteins | ||||
| TG (mg/dL) | 237.3 ± 17.5a | 215.3 ± 17.5a | 173.7 ± 17.5b | 0.002 |
| TC (mg/dL) | 209.0 ± 7.9 | 212.1 ± 7.9 | 207.9 ± 7.9 | 0.60 |
| LDL-C (mg/dL) | 123.3 ± 7.6 | 127.6 ± 7.6 | 130.3 ± 7.6 | 0.21 |
| HDL-C (mg/dL) | 42.6 ± 1.9 | 42.7 ± 1.9 | 43.2 ± 1.9 | 0.76 |
| non-HDL-C (mg/dL) | 166.4 ± 7.1 | 169.4 ± 7.1 | 164.7 ± 7.1 | 0.54 |
| non-HDL-C:HDL-C | 4.03 ± 0.2 | 4.04 ± 0.2 | 3.95 ± 0.2 | 0.74 |
| LDL-C:HDL-C | 2.94 ± 0.2 | 3.00 ± 0.2 | 3.11 ± 0.2 | 0.15 |
| TC:HDL-C | 5.03 ± 0.2 | 5.04 ± 0.2 | 4.95 ± 0.2 | 0.74 |
| Glucose metabolism | ||||
| Glucose (mg/dL) | 96.1 ± 2.0 | 98.0 ± 1.9 | 99.2 ± 1.9 | 0.14 |
| Insulin (μIU/mL) | 14.6 ± 1.4 | 15.5 ± 1.4 | 15.0 ± 1.4 | 0.31 |
| HOMA-IR | 3.55 ± 0.4 | 3.75 ± 0.4 | 3.64 ± 0.4 | 0.46 |
| QUICKI | 0.14 ± 0.002 | 0.14 ± 0.002 | 0.14 ± 0.002 | 0.36 |
| Markers of inflammation (plasma protein concentrations) | ||||
| hs-CRP (mg/L) | 1.45 ± 0.2 | 1.32 ± 0.2 | 1.29 ± 0.2 | 0.72 |
| IL-1β (pg/mL) | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.02 | 0.89 |
| IL-6 (pg/mL) | 0.87 ± 0.15 | 0.85 ± 0.15 | 0.87 ± 0.15 | 0.89 |
| TNF-α (pg/mL) | 1.16 ± 0.07 | 1.07 ± 0.07 | 1.10 ± 0.07 | 0.20 |
| Markers of inflammation (normalized gene expression in isolated PBMCs) | ||||
| IL-1β expression | 1.10 ± 0.09 | 1.06 ± 0.09 | 1.08 ± 0.09 | 0.92 |
| IL-6 expression | 0.73 ± 0.16 | 0.73 ± 0.16 | 0.69 ± 0.16 | 0.70 |
| TNF-α expression | 1.00 ± 0.07 | 0.94 ± 0.07 | 0.97 ± 0.07 | 0.82 |
| Liver enzymes (IU/L) | ||||
| AST | 20.3 ± 1.1 | 21.1 ± 1.1 | 21.9 ± 1.1 | 0.18 |
| ALT | 27.4 ± 3.1 | 30.4 ± 3.1 | 32.4 ± 3.1 | 0.11 |
| Erythrocyte omega-3 fatty acid content (% by weight) | ||||
| EPA | 0.57 ± 0.09a | 1.15 ± 0.09b | 2.30 ± 0.09c | <0.0001 |
| DPA | 2.74 ± 0.08a | 3.04 ± 0.08b | 3.40 ± 0.08c | <0.0001 |
| DHA | 4.39 ± 0.15a | 5.34 ± 0.15b | 6.49 ± 0.15c | <0.0001 |
| Omega-3 index | 4.96 ± 0.21a | 6.49 ± 0.21b | 8.79 ± 0.21c | <0.0001 |
All values are as means ± SEMs. LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; TC, total cholesterol; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HOMA-IR, homeostatic model of insulin resistance; QUICKI, quantitative insulin-sensitivity check index; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid (n−3); DHA, docosahexaenoic acid; PBMCs, peripheral blood mononuclear cells. Values with different superscript letters are significantly different, P < 0.05 (Tukey-adjusted values from post hoc tests).
