Abstract
The IGFs and the IGF type 1 receptor (IGF-1R) are essential mediators of normal mammary gland development in mice. IGF-I and the IGF-1R have demonstrated functions in formation and proliferation of terminal end buds and in ductal outgrowth and branching during puberty. To study the functions of IGF-1R during pregnancy and lactation, we established transgenic mouse lines expressing a human dominant-negative kinase dead IGF-1R (dnhIGF-1R) under the control of the whey acidic protein promoter. We provide evidence that the IGF-1R pathway is necessary for normal epithelial proliferation and alveolar formation during pregnancy. Furthermore, we demonstrate that the whey acidic protein-dnhIGF-1R transgene causes a delay in alveolar differentiation including lipid droplet formation, lumen expansion, and β-casein protein expression. Analysis of IGF-1R signaling pathways showed a decrease in P-IGF-1R and P-Akt resulting from expression of the dnhIGF-1R. We further demonstrate that disruption of the IGF-1R decreases mammary epithelial cell expression of the signaling intermediates insulin receptor substrate (IRS)-1 and IRS-2. No alterations were observed in downstream signaling targets of prolactin and progesterone, suggesting that activation of the IGF-1R may directly regulate expression of IRS-1/2 during alveolar development and differentiation. These data show that IGF-1R signaling is necessary for normal alveolar proliferation and differentiation, in part, through induction of signaling intermediates that mediate alveolar development.
Development of the murine mammary gland occurs in well-defined stages characterized by morphological changes under the control of circulating and locally produced hormones and growth factors. Although mammary developmental phases include embryonic, prepubertal, pubertal, pregnancy, lactation, and involution, the majority of development is initiated postnatally upon ovarian hormone stimulation. During pregnancy, mammary glands undergo further development primarily under the stimulation of progesterone and prolactin. In the first half of pregnancy, mammary epithelial development is characterized by tertiary branching, the formation of alveolar buds, and extensive proliferation; in the second half of pregnancy, alveolar proliferation continues but is accompanied by differentiation to form secretory alveoli (1).
Studies on the functions of the IGF and IGF type 1 receptor (IGF-1R) in postnatal mammary gland development have revealed essential roles for IGF-I and the IGF-1R in pubertal-induced epithelial growth and for IGF-I and IGF-II in pregnancy-induced alveolar proliferation (for review, see Ref. 2). Expression of IGF-I in the mammary fat pad is regulated predominantly by GH (3). Mammary glands of igf-1 null mice have reduced terminal end bud formation and severely compromised ductal outgrowth, even after stimulation with estrogen and progesterone to compensate for ovarian defects in these mice (4). In addition to its stromal expression, IGF-I is expressed in epithelial cells at specific times including in the terminal end buds during puberty and throughout alveoli and ducts during late pregnancy (5). Epithelial-specific loss of IGF-I during pubertal growth results in decreased ductal branching (6). During early pregnancy, when IGF-I is expressed predominantly in stroma, 50% reduction of IGF-I in IGF-I (+/−) mice results in reduced alveolar budding and decreased alveolar density with compensatory hyperplasia in mammary epithelial cells (MECs) (6).
In contrast to IGF-I, IGF-II is expressed in a nonuniform pattern in mammary epithelium throughout pregnancy (5, 7). Moreover, IGF-II, but not IGF-I, is a downstream target of prolactin signaling (8, 9), and IGF-II (−/−) epithelial cells show deficits in alveologenesis when transplanted into cleared fat pads of wild-type mice (8).
Both IGF-I and IGF-II bind to the IGF-1R to activate downstream signaling. IGF-II also binds to a splice variant of the insulin receptor (IR) known as IR-A. However, recent studies found that the IGF-1R is more active in mediating downstream signaling than IR in MECs (10). The IGF-1R is important for proliferation and outgrowth of pubertal mammary epithelium (11); however, the functions of IGF-1R in mammary gland development during pregnancy and lactation have not been addressed in previous studies. To address the functions of signaling through the IGF-1R in MECs during pregnancy and lactation, we generated transgenic mice expressing a kinase-dead dominant-negative human IGF-1R (dnhIGF-1R) in mammary epithelium from the whey acid protein (WAP) promoter, which is activated from midpregnancy through lactation (12). We present data showing that the IGF-1R is essential for normal alveolar proliferation and differentiation in midpregnancy. Moreover, we demonstrate that IGF-1R signaling regulates expression of IRS-1 and IRS-2, signaling intermediates essential for alveolar development.
Materials and Methods
Animals and genotyping
Transgenic lines were established by pronuclear injections into FVB mouse embryos. The construct used for pronuclear injections contained the WAP promoter (12) and a kinase-dead human igf1r gene described previously (13, 14). We obtained three independent transgenic lines we used for further analysis. Animal care was provided by the veterinary staff of the Division of Animal Resources in the University of Medicine and Dentistry of New Jersey (UMDNJ) Cancer Center at New Jersey Medical School. For tissue harvest, animal euthanasia was performed with CO2. All animal protocols were approved by the UMDNJ Institutional Animal Care and Use Committee.
For genotyping, genomic DNA was isolated from tail clips of 3- to 4-wk-old mice according to a standard protocol (15). PCR was performed using genomic DNA as a template for 32 cycles at 55 C. The forward primer was: 5′-AGA CAG CCA TCA GTC ACT TGC-3′; The reverse primer was: 5′-CGG AGC CAG ACT TCA TTC CTT-3′. Negative female littermates were used for wild-type controls except for MEC in vitro experiments. For consistency in gland development at the initiation of pregnancy, 10-wk virgin female mice were used for mating in all analyses. The morning of vaginal plug detection was designated as pregnancy d 0.5.
For pup weight analyses, litters were normalized to eight pups per dam at post-parturition day (PPD) 1. Total pup weights of each litter were obtained from PPD 2 until PPD 19. Average single pup weight gain was used for statistical analysis.
Whole-mount analysis and histology
The number 4 inguinal mammary glands used for whole-mount or histological analyses were harvested from FVB wild-type mice and WAP-dnhIGF-1R mice at 10 wk of age (virgin), at pregnancy d 10.5 (P10.5), P12.5, P14.5, P17.5, or at lactation d 2 (L2) or L5. For whole-mount staining, mammary glands were fixed on glass slides in 4% paraformaldehyde. Glands were stained in Carmine Alum overnight at room temperature. Glands were rinsed in graded alcohols, cleared in xylenes, and mounted with xylene-based Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI). For histological procedures, glands were fixed in 4% paraformaldehyde for 2 h and paraffin embedded. Sections were cut at 5 μm and used for hematoxylin and eosin (H&E) staining according to standard protocols.
Immunofluorescence
Paraffin sections were deparaffinized with xylenes, hydrated through graded alcohols, and rinsed with PBS. Antigen retrieval was performed for 20 min in 0.1 m sodium citrate, pH 6.0, in a microwave oven. The sections were blocked with PGB superblock (10% normal goat serum and 10% BSA in 0.5 m PBS) for 1 h at room temperature and incubated with primary antibodies in PGB diluent (PGB superblock with 1% Triton-X-100) in a humidifier box at 4 C overnight followed by incubation with fluorescence-conjugated secondary antibodies. Sections were rinsed with PBS and mounted with water-based mounting media (M01; Biomeda Corp., Foster City, CA).
