Abstract
Survival is reportedly worse in cancer patients concurrently diagnosed with deep venous thrombosis. However, information on specific malignancies is limited. From a cohort study of male U.S. veterans we identified incident cancer cases (n=412,008) and compared survival patterns among those with versus without a history of deep venous thrombosis. Using Cox-proportional hazard models, we estimated hazard ratios (HR) and 95% confidence intervals as measures of the relative risk of dying. Individuals with (versus without) a concomitant deep venous thrombosis and cancer diagnosis had a higher risk of dying (HR=1.38; 1.28–1.49). The most prominent excess mortality (HRs=1.29–2.55) was observed among patients diagnosed with deep venous thrombosis at the time of diagnosis of lung-, gastric-, prostate-, bladder-, or kidney cancer. Increased risk of dying was also found among cancer patients diagnosed with deep venous thrombosis 1 year (HR=1.14; 1.07–1.22), 1–5 years (HR=1.14; 1.10–1.19) and >5 years (HR=1.27; 1.23–1.31) before cancer; this was true for most cancer sites (HRs=1.17–1.64). In summary, antecedent deep venous thrombosis confers a worse prognosis upon cancer patients. Advanced stage at diagnosis, treatment effects, lifestyle factors, and comorbidity could explain differences by cancer site and time frame between a prior deep venous thrombosis diagnosis and cancer outcome.
Keywords: cancer, deep venous thrombosis, survival
INTRODUCTION
Deep venous thrombosis of the lower extremities (DVT) is common in the U.S., resulting in approximately 600,000 hospitalizations per year1. Risk factors for DVT include surgery, long periods of immobility, obesity and some medical conditions, including cancer2. Cancer can increase the risk of thromboembolism by causing abnormalities in blood flow; compression of blood flow or invasion of blood vessels by the tumor; activation of coagulation through interactions with platelets, clotting factors and the fibrinolytic system; immobility due to cancer related debility; or endothelial damage due to chemotherapeutic treatments. Approximately 15% of all cancer patients will develop thromboembolic disease3, which is the second leading cause of death in these patients4. Most diagnoses occur in the 4 months following diagnosis of metastatic disease5. In addition, it is estimated that around 7% of patients with an unexplained (idiopathic) DVT will later be diagnosed with cancer6–9. Consequently, screening for underlying malignancy in patients presenting with idiopathic DVT is commonplace. Indeed, cancer diagnosis rates are highest in the first few months following DVT7,8,10 but may remain elevated for 10 or more years8,10, indicating the operation of some shared genetic or environmental etiology.
Using data from Denmark, Sorensen et al. found that cancer patients diagnosed with thromboembolism, at or just preceding cancer diagnosis, had more advanced disease and poorer overall survival than cancer patients without a history of thromboembolism11. Other studies have also identified venous thromboembolism as a negative predictor of survival in solid tumours11–16. However, Sorensen et al. found that a diagnosis of thromboembolism made more than one year before cancer diagnosis was also associated with somewhat higher mortality. Given that there is substantial biological and clinical heterogeneity among different types of malignancies, DVT may not be associated with increased mortality for all cancers. To our knowledge, few studies have investigated the clinical impact of antecedent DVT on the prognosis of different types of tumors.
Using a cohort of more than 4 million white and black male U.S. veterans, we identified all incident cancer cases (n=412,008) and evaluated overall survival patterns for specific malignancies among veterans with versus without a concomitant/antecedent diagnosis of DVT.
MATERIALS AND METHODS
Using the U.S. Veterans Affairs (VA) database of computerized discharge records for inpatient visits (July 1, 1969– September 30, 1996) at U.S. VA hospitals17, we selected patients with a diagnosis of cancer. The VA database has 26 million hospital discharge records from 5,790,493 veterans with one or more hospital visits. Due to limited numbers, persons aged ≤18 or >100 years (n= 2,969, 0.05%), those with a race other than black or white (n=135,651, 2.3%) and females (n=112,527, 1.9%) were excluded from the study. In addition, cancer patients who developed DVT (ICDA-8=451.0 for 1969–1980 data or ICD-9-CM=453 for 1981–1996 data) after diagnosis of cancer were excluded (n=70,864). Therefore, the current investigation includes 412,008 patients with a hospital discharge diagnosis of cancer. Patients’ cancers were classified into larynx, lung, buccal, esophagus, stomach, pancreas, colorectal, liver, prostate, bladder, and kidney cancers, non-Hodgkin lymphoma, leukemia, and multiple myeloma, based on the International Classification of Diseases, adapted for the U.S. (8th revision for 1969–1980 data)18 and ICD-9-CM for 1981–1996 data19.
VA cancer patients with a DVT diagnosis, as defined above, on the same discharge summary as their cancer diagnosis or on an earlier discharge summary were eligible to be cases. We compared survival between cancer patients with a DVT and cancer patients without a DVT diagnosis. Patients diagnosed with DVT in the 3-month period before cancer diagnosis are classified as having DVT “at cancer diagnosis”. Patients diagnosed with DVT 3 months to 1 year before cancer diagnosis are classified as having DVT “1 year” before cancer diagnosis. Patients diagnosed with DVT 1 to 5 years or more than 5 years before cancer diagnosis are classified as having DVT “1–5 years” or “>5 years” before cancer diagnosis, respectively.
