Abstract
In recent years, vitamin D has received increased attention due to the resurgence of vitamin D deficiency and rickets in developed countries together with the identification of extraskeletal vitamin D receptor-mediated actions, suggesting unexpected benefits of vitamin D in health and diseases. Although there is increased awareness of the importance of vitamin D, the role of vitamin D in extraskeletal health has been a matter of debate. In this review, we will summarize what is known and indicate the questions that remain and need to be addressed.
The importance of vitamin D for curing rickets has been known for over 80 yr. In recent years, there has been renewed interest in vitamin D because of its many other suggested roles in the skin, in the immune system, and in cancer prevention and treatment. This review will first briefly summarize the mechanisms by which vitamin D maintains calcium homeostasis followed by a consideration of the role of vitamin D in extraskeletal health.
Vitamin D and the Maintenance of Calcium Homeostasis
Vitamin D is important for the development and maintenance of bone as well as for the maintenance of normal calcium and phosphorus homeostasis (1–3). The causal link between vitamin D deficiency during bone development and rickets and in adults between vitamin D deficiency and secondary hyperparathyroidism that can result in accelerated bone loss and increased risk of fracture is well documented (4, 5). Vitamin D is synthesized in the skin from 7-dehydrocholesterol by UV irradiation. The synthesis of vitamin D in the skin, which is the most important source of vitamin D, depends of the intensity of UV irradiation, which varies with season and latitude (6). Although vitamin D can also be taken in the diet, few foods (which include fortified dairy products and fish oils) contain appreciable amounts of vitamin D. The hormonally active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), is produced by two sequential hydroxylations [25 hydroxylation in the liver, which results in the formation of 25-hydroxyvitamin D3 (25(OH)D3), the major circulating form of vitamin D, and by 25-hydroxyvitamin D3 1α hydroxylation in the proximal renal tubule] (1–3, 7). 25-Hydroxyvitamin D3 24 hydroxylase [CYP 24 or 24(OH)ase] limits the amount of 1,25(OH)2D3 by accelerating the catabolism of 1,25(OH)2D3 in target tissues to 1,24,25(OH)3D3, ultimately resulting in the formation of calcitroic acid, and also by producing 24,25(OH)2D3, thus decreasing the 25(OH)D3 substrate available for 1α hydroxylation. Elevated parathyroid hormone (PTH) resulting from hypocalcemia induces 1,25(OH)2D3 synthesis in the kidney. 1,25(OH)2D3 in turn acts at the parathyroid gland to suppress PTH production. 1α(OH)ase is negatively regulated by 1,25(OH)2D3. 24(OH)ase is reciprocally regulated [stimulated by 1,25(OH)2D3 and inhibited by PTH and low calcium] (7).
The actions of 1,25(OH)2D3, similar to other steroid hormones, are mediated by a nuclear receptor [vitamin D receptor (VDR)]. 1,25(OH)2D3 occupied VDR heterodimerizes with the retinoid X receptor and together with coregulatory proteins interacts with vitamin D response elements predominantly, but not exclusively, in the promoter region of target genes and modulates their transcription (1, 2, 8, 9). The phenotype of VDR knockout (KO) mice includes rickets, osteomalacia, and secondary hyperparathyroidism and thus represents a mouse model of vitamin D-dependent rickets type II (10–13). When VDR KO mice are fed a diet high in calcium, phosphorus, and lactose, serum calcium and PTH are normalized and osteomalacia and rickets are prevented (14). These findings in the VDR KO mice suggest that a major defect from the loss of VDR activity is a defect in intestinal calcium and phosphate absorption, which is the primary cause of decreased bone mineralization. Thus, the principal function of vitamin in mineral homeostasis is to increase calcium and phosphorus absorption from the intestine. If normal serum calcium is unable to be maintained by intestinal absorption, increased PTH induces the synthesis of 1,25(OH)2D3, and together PTH and 1,25(OH)2D3 mobilize bone calcium and increase calcium reabsorption from the renal distal tubule (15, 16). Thus, it is clear that, through these mechanisms, vitamin D is vital for mineral homeostasis. Considering these findings and what has been observed in the VDR KO mice, are there extraskeletal biological systems where 1,25(OH)2D3 and VDR generate significant biological responses that can affect health and disease?
