Abstract
Sexually naïve, hormone-primed, C57BL/6J female mice are not receptive to mating attempts by conspecific males. Repeated experience with sexually active males and concurrent treatment with estradiol and progesterone gradually increases female receptivity over the course of five trials to maximal levels. Ovarian hormones activate their cognate nuclear steroid receptors estrogen receptor-α and progesterone receptor to induce female sexual receptivity. Nuclear receptors recruit coactivators of transcription that include histone acetyltransferases to hormone responsive genes. In this set of studies, we found that the histone deacetylase inhibitor sodium butyrate enhances the experiential acquisition of receptivity. Evidence is provided that the actions of sodium butyrate on receptivity require activated estrogen receptor-α and progesterone.
Ovarian hormones are essential for sexual receptivity in adult female rodents and are produced maximally during behavioral estrus, yet the comprehensive molecular mechanisms downstream of hormone activity that promote receptivity are far from understood. Females are not receptive outside of proestrus or after the removal of endogenous gonadal hormones through ovariectomy (OVX); they rarely display the receptive posture of lordosis and actively avoid copulatory contacts (1–4). Both high pharmacological levels of exogenous estradiol replacement, and low physiological levels of estradiol combined with progesterone replacement, can induce receptivity in OVX female rodents (4–8). However, rats and C57BL/6J mice differ in their response to hormone replacement. Sexually inexperienced OVX adult mice reared and tested under normal laboratory procedures are unreceptive to males, even when given hormone replacement that induces receptivity in sexually naive rats (9–11). Receptivity in hormone replaced OVX mice gradually increases over repeated days of sex experience with a sexually active male (11–14). The mechanisms underlying this sex-experience learning requirement in mice have not been directly investigated.
Chromatin modifications involved in the regulation of gene expression are implicated in learning and memory and in mood. Most research in this area focuses on the promoter regions of genes of interest, and investigates cytosine (DNA) methylation, histone acetylation, and histone methylation. These modifications regulate chromatin structure and the binding of transcriptional coregulators. Acetylated histones are particularly associated with transcriptionally active euchromatin. Histone acetyl-lysine residues serve as docking sites for bromodomain containing coregulators (15) and increased acetylation of nearby histones is positively correlated with the recruitment of RNA polymerase II to the transcriptional start site of genes (16–19). Posttranslational acetylation of transcription factors also has functional consequences on their activities (20–22). Histone deacetylase (HDAC) enzymes dynamically remove acetyl groups, and HDAC are generally considered to be transcriptional corepressors (16, 23). Interestingly, in vivo pharmacological studies with HDAC inhibitor drugs have positive effects on rodent behaviors modeling cognition, mood, and neurodegeneration (24–27). Sodium butyrate (SB), a general class I and class II HDAC inhibitor, causes hyperacetylation of histones in the brain and affects rodent behavior in learning and memory tasks, anxiety, and depression (28–32). It is worth noting that ovarian hormones also affect learning and memory and mood (33–36). In fact, estradiol and SB may work synergistically to have antidepressant-like effects (37).
Here we test the hypothesis that ovarian hormones prime neurological circuits for the acquisition of lordosis behavior, in part by increasing acetylation of chromatin by way of nuclear hormone receptor activation. In the first experiment (experiment 1), we found that sexually naive OVX female mice primed with estradiol benzoate (EB) and progesterone (P) and treated with the HDAC inhibitor SB are faster to acquire receptivity and display higher average lordosis quotients (LQ) than control mice. In the second experiment (experiment 2), we noted that SB treatment has no effect on lordosis behavior without a functional estrogen receptor (estrogen receptor-α, ERα). Lastly (experiments 3 and 4), we showed that SB treatment does not promote lordosis independently of ovarian hormones.
Materials and Methods
Animals
The first three experiments were conducted with sexually naive adult females in the C57BL/6J background strain bred in the animal facilities at the University of Virginia. For the final study, females were ordered from the Jackson Labs (Bar Harbor, ME). Experiment 2 used ERα gene knockout (ERαKO) and wild-type (WT) female littermates in the same C57BL/6J background (38). ERαKO and WT littermates were genotyped by PCR amplification of Esr1 as described (38). Females were ovariectomized under general isoflurane anesthesia at 60–80 d old and housed in groups of three to four (experiments 1 and 2) or five (experiments 3 and 4) in a 12-h light,12-h dark cycle (lights on 2400 h, lights off 1200 h EST) and given food (diet no. 7912; Harlan-Teklad, Indianapolis, IN) and water ad libitum. All procedures and experiments were conducted with the approval of and in compliance with the Animal Care and Use Committee of the University of Virginia.