Dates of death were obtained from VA hospital records and by linkage to Social Security Administration Death Master File20. Follow-up started at the date of cancer diagnosis and ended at either death or end of follow-up (October 1st 1996), whichever occurred first.
An exemption from institutional review board review was obtained from the National Institutes of Health Office of Human Subjects Research because these data were analyzed without the use of personal identifiers. Since no patients were contacted in this study, informed patient consent was not required.
Statistical Methods
Cox proportional hazard models (PROC PHREG, SAS version 9.1; SAS Institute, Cary, NC) were used to compare mortality in cancer patients with and without a concomitant or an antecedent diagnosis of DVT. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated. Adjustment variables included in the models were race (white Caucasian, African American), age (as a continuous variable), calendar period of cancer diagnosis (<1985, ≥1985) and the number of hospital visits (as a continuous variable).
Survival curves were estimated with the Kaplan-Meier method, using direct adjustment for the covariates in our multivariate Cox models21. Survival curves are presented for all cancers combined and for the most common cancers, i.e., lung, colorectal, and prostate cancers. The proportional hazards assumption was upheld for lung, colorectal and prostate cancer. For total cancer, the proportional hazards assumption was not met for one of the exposure groups (1–5 years). However, the degree of non-proportionality was modest (i.e., the relative risks for this group remained greater than 1.0 for the duration of the study period) and thus did not materially affect our conclusions. We also observed that hazards were not proportional for the potential confounders over follow-up time. However, modelling the non-proportionality did not impact the estimated associations between DVT and mortality. As the choice of models with or without modelling for non proportionality did not affect our conclusions, we report the results from models without modelling for non-proportionality.
RESULTS
We identified a total of 412,008 veterans with an incident diagnosis of cancer. Among these, 10,461 (2.5%) had a hospital discharge diagnosis of DVT at or before the diagnosis of cancer: DVT was reported in 845 (0.2%) patients at cancer diagnosis, 1,250 (0.3%) at one year before diagnosis, 3,347 (0.8%) at 1–5 years before cancer diagnosis and 5,019 (1.2%) at more than 5 years before the diagnosis of cancer. The characteristics of the patients are given in Table 1.
Table 1.
Characteristics of the study participants: male veterans diagnosed with cancer in one out of 142 U.S. Veterans Affairs Hospitals who had at least one hospital admission between July 1, 1969 and September 30, 1996 with a follow-up of more than one year.
| Characteristics | Cancer patients without a history of DVT (N=463,696) |
Cancer patients with DVT at diagnosis* (N=845) |
Cancer patients with DVT 1 yr before diagnosis† (N=1,250) |
Cancer patients with DVT 1–5 yrs before diagnosis (N=3,347) |
Cancer patients with DVT>5 yrs before diagnosis (N=5,019) |
|---|---|---|---|---|---|
| Age | |||||
| Mean age in years | 65.0 | 63.3 | 64.8 | 65.7 | 67.4 |
| < 60 years | 129,509 (27.9%) | 292 (34.6%) | 374 (29.9%) | 850 (25.4%) | 865 (17.2%) |
| ≥ 60 years | 334,187 (72.1%) | 553 (65.4%) | 876 (70.1%) | 2,497 (74.6%) | 4,154 (82.8%) |
| Race | |||||
| White Caucasian | 375,120 (80.9%) | 703 (83.2%) | 1,037 (83.0%) | 2,793 (83.4%) | 4,090 (81.5%) |
| African American | 88,576 (19.1%) | 142 (16.8%) | 213 (17.0%) | 554 (16.6%) | 929 (18.5%) |
| Calendar period of cancer diagnosis | |||||
| < 1985 | 213,638 (46.1%) | 386 (45.7%) | 567 (45.4%) | 1,664 (49.7%) | 1,476 (29.4%) |
| ≥ 1985 | 250,058 (53.9%) | 459 (54.3%) | 683 (54.6%) | 1,683 (50.3%) | 3,543 (70.6%) |
| Mean person years of post-cancer follow-up | 3.0 | 2.3 | 2.8 | 3.0 | 2.3 |
| Number of hospitalizations | 8.7 | 9.2 | 10.6 | 12.8 | 15.2 |
Deep venous thrombosis (DVT) diagnosed within the 3-month period prior to cancer diagnosis.
DVT diagnosed between 3 months and 1 year before cancer diagnosis.
Overall, cancer patients who were diagnosed with DVT at cancer diagnosis had a poorer outcome compared with cancer patients without a diagnosis of DVT (Table 2). More specifically, patients with lung (HR=1.29), gastric (HR=2.35), prostate (HR=1.34), bladder (HR=2.55), and kidney cancer (HR= 1.95) and with a concomitant DVT diagnosis had significantly higher mortality than those with the same cancer without a DVT diagnosis.
Table 2.