Extraskeletal Effects of Vitamin D
The possibility of extraskeletal effects of 1,25(OH)2D3 was noted with the discovery in 1979 through the 1980s of the presence of VDR in tissues and cells that were not involved in maintaining calcium homeostasis, including pancreas, skin, placenta, brain, and activated T cells (1, 17–19). VDR is not found in every cell or tissue (for example, VDR has been reported to be undetectable in striated and smooth muscle) (20, 21), supporting a role for VDR at specific extraskeletal sites. VDRs were also noted in a number of cancer cells, including breast, prostate, and colon cancer cells (22–25). When these cancer cells were incubated with 1,25(OH)2D3, their cellular proliferation was inhibited (24, 25). Leukemia cells were also found to express VDR, and when incubated with 1,25(OH)2D3, they differentiated to normal macrophages (26). In addition, in 1979, evidence of extrarenal 1α(OH)ase was first found in placenta followed by the identification of 1α hydroxylation in macrophages (27, 28). The question that remained was the biological significance of the presence of VDR and 1α(OH)ase in different tissues.
Extrarenal 1α(OH)ase
Monocytes/macrophages
It had been thought that the hypercalcemia and hypercalciuria in patients with sarcoidosis was due to enhanced responsiveness of the intestine to vitamin D. When elevated serum 1,25(OH)2D3 occurred in a hypercalcemic anephric patient with sarcoidosis, this established an extrarenal site for the production of 1,25(OH)2D3 (29). Subsequently, it was demonstrated that macrophages from patients with sarcoidosis are the source of 1,25(OH)2D3 and that monocyte/macrophage 1α(OH)ase is regulated differently than renal 1α(OH)ase [it is not suppressed by elevated 1,25(OH)2D3 or calcium] (28, 30). These findings indicated the in vivo significance of 1α(OH)ase in macrophages and the mechanisms responsible for the hypercalcemia of granulomatous disorders.
Placenta
In placenta, 1α(OH)ase is expressed in both fetal trophoblasts and maternal decidual cells beginning early in gestation (31). Maternal killer cells from the decidua show decreased synthesis of cytokines, such as TNF and IL-6 in response to 1,25(OH)2D3, suggesting that 1,25(OH)2D3 may act as an autocrine/paracrine regulator of immunity at the fetal-maternal interface (32). Induction of the mRNA for cathelicidin, an antimicrobial peptide, by 1,25(OH)2D3 in decidual cells has also been reported (33). It has been suggested that the immunosuppressive effects of 1,25(OH)2D3 allow for proper trophoblast invasion without a maternal immune response and thus for successful implantation (32). Although the placenta was one of the first documented sources of extrarenal 1α(OH)ase activity and 1,25(OH)2D3 has been shown to modulate decidual immune cell function, the role of placental 1α(OH)ase is speculative at this time. It is of interest, however, that in 1α(OH)ase KO mice, in addition to rickets, reproductive and immune defects have been noted (34), supporting the suggested role for 1,25(OH)2D3 as an autocrine/paracrine regulator of immunity during pregnancy. Although it has been reported that 1,25(OH)2D3 can be synthesized at sites other than kidney, macrophages, and placenta, the role of 1α(OH)ase under normal conditions at other extrarenal sites has been a matter of debate.
The Role of VDR in Tissues Not Involved in Maintaining Calcium Homeostasis
VDR in cancer cells: Is there a role for vitamin D in cancer prevention and treatment?