P values are for the main effect of treatment based on the MIXED procedure (version 9.2; SAS Institute Inc, Cary, NC). Lipids and lipoproteins are the average of 2 samples taken on 2 separate days.
Neither dose of omega-3 fatty acids had any effects on glucose, insulin, inflammatory markers, or inflammatory gene expression in isolated PBMCs relative to placebo (Table 3). Endpoint values for calculated measures of glucose metabolism did not differ by treatment. Liver enzymes (alanine aminotransferase and aspartate aminotransferase) and body weight (or BMI) were also unchanged.
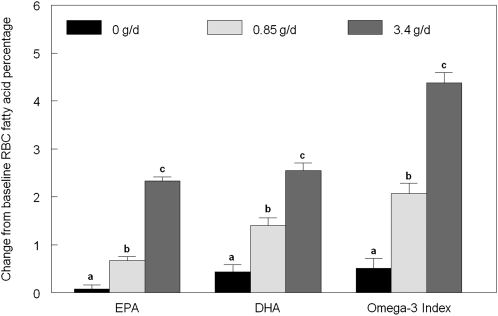
Erythrocyte omega-3 fatty acids
The omega-3 fatty acids EPA, docosapentaenoic acid, and DHA increased in a dose-dependent manner (Table 3, Figure 2). The increase in EPA and DHA concentrations resulted in a significant increase in omega-3 index of 32% for the 0.85-g/d dose and 79% for the 3.4-g/d dose. This effect was achieved via modest displacement of monounsaturated and omega-6 polyunsaturated fatty acids (see supplementary tables under “Supplemental data” in the online issue for full fatty acid analysis).
FIGURE 2.
Mean (±SEM) changes in red blood cell (RBC) long-chain omega-3 fatty acids and omega-3 index. Change scores for each treatment were calculated as the end of treatment period value minus the baseline value and compared by using the MIXED procedure (SAS version 9.2; SAS Institute Inc, Cary, NC). Different lowercase letters within outcomes indicate significant differences between treatment groups, P < 0.05 (Tukey-adjusted post hoc pairwise comparisons). EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
Endothelial function and reactive hyperemia indexes
Endothelial function, as measured by FMD, RHI, or F-RHI, was not affected by either dose of omega-3 fatty acids relative to placebo. There were no effects on arterial stiffness (AI or AI adjusted for heart rate) or on hyperemic flow measured by Doppler ultrasound. Like FMD, the EndoPAT reactive hyperemia scores measure nitric oxide–dependent dilation (41) but may be more indicative of microvascular changes (39) than FMD, which measures changes in brachial artery diameter. No measures of vascular endothelial function or measures of artery stiffness were affected by treatment.
Although there were no significant treatment effects on vascular function or hyperemic response, several of these variables showed small but significant period effects (Table 4). Resting and peak brachial artery diameters increased after the first visit by ≈2%, and this was accompanied by a reduction in some hyperemic indexes. RHI decreased 13% after the first visit. However, no period effects on FMD or the magnitude of flow-induced change in artery diameter (mm) were observed.
TABLE 4.