Antibodies and reagents used for immunostaining included primary antibodies to Ki67 (1:500, VP-K451; Vector Laboratories, Burlingame, CA), CK14 (1:200, MAB3164; R&D System, Minneapolis, MN), E-cadherin (1:1000, 13–1900; Invitrogen, Carlsbad, CA), ZO-1 (1:50, 339100; Invitrogen), β-casein (1:100, mouse monoclonal, a gift from Dr. Margaret C. Neville), adipophilin (ADPH) (1:1000, RDI-PROGP40; Fitzgerald, Concord, MA), IgG (H&L) (1:200, RDI-706165148; Fitzgerald), signal transducer and activator of transcription (STAT)5A (1:250, sc-1081; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and wheat germ agglutinin (WGA) conjugates (1:1000, W11261; Molecular Probes, Eugene, OR). 4′,6-Diamidino-2-phenylindole (DAPI) (1:5000, 5 min at room temperature (rt); Sigma, St. Louis, MO) was used to detect nuclei.
Quantification of Ki67 cells was performed on immunostained sections from three wild-type and three transgenic glands from individual animals. Nine photomicrograph images were taken from each of three nonadjacent sections from each gland. Regions were captured in the same area on each section on the distal side of the lymph node for consistency. The captured images were used to count Ki67+ and DAPI+ epithelial cells in each region; the investigator was blinded to genotype. The average of Ki67+/DAPI+ epithelial cells for each of the three glands per genotype was used for statistical analysis. Quantification of lumen size was performed on WGA stained sections using a similar blinded strategy of nine images from each of three wild-type and transgenic glands taken from individual animals. Lumen size was calculated by measuring the longest diameter (R1) and shortest diameter (R2). Lumen area was calculated using the formula 3.14*0.5R1*0.5R2 by IPLab imaging software (Scientific Image Processing version 3.9.5 r4, BD Biosciences, Franklin Lakes, NJ).
Mammary epithelial cell isolation and ligand treatment
Primary MECs were isolated from WAP-dnhIGF-1R female mice and wild-type FVB female mice at P10.5, P12.5, P14.5, or P17.5. For acute ligand treatment experiments, MECs were harvested from glands between P12.5 to P14.5 for midpregnancy, or P15.5 to P18.5 for late pregnancy. MECs were pooled from glands isolated from one to two animals for midpregnancy and from one animal each for late pregnancy analyses. For tissue harvest, female mice were euthanized by CO2. MEC organoid isolation and acute ligand treatment were performed as described previously (10, 16).
Western immunoblotting and immunoprecipitations
Total protein (40 μg) was used for Western immunoblotting as described previously (10). Antibodies to STAT5 (9363), P-STAT5 (Tyr694) (9351), Akt (9272), and P-Akt (Ser473) (3787) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies to IGF-1R were obtained from Cell Signaling Technology (no. 3027) and Santa Cruz Biotechnology (sc-713). Insulin receptor substrate (IRS)-1/2 antibodies were obtained from Upstate Biotechnology (Lake Placid, NY; 06–248, 06–506, respectively). Other antibodies were to β-casein (sc-17969, 1:500 dilution) and to STAT5A (L-20; sc-1081, 1:250 dilution) from Santa Cruz Biotechnology, to cytokeratin 8 (CK8) from Developmental Studies Hybridoma Bank (Troma-1; Iowa City, IA), and to β-actin from Sigma (A5441; 1:5000 dilution; St. Louis, MO). All antibodies were used at 1:1000 dilution except where indicated.
For immunoprecipitation studies, each sample was diluted to 750 μl final volume in lysis buffer. Preclearing was performed with Protein A/G Agarose (Thermo Scientific; Rockford, IL) before incubation with either anti-IGF-1Rβ (C20; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-P-tyrosine (1:1000, 9411; Cell Signaling Technology) at 4 C overnight with end-over-end rotation. 60–100 μl of 50% protein A/G slurry was used to precipitate IGF-1R or P-tyrosine proteins. Samples were centrifuged and pellets washed with 50 mm Tris (pH 7.4) and used for Western blot analysis to detect either P-tyrosine (see above) or IRβ (1:200; rabbit polyclonal antibody C19; Santa Cruz Biotechnology). Secondary antibodies used for detection were either goat antimouse horseradish peroxidase or goat antirabbit horseradish peroxidase (1:5000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA).
RNA isolation and quantitative PCR (Q-PCR)
Total RNA isolation from MECs was performed using the RNeasy Mini Kit (74104; QIAGEN, Valencia, CA) following the protocol provided. The RNA concentration and quality were measured using NanoDrop (ND-1000). Both reference cDNA and sample cDNA were synthesized using SuperScript II Reverse Transcriptase (Invitrogen) according to manufacturer's instructions. cDNA was quantified using NanoDrop (ND-1000) and stored at −20 C. Q-PCR were performed on Applied Biosystems 7300 Real-time PCR system using associated Sequence Detection Systems software version 1.3.1 (SDS1.3.1, Foster City, CA). The thermal profile for all reactions was as follows: 50 C for 2 min, 95 C for 10 min, 40 cycles of 95 C for 15 sec and 60 C (annealing temperature) for 1 min.
The primers to IRS-1 (QT00251657), IRS-2 (QT00286097), STAT5A (QT00164367), STAT5B (QT00126126), β-casein (QT01055838), and receptor activator of nuclear factor κB ligand (RANKL) (QT00147385) were from QIAGEN. Primers to β-actin (forward: 5′-GTACGTAGCCATCCA-3′; reverse: 5′-TCTCCGGAGTCCATCACAATG-3′) were from Integrated DNA Technologies (San Diego, CA). QuantiTect SYBR Green PCR Kit (204143 + 204163) was from QIAGEN, and calibrator samples for mouse or human were Universal Mouse Reference RNA (750600) and Universal Human Reference RNA (750500), respectively, from Stratagene (La Jolla, CA). Reaction volume was 20 μl containing 1 × QuantiTect SYBR Green PCR Master Mix, 250 ng cDNA, and 1 × QuantiTect primer assay mix.
Statistical analyses
Statistical differences were determined using Student's t test for two-group comparisons. P < 0.05 was considered to be significant. All results were reproducible across multiple experiments.
Results
Mammary epithelial expression of a dnhIGF-1R during pregnancy decreases branching outgrowth and alveolar density
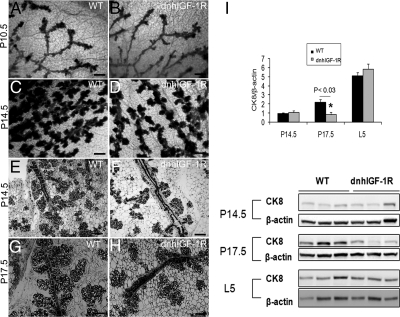
To address the functions of IGF-1R signaling on mammary gland development during pregnancy, we generated transgenic mouse lines using the WAP gene promoter to drive expression of a human kinase dead igf1r gene. Consistent with lack of WAP promoter expression before midpregnancy (12, 17), no morphological differences were observed between wild-type and dnhIGF-1R glands isolated either from virgin mice at 10 wk (data not shown) or from mice at P10.5 (Fig. 1, A and B). However, dnhIGF-1R transgenic glands analyzed at P14.5 showed decreased alveolar outgrowth (Fig. 1, C and D). H&E-stained sections of glands at P14.5 similarly revealed alveolar buds with reduced outgrowth distributed along the main ducts of dnhIGF-1R transgenic glands (Fig. 1, E and F). Reduced outgrowth was still apparent in dnhIGF-1R glands at P17.5 (Fig. 1, G and H). These results suggest that expression of the dnhIGF-1R inhibited branching outgrowth and resulted in reduced alveolar formation and differentiation.