SB treatment
A week after surgery, females were administered (ad libitum) SB (Sigma, St. Louis, MO) in their drinking water (8 mg/ml) for the duration of the experiments. This route of administration and dose of SB hyperacetylates histones in neurons (39, 40). Intake of SB by group-housed females was estimated by weighing water bottles. The average daily consumption of drug by the treated individuals was 41.2 ± 7.15 mg (mean ± sd) per day. The SB-treated females drank an average of 5.14 ± 0.89 ml/d (range 3.93–6.93 ml/d), slightly more than the 4.50 ± 0.76 ml/d consumed by the control females drinking water. The dose and volume of SB was within the range previously reported in the literature (39, 40).
Female sexual behavior
Starting 1 wk after the start of SB treatment, female receptivity tests were conducted every fourth day for five (experiments 1, 2. and 4) or six (experiment 3) trials. OVX females were injected (sc) with EB (0.5 μg EB in 0.05 ml sesame oil; Sigma) 2 d before, and P (830 μg in 0.05 ml sesame oil; Sigma) 3–6 h before, each trial as previously described (41). After lights off (between 1200 and 1800 h EST) hormone-primed females were tested for receptivity by placing them with a C57BL/6J male in his home cage. Males were screened for high sexual activity from a group of 20–30 sexually experienced males. In the rare case in which a male did not mount during the test, he was replaced so that all females were tested with a highly active male. Ejaculating males were not used again for the remainder of the trial day. An observer blind to treatment (and genotype in experiment 2) recorded each mount attempt by the male and each receptive stand by the female. A stand was scored if a female did not sit or run away to avoid a mount attempt and instead braced steady with all four limbs during a mount attempt. The observer stopped trials after 20 mounts, 15 min, or an ejaculation by the male, whichever happened first. Degree of individual female sexual receptivity in every trial was measured by the LQ [LQ = (stands/mounts) 100].
Elevated plus maze (EPM)
After their last receptivity trials, the females from experiments 1 and 2 were maintained on SB or control drinking water. Behavior in the) was tested for 5 min under bright illumination the day after the last receptivity test during the light portion of the day (between 0800 and 1200 h EST). The number of entries into closed and opened arms, time spent in open arms, time spent in closed arms, and time spent in the center of the maze were measured.
Western immunoblots
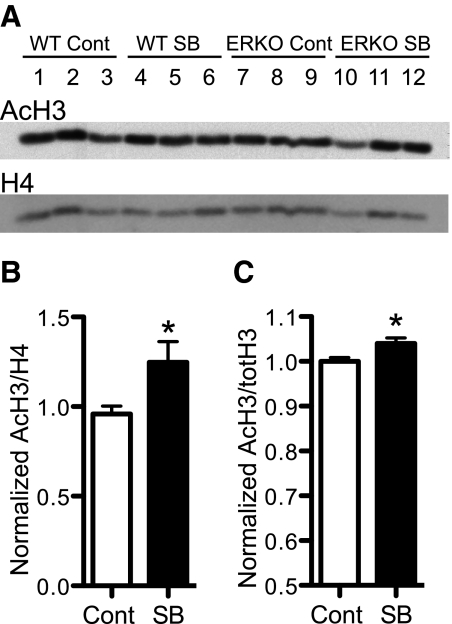
Whole frozen brains from experiment 2 (below) were homogenized using a Tissue-Tearor (Biospec Products, Inc., Bartlesville, OK) in cold radioimmunoprecipitation assay buffer (0.05 m Tris; 0.9% NaCl; 5 mm EDTA, pH 7.4) with 10 μl/ml protease inhibitor cocktail (Sigma) and phenylmethylsulfonyl fluoride (1 mm). Cells were lysed in Pyrex Tenbroeck tissue grinders (Corning Inc., Corning, NY) with 12 strokes, nuclei isolated by centrifugation, and histone acid extracted and trichloroacetic acid precipitated as previously described (42). Dried precipitates were boiled in 2× Laemmli buffer (4% sodium dodecyl sulfate; 20% glycerol; 120 mm Tris, pH 6.8) for 5 min to resuspend. Samples were diluted into complete Laemmli buffer (1× with 200 mm dithiothreitol and 0.02% bromophenol blue), and 5 μg of total protein was electrophoresed in 16% polyacrylamide-sodium dodecyl sulfate gels and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA) as described (42). Membranes were blocked with 5% milk in Tris-buffered saline (50 mm Tris-HCl; 150 mm NaCl, pH 7.4) with 0.1% Tween 20 (TBST) overnight at 4 C. Blots were incubated at room temperature for 1 h in primary antibody (1:20,000 in 5% milk TBST) against acetyl-H3K9/14 (rabbit polyclonal IgG; Millipore-Upstate, Lake Placid, NY; no. 06-599), rinsed with TBST, and incubated at room temperature in ImmunoPure horseradish peroxidase-conjugated goat antirabbit (Pierce, Rockford, IL) secondary antibody (1:10,000 in TBST) for 1 h. Blots were developed on film with chemiluminescent substrate as described (42). For loading controls, blots were reprobed using the total Anti-Histone H4 (Millipore-Upstate; rabbit polyclonal IgG; no. 07-108) as the primary antibody (1:10,000 in 5% milk TBST). The band intensities were measured on a densitometer (GS-800 calibrated densitometer, no. 170-7983; Bio-Rad Laboratories) and quantified using ImageQuant TL software (GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK).