In addition to the presence of VDR in cancer cells and the inhibition of proliferation by 1,25(OH)2D3, compelling evidence for a role for vitamin D and 1,25(OH)2D3 in cancer prevention and treatment is from animal studies. It has been demonstrated that rats fed diets low in vitamin D and calcium develop significantly more mammary tumors when treated with 7,12 dimethylbenz(a)anthracene (DMBA) than rats fed control diets with adequate vitamin D and calcium (35). In other in vivo studies using N-methyl-N-nitrosourea (NMU), an inhibition of the progression of mammary tumor growth is observed in rats treated with 1,25(OH)2D3 or analogs of 1,25(OH)2D3 after NMU treatment (36). When rats are treated with 1,25(OH)2D3 or analogs of 1,25(OH)2D3 before treatment with NMU, tumor incidence is reduced or prevented (37). In addition, the incidence of preneoplastic mammary lesions and the development of estrogen receptor negative tumors in response to DMBA is higher in VDR KO mice compared with wild-type mice (38). In response to DMBA, VDR KO mice also display increased sensitivity to development of a variety of skin tumors and tumors in the lymph nodes (38, 39). The studies in the VDR KO mice provide direct evidence in vivo that VDR ablation can enhance sensitivity to tumorigenesis. 1,25(OH)2D3 has also been shown to delay the development of prostate interepithelial neoplasm in the Nkx3.1;Pten mutant mouse (a putative model for prostate carcinogenesis) (40) and to have tumor inhibitory activity in models of colorectal adenoma (41). 1,25(OH)2D3 and 1,25(OH)2D3 analogs have been reported to potentiate the antitumor actions of traditional anticancer agents (42–44). A number of cellular mechanisms have been proposed for the anticancer activity of 1,25(OH)2D3 (42, 44). These preclinical studies provide evidence supporting tumor inhibitory activity of 1,25(OH)2D3. At the least, understanding the mechanisms involved can potentially lead to the identification of new targets for anticancer treatment. However, the role of vitamin D, 1,25(OH)2D3, or 1,25(OH)2D3 analogs to treat cancer patients is uncertain at this time. The number of completed clinical trials is limited. Most of the clinical trials have been conducted in prostate cancer patients (fewer studies have been conducted in patients with other cancers) and in patients with advanced cancer that have not responded to traditional anticancer therapy (42, 44). A limitation of previous clinical trials is that it may not be possible to observe significant vitamin D anticancer effects in patients with very advanced disease who have failed other therapies. In the future, well designed, large scale clinical trials are needed to assess whether or not dietary vitamin D, 1,25(OH)2D3, or 1,25(OH)2D3 analogs, perhaps in combination with traditional chemotherapeutic agents and early in disease, have a role as anticancer agents. The evidence in the laboratory indicates that 1,25(OH)2D3 generates biological responses that result in the inhibition of the disease process of cancer. To demonstrate the suggested benefit of vitamin D, new, large scale clinical trials are needed.
VDR in keratinocytes
Studies in keratinocytes indicated that 1,25(OH)2D3 causes a marked decrease in proliferation and an increase in differentiation (45). This led to the concept that 1,25(OH)2D3 and/or its less calcemic analogs could be used to treat psoriasis. Topically applied, 1,25(OH)2D3 and 1,25(OH)2D3 analogs have been developed as a therapy for psoriasis (46). Thus, at least for psoriasis, 1,25(OH)2D3 and its analogs do have therapeutically relevant effects on the skin.
Vitamin D and the cardiovascular system
Studies in VDR KO mice and 1α(OH)ase KO mice have shown that these mice develop hypertension and cardiac hypertrophy, which is associated with an increase in renin (47, 48). Data obtained in the 1α(OH)ase KO mice indicate that the protective role of 1,25(OH)2D3 against cardiovascular abnormalities involves repression of renin biosynthesis by a calcium and phosphorus-independent mechanism (48). Thus, these findings in mice indicate that vitamin D does have a beneficial effect on the cardiovascular system. Clinical and epidemiological studies are also suggestive of an effect of vitamin D on cardiac function. In most of these studies, associations are reported [for example, between low serum 25(OH)D and hypertension] (49, 50), and large scale intervention studies have not been done. Thus, conclusive clinical evidence of a role of vitamin D in cardiovascular health is not yet available.