Effects of treatment on measures of endothelial function (n = 26)1
| EPA+DHA |
P value2 |
||||
| 0 g/d | 0.85 g/d | 3.4 g/d | For treatment effect | For period effect | |
| Reactive hyperemia outcomes from FMD | |||||
| FMD (% change in artery diameter) | 5.00 ± 0.48 | 4.03 ± 0.48 | 4.14 ± 0.48 | 0.11 | 0.73 |
| ΔArtery diameter (mm, peak-base) | 0.24 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.07 | 0.80 |
| Peak flow:resting flow3 | 6.28 ± 0.44 | 6.59 ± 0.45 | 6.84 ± 0.44 | 0.55 | 0.001 |
| RHI from EndoPAT | |||||
| RHI | 1.84 ± 0.10 | 1.82 ± 0.10 | 1.86 ± 0.10 | 0.86 | 0.02 |
| Framingham RHI | 0.28 ± 0.06 | 0.27 ± 0.07 | 0.33 ± 0.07 | 0.66 | 0.17 |
| Pulse wave properties and HR from EndoPAT | |||||
| AI | −9.33 ± 1.6 | −9.52 ± 1.6 | −9.25 ± 1.6 | 0.97 | 0.56 |
| AI standardized for HR of 75 bpm | −16.9 ± 1.6 | −17.5 ± 1.5 | −18.1 ± 1.5 | 0.53 | 0.91 |
| Heart rate (beats/min)4 | 62.9 ± 1.6 | 62.7 ± 1.6 | 61.0 ± 1.6 | 0.09 | 0.03 |
| Resting artery diameter and blood flow values (Doppler ultrasound) | |||||
| Artery diameter (mm)5 | 4.83 ± 0.13 | 4.92 ± 0.13 | 4.87 ± 0.13 | 0.09 | 0.005 |
| Velocity time integral (m)5 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.88 | 0.003 |
| Maximum velocity (m/s) | 0.98 ± 0.06 | 0.95 ± 0.06 | 1.00 ± 0.06 | 0.49 | 0.10 |
| Average flow velocity (m/s)5 | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.84 | 0.01 |
| Flow (mL/min)5 | 201 ± 17.5 | 207 ± 17.7 | 206 ± 17.5 | 0.94 | 0.0007 |
| Postocclusion artery diameter and blood flow values (Doppler ultrasound) | |||||
| Artery diameter (mm)5 | 5.07 ± 0.13 | 5.10 ± 0.13 | 5.06 ± 0.13 | 0.55 | 0.01 |
| Velocity time integral (m) | 0.97 ± 0.05 | 0.99 ± 0.05 | 1.02 ± 0.05 | 0.45 | 0.43 |
| Maximum velocity (m/s) | 1.76 ± 0.07 | 1.76 ± 0.07 | 1.78 ± 0.07 | 0.83 | 0.22 |
| Average flow velocity (m/s) | 1.02 ± 0.04 | 1.01 ± 0.04 | 1.02 ± 0.04 | 0.83 | 0.57 |
| Flow (mL/min) | 1193 ± 77 | 1219 ± 77 | 1238 ± 77 | 0.68 | 0.47 |
Values are expressed as means ± SEMs. EndoPAT, Itamar Medical Ltd, Caesarea, Israel. RHI, Reactive Hyperemia Index; AI, Augmentation Index; HR, heart rate; FMD, flow-mediated dilation; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
P values are for the main effect of treatment and period based on the MIXED procedure with both fixed effects in the model when period effects were significant (version 9.2; SAS Institute Inc, Cary, NC). When the period was nonsignificant, it was removed from the model to determine treatment effects. None of the Tukey-adjusted P values for pairwise comparisons for treatment effects were significant (P > 0.05).
First-visit values were significantly greater than visit 2 and visit 3 values (P < 0.005, Tukey-adjusted).
First-visit values were significantly lower than visit 3 values (P = 0.04, Tukey-adjusted).
First-visit values were significantly lower than visit 2 and visit 3 values (P < 0.05, Tukey-adjusted).