Hazard ratio (HR) estimates and 95% confidence intervals (CIs) comparing mortality risk among cancer patients with antecedent deep venous thrombosis (DVT) versus cancer patients without a history of DVT
| No history of DVT (reference group) |
DVT at diagnosis* | DVT 1 yr before diagnosis† |
DVT 1–5 yrs before diagnosis |
DVT>5 yrs before diagnosis |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer | N | N | HR (95%CI) | N | HR (95%CI) | N | HR (95%CI) | N | HR (95%CI) |
| Total | 401,547 | 845 | 1.38 (1.28–1.49) | 1,250 | 1.14 (1.07–1.22) | 3,347 | 1.14 (1.10–1.19) | 5,019 | 1.27 (1.23–1.31) |
| Larynx | 16,397 | 10 | 1.32 (0.63–2.77) | 22 | 1.56 (0.99–2.48) | 88 | 1.33 (1.02–1.65) | 160 | 1.18 (0.97–1.44) |
| Lung | 129,521 | 219 | 1.29 (1.12–1.48) | 267 | 1.20 (1.05–1.36) | 898 | 1.13 (1.07–1.20) | 1,391 | 1.21 (1.14–1.28) |
| Buccal | 34,463 | 31 | 1.22 (0.83–1.77) | 61 | 0.87 (0.64–1.17) | 254 | 1.03 (0.89–1.19) | 336 | 1.28 (1.13–1.45) |
| Esophagus | 12,170 | 16 | 0.97 (0.56–1.67) | 30 | 1.47 (1.01–2.13) | 73 | 1.16 (0.90–1.48) | 124 | 1.27 (1.05–1.54) |
| Gastric | 9,728 | 13 | 2.35 (1.33–4.14) | 23 | 1.88 (1.21–2.91) | 70 | 1.24 (0.96–1.60) | 107 | 1.18 (0.95–1.46) |
| Pancreas | 10,631 | 51 | 1.25 (0.93–1.67) | 29 | 1.32 (0.90–1.94) | 76 | 0.92 (0.73–1.17) | 115 | 1.64 (1.36–1.98) |
| Colorectal | 43,383 | 71 | 1.05 (0.80–1.39) | 122 | 1.28 (1.04–1.58) | 325 | 1.27 (1.12–1.44) | 490 | 1.33 (1.20–1.49) |
| Liver | 6,262 | 11 | 1.68 (0.93–3.03) | 14 | 0.89 (0.51–1.57) | 53 | 1.32 (1.00–1.76) | 75 | 1.19 (0.93–1.52) |
| Prostate | 77,238 | 140 | 1.34 (1.10–1.62) | 270 | 1.30 (1.12–1.50) | 622 | 1.11 (1.00–1.23) | 878 | 1.17 (1.07–1.28) |
| Bladder | 22,949 | 34 | 2.55 (1.77–3.67) | 58 | 0.68 (0.47–0.97) | 154 | 1.15 (0.94–1.41) | 218 | 1.21 (1.02–1.45) |
| Kidney | 10,799 | 21 | 1.95 (1.23–3.10) | 38 | 1.42 (1.23–2.06) | 68 | 1.35 (1.03–1.77) | 149 | 1.39 (1.13–1.72) |
| Non-Hodgkin lymphoma | 11,388 | 32 | 1.33 (0.91–1.94) | 50 | 1.32 (0.97–1.80) | 75 | 1.09 (0.83–1.42) | 129 | 1.36 (1.11–1.66) |
| Leukemia | 1,124 | 24 | 1.24 (0.77–1.99) | 43 | 1.19 (0.86–1.64) | 102 | 1.23 (1.00–1.53) | 155 | 1.37 (1.14–1.64) |
| Multiple myeloma | 5,404 | 8 | 1.15 (0.52–2.57) | 22 | 1.09 (0.69–1.74) | 58 | 1.02 (0.77–1.36) | 65 | 1.27 (0.95–1.69) |
All models are multivariable Cox regression models that include yes-no history of DVT, race (white Caucasian, African American), age (continuous), calendar period (<1985, ≥1985) and the number of hospital visits (continuous).
Underlined results were significant at p<0.05.
DVT diagnosed within the 3-month period prior to cancer diagnosis.
DVT diagnosed between 3 months and 1 year before cancer diagnosis.
We also found patients with lung, esophageal, gastric, colorectal, prostate, and kidney cancer with (versus without) a diagnosis of DVT 1 year prior to their cancer diagnosis to have a poorer outcome. Among patients diagnosed with a DVT 1–5 years before a diagnosis of laryngeal, lung, colorectal, liver, prostate, or kidney cancer or leukemia, we observed a significantly higher mortality rate than patients with those cancers but without a history of DVT. Mortality was also elevated for esophageal, gastric and bladder cancer patients with (versus without) a diagnosis of DVT 1–5 years before their cancer diagnosis. For virtually all cancer types, patients with (versus without) a diagnosis of DVT more than 5 years before the diagnosis of their cancer had a worse prognosis.