Vitamin D and the immune system
A role for vitamin D in the immune system was suggested by early studies indicating the presence of VDR in activated T cells (19). 1,25(OH)2D3 inhibits lymphocyte proliferation and activation and IL-2 and interferon (IFN)γ are decreased after activated T cells are exposed to 1,25(OH)2D3 (51). 1,25(OH)2D3 has also been shown to inhibit the differentiation and survival of dendritic cells, resulting in impaired alloreactive T cell activation (51). The inhibition of maturation and differentiation of dendritic cells results in a decrease in IL-12 and an increase in IL-10 secretion (51). IL-17, which is involved in the pathogenesis of autoimmune inflammation and has been implicated in numerous autoimmune diseases, is inhibited by 1,25(OH)2D3 (52). These in vitro studies provide evidence that 1,25(OH)2D3 is a modulator of the immune system. With regard to in vivo physiological significance, animal studies have shown that 1,25(OH)2D3 can protect against a number of experimental autoimmune diseases, including experimental autoimmune encephalomyelitis (EAE) [the murine model of multiple sclerosis (MS)], systemic lupus erythematosus, inflammatory bowel disease (IBD), and autoimmune thyroiditis (53, 54). At least in EAE, systemic lupus erythematosus, and IBD, dietary calcium is required for the 1,25(OH)2D3 suppressive effects (53, 55). 1,25(OH)2D3 inhibits induction of EAE when given before immunization (56, 57). In addition, when EAE mice with paralysis are treated with 1,25(OH)2D3, treatment results in a reversal of paralysis, and this improvement is observed throughout the course of treatment (Fig. 1) (57, 58). Inhibition of EAE by 1,25(OH)2D3 has been reported to be dependent on IL-10 and to be associated with an inhibition of IL-12 and IL-17 (58–60). Studies in VDR KO mice have indicated that VDR is necessary for 1,25(OH)2D3 to suppress EAE (61). Hypercalcemia itself, induced by PTH, blocks EAE in female but not male mice (62). Further, UV irradiation suppresses EAE independent of vitamin D (63). Although vitamin D may play a protective role, the reduction of MS at high sunlight regions may not be via production of vitamin D (63, 64). VDR KO mice also develop more severe IBD (65). IBD is due, at least in part, to an immune-mediated attack that results in IL-17 and IFNγ overproduction. The enhanced severity of IBD in the VDR KO mice has been associated with increased numbers of IL-17 and IFNγ secreting T cells and a reduction in regulatory T cells in the KO mice (66). 1,25(OH)2D3 and 1,25(OH)2D3 analogs also prevent autoimmune diabetes in nonobese diabetic (NOD) mice and inhibit the progression of autoimmune diabetes when administered after disease onset (Fig. 2) (53, 67, 68). Decreased numbers of effector T cells and induction of regulatory T cells have been associated with the protective effect of 1,25(OH)2D3 in the NOD mouse model (68). These in vivo studies in mouse models are convincing and suggest that vitamin D has a role in protection against these diseases. Whether vitamin D, 1,25(OH)2D3, or vitamin D analogs are effective in humans with autoimmune diseases in reducing symptoms perhaps together with traditional therapies or if vitamin D supplementation is protective if given early in life is not known at this time. Recent clinical studies are suggestive of a protective role of vitamin D. For example, in a study by Munger et al. (69), there was an inverse relationship between 25(OH)D3 levels and MS, and this relationship was particularly strong when 25(OH)D levels were measured before the age of 20, suggesting that vitamin D supplements in adolescents and young adults may be important for those with a family history of MS.
Fig. 1.