Fig. 1.
WAP-dnhIGF-1R expression decreases mammary ductal branch outgrowth and alveolar density during pregnancy. A and B, Whole-mount stained glands at P10.5 showing no morphological difference between wild-type (WT) glands and WAP-dnhIGF-1R glands. C and D, Whole-mount stained glands from wild-type and WAP-dnhIGF-1R glands at P14.5. E–H, H&E-stained sections from wild-type and WAP-dnhIGF-1R glands at P14.5 (E and F) and P17.5 (G and H). I, Western blot analysis and quantification of CK8 expression in protein extracted from wild-type or WAP-dnhIGF-1R whole glands at P14.5, P17.5, and L5. Graph shows levels of CK8 adjusted to β-actin; n = 3 animals from each genotype; *, P < 0.03. Scale bars, 100 μm in A–D; 40 μm in E–I.
To further address the effects of dnhIGF-1R on alveolar development, we quantified alveolar epithelial density by determining levels of CK8, an epithelial cell marker, at P14.5, P17.5, and L5 by Western immunoblotting; CK8 expression was decreased in dnhIGF-1R glands compared with wild-type glands at P17.5 (Fig. 1I).
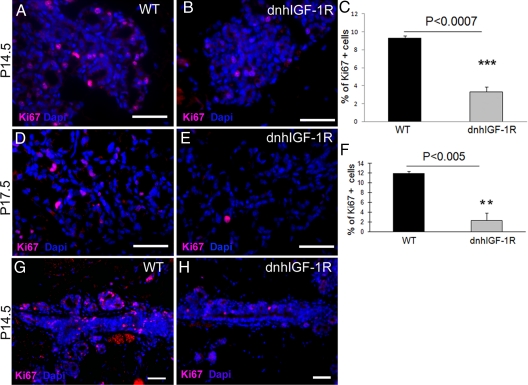
To determine whether the decreased outgrowth and subsequent reduction in alveolar epithelium in the dnhIGF-1R glands was due to reduced proliferation, we analyzed expression of Ki67 in sections from wild-type and dnhIGF-1R mammary glands. The number of Ki67-positive epithelial cells was decreased in transgenic glands at both P14.5 (Fig. 2, A–C) and P17.5 (Fig. 2, D–F). In addition, many luminal cells were seen in mitosis in wild-type glands, but not in transgenic mammary glands at P14.5 (Supplemental Fig. 1). Consistent with the reduced outgrowth of alveolar buds along the ducts, we found reduced numbers of Ki67-positive epithelial cells along the ducts in dnhIGF-1R glands at P14.5 (Fig. 2, G and H). In contrast, we found no difference in levels of cleaved caspase 3 between wild-type glands and dnhIGF-1R glands based on Western blot analysis at P14.5 and P17.5 (Supplemental Fig. 2). These results support the conclusion that the reduction in epithelium resulting from dnhIGF-1R transgene expression during alveolar development was primarily due to decreased epithelial cell proliferation and not increased apoptosis.
Fig. 2.
WAP-dnhIGF-1R expression decreases mammary epithelial cell proliferation during pregnancy. A, B, D, and E, Representative photomicrographs used for quantification of Ki67-positive cells in wild-type (WT) glands (A and D) and WAP-dnhIGF-1R glands (B and E) at P14.5 (A and B) and P17.5 (D and E). DAPI was used to detect nuclei (blue). C and F, Quantification of Ki67-positive cells at P14.5 (C) and P17.5 (F) expressed as a percentage of DAPI-positive epithelial cells. G and H, Immunofluorescent images showing reduced Ki67 staining along ducts at sties of alveolar bud outgrowths at P14.5. Scale bars, 50 μm (red autofluorescence of blood cells seen in panel G). **, P < 0.01; ***, P < 0.01.
WAP-dnhIGF-1R expression delays alveolar differentiation and lumen expansion of alveoli
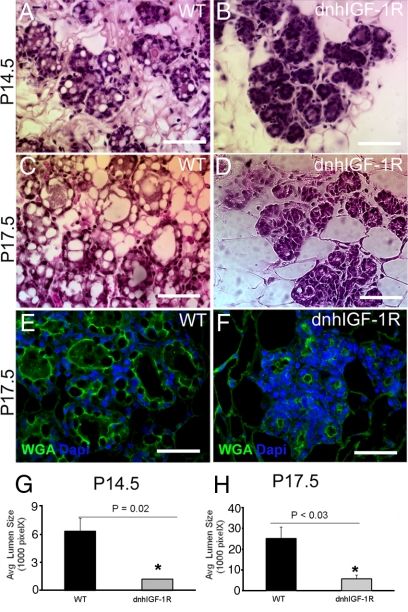
Mammary gland development in the second half of pregnancy is characterized by extensive proliferation as well as differentiation of alveolar buds to form clusters of secretory lobuloalveolar units (1). Lipid droplet formation and lumen size are characteristic markers for alveolar differentiation. H&E-stained sections of mammary glands from wild-type and transgenic glands at P14.5 and P17.5 showed many apparent lipid droplets present in luminal cells and expanded alveolar lumens at P14.5 and P17.5 in wild-type glands (Fig. 3, A and C). However, in WAP-dnhIGF-1R glands many regions of alveoli had small lumens and very few apparent lipid droplets (Fig. 3, B and D). Measurements of lumen size in wild-type and transgenic gland sections stained for WGA confirmed decreased lumen size in WAP-dnhIGF-1R glands at P14.5 and P17.5 (Fig. 3, E–H).
Fig. 3.
WAP-dnhIGF-1R expression causes deficits in alveolar differentiation during pregnancy. A–D, Representative images of H&E-stained alveoli from wild-type (WT) (A and C) or dnhIGF-1R (B and D) glands at P14.5 (A and B) and at P17.5 (C and D). E and F, Images showing immunofluorescence for WGA (green) and DAPI (blue) in wild-type (E) or dnhIGF-1R (F) glands at P17.5. G and H, Quantification of alveolar lumen size at P14.5 and P17.5. Scale bar, 50 μm. *, P < 0.03.
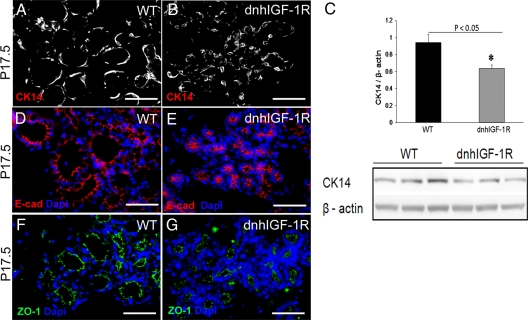
To determine whether expression of the dnhIGF-1R transgene disrupted cellular organization of alveoli, we analyzed myoepithelial (CK14) and luminal (CK18) cell marker expression on sections from P14.5 and P17.5 glands. Myoepithelial cells were elongated and seen surrounding epithelial cells in wild-type glands (Fig. 4A). Myoepithelial cells in WAP-dnhIGF-1R glands appeared less elongated possibly due to the compressed size of the alveolar structures (Fig. 4B). Immunoblotting for CK14 from acutely isolated MECs also revealed reduced levels of CK14 at P17.5 (Fig. 4C) reflecting either less CK14 protein per cell or fewer myoepithelial cells overall.
Fig. 4.