Regression modeling
Triglyceride values as a predictor of triglyceride changes in response to 3.4 g EPA+DHA/d
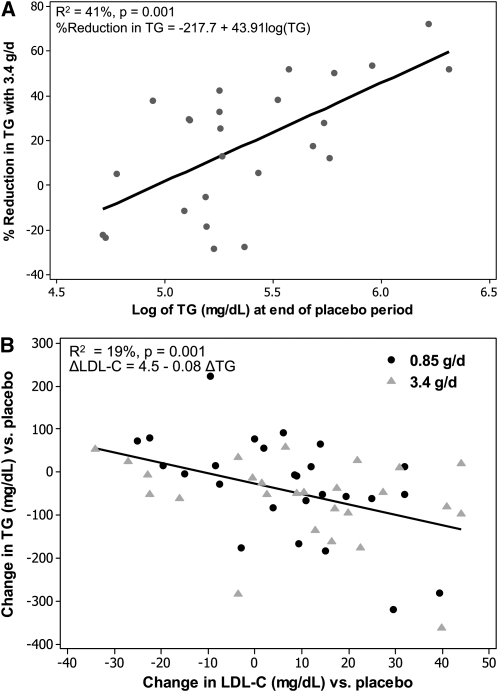
Previous studies have shown a relation between triglyceride values and the percentage reduction in triglycerides in response to 3.4 g EPA+DHA/d (reviewed in 12). Therefore, regression modeling was used to assess whether this relation could be modeled on an individual basis within our sample. Log-transformed triglyceride values at the end of the placebo period were linearly related to the percentage reduction in triglycerides achieved by participants after 3.4 g EPA+DHA/d (R2 = 41%, P = 0.001; Figure 3). The Pearson's r for this correlation was 0.64.
FIGURE 3.
Scatterplots for selected regression analyses (version 16.1; Minitab, State College, PA). A: Percentage reduction in triglyceride (TG) values in response to 3.4 g eicosapentaenoic acid + docosahexaenoic acid/d compared with TG values after placebo treatment. The points (n = 26) are plotted as the percentage reduction in TG values achieved with 3.4 g eicosapentaenoic acid + docosahexaenoic acid/d (relative to placebo) compared with log-transformed TG values after the placebo period. TG values at the end of the placebo treatment period significantly predicted the percentage reduction achieved after 3.4 g/d [R2 = 41%; percentage reduction in TG = −217.7 + 43.91 log(TG); P = 0.001]. B: Correlation between TG reduction and increases in LDL cholesterol (LDL-C). The points (n = 26 per treatment group) are plotted as the change relative to placebo for TG and LDL-C, and the regression was modeled as the change in LDL-C predicted by the change in TG across both the 0.85- and 3.4-g/d groups. The change in TG significantly predicted the change in LDL-C (R2 = 19%; ΔLDL-C = 4.47–0.0784 ΔTG; P = 0.001). The slope of the regression did not differ significantly by treatment. One outlier data point was removed from the calculation of average placebo LDL-C for one subject in the final model (suspected technical error).
Change in triglycerides as a predictor of change in LDL cholesterol and FMD across treatments
The change in triglyceride values resulting from omega-3 treatment relative to placebo treatment was assessed as a predictor of changes in LDL cholesterol and FMD relative to placebo. The slope of the line did not differ by treatment. Combining across the 2 omega-3 treatments, the change in triglycerides was inversely correlated with the change in LDL cholesterol (P = 0.003); it predicted 12% of the change in LDL cholesterol relative to placebo. The model was also tested after one of the cholesterol measures that was used to calculate the average LDL cholesterol for the placebo period for one subject was excluded (suspected technical error), and the regression R-squared increased to 19% (P = 0.001; Figure 3). The data points for this participant had the largest residuals in the original model. The model predicts for this sample that each additional 13-mg/dL reduction in triglycerides was associated with an increase in LDL cholesterol of 1 mg/dL.
Pooling across both doses, the reduction in triglyceride values after fish-oil treatment was significantly predictive of FMD improvement. The change in triglyceride value predicted ≈10% of the change in FMD (P = 0.04). Changes in triglycerides did not predict the changes in EndoPAT RHI, F-RHI, and AI (data not shown).
Other regression models examined
Changes in LDL cholesterol, HDL cholesterol, hs-CRP, and the omega-3 index were not significant, independent predictors of the change in FMD (data not shown), and changes in the omega-3 index were not predictive of changes in inflammatory status in this sample (data not shown). However, there was very little range in within-subject inflammatory changes in this study, which limited regression modeling.