1,25(OH)2D3 treatment reverses ongoing EAE. EAE was induced in C57BL/6 mice, and the mice were scored daily (1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; and 4, hind limb and forelimb paralysis). At d 15 (peak of disease), mice were randomized, fed a diet containing no vitamin D, and treated with either vehicle (black circles) or 50 ng 1,25(OH)2D3 (open circles) (indicated by the line, d 15- 30). Treatment with 1,25(OH)2D3 reversed paralysis and protected against ongoing EAE. *, P < 0.05; **, P < 0.01. Studies using infiltrating mononuclear cells isolated from the spinal cord and brain after 3 d treatment with 1,25(OH)2D3 (arrowhead) showed that improvement in the clinical score after 1,25(OH)2D3 treatment is associated with inhibition of production of IL-17 (data not shown). From Joshi et al. (58).
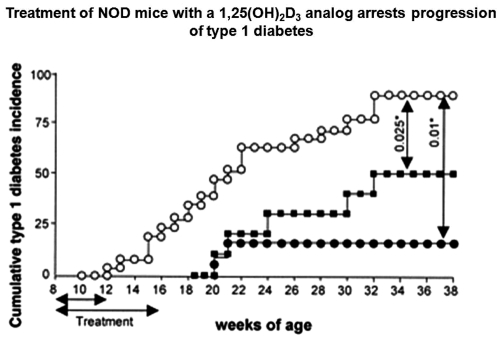
Fig. 2.
1,25(OH)2D3 analog, RO26-2198 administered to NOD mice inhibits type 1 diabetes development. NOD mice were treated five times per week with vehicle (open circles) or with 0.03 μg/kg 1,25(OH)2D3 analog RO26-2198 from eight to 16 wk of age (black circles) or from eight to 12 wk of age (black squares). Note that the short course of treatment arrested the clinical onset of diabetes in the NOD mice. RO26-2198 did not induce hypercalcemia even after 40 administrations (data not shown). From Gregori et al. (68), with permission.
Innate immunity
Recent studies have suggested that vitamin D can also modulate innate immunity. 1,25(OH)2D3 has been shown to induce the antimicrobial peptide cathelicidin with subsequent killing of bacteria, including mycobacterium tuberculosis (70). Whether there is a beneficial effect of vitamin D in tuberculosis patients or a subset of tuberculosis patients remains to be determined.
Conclusion
The recent Institute of Medicine recommendation related to calcium and vitamin D supported their key role in skeletal health but concluded that “it is not yet compelling that either nutrient confers benefits for extraskeletal health” (71). As indicated in this review, the evidence in the laboratory, including the use of animal models, indicates that 1,25(OH)2D3 generates a number of extraskeletal biological responses. These responses include inhibition of cancer progression, effects on the cardiovascular system and the skin, and inhibition of certain autoimmune diseases. Although there are many major differences between animal models and human disease, it is likely that many genes function similarly in humans and animals. In addition, findings related to 1,25(OH)2D3 effects in animal models may suggest mechanisms involving similar pathways in humans that could lead to the identification of new therapies. Although large-scale clinical trials are needed and, unlike vitamin D deficiency and rickets, a causal link between vitamin D deficiency and specific diseases, including cancer and autoimmune diseases, has not yet been proven, convincing evidence in the laboratory of beneficial effects of 1,25(OH)2D3 beyond bone cannot be dismissed.
Acknowledgments
This work was supported by the National Institutes of Health Grant DK-38961-22 (to S.C.) and the Wisconsin Alumni Research Foundation (to H.F.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DMBA
- 7,12 Dimethylbenz(a)anthracene
- EAE
- experimental autoimmune encephalomyelitis
- IBD
- inflammatory bowel disease
- IFN
- interferon
- KO
- knockout
- MS
- multiple sclerosis
- NMU
- N-methyl-N-nitrosourea
- NOD
- nonobese diabetic
- 24(OH)ase
- 24 hydroxylase
- 25(OH)D3
- 25-hydroxyvitamin D3
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- PTH
- parathyroid hormone
- VDR
- vitamin D receptor.