WAP-dnhIGF-1R expression alters myoepithelial cell morphology and CK14 expression but does not effect E-cadherin and ZO-1 expression in alveoli. A and B, Immunofluorescence for CK14 in wild-type (WT) (A) and dnhIGF-1R (B) glands at P17.5. C, Western blot analysis of CK14 expression in isolated MECs from wild-type and dnhIGF-1R glands at P17.5 after adjusting to levels of β-actin. D–G, Immunofluorescence for E-cadherin (E-cad; red, D and E) or ZO-1 (green; F and G) on sections from wild-type (D and F) or dnhIGF-1R (E and G) glands at P17.5. Sections were stained with DAPI to detect nuclei (blue). Scale bars, 50 μm. *, P < 0.05.
To determine whether dnhIGF-1R expression altered epithelial cell polarization, we analyzed expression of the cell junction proteins, E-cadherin and ZO-1. Both E-cadherin (Fig. 4, D and E) and ZO-1 (Fig. 4, F and G) localized to the apical side of the epithelial cells in wild-type glands and WAP-dnhIGF-1R glands at P17.5, suggesting that dnhIGF-1R expression had no effect on cell junction protein expression or epithelial cell polarization.
WAP-dnhIGF-1R expression delays alveolar differentiation
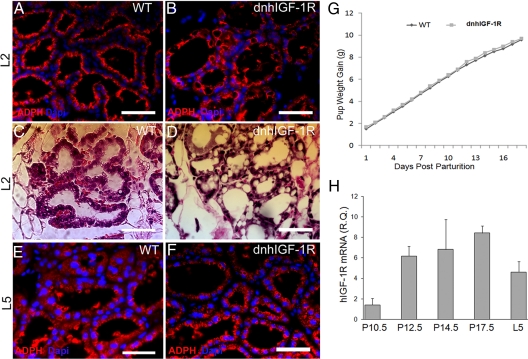
To further understand the effects of dnhIGF-1R expression on alveolar development, we examined mammary glands at L2. ADPH immunostaining was used to confirm lipid droplets seen by H&E staining as described in previous characterizations in mammary glands during pregnancy and lactation (1, 18–20). In wild-type glands, the large lipid droplets apparent in late pregnancy had disappeared and were replaced by small lipid droplets present at the apical surface of the epithelial cells consistent with active milk secretion (Fig. 5, A and C). In contrast, the dnhIGF-1R glands retained large lipid droplets in the cytoplasm (Fig. 5, B and D) similar to the alveolar morphology of wild-type glands at P17.5, suggesting that alveolar development in dnhIGF-1R glands was delayed (compare Figs 5D and 3C). ADPH immunostaining on sections from L5 glands revealed similar lipid droplet morphology in wild-type and dnhIGF-1R glands, suggesting normal milk secretion in the dnhIGF-1R glands by L5 (Fig. 5, E and F).
Fig. 5.
WAP-dnhIGF-1R expression delays alveolar differentiation during pregnancy but does not effect pup weight gain during lactation. A–D, Sections from wild-type (WT) (A and C) and dnhIGF-1R (B and D) glands at L2 were analyzed for lipid droplet formation by ADPH immunofluorescence (red, A and B) or by H&E-staining (C and D). E and F, ADPH immunofluorescence (red) in sections from wild-type (E) and dnhIGF-1R (F) glands at L5. G, Analysis of average pup weight gain from PPD 2–PPD 19 litters from wild-type (n = 5) and dnhIGF-1R (n = 14) dams. H, Relative Q-PCR analysis showing human IGF-1R transgene expression in purified MECs during pregnancy at P10.5, P12.5, P14.5, P17.5, and lactation at L5 after normalization to β-actin. n = 2 for P10.5 and n = 3 for all other time points. Scale bars in A–F, 50 μm. DAPI (blue) was used to detect nuclei in A and B and E and F.
To determine whether the deficits in alveolar development and differentiation compromised milk production or quality, we measured average pup weight gain. Pup numbers were normalized to eight pups per litter at PPD1 for both wild-type and dnhIGF-1R dams. The total pup weight of each litter was measured starting from PPD2 to PPD19. No significant difference was found in pup weights between wild-type and dnhIGF-1R dams (Fig. 5G).
To address whether the recovery observed both morphologically and functionally was due to gradual loss of transgene expression either from expansion of epithelial cells not expressing the transgene or due to IGF signaling regulating WAP promoter expression (21), we performed Q-PCR using primers specific for the human IGF-1R using RNA from isolated MECs at P10.5 through P17.5 and from whole glands at L5. The dnhIGF-1R transgene was induced between P10.5 and P12.5 and remained expressed in WAP-dnhIGF-1R glands through L5 (Fig. 5H). The peak transgene expression was at P17.5, which is consistent with the temporal induction of WAP gene expression (1). At the protein level, we also observed a significant increase in the IGF-1R by P17.5, supporting the increase in transgene expression by this time (data not shown). Interestingly, transgene expression was moderately reduced between P17.5 and L5 likely due to analysis of RNA extracted from whole glands at L5 vs. from epithelial cells at P17.5 and to the excess of milk protein mRNA during lactation ages. These results suggest that the transgene was functional from P12.5 through lactation and that loss of transgene expression does not explain the recovery observed in alveolar differentiation at L5.
dnhIGF-1R expression decreases P-IGF-1Rβ and P-Akt at P17.5
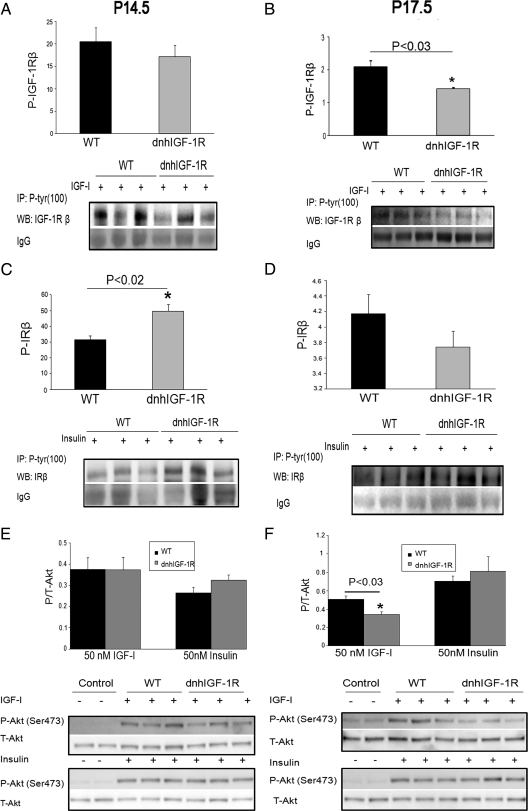
To address disruption of the IGF-1R and of downstream signaling pathways during pregnancy in the dnhIGF-1R glands, we analyzed IGF-1R and Akt phosphorylation in extracts from whole glands at P14.5 and P17.5. Immunoprecipitation of the IGF-1Rβ followed by immunodetection for P-tyrosine revealed a trend for decreased P-IGF-1R expression in dnhIGF-1R glands at P17.5, which did not reach statistical significance (Supplemental Fig. 3A). Interestingly, a similar analysis of the IRβ in the same glands showed significantly increased P-IR at P17.5 (Supplemental Fig. 3B). These data suggested that the IR pathway may be up-regulated to compensate for the defects in the IGF-1R pathway. However, because IR is highly expressed in fat cells, the increased P-IR may reflect the increased percentage of fat/stroma to epithelium at P17.5 because the percentage of epithelium is decreased in the transgenic glands at this time (see Fig. 1I). Thus, in subsequent studies to analyze the effect of the dnhIGF-1R on receptor activation and downstream signaling, we used acutely isolated MECs stimulated with IGF-I or insulin to activate receptor signaling.