DISCUSSION
The results of this study corroborate the effectiveness of pharmacologic doses of omega-3 fatty acids in reducing triglyceride concentrations in persons with moderate hypertriglyceridemia. Relative to the placebo treatment, fasting triglycerides were reduced by 27% after the 3.4-g/d dose, consistent with previous reports (12, 42–46). The percentage reduction achieved was related to the subjects’ triglyceride concentrations after the placebo dose so that, on average, the subjects with the highest triglycerides achieved the greatest percentage reduction after the higher dose. The lower dose (0.85 g/d) did not alter lipid values, and fasting measures of glucose metabolism were not altered by either dose relative to placebo.
As expected, the omega-3 fatty acid composition of erythrocytes increased in a dose-responsive manner, confirming compliance and uptake of omega-3 fatty acids into cellular membranes. After 8 wk of supplementation, the 3.4-g/d dose achieved an average omega-3 index associated with a reduced CVD risk (> 8%) (33). Because the duration of supplementation was only 8 wk, these values do not reflect maximum omega-3 uptake into RBC fatty acids (47). The time between endpoint measures was ≥14 wk (8-wk treatment + 6-wk washout) to minimize potential carryover between doses.
Despite the dose-response uptake of omega-3 fatty acids into membranes, we did not observe improvements in any measure of inflammation, including circulating concentrations of hs-CRP and inflammatory cytokines or gene expression of inflammatory cytokines in isolated peripheral blood mononuclear cells. Although there is extensive evidence of the antiinflammatory effects of omega-3 fatty acids in epidemiologic (48, 49), animal, and in vitro (19, 50) and ex vivo (51, 52) studies, many clinical studies (including this one) have found no effect of supplemental omega-3 fatty acids on circulating markers of inflammation, such as hs-CRP (25, 30, 53, 54). Longer treatment duration or alternate routes of administration may be necessary (55). However, most evidence suggests that omega-3 fatty acids suppress provoked inflammatory responses, and the use of a standardized inflammatory challenge for in vivo human research is a promising strategy for future studies (55, 56).
The FMD values that we obtained with lower arm occlusion (≈4–5%) indicate relatively impaired endothelial function that is similar to values seen in persons with type 2 diabetes (24, 57); however, endothelial function was not affected by either dose of omega-3 fatty acids. Our findings agree with those of Stirban et al (24), who found no change in fasting FMD after supplementation with 1.7 g EPA+DHA/d for 6 wk. However, in that study, treatment with omega-3 fatty acids attenuated the postprandial decrease in endothelial function after a high-fat meal (24), which suggested that the effects of omega-3 supplementation on FMD may be more evident during an oxidative, inflammatory, and/or lipemic challenge.
Earlier work reported improved fasting FMD when subjects with hypercholesterolemia were treated with 3.4 g EPA+DHA/d for 4 mo (28). However, in this study, the vasodilatory response was reported as a millimeter change, whereas the percentage FMD calculated from these data are unexpectedly low relative to typical values (1–3%) (38, 58). In adults with lupus (30) and peripheral arterial disease (25), FMD improved after 12–24 wk of 2 to 3.4 g EPA+DHA/d, despite no effect on traditional markers on inflammation (25, 30). Other studies have shown no vascular effects of 4 g EPA+DHA/d given for 6 to 8 wk (26, 29).
Omega-3 fatty acids had no effect on EndoPAT scores or hyperemic responses. These findings agree with a recent report, which showed that RHI and F-RHI did not change in obese adolescents after supplementation with 1.2 g EPA+DHA/d for 12 wk (59). Because we found no effect on any vascular endpoint with omega-3 fatty acid treatment, we concluded that neither dose affected fasting endothelium dependent vasodilation, whether microvascular or macrovascular, after 8 wk.
In our study, reductions in fasting triglycerides after fish-oil treatment (relative to placebo) were associated with both increases in LDL cholesterol (R2 = 19%, P = 0.001) and, to a lesser extent, improvements in FMD (R2 = 10%, P = 0.04). The association between reductions in triglycerides and increases in LDL cholesterol corresponded to a Pearson's correlation (r) of 0.44 and is consistent with other reports (12, 60). Changes in LDL cholesterol across the 2 treatments were not predictive of changes in FMD, which suggests that any increase in LDL cholesterol within subjects did not nullify potential vascular benefits resulting from triglyceride reductions. Many exploratory regression models for predicting change in endothelial function were tested, so the finding that triglyceride reductions predict improvements in FMD is only hypothesis generating.