References
- 1. DeLuca HF. 2008. Evolution of our understanding of vitamin D. Nutr Rev 66:S73–S87 [DOI] [PubMed] [Google Scholar]
- 2. Bikle D, Adams J, Christakos S. 2009. Vitamin D: production, metabolism, mechanism of action, and clinical Requirements. In: Rosen C. ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 7th ed. Washington, DC: American Society for Bone and Mineral Research; 141–149 [Google Scholar]
- 3. Norman AW. 1979. Vitamin D: the calcium homeostatic steroid hormone. New York: Academic Press [Google Scholar]
- 4. Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 5. Lips P. 2001. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501 [DOI] [PubMed] [Google Scholar]
- 6. Webb AR, Kline L, Holick MF. 1988. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab 67:373–378 [DOI] [PubMed] [Google Scholar]
- 7. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. 2010. Vitamin D: metabolism. Endocrinol Metab Clin North Am 39:243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. 2001. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord 2:203–216 [DOI] [PubMed] [Google Scholar]
- 9. Pike JW, Meyer MB. 2010. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3). Endocrinol Metab Clin North Am 39:255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. 1997. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA 94:9831–9835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. 1997. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 16:391–396 [DOI] [PubMed] [Google Scholar]
- 12. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. 2008. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 29:726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malloy PJ, Feldman D. 2010. Genetic disorders and defects in vitamin D action. Endocrinol Metab Clin North Am 39:333–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amling M, Priemel M, Holzmann T, Chapin K, Rueger JM, Baron R, Demay MB. 1999. Rescue of the skeletal phenotype of vitamin D receptor-ablated mice in the setting of normal mineral ion homeostasis: formal histomorphometric and biomechanical analyses. Endocrinology 140:4982–4987 [DOI] [PubMed] [Google Scholar]
- 15. Garabedian M, Tanaka Y, Holick MF, DeLuca HF. 1974. Response of intestinal calcium transport and bone calcium mobilization to 1,25-dihydroxyvitamin D3 in thyroparathyroidectomized rats. Endocrinology 94:1022–1027 [DOI] [PubMed] [Google Scholar]
- 16. Friedman PA, Gesek FA. 1993. Vitamin D3 accelerates PTH-dependent calcium transport in distal convoluted tubule cells. Am J Physiol 265:F300–F308 [DOI] [PubMed] [Google Scholar]
- 17. Bouillon R, Okamura WH, Norman AW. 1995. Structure-function relationships in the vitamin D endocrine system. Endocr Rev 16:200–257 [DOI] [PubMed] [Google Scholar]
- 18. Stumpf WE, Sar M, Reid FA, Tanaka Y, DeLuca HF. 1979. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 206:1188–1190 [DOI] [PubMed] [Google Scholar]
- 19. Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. 1983. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab 57:1308–1310 [DOI] [PubMed] [Google Scholar]
- 20. Stumpf WE. 1995. Vitamin D sites and mechanisms of action: a histochemical perspective: reflections on the utility of autoradiography and cytopharmacology for drug targeting. Histochem Cell Biol 104:417–427 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, DeLuca HF. 2011. Is the vitamin D receptor found in muscle? Endocrinology 152:354–363 [DOI] [PubMed] [Google Scholar]
- 22. Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1979. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet 2:1335–1336 [DOI] [PubMed] [Google Scholar]
- 23. Frampton RJ, Suva LJ, Eisman JA, Findlay DM, Moore GE, Moseley JM, Martin TJ. 1982. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res 42:1116–1119 [PubMed] [Google Scholar]
- 24. Frampton RJ, Omond SA, Eisman JA. 1983. Inhibition of human cancer cell growth by 1,25-dihydroxyvitamin D3 metabolites. Cancer Res 43:4443–4447 [PubMed] [Google Scholar]