To uncover how transgene expression affects the activity of IGF-1R and IR, isolated MECs were treated acutely with IGF-I or insulin for 15 min at 37 C. Results from these studies showed that P-IGF-1R expression decreased in IGF-I-treated MECs at P14.5 but the decrease was not statistically significant, similar to the whole-gland analysis (Fig. 6A). However, P-IGF-1R levels were decreased significantly in IGF-I-treated MECs isolated from dnhIGF-1R glands at P17.5 (Fig. 6B) a time when transgene expression was highest. Interestingly, P-IR was significantly increased in insulin-treated MECs at P14.5 but not at P17.5 (Fig. 6, C and D). No change in total IR expression was observed at either time point (data not shown). These data confirmed decreased IGF-1R signaling in MECs during pregnancy due to expression of the WAP-dnhIGF-1R transgene.
Fig. 6.
WAP-dnhIGF-1R expression reduces P-IGF-1R and P-Akt in late pregnant MECs. A–D, Immunoprecipitation using anti-P-tyrosine (100) and Western blot analyses to detect IGF-1Rβ (A and B) or IRβ (C and D) after acute (15 min) IGF-I or insulin stimulation of purified MECs from midpregnant (P12.5–P14.5; A and C) and late pregnant (P15.5–P18.5; B and D) glands. Graphs show quantification after normalization to IgG bands. E and F, Western blot analysis and quantification of P-Akt/total Akt expression in MEC samples shown in panels A–D. Control samples were treated with saline in the absence of ligand. n = 3 for each genotype. For midpregnant samples, each lane represents MECs isolated from one to two animals; for late-pregnant samples, each lane represents MECs isolated from individual animals. Panels B and F, *, P < 0.03; panel C, *, P < 0.02. WT, Wild type.
Our previously published studies demonstrated IGF-1R activation of Akt in isolated MECs treated with IGF ligands (10). Consistent with changes in P-IGF-1R, P-Akt was decreased in P17.5 dnhIGF-1R MECs (Fig. 6F), but not in P14.5 MEC (Fig. 6E), stimulated with IGF-I. No significant changes were observed in P-Erk1/2 in the same samples (data not shown). In contrast to the IGF-I-treated MECs, there were no changes in P-Akt in insulin-treated MECs from dnhIGF-1R glands at either P14.5 or P17.5, although insulin stimulation increased P-Akt equally in wild-type and dnhIGF-1R MECs vs. unstimulated control MECs (Fig. 6, E and F). Taken together, these data suggest that the dnhIGF-1R transgene specifically decreased P-Akt induced by IGF-1R activation. Moreover, the increase in P-IR at P14.5 was not reflected by changes in P-Akt.
dnhIGF-1R decreases expression of IRS-1/2 expression during pregnancy
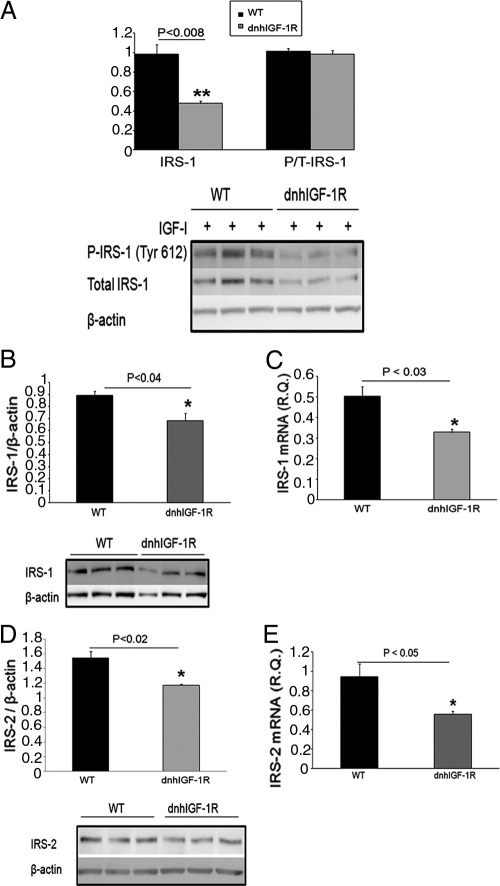
The IRS proteins mediate IGF-1R and IR signaling to phosphatidylinositol 3-kinase/Akt in most tissues including the mammary gland (22). Previous studies have shown induction of IRS-1 and IRS-2 during alveolar differentiation and lactation (23). Because we found decreased P-IGF-1R and P-Akt in IGF-I-treated dnhIGF-1R MECs during pregnancy, we investigated whether expression of the transgene also decreased P-IRS-1/2 in the pregnant gland. P-IRS-1 (tyr 612) was decreased in P14.5 dnhIGF-1R MECs treated with either IGF-I (Fig. 7A). However, total levels of IRS-1 were also decreased so no difference was seen in the ratio of P-IRS-1/total IRS-1 between dnhIGF-1R MECs and wild-type MECs (Fig. 7A). Similar results were seen in P17.5 MECs (data not shown). To eliminate potential regulation of IRS protein levels during ligand stimulation, we analyzed protein isolated directly from non-ligand-treated MECs at P14.5 and found a similar decrease in IRS-1 and IRS-2 in dnhIGF-1R glands (Fig. 7, B and D).
Fig. 7.
WAP-dnhIGF-1R expression decreases IRS-1 and IRS-2 protein and mRNA expression at P14.5. A, Western blot analysis and quantification of total IRS-1 and P-IRS-1(tyr612)/total IRS-1 in IGF-I stimulated MECs from P12.5–P14.5. B and C, Western blot analysis and quantification of protein levels (B) and Q-PCR analysis of mRNA expression (C) of IRS-1 in isolated MECs at P14.5 after normalization to β-actin. D and E, Western blot analysis and quantification of protein levels (D) and Q-PCR analysis of mRNA expression (E) of IRS-2 in isolated MECs at P14.5 after normalization to β-actin. n = 3 for each genotype; **, P < 0.008 (A); *, P < 0.04 (B); *, P < 0.03 (C); *, P < 0.02 (D); *, P < 0.05 (E). WT, Wild type.