We were surprised to find significant period effects for hyperemic outcomes, heart rate, and artery diameter, which indicated that the response to the hyperemic stimulus changed in a systematic way with repeated exposure. This underscores the importance of placebo control in vascular studies and suggests that participants should be exposed to the testing conditions before the collection of endpoint measurements.
Study strengths
Our study population is unique in that our participants were recruited specifically based on the criterion of moderately elevated triglycerides. The subjects were otherwise healthy; were nonsmokers; and were not taking drugs for hypertension, elevated cholesterol, or inflammation or taking supplements. Although this sample is not typical of the population with moderate hypertriglyceridemia, these stringent inclusion and exclusion criteria may reduce potential variability in treatment effects. FMD values, which are highly dependent on analytic precision, were obtained following established guidelines (61). All scans were collected by one sonographer with expertise in vascular ultrasound. Our crossover design allowed us to compare the effects of 2 clinically important doses within the same participants.
Study limitations
The predominantly white male sample, treatment duration, absence of pretreatment endothelial function testing, and sample size (although typical of endothelial function studies) are potential limitations. Whereas triglyceride effects are known to occur very quickly (<2 wk), it may be that >8 wk is required to observe improvements in inflammation and endothelial function as measured by FMD and EndoPAT (25, 30). Our design required 9 mo of participation from subjects (including washout periods); despite this, our retention rate was high (only one voluntary dropout).
Conclusions
We found that 3.4 g EPA+DHA/d reduced fasting triglycerides by 27% in subjects with moderately elevated triglycerides, and this response was proportional to each individual's degree of triglyceride elevation in regression modeling. In contrast, no effects of 0.85 or 3.4 g EPA+DHA/d on endothelial function, insulin, glucose, or inflammation relative to placebo were observed. In regression models, reductions in triglycerides predicted increases in LDL cholesterol and FMD. Future studies should continue to examine whether omega-3 fatty acids have dose-response effects on adaptive responses to postprandial and inflammatory challenges.
Supplementary Material
Acknowledgments
We thank our research participants for their dedication to the project. Many members of Vascular Health Interventions Laboratory helped with the execution of this project and with the analysis of the data, including William Turbitt, Molly Crispell, Angela El Adas, and Lisa Groves. Technical support for the gene expression work was provided by Daniel Hannon. We are also grateful to the nursing and clinician staff of the General Clinical Research Center of The Pennsylvania State University, which was supported by NIH grant M01 RR 10732.
The authors’ responsibilities were as follows—ACS-R, PMK-E, and SGW: designed the research; ACS-R: conducted the research; PRW: performed all sonography; JPVH: provided essential materials for the gene expression studies; ACS-R and SGW: performed the statistical analyses; and ACS-R, WSH, PMK-E, and SGW: wrote the manuscript. All authors take responsibility for the manuscript's final content. Financial supporters had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. WSH is a scientific advisor to companies with interests in fatty acids, including Monsanto, Unilever, Neptune, Omthera, and GlaxoSmithKline, and was a speaker for the latter. In addition, he is the owner of OmegaQuant, LLC, a company that offers blood omega-3 fatty testing. None of the other authors had a conflict of interest to declare.