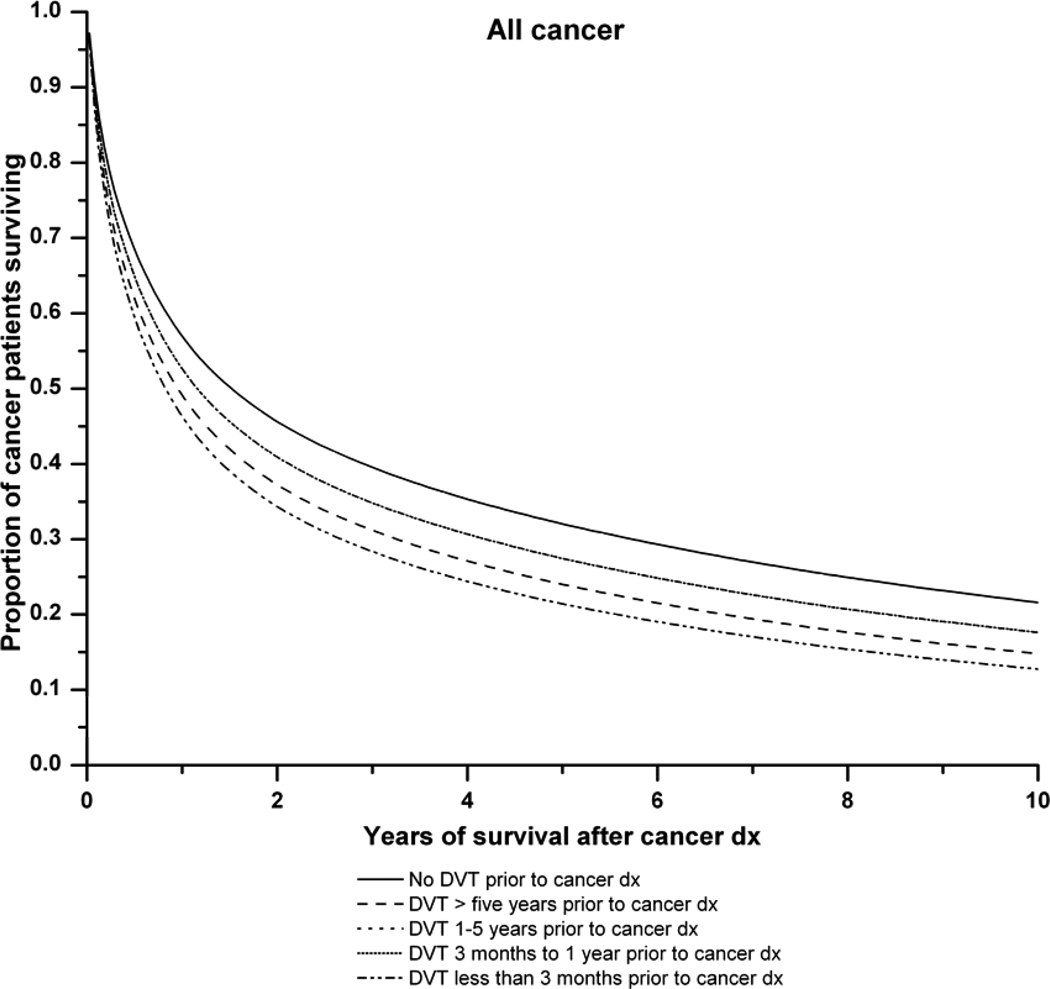
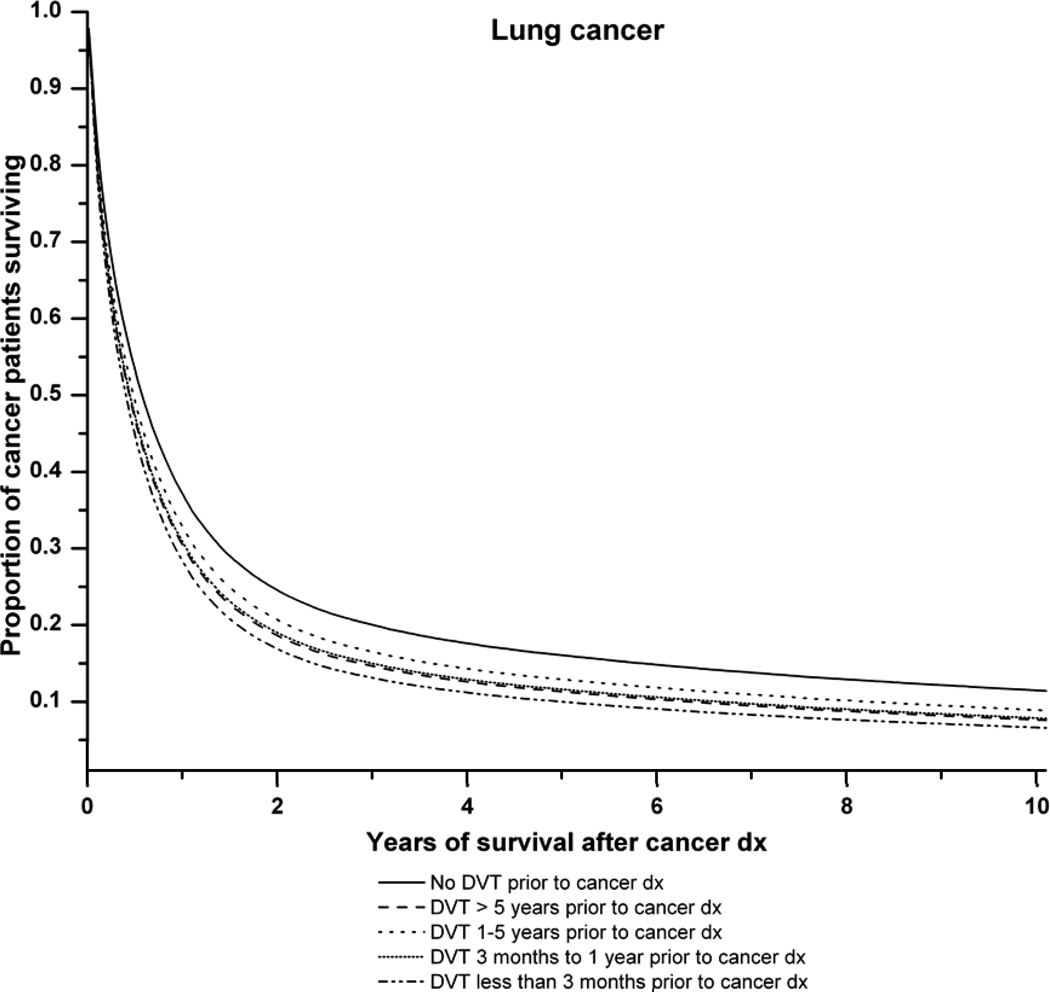
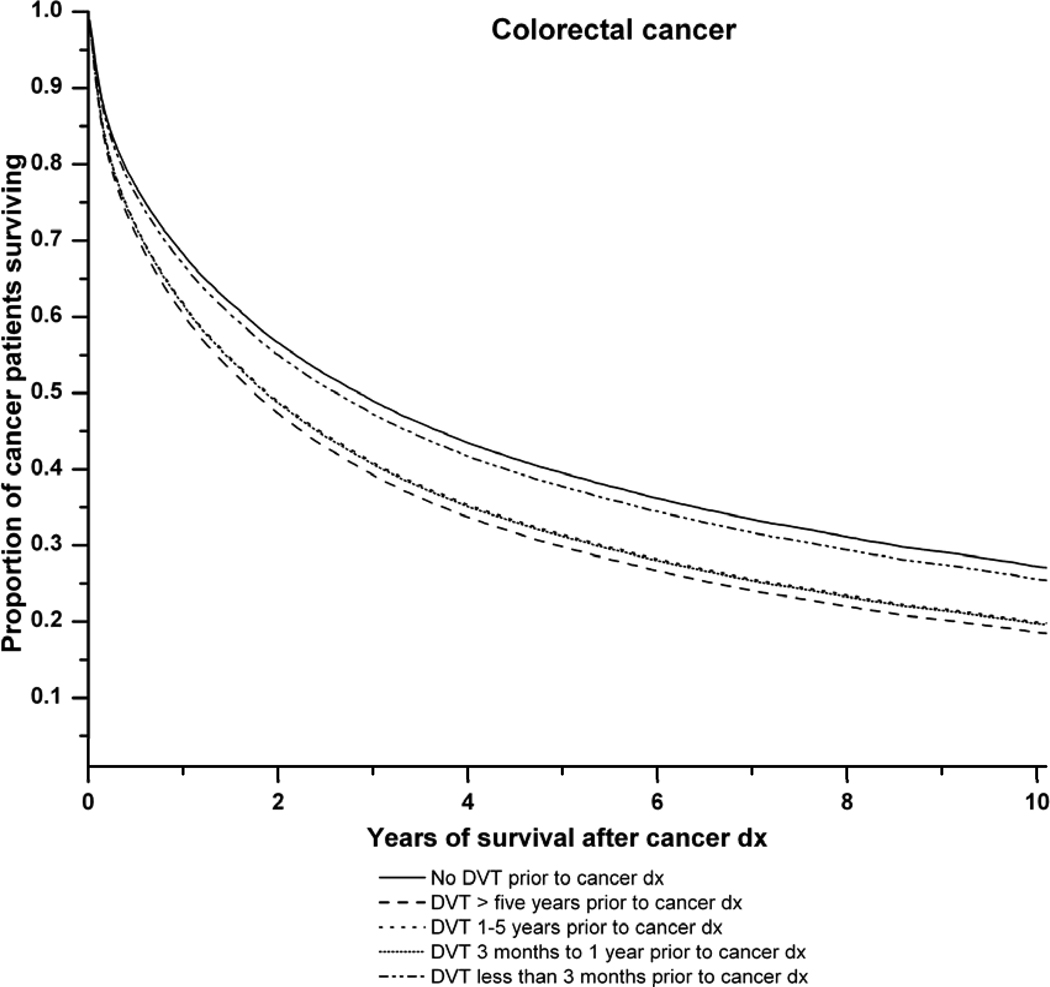
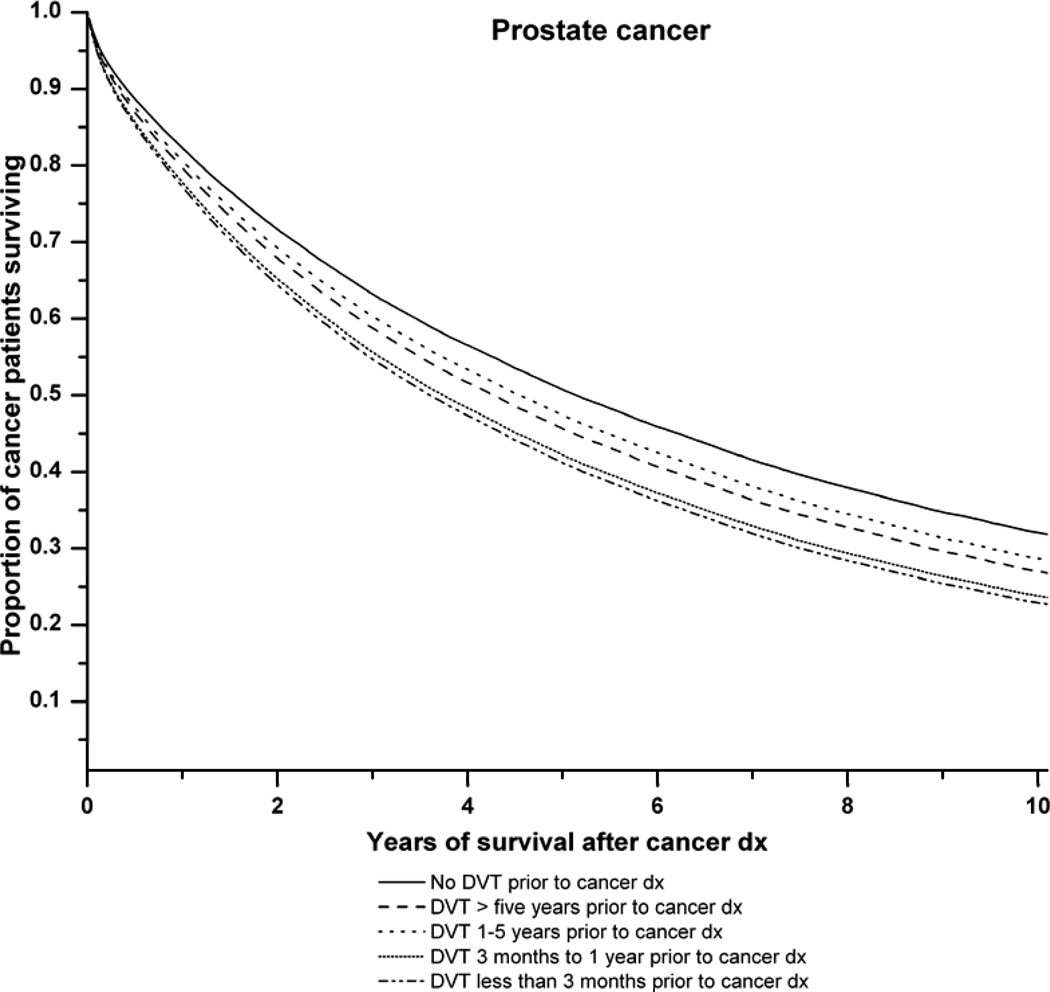
Figure 1 illustrates the survival curves for all cancers combined and for lung, colorectal and prostate cancer in relation to the time period of DVT diagnosis before cancer. Antecedent DVT increased the risk of death for each cancer type.
Figure 1.
A: Survival curves for all cancer patients with and without a history of deep venous thrombosis of the lower extremities (DVT). Adjusted survival curves are presented. The solid line represents cancer patients with no history of DVT. The dashed lines represent cancer patients with: DVT more than 5 years prior to cancer diagnosis ( ), DVT 1–5 years before cancer diagnosis (
), DVT 1–5 years before cancer diagnosis ( ), DVT 1 year before cancer diagnosis (
), DVT 1 year before cancer diagnosis ( ) and DVT at cancer diagnosis (
) and DVT at cancer diagnosis ( ). The lines for DVT 1–5 years before cancer diagnosis and DVT 1 year before cancer diagnosis are superimposed. P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
). The lines for DVT 1–5 years before cancer diagnosis and DVT 1 year before cancer diagnosis are superimposed. P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