- 25. Skowronski RJ, Peehl DM, Feldman D. 1993. Vitamin D and prostate cancer: 1,25 dihydroxyvitamin D3 receptors and actions in human prostate cancer cell lines. Endocrinology 132:1952–1960 [DOI] [PubMed] [Google Scholar]
- 26. Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. 1981. Differentiation of mouse myeloid leukemia cells induced by 1 α,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA 78:4990–4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1979. 1α, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature 281:317–319 [DOI] [PubMed] [Google Scholar]
- 28. Adams JS, Sharma OP, Gacad MA, Singer FR. 1983. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 72:1856–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. 1981. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med 305:440–443 [DOI] [PubMed] [Google Scholar]
- 30. Stoffels K, Overbergh L, Bouillon R, Mathieu C. 2007. Immune regulation of 1α-hydroxylase in murine peritoneal macrophages: unravelling the IFNγ pathway. J Steroid Biochem Mol Biol 103:567–571 [DOI] [PubMed] [Google Scholar]
- 31. Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. 2002. The ontogeny of 25-hydroxyvitamin D(3) 1α-hydroxylase expression in human placenta and decidua. Am J Pathol 161:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. 2006. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod 75:816–822 [DOI] [PubMed] [Google Scholar]
- 33. Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. 2009. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. 2001. Targeted ablation of the 25-hydroxyvitamin D 1α -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 98:7498–7503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobson EA, James KA, Newmark HL, Carroll KK. 1989. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz (a) anthracene in female Sprague-Dawley rats. Cancer Res 49:6300–6303 [PubMed] [Google Scholar]
- 36. Colston KW, Mackay AG, James SY, Binderup L, Chander S, Coombes RC. 1992. EB1089: a new vitamin D analogue that inhibits the growth of breast cancer cells in vivo and in vitro. Biochem Pharmacol 44:2273–2280 [DOI] [PubMed] [Google Scholar]
- 37. Mehta R, Hawthorne M, Uselding L, Albinescu D, Moriarty R, Christov K, Mehta R. 2000. Prevention of N-methyl-N-nitrosourea-induced mammary carcinogenesis in rats by 1α-hydroxyvitamin D(5). J Natl Cancer Inst 92:1836–1840 [DOI] [PubMed] [Google Scholar]
- 38. Zinser GM, Suckow M, Welsh J. 2005. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol 97:153–164 [DOI] [PubMed] [Google Scholar]
- 39. Zinser GM, Sundberg JP, Welsh J. 2002. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 23:2103–2109 [DOI] [PubMed] [Google Scholar]
- 40. Banach-Petrosky W, Ouyang X, Gao H, Nader K, Ji Y, Suh N, DiPaola RS, Abate-Shen C. 2006. Vitamin D inhibits the formation of prostatic intraepithelial neoplasia in Nkx3.1;Pten mutant mice. Clin Cancer Res 12:5895–5901 [DOI] [PubMed] [Google Scholar]
- 41. Huerta S, Irwin RW, Heber D, Go VL, Koeffler HP, Uskokovic MR, Harris DM. 2002. 1α,25-(OH)(2)-D(3) and its synthetic analogue decrease tumor load in the Apc(min) Mouse. Cancer Res 62:741–746 [PubMed] [Google Scholar]
- 42. Krishnan AV, Feldman D. 2011. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 51:311–336 [DOI] [PubMed] [Google Scholar]
- 43. Wang Q, Yang W, Uytingco MS, Christakos S, Wieder R. 2000. 1,25-Dihydroxyvitamin D3 and all-trans-retinoic acid sensitize breast cancer cells to chemotherapy-induced cell death. Cancer Res 60:2040–2048 [PubMed] [Google Scholar]
- 44. Deeb KK, Trump DL, Johnson CS. 2007. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 7:684–700 [DOI] [PubMed] [Google Scholar]
- 45. Bikle D. 2009. Nonclassic actions of vitamin D. J Clin Endocrinol Metab 94:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holick MF. 1998. Clinical efficacy of 1,25 dihydroxyvitamin D3 and its analogs in the treatment of psoriasis. Retinoids 14:7–12 [Google Scholar]
- 47. Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. 2005. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endcorinol Metab 288:E125–E132 [DOI] [PubMed] [Google Scholar]