REFERENCES
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356–9 [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Maksoud MF, Hokanson JE. The complex role of triglycerides in cardiovascular disease. Semin Vasc Med 2002;2:325–33 [DOI] [PubMed] [Google Scholar]
- 3.National Cholesterol Education Program Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421 [PubMed] [Google Scholar]
- 4.Jeppesen J, Hein HO, Suadicani P, Gyntelberg F. Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 1998;97:1029–36 [DOI] [PubMed] [Google Scholar]
- 5.Gotto AM., Jr Triglyceride: the forgotten risk factor. Circulation 1998;97:1027–8 [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev 2005;13:322–7 [PubMed] [Google Scholar]
- 7.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213–9 [PubMed] [Google Scholar]
- 8.Carey VJ, Bishop L, Laranjo N, Harshfield BJ, Kwiat C, Sacks FM. Contribution of high plasma triglycerides and low high-density lipoprotein cholesterol to residual risk of coronary heart disease after establishment of low-density lipoprotein cholesterol control. Am J Cardiol 2010;106(6):757–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarwar N, Sandhu MS, Ricketts SL, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet 2010;375(9726):1634–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302(18):1993–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy 2007;27:715–28 [DOI] [PubMed] [Google Scholar]
- 12.Skulas-Ray AC, West SG, Davidson MH, Kris-Etherton PM. Omega-3 fatty acid concentrates in the treatment of moderate hypertriglyceridemia. Expert Opin Pharmacother 2008;9:1237–48 [DOI] [PubMed] [Google Scholar]
- 13.Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol 2006;98(4A):27i–33i [DOI] [PubMed] [Google Scholar]
- 14.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 15.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2(8666):757–61 [DOI] [PubMed] [Google Scholar]
- 16.Leaf A, Xiao YF, Kang JX, Billman GE. Membrane effects of the n−3 fish oil fatty acids, which prevent fatal ventricular arrhythmias. J Membr Biol 2005;206:129–39 [DOI] [PubMed] [Google Scholar]
- 17.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n−3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n−3 fish oils. Circulation 2003;107:2646–52 [DOI] [PubMed] [Google Scholar]
- 18.Calder PC. n−3 Fatty acids and cardiovascular disease: evidence explained and mechanisms explored. Clin Sci (Lond) 2004;107:1–11 [DOI] [PubMed] [Google Scholar]
- 19.Oh da Y, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142(5):687–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calder PC. Polyunsaturated fatty acids, inflammatory processes and inflammatory bowel diseases. Mol Nutr Food Res 2008;52:885–97 [DOI] [PubMed] [Google Scholar]
- 21.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 2002;21:495–505 [DOI] [PubMed] [Google Scholar]
- 22.West SG, Hecker KD, Mustad VA, et al. Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia 2005;48:113–22 [DOI] [PubMed] [Google Scholar]
- 23.Vogel RA, Corretti MC, Plotnick GD. The postprandial effect of components of the Mediterranean diet on endothelial function. J Am Coll Cardiol 2000;36:1455–60 [DOI] [PubMed] [Google Scholar]
- 24.Stirban A, Nandrean S, Gotting C, et al. Effects of n−3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr 2009;91(3):808–13 [DOI] [PubMed] [Google Scholar]
- 25.Schiano V, Laurenzano E, Brevetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr 2008;27:241–7 [DOI] [PubMed] [Google Scholar]
- 26.Woodman RJ, Mori TA, Burke V, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 2003;166:85–93 [DOI] [PubMed] [Google Scholar]
- 27.Engler MM, Engler MB, Malloy M, et al. Docosahexaenoic acid restores endothelial function in children with hyperlipidemia: results from the EARLY study. Int J Clin Pharmacol Ther 2004;42:672–9 [DOI] [PubMed] [Google Scholar]
- 28.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol 2000;35:265–70 [DOI] [PubMed] [Google Scholar]
- 29.Dyerberg J, Eskesen DC, Andersen PW, et al. Effects of trans- and n−3 unsaturated fatty acids on cardiovascular risk markers in healthy males. An 8 weeks dietary intervention study. Eur J Clin Nutr 2004;58:1062–70 [DOI] [PubMed] [Google Scholar]
- 30.Wright SA, O'Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis 2008;67:841–8 [DOI] [PubMed] [Google Scholar]