B: Survival curves for lung cancer patients with, and without, a history of deep venous thrombosis (DVT). Adjusted survival curves are presented. The solid line represents lung cancer patients with no history of DVT. The dashed lines represent lung cancer patients with: DVT more than 5 years prior to cancer diagnosis ( ), DVT 1–5 years before cancer diagnosis (
), DVT 1–5 years before cancer diagnosis ( ), DVT 1 year before cancer diagnosis (
), DVT 1 year before cancer diagnosis ( ) and DVT at cancer diagnosis (
) and DVT at cancer diagnosis ( ). P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
). P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
C: Survival curves for colorectal cancer patients with, and without, a history of deep venous thrombosis (DVT). Adjusted survival curves are presented. The solid line represents colorectal cancer patients with no history of DVT. The dashed lines represent colorectal cancer patients with: DVT more than 5 years prior to cancer diagnosis ( ), DVT 1–5 years before cancer diagnosis (
), DVT 1–5 years before cancer diagnosis ( ), DVT 1 year before cancer diagnosis (
), DVT 1 year before cancer diagnosis ( ) and DVT at cancer diagnosis (
) and DVT at cancer diagnosis ( ). P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
). P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
D: Survival curves for prostate cancer patients with, and without, a history of deep venous thrombosis (DVT). Adjusted survival curves are presented. The solid line represents prostate cancer patients with no family of DVT. The dashed lines represent prostate cancer patients with: DVT more than 5 years prior to cancer diagnosis ( ), DVT 1–5 years before cancer diagnosis (
), DVT 1–5 years before cancer diagnosis ( ), DVT 1 year before cancer diagnosis (
), DVT 1 year before cancer diagnosis ( ) and DVT at cancer diagnosis (
) and DVT at cancer diagnosis ( P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
P-value (likelihood ratio test) for difference in survival according to pre-existing DVT status: P<0.0001.
DISCUSSION
In this nationwide study including over 400,000 male U.S. veterans with a diagnosis of cancer, we found that patients with a concomitant or an antecedent diagnosis of DVT had poorer survival than cancer patients without a history of DVT. The most prominent excess mortality patterns were found among patients diagnosed with DVT concomitantly with lung, gastric, prostate, bladder, or kidney cancer. However, significantly increased mortality was also found among cancer patients diagnosed with (versus without) DVT more than 5 years prior to cancer diagnosis.