- 48. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. 2008. Calcium independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1a-hydroxylase knockout mice. Kidney International 74:170–179 [DOI] [PubMed] [Google Scholar]
- 49. Scragg R, Sowers M, Bell C. 2007. Serum 25-hydroxyvitamin D, ethnicity and blood pressure in the third national health and nutrition examination survey. Amer J Hypertens 20:713–719 [DOI] [PubMed] [Google Scholar]
- 50. Feneis JF, Arora RR. 2010. The role of vitamin D in blood pressure homeostasis. Am J Therapeutics 17:e221–e229 [DOI] [PubMed] [Google Scholar]
- 51. Mathieu C, Adorini L. 2002. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol Med 8:174–179 [DOI] [PubMed] [Google Scholar]
- 52. Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, Nishimura T. 2010. 1α,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett 134:7–16 [DOI] [PubMed] [Google Scholar]
- 53. DeLuca HF, Cantorna MT. 2001. Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585 [DOI] [PubMed] [Google Scholar]
- 54. Raghuwanshi A, Joshi SS, Christakos S. 2008. Vitamin D and multiple sclerosis. J Cell Biochem 105:338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cantorna MT, Humpal-Winter J, DeLuca HF. 1999. Dietary calcium is a major factor in 1,25-dihydroxycholecalciferol suppression of experimental autoimmune encephalomyelitis in mice. J Nutr 129:1966–1971 [DOI] [PubMed] [Google Scholar]
- 56. Lemire JM, Archer DC. 1991. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest 87:1103–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cantorna MT, Hayes CE, DeLuca HF. 1996. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA 93:7861–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Joshi S, Youssef S, Gaffen S, Steinman L, Christakos S. 2008. A key mechanism underlying the immunosuppressive effects of vitamin D: 1,25dihydroxyvitamn D3 is a transcriptional modulator of IL-17. J Bone Miner Res 23:S105 [Google Scholar]
- 59. Spach KM, Nashold FE, Dittel BN, Hayes CE. 2006. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol 177:6030–6037 [DOI] [PubMed] [Google Scholar]
- 60. Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. 2000. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1,25-dihydroxyvitamin D(3). Eur J Immunol 30:498–508 [DOI] [PubMed] [Google Scholar]
- 61. Meehan TF, DeLuca HF. 2002. The vitamin D receptor is necessary for 1α,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys 408:200–204 [DOI] [PubMed] [Google Scholar]
- 62. Meehan TF, Vanhooke J, Prahl J, DeLuca HF. 2005. Hypercalcemia produced by parathyroid hormone suppresses experimental autoimmune encephalomyelitis in female but not male mice. Arch Biochem Biophys 442:214–221 [DOI] [PubMed] [Google Scholar]
- 63. Becklund BR, Severson KS, Vang SV, DeLuca HF. 2010. UV radiation suppresses experimental autoimmune encephalomyelitis independent of vitamin D production. Proc Natl Acad Sci USA 107:6418–6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldberg P. 1974. Multiple sclerosis: vitamin D and calcium as environmental determinants of prevalence (a viewpoint). Part I: sunlight, dietary factors and epidemiology. Int J Environ Stud 6:19–27 [Google Scholar]
- 65. Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. 2003. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol 17:2386–2392 [DOI] [PubMed] [Google Scholar]
- 66. Cantorna MT. 2010. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc 69:286–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mathieu C, Waer M, Laureys J, Rutgeerts O, Bouillon R. 1994. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 37:552–558 [DOI] [PubMed] [Google Scholar]
- 68. Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. 2002. A 1α,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 69. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. 2006. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 296:2832–2838 [DOI] [PubMed] [Google Scholar]
- 70. Liu PT, Stenger S, Tang DH, Modlin RL. 2007. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179:2060–2063 [DOI] [PubMed] [Google Scholar]
- 71. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]