- 31.Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr 2007;85:1267–74 [DOI] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560–72 [DOI] [PubMed] [Google Scholar]
- 33.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med 2004;39:212–20 [DOI] [PubMed] [Google Scholar]
- 34.West SG, Wagner P, Schoemer SL, et al. Biological correlates of day-to-day variation in flow-mediated dilation in individuals with Type 2 diabetes: a study of test-retest reliability. Diabetologia 2004;47:1625–31 [DOI] [PubMed] [Google Scholar]
- 35.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10 [DOI] [PubMed] [Google Scholar]
- 36.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27(6):1487–95 [DOI] [PubMed] [Google Scholar]
- 37.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol 2004;44(11):2137–41 [DOI] [PubMed] [Google Scholar]
- 38.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117(19):2467–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009;19:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525:263–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003;146:168–74 [DOI] [PubMed] [Google Scholar]
- 42.Lungershausen YK, Abbey M, Nestel PJ, Howe PR. Reduction of blood pressure and plasma triglycerides by omega-3 fatty acids in treated hypertensives. J Hypertens 1994;12:1041–5 [PubMed] [Google Scholar]
- 43.Grundt H, Nilsen DW, Hetland O, et al. Improvement of serum lipids and blood pressure during intervention with n−3 fatty acids was not associated with changes in insulin levels in subjects with combined hyperlipidaemia. J Intern Med 1995;237:249–59 [DOI] [PubMed] [Google Scholar]
- 44.Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther 2007;29:1354–67 [DOI] [PubMed] [Google Scholar]
- 45.Calabresi L, Donati D, Pazzucconi F, Sirtori CR, Franceschini G. Omacor in familial combined hyperlipidemia: effects on lipids and low density lipoprotein subclasses. Atherosclerosis 2000;148:387–96 [DOI] [PubMed] [Google Scholar]
- 46.Mackness MI, Bhatnagar D, Durrington PN, et al. Effects of a new fish oil concentrate on plasma lipids and lipoproteins in patients with hypertriglyceridaemia. Eur J Clin Nutr 1994;48:859–65 [PubMed] [Google Scholar]
- 47.Harris WS. The omega-3 index as a risk factor for coronary heart disease. Am J Clin Nutr 2008;87(suppl):1997S–2002S [DOI] [PubMed] [Google Scholar]
- 48.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n−3 and n−6 fatty acids in relation to inflammatory markers among US men and women. Circulation 2003;108:155–60 [DOI] [PubMed] [Google Scholar]
- 49.He K, Liu K, Daviglus ML, et al. Associations of dietary long-chain n−3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 2009;103:1238–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudert CA, Weylandt KH, Lu Y, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA 2006;103:11276–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids 2008;79:101–8 [DOI] [PubMed] [Google Scholar]
- 52.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n−3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr 1996;63:116–22 [DOI] [PubMed] [Google Scholar]
- 53.Chan DC, Watts GF, Barrett PH, Beilin LJ, Mori TA. Effect of atorvastatin and fish oil on plasma high-sensitivity C-reactive protein concentrations in individuals with visceral obesity. Clin Chem 2002;48:877–83 [PubMed] [Google Scholar]
- 54.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 2003;35(7):772–81 [DOI] [PubMed] [Google Scholar]
- 55.Pluess TT, Hayoz D, Berger MM, et al. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med 2007;33:789–97 [DOI] [PubMed] [Google Scholar]
- 56.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock 2005;24(Suppl 1):94–100 [DOI] [PubMed] [Google Scholar]
- 57.Wong CY, Yiu KH, Li SW, et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with Type 2 diabetes mellitus. Diabet Med 2009;27(1):54–60 [DOI] [PubMed] [Google Scholar]
- 58.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004;109:613–9 [DOI] [PubMed] [Google Scholar]
- 59.Dangardt F, Osika W, Chen Y, et al. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis 2010;212(2):580–5 [DOI] [PubMed] [Google Scholar]
- 60.Balk E, Chung M, Lichtenstein A, et al. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evid Rep Technol Assess (Summ) 2004;Mar:1–6 [PMC free article] [PubMed] [Google Scholar]
- 61.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–65 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.