To address whether the decrease in IRS-1/2 was reflected by changes in mRNA expression, real-time PCR was performed on RNA isolated from MECs at P14.5. IRS-1/2 mRNA levels were lower in dnhIGF-1R glands than in wild-type glands at P14.5 (Fig. 7, C and E). These data indicate that dnhIGF-1R transgene expression decreased IRS-1/2 mRNA and protein expression in MECs during pregnancy. However, consistent with the morphological recovery, levels of IRS1 and IRS2 recovered by L5 (Supplemental Fig. 4).
dnhIGF-1R expression decreases β-casein protein expression at P14.5
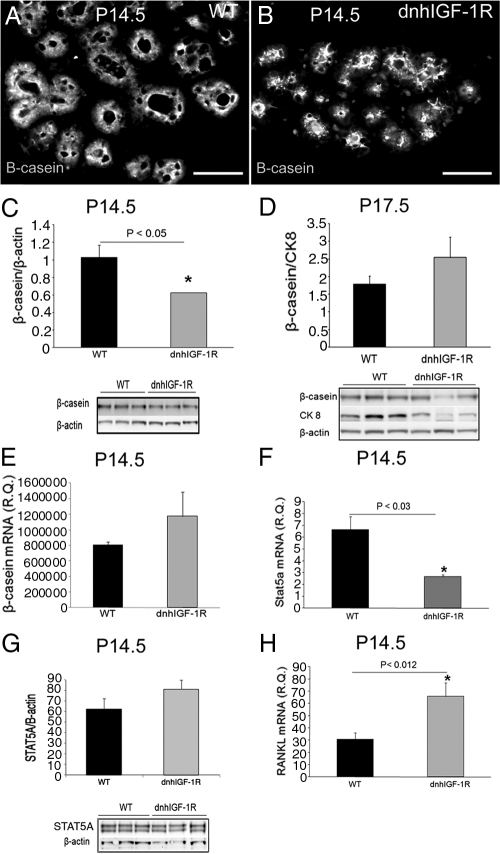
Data presented thus far demonstrate that dnhIGF-1R expression resulted in deficits in alveolar differentiation and in IGF-1R signaling during alveolar differentiation. To address whether those deficits affected regulation of milk production, we examined expression of the milk protein β-casein during pregnancy and lactation. β-Casein expression was analyzed initially at the cellular level. β-Casein was observed in epithelial cells of alveoli in wild-type glands at P14.5; however, in dnhIGF-1R glands β-casein was concentrated toward the lumen (Fig. 8, A and B). Quantification of β-casein protein expression showed a decrease in dnhIGF-1R glands at P14.5 but not at P17.5 (Fig. 8, C and D). In contrast to the change in protein levels, Q-PCR analysis showed no change in β-casein mRNA at P14.5 (Fig. 8E).
Fig. 8.
WAP-dnhIGF-1R expression decreases β-casein protein expression and STAT5A mRNA expression at P14.5. A and B, Immunofluorescence staining for β-casein on sections from wild-type (WT) (A) and dnhIGF-1R (B) glands at P14.5. C and D, Western blot analysis and quantification of β-casein protein in whole glands at P14.5 after normalization to β-actin expression (C) and at P17.5 after normalization to CK8 to adjust for reduced number of epithelial cells at this time (D). E and F, Q-PCR analysis of β-casein (F) and STAT5A (G) mRNA expression in isolated MECs at P14.5 after normalization to β-actin. G, Western blot analysis and quantification of STAT5A protein in isolated MECs at P14.5 after normalization to β-actin expression. H, Q-PCR analysis of RANKL mRNA expression in isolated MECs at P14.5 after normalization to β-actin. n = 3 for each genotype; *, P < 0.05 (C and F); *, P < 0.02 (H).
The prolactin signaling pathway regulates milk protein β-casein expression by phosphorylating STAT5A and STAT5B, resulting in formation of STAT5 dimers, which then activate the transcription of β-casein (24). STAT5 has been identified as a core regulator for milk protein gene expression (25–27). Analysis of STAT5A and STAT5B mRNA expression revealed a decrease in STAT5A (Fig. 8F), but not STAT5B (data not shown), in dnhIGF-1R MECs at P14.5. Although STAT5A mRNA expression was decreased, expression of STAT5A protein was similar in wild-type and dnhIGF-1R transgenic glands (Fig. 8G). Analysis of STAT5A expression in tissue sections also showed similar nuclear localization of STAT5A in epithelial cells between the two genotypes at P14.5 (Supplemental Figure 5). These results indicate that sufficient STAT5A protein was available and active for induction of β-casein mRNA transcription.
The previous data suggest that IRS-1 and IRS-2 are coordinately regulated at midpregnancy and require normal signaling through the IGF-1R. How these essential mediators of signal transduction and alveolar differentiation are regulated during pregnancy is not known. Prolactin and progesterone both have critical roles in alveolar proliferation and differentiation (28) and, as such, are likely candidates for regulating IRS-1 and -2. However, because β-casein mRNA expression was unaltered by the dnhIGF-1R transgene, it is unlikely that signaling from the prolactin receptor was significantly altered by disrupting IGF-1R signaling. However, previous studies indicated estrogen/progesterone regulation of IRS-1/2 at various levels (23, 29, 30). To determine whether disrupting IGF-1R compromised progesterone signaling, we analyzed expression of receptor activator of nuclear factor κB ligand (RANKL), a progesterone receptor target in mammary epithelium (28, 31–33). Rather than the expected decrease in RANKL mRNA levels we predicted if progesterone signaling was inhibited, RANKL mRNA levels were increased in MECs from P14.5 dnhIGF-1R glands (Fig. 8H). Taken together, these data suggest that reduction of IRS-1 and -2 by expression of the dnhIGF-1R was not due to compromised prolactin or progesterone signaling.
Discussion
Our results demonstrate that IGF-1R signaling is essential for normal mammary epithelial growth during mid- to late pregnancy. The effect of IGF-1R disruption includes decreased proliferation as well as compromised differentiation of the developing alveoli. The proliferative and morphological defects observed likely are a direct consequence of the delay in expression of the signaling intermediates IRS-1 and IRS-2, which are required for normal alveolar development. Although alveolar development was compromised during pregnancy, alveolar differentiation was restored by L5, and pups from dnhIGF-1R dams showed normal weight gain postnatally. The recovery from the deficits observed during pregnancy due to decreased IGF-1R signaling may be due to a transient requirement for the IGF-1R in initial stages of alveolar proliferation and differentiation. However, because the knockdown of IGF-1R signaling was partial rather than a complete knockdown, it is also possible that actions of another pathway were sufficient to compensate for decreased IGF-1R signaling and promote recovery.
The IR is the most likely receptor that might compensate for disruption in IGF-1R signaling. Our previous studies demonstrated IR expression is much higher than IGF-1R in MECs during pregnancy (10). Moreover, the expression of the IR-B isoform increases in MECs during late pregnancy (10, 34). These findings and our observations, which include increased P-IR in the dnhIGF-1R glands at midpregnancy, suggest a potential compensatory response by the IR. However, our data also support functions of the IGF-1R which are independent of the IR at mid-pregnancy. The increased P-IR with expression of the dnhIGF-1R transgene at P14.5 and lack of reduction in P-IR at P17.5 when P-IGF-1R is significantly reduced also suggests that the transgene predominantly forms hybrids with the IGF-1R and not the IR. Although IGF-1R/IR hybrid receptors are known to exist in many cell types including MECs, our previous data demonstrated that the amount of IGF-1R/IR hybrid receptors is low in primary MECs during pregnancy (10). Moreover, these data further support our hypothesis that formation of the hybrid receptors in MECs is not regulated entirely by a stochastic process (10).