Previous studies have consistently found that DVT risk is increased in patients with cancer7,8,10 and that DVT is associated with increased mortality among cancer patients4. Our findings are in keeping with some12,13, but not all16, studies reporting poorer survival in cancer patients who are diagnosed with a DVT at or shortly after diagnosis. We found mortality to be significantly higher for cancer patients with concomitant DVT who had specific malignancies, including lung, gastric, prostate, bladder, and kidney cancers. Prior studies have reported concomitant DVT and cancer diagnoses to reflect a more advanced cancer stage5,22. However, it is also possible that there is an influence of underlying co-morbidity and adverse lifestyle factors. Unfortunately, we did not have access to detailed clinical information for individual patients, so we were unable to obtain information about cancer stage from the dataset to confirm whether or not the poorer prognosis is due to advanced cancer stage. In contrast to prior findings11, we did not find survival to be ‘particularly poor’ in all cancer patients with concomitant DVT. In fact, mortality was not significantly higher in patients with colorectal, laryngeal, esophageal, or pancreatic cancer; leukemia; or multiple myeloma who had a concomitant DVT although this could have been due to the small number of patients in some categories.
We observed poorer survival for lung, esophageal, gastric, colorectal, prostate, and kidney cancer patients diagnosed with a DVT in the year before cancer diagnosis. It is accepted that idiopathic DVT may be a marker of undetected cancer. In a large cohort study of 17,475 patients with acute venous thromboembolism 1.2% had underlying malignancy. The most common cancers detected were lung, colorectal, prostate and hematologic cancers23. It has been suggested that an in-depth clinical examination of patients presenting with an unexplained DVT might reduce mortality from some cancers24. However, it is important to take into consideration the fact that the absolute increase in mortality for specific malignancies is low, thus, cancer screening procedures would need to be tailored and aimed to provide optimal medical risk-benefit for individuals, while being cost-effective.
In keeping with the findings of Sorensen et al.11, we found mortality to be significantly higher among cancer patients who were diagnosed with DVT several years before a diagnosis of cancer (versus those without a history of DVT), irrespective of the cancer site. Several potential explanations arise. There could be similar genetic or environmental risk factors associated with risk of DVT and with poorer cancer survival. For example, smoking is a recognized risk factor for DVT25, and smoking intensity is correlated with poorer survival in lung cancer patients26. Another potential explanation for the decreased survival among cancer patients with a prior DVT might be that co-morbidity, such as cardiovascular disease, influences clinical decision making for cancer patients or that these conditions could have a direct or indirect impact upon cancer survival. Unfortunately, we were unable to adjust for co-morbidity in the analyses.
Our study has several strengths, including its large population size, enabling detection of small increases in risk of death amongst cancer patients with antecedent or concomitant DVT, a long follow-up period in which to assess outcome and the use of hospitalization data to define DVT clinically, eliminating recall bias. In addition, the study represents approximately 10% of the U.S. population, and access to health care is not dependent on income. However, the study also has limitations. Since only hospitalization data were utilized and since DVT can be treated with low-molecular weight heparin in an outpatient setting27, DVT may have been underascertained in the dataset. However, treatment for DVT was predominantly carried out in an inpatient setting during the study period (1969–1996), and thus it is unlikely that a large proportion of cases received treatment in an outpatient setting. In addition, it is possible that patients were investigated or treated in non-VA hospitals potentially leading to underascertainment of DVT, cancer, or both. Comorbidity has been shown to be higher in male veterans than in the general population28, which may limit the generalizability of our data. Another limitation was the lack of information in the dataset about the cause of death and potential confounders, such as smoking, physical activity or anthropometry, that could affect DVT risk and possibly cancer survival. In addition, our study was limited to male U.S. veterans which limit the generalizability of this data.
In conclusion, we evaluated patterns of survival in cancer patients with and without DVT. We observed poorer survival for cancer patients with concomitant DVT. For certain cancers (lung, esophagus, gastric, colorectal, prostate, or kidney cancer), patients diagnosed with a DVT in the year before cancer diagnosis had a worse prognosis. Outcome was also worse for cancer patients with a diagnosis of DVT more than 5 years before cancer diagnosis, suggesting that these patients may have had poor health or other comorbid conditions. Our findings need to be assessed in future studies. If confirmed, efforts aimed at uncovering underlying mechanisms might provide novel clues on cancer pathogenesis with potential impact on clinical management and therapy.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NCI. The authors thank the Medical Administration Service of the Veterans Health Services and Research Administration, USA, which provided the data on which this study is based. The Research and Development Office, Northern Ireland, sponsored Dr Lesley Anderson’s participation in the Cancer Prevention Fellowship Program, Office of Preventative Oncology, Division of Cancer Prevention, National Cancer Institute, USA, through the Ireland-Northern Ireland-USA Consortium.
Footnotes
Conflict of interest: None to declare.