IGF-1R regulates Akt signaling during alveolar development
Our data support the hypothesis that the IGF-1R has essential functions in regulating normal alveolar proliferation and differentiation during pregnancy through phosphatidylinositol 3-kinase/Akt activation. This is consistent with a previous report showing induction of P-Akt in mammary tissue after systemic injection of IGF-I (22, 35). Ablation of akt1 in mice delays epithelial cell differentiation and, in particular, impairs lipid synthesis and milk production during pregnancy and lactation (36, 37). However, Akt2 compensates for additional functions of Akt1; loss of one allele of akt2 in an akt1 null background results in a more severe phenotype in STAT5 activation and mammary alveolar differentiation (38). Consistent with these findings, expression of a constitutively active Akt in mammary epithelium (mouse mammary tumor virus-myr-Akt) results in precocious lipid droplet formation and alveolar differentiation during pregnancy (39). Here, we show that expression of the dnhIGF-1R transgene reduces Akt activation and decreases alveolar differentiation and lipid droplet formation, a phenotype similar to the phenotypes reported with loss of Akt1 (36, 37). However, in our model, alveolar differentiation recovers by early lactation, and milk production is not impaired, possibly by actions of the IR through Akt during late pregnancy and lactation. It is also of note that in the Akt mutant studies, reduction of β-casein expression (mRNA and protein) was observed only in Akt1−/−;Akt2+/− glands but not in Akt1−/− glands (38). Our analysis supports a role for the IGF-1R in regulating β-casein protein in the absence of a change in mRNA expression consistent with reports of insulin at micromolar concentrations regulating β-casein mRNA translation in coordination with prolactin (40). Thus, although some aspects of the dnIGF-1R phenotype correlate with Akt1/2 deletion, the effects on terminal differentiation and milk protein gene expression are less severe. This suggests either that the partial knockdown of IGF-1R function is insufficient to significantly impair milk protein gene expression or that the IGF-1R has essential functions predominantly in alveolar proliferation and the early transition to differentiation at midpregnancy. The pathways mediating IGF-1R effects on alveolar proliferation are less clear because loss of Akt isoforms do not appear to reduce epithelial proliferation or ductal branch outgrowth during pregnancy, including in the Akt1−/−;Akt2+/− glands (36–38). It is possible that the lack of a proliferative phenotype in the Akt isoform deletions reflects the ability of the other Akt2 allele or of Akt3 to compensate for this function but not for lipid synthesis.
Our finding that the IGF-1R regulates alveolar proliferation at midpregnancy is of interest in light of the previous literature on IGF ligands and hormone-regulated proliferation during alveolar development. Analysis of oophorectomized igf-1−/− mice treated with hormones demonstrated that IGF-I works coordinately with estradiol and progesterone to stimulate alveolar development (41). We previously showed that IGF-I promotes expression of G1 and G2 cyclins in coordination with epidermal growth factor (EGF) and is essential for EGF-related ligands to promote S-phase entry in MECs in the intact gland ex vivo (42, 43). A recent report by Beleut et al. (31) supports an essential role for progesterone-mediated MEC proliferation via two distinct mechanisms: the first is cyclin D1 dependent and the second is via RANKL acting in a paracrine fashion. However, expression of RANKL mRNA was actually increased in the dnhIGF-1R MECs. Moreover, patterning of PR expression in the MECs appeared normal in the transgenic epithelium (data not shown). Although we cannot rule out an effect of IGF-1R disruption on translation or stability of RANKL protein, our data suggest that IGF-1R regulation of MEC proliferation is via mechanisms independent of RANKL and possibly through regulation of cell cycle coordinately with progesterone or other growth factors such as the EGF-related ligands.
IGF-1R is necessary for IRS-1/2 expression in MECs during pregnancy
Our study demonstrated that IRS-1/2 protein as well as mRNA expression decreased in dnhIGF-1R MECs during pregnancy. IRS-1/2 expression increases dramatically during pregnancy and lactation and is rapidly lost during involution (23). Deletion of either IRS-1 or IRS-2 results in modest deficits in pup weight gain during lactation, and loss of IRS-1 is associated with a compensatory increase in IRS-2 (22). IRS-1 knockout mice have a reduction in insulin- and IGF-dependent mammary gland-specific activation of Akt phosphorylation (22). Data presented here support the conclusion that IGF-1R signaling is necessary for normal IRS-1/2 mRNA expression. Overall, we provide new evidence supporting an essential role for the IGF-1R in coordinate regulation of IRS-1 and IRS-2 and in alveolar proliferation and differentiation in the mammary epithelium during midpregnancy.
Acknowledgments
We thank Peter Carroll and David Lagunoff (New Jersey Medical School, Newark, New Jersey) for assistance with embedding, sectioning, and histology. The fluorescein-conjugated mouse monoclonal antibody to β-casein was a generous gift from Dr. Margaret Neville (Denver Health Sciences Center, Denver, Colorado). We also thank Stacey Cifelli and Lauren Rota (New Jersey Medical School, Newark, New Jersey) for technical assistance and Dr. Margaret Neville (Denver Health Sciences Center, Denver, Colorado) for helpful discussions.
This work was supported by National Institutes of Health Grant DK060612 (to T.L.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADPH
- Adipophilin
- CK8
- cytokeratin 8
- DAPI
- 4′,6-diamidino-2-phenylindole
- dnhIGF-1R
- kinase-dead dominant-negative human IGF-1R
- EGF
- epidermal growth factor
- H&E
- hematoxylin and eosin
- IGF-1R
- IGF type 1 receptor
- IR
- insulin receptor
- IRS
- insulin receptor substrate
- L2
- lactation d 2
- MEC
- mammary epithelial cells
- P10.5
- pregnancy d 10.5
- PPD
- post-parturition day
- Q-PCR
- quantitative PCR
- RANKL
- receptor activator of nuclear factor κB ligand
- STAT
- signal transducer and activator of transcription
- WAP
- whey acid protein
- WGA
- wheat germ agglutinin.
References
- 1. Anderson SM, Rudolph MC, McManaman JL, Neville MC. 2007. Key stages in mammary gland development. Secretory activation in the mammary gland: it's not just about milk protein synthesis! Breast Cancer Res 9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rowzee AM, Lazzarino DA, Rota L, Sun Z, Wood TL. 2008. IGF ligand and receptor regulation of mammary development. J Mammary Gland Biol Neoplasia 13:361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walden PD, Ruan W, Feldman M, Kleinberg DL. 1998. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology 139:659–662 [DOI] [PubMed] [Google Scholar]
- 4. Ruan W, Kleinberg DL. 1999. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology 140:5075–5081 [DOI] [PubMed] [Google Scholar]
- 5. Richert MM, Wood TL. 1999. The insulin-like growth factors (IGF) and IGF type I receptor during postnatal growth of the murine mammary gland: sites of messenger ribonucleic acid expression and potential functions. Endocrinology 140:454–461 [DOI] [PubMed] [Google Scholar]
- 6. Loladze AV, Stull MA, Rowzee AM, Demarco J, Lantry JH, III, Rosen CJ, Leroith D, Wagner KU, Hennighausen L, Wood TL. 2006. Epithelial-specific and stage-specific functions of insulin-like growth factor-I during postnatal mammary development. Endocrinology 147:5412–5423 [DOI] [PubMed] [Google Scholar]