REFERENCES
- 1.Stein PD, Beemath A, Olson RE. Trends in the incidence of pulmonary embolism and deep venous thrombosis in hospitalized patients. Am J Cardiol. 2005;95:1525–1526. doi: 10.1016/j.amjcard.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. doi: 10.1053/j.seminhematol.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10(2):499–530. doi: 10.1016/s0889-8588(05)70349-x. [DOI] [PubMed] [Google Scholar]
- 4.Donati MB. Cancer and thrombosis. Haemostasis. 1994;24(2):128–131. doi: 10.1159/000217092. [DOI] [PubMed] [Google Scholar]
- 5.White RH, Chew HK, Zhou H, Parikh-Patel A, Harris D, Harvey D, Wun T. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch Intern Med. 2005;165(15):1782–1787. doi: 10.1001/archinte.165.15.1782. [DOI] [PubMed] [Google Scholar]
- 6.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E, Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Oudega R, Moons KG, Karel NH, van Nierop FL, Hoes AW. Deep vein thrombosis in primary care: Possible malignancy? Br J Gen Pract. 2006;56:693–696. [PMC free article] [PubMed] [Google Scholar]
- 8.Murchison JT, Wylie L, Stockton DL. Excess risk of cancer in patients with primary venous thromboembolism: A national, population-based cohort study. Br J Cancer. 2004;91:92–95. doi: 10.1038/sj.bjc.6601964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hettiarachchi RJ, Prins MH, Lensing AW, Buller HR. Low molecular weight heparin versus unfractionated heparin in the initial treatment of venous thromboembolism. Curr Opin Pulm Med. 1998;4(4):220–225. doi: 10.1097/00063198-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Baron JA, Gridley G, Weiderpass E, Nyren O, Linet M. Venous thromboembolism and cancer. Lancet. 1998;351:1077–1080. doi: 10.1016/S0140-6736(97)10018-6. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. doi: 10.1056/NEJM200012213432504. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu R, Pan CX, Wun T, Harvey D, Zhou H, White RH, Chew HK. The incidence of venous thromboembolism and its effect on survival among patients with primary bladder cancer. Cancer. 2010;116(11):2596–2603. doi: 10.1002/cncr.25004. [DOI] [PubMed] [Google Scholar]
- 13.Chew HK, Davies AM, Wun T, Harvey D, Zhou H, White RH. The incidence of venous thromboembolism among patients with primary lung cancer. J Thromb Haemost. 2008;6(4):601–608. doi: 10.1111/j.1538-7836.2008.02908.x. Epub 2008 Jan 17. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH. Venous thromboembolism in ovarian cancer. Gynecol Oncol. 2007;105(3):784–790. doi: 10.1016/j.ygyno.2007.02.024. Epub 2007 Apr 6. [DOI] [PubMed] [Google Scholar]
- 15.Chew HK, Wun T, Harvey DJ, Zhou H, White RH. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J Clin Oncol. 2007;25(1):70–76. doi: 10.1200/JCO.2006.07.4393. [DOI] [PubMed] [Google Scholar]
- 16.Alcalay A, Wun T, Khatri V, Chew HK, Harvey D, Zhou H, White RH. Venous thromboembolism in patients with colorectal cancer: incidence and effect on survival. J Clin Oncol. 2006;24(7):1112–1118. doi: 10.1200/JCO.2005.04.2150. [DOI] [PubMed] [Google Scholar]
- 17.Boyko EJ, Koepsell TD, Gaziano JM, Horner RD, Feussner JR. US department of veterans affairs medical care system as a resource to epidemiologists. Am J Epidemiol. 2000;151:307–314. doi: 10.1093/oxfordjournals.aje.a010207. [DOI] [PubMed] [Google Scholar]
- 18.Public Health Service Publication. The international classifications of diseases, 8th revision. 1967
- 19.The international classification of diseases, 9th revision, clinical modification. 2nd edition. Ann Arbour, MI: Edward Brothers; 1980. [Google Scholar]
- 20.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the department of veterans affairs. Popul Health Metr. 2006;10(4):2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML. APPROACH (Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease) Investigators. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286(12):1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338:1169–1173. doi: 10.1056/NEJM199804233381701. [DOI] [PubMed] [Google Scholar]
- 23.Trujillo-Santos J, Prandoni P, Rivron-Guillot K, Román P, Sánchez R, Tiberio G, Monreal M RIETE Investigators. Clinical outcome in patients with venous thromboembolism and hidden cancer: findings from the RIETE Registry. J Thromb Haemost. 2008;6(2):251–255. doi: 10.1111/j.1538-7836.2008.02837.x. Epub 2007 Nov 15. [DOI] [PubMed] [Google Scholar]
- 24.Monreal M, Trujillo-Santos J. Screening for occult cancer in patients with acute venous thromboembolism. Curr Opin Pulm Med. 2007;13:368–371. doi: 10.1097/MCP.0b013e3282058b6f. [DOI] [PubMed] [Google Scholar]
- 25.Kim V, Spandorfer J. Epidemiology of venous thromboembolic disease. Emerg Med Clin North Am. 2001;19:839–859. doi: 10.1016/s0733-8627(05)70221-2. [DOI] [PubMed] [Google Scholar]
- 26.Knoke JD, Shanks TG, Vaughn JW, Thun MJ, Burns DM. Lung cancer mortality is related to age in addition to duration and intensity of cigarette smoking: An analysis of CPS-I data. Cancer Epidemiol Biomarkers Prev. 2004;13:949–957. [PubMed] [Google Scholar]
- 27.Matsagas MI. Outpatient treatment of venous thromboembolism using low molecular weight heparins. an overview. Int Angiol. 2004;23:305–316. [PubMed] [Google Scholar]
- 28.Kazis LE, Miller DR, Clark J, Skinner K, Lee A, Rogers W, Spiro A, 3rd, Payne S, Fincke G, Selim A, Linzer M. Health-related quality of life in patients served by the department of veterans affairs: Results from the veterans health study. Arch Intern Med. 1998;158:626–632. doi: 10.1001/archinte.158.6.626. [DOI] [PubMed] [Google Scholar]