- 7. Wood T, Richert M, Stull M, Allar M. 2000. The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands. J Mammary Gland Biol Neoplasia 5:31–42 [DOI] [PubMed] [Google Scholar]
- 8. Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. 2002. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell 3:877–887 [DOI] [PubMed] [Google Scholar]
- 9. Hovey RC, Harris J, Hadsell DL, Lee AV, Ormandy CJ, Vonderhaar BK. 2003. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol Endocrinol 17:460–471 [DOI] [PubMed] [Google Scholar]
- 10. Rowzee AM, Ludwig DL, Wood TL. 2009. Insulin-like growth factor type 1 receptor and insulin receptor isoform expression and signaling in mammary epithelial cells. Endocrinology 150:3611–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonnette SG, Hadsell DL. 2001. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 142:4937–4945 [DOI] [PubMed] [Google Scholar]
- 12. Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res 25:4323–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernández AM, Dupont J, Farrar RP, Lee S, Stannard B, Le Roith D. 2002. Muscle-specific inactivation of the IGF-I receptor induces compensatory hyperplasia in skeletal muscle. J Clin Invest 109:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kato H, Faria TN, Stannard B, Roberts C, Jr, LeRoith D. 1993. Role of tyrosine kinase activity in signal transduction by the insulin-like growth factor-I (IGF-I) receptor: characterization of kinase-deficient IGF-I receptors and the action of an IGF-I-mimetic antibody (aIR-3). J Biol Chem 268:2655–2661 [PubMed] [Google Scholar]
- 15. Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29:52–54 [DOI] [PubMed] [Google Scholar]
- 16. Imagawa W, Yang J, Guzman RC, Nandi S. 2000. Collagen gel method for the primary culture of mouse mammary epithelium. In: Ip MM, Asch BB. eds., Methods in mammary gland biology and breast cancer research. New York: Kluwer Academic/Plenum Publishers; 111–123 [Google Scholar]
- 17. Burdon T, Sankaran L, Wall RJ, Spencer M, Hennighausen L. 1991. Expression of a whey acidic protein transgene during mammary development. Evidence for different mechanisms of regulation during pregnancy and lactation. J Biol Chem 266:6909–6914 [PubMed] [Google Scholar]
- 18. McManaman JL, Russell TD, Schaack J, Orlicky DJ, Robenek H. 2007. Molecular determinants of milk lipid secretion. J Mammary Gland Biol Neoplasia 12:259–268 [DOI] [PubMed] [Google Scholar]
- 19. Russell TD, Palmer CA, Orlicky DJ, Fischer A, Rudolph MC, Neville MC, McManaman JL. 2007. Cytoplasmic lipid droplet accumulation in developing mammary epithelial cells: roles of adipophilin and lipid metabolism. J Lipid Res 48:1463–1475 [DOI] [PubMed] [Google Scholar]
- 20. Russell TD, Palmer CA, Orlicky DJ, Bales ES, Chang BH, Chan L, McManaman JL. 2008. Mammary glands of adipophilin-null mice produce an amino-terminally truncated form of adipophilin that mediates milk lipid droplet formation and secretion. J Lipid Res 49:206–216 [DOI] [PubMed] [Google Scholar]
- 21. Booth BW, Boulanger CA, Smith GH. 2007. Alveolar progenitor cells develop in mouse mammary glands independent of pregnancy and lactation. J Cell Physiol 212:729–736 [DOI] [PubMed] [Google Scholar]
- 22. Hadsell DL, Olea W, Lawrence N, George J, Torres D, Kadowaki T, Lee AV. 2007. Decreased lactation capacity and altered milk composition in insulin receptor substrate null mice is associated with decreased maternal body mass and reduced insulin-dependent phosphorylation of mammary Akt. J Endocrinol 194:327–336 [DOI] [PubMed] [Google Scholar]
- 23. Lee AV, Zhang P, Ivanova M, Bonnette S, Oesterreich S, Rosen JM, Grimm S, Hovey RC, Vonderhaar BK, Kahn CR, Torres D, George J, Mohsin S, Allred DC, Hadsell DL. 2003. Developmental and hormonal signals dramatically alter the localization and abundance of insulin receptor substrate proteins in the mammary gland. Endocrinology 144:2683–2694 [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev 11:179–186 [DOI] [PubMed] [Google Scholar]
- 25. Kazansky AV, Raught B, Lindsey SM, Wang YF, Rosen JM. 1995. Regulation of mammary gland factor/Stat5a during mammary gland development. Mol Endocrinol 9:1598–1609 [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Robinson GW, Hennighausen L. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol Endocrinol 10:1496–1506 [DOI] [PubMed] [Google Scholar]
- 27. Rosen JM, Wyszomierski SL, Hadsell D. 1999. Regulation of milk protein gene expression. Annu Rev Nutr 19:407–436 [DOI] [PubMed] [Google Scholar]
- 28. Hennighausen L, Robinson GW. 2005. Information networks in the mammary gland. Nat Rev Mol Cell Biol 6:715–725 [DOI] [PubMed] [Google Scholar]
- 29. Santos SJ, Haslam SZ, Conrad SE. 2008. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology 149:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vassen L, Wegrzyn W, Klein-Hitpass L. 1999. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol Endocrinol 13:485–494 [DOI] [PubMed] [Google Scholar]
- 31. Beleut M, Rajaram RD, Caikovski M, Ayyanan A, Germano D, Choi Y, Schneider P, Brisken C. 2010. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc Natl Acad Sci USA 107:2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haslam SZ, Drolet A, Smith K, Tan M, Aupperlee M. 2008. Progestin-regulated luminal cell and myoepithelial cell-specific responses in mammary organoid culture. Endocrinology 149:2098–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. 2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berlato C, Doppler W. 2009. Selective response to insulin versus IGF-I and IGF-II and up-regulation of insulin-receptor splice variant B in the differentiated mouse mammary epithelium. Endocrinology 150:2924–2933 [DOI] [PubMed] [Google Scholar]
- 35. Lee AV, Taylor ST, Greenall J, Mills JD, Tonge DW, Zhang P, George J, Fiorotto ML, Hadsell DL. 2003. Rapid induction of IGF-IR signaling in normal and tumor tissue following intravenous injection of IGF-I in mice. Horm Metab Res 35:651–655 [DOI] [PubMed] [Google Scholar]
- 36. Boxer RB, Stairs DB, Dugan KD, Notarfrancesco KL, Portocarrero CP, Keister BA, Belka GK, Cho H, Rathmell JC, Thompson CB, Birnbaum MJ, Chodosh LA. 2006. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab 4:475–490 [DOI] [PubMed] [Google Scholar]
- 37. Maroulakou IG, Oemler W, Naber SP, Klebba I, Kuperwasser C, Tsichlis PN. 2008. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J Cell Physiol 217:468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen CC, Boxer RB, Stairs DB, Portocarrero CP, Horton RH, Alvarez JV, Birnbaum MJ, Chodosh LA. 2010. Akt is required for Stat5 activation and mammary differentiation. Breast Cancer Res 12:R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwertfeger KL, McManaman JL, Palmer CA, Neville MC, Anderson SM. 2003. Expression of constitutively activated Akt in the mammary gland leads to excess lipid synthesis during pregnancy and lactation. J Lipid Res 44:1100–1112 [DOI] [PubMed] [Google Scholar]
- 40. Choi KM, Barash I, Rhoads RE. 2004. Insulin and prolactin synergistically stimulate β-casein messenger ribonucleic acid translation by cytoplasmic polyadenylation. Mol Endocrinol 18:1670–1686 [DOI] [PubMed] [Google Scholar]
- 41. Ruan W, Monaco ME, Kleinberg DL. 2005. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology 146:1170–1178 [DOI] [PubMed] [Google Scholar]
- 42. Stull MA, Richert MM, Loladze AV, Wood TL. 2002. Requirement for insulin-like growth factor-I in epidermal growth factor-mediated cell cycle progression of mammary epithelial cells. Endocrinology 143:1872–1879 [DOI] [PubMed] [Google Scholar]
- 43. Stull MA, Rowzee AM, Loladze AV, Wood TL. 2004. Growth factor regulation of cell cycle progression in mammary epithelial cells. J Mammary Gland Biol Neoplasia 9:15–26 [DOI] [PubMed] [Google Scholar]