To measure hypothalamic histone acetylation, hypothalami were dissected from brains collected in experiment 4, and the above procedures were repeated with the following changes. Whole hypothalamus lysates were prepared by boiling homogenates in 2× Laemmli buffer, followed by centrifugation to pellet out insoluble material. After acetylated histone H3 detection, blots were stripped and reprobed with total Histone H3 antibody (Cell Signaling Technology, Danvers, MA; no. 9715) as the primary (1:5000 in 3% milk TBST) for loading controls.
Experiment 1: effects of HDAC inhibitor treatment on lordosis
For the first experiment, we tested whether acetylation plays a role in the activation and acquisition of lordosis under proestrus-like hormone conditions. Twelve sexually naive OVX C57BL/6J females were treated with SB in their drinking water, and 11 control females received regular water. All females were hormone primed and tested for lordosis receptivity every fourth day for five trials.
Experiment 2: ERα and HDAC inhibition effects on lordosis
Histone acetylation is biochemically downstream of ER activation, and transcriptional coregulators with bromodomains can dock at acetyl-lysines (15, 18). Here we assessed whether global hyperacetylation could rescue the lack of receptivity in ERαKO mice. Twenty OVX adult ERαKO, and 20 of their homozygous WT littermates (38), were given either SB treated or control drinking water. The final group sizes were 10 per group with four groups: ERαKO control, ERαKO SB, WT control, and WT SB. All females were hormone primed and tested for lordosis as described in experiment 1. Two days after the last sex behavior test, females received EB injections followed 48 h later by P injections. Brains were collected 3–6 h after P injections, immediately frozen in crushed dry ice, and stored at −80 C until histones were extracted.
Experiment 3: hormone-independent effects of HDAC inhibition on lordosis
Because ERα activation is critical for both ligand-dependent and ligand-independent activation of receptivity, we asked whether SB treatment could eliminate the need for hormone replacement. Ten OVX adult C57BL/6J females were treated with SB drinking water for the duration of the experiment, and all were tested for lordosis every fourth day for six trials. Five females in group 1 were hormone primed with EB + P for the first five trials and injected with vehicle only on the sixth trial. In group 2, five females were injected with vehicle for the first five trials and EB + P primed for the final test.
Experiment 4: P and HDAC inhibition effects on lordosis
In this final experiment, we asked whether P is required for lordosis in SB-treated females. Thirty OVX adult C57BL/6J females were divided into three treatment groups (n = 10 in each) and tested for acquisition of lordosis receptivity. One group (EB + P) was tested with EB and P replacement and given control drinking water. The other two groups were tested with only EB replacement (and oil vehicle instead of P). One of these groups was drinking regular water (EB), and the other group received SB treated drinking water (EB + SB). Four days after the last behavioral test and 48 h after an EB injection, whole hypothalami were dissected and frozen from the EB and EB + SB groups to measure acetylation levels. To ensure that all females were consuming SB before brain collection, females were deprived of drinking water overnight and allowed to drink for 1–2 h before the animals were killed. Based on water consumption, the EB + SB group had an SB intake of 24.5 ± 9.2 mg (∼1 g/kg) during this period, near the 1.2 g/kg reported in the literature for acute injections (28, 30, 37).
Statistics
Repeated-measures ANOVA were used to detect increased LQ over trial 1, baseline, within each treatment group. One-way, repeated-measures ANOVA were used to determine which treatments influenced receptivity in experiments 1, 3, and 4. A two-way repeated-measures ANOVA was used to test the effects of SB, genotype, and trial in experiment 2. For analysis of experiment 2, trial 5 sex behavior, EPM behavior, and H3 acetylation, we used two-way ANOVA. In all experiments ANOVA were followed by Fisher's least significant differences multiple-comparison tests. The effects of SB on EPM behavior and histone acetylation were assessed by Student's t test.
Results
Experiment 1: HDAC inhibitor treatment accelerates and enhances the acquisition of lordosis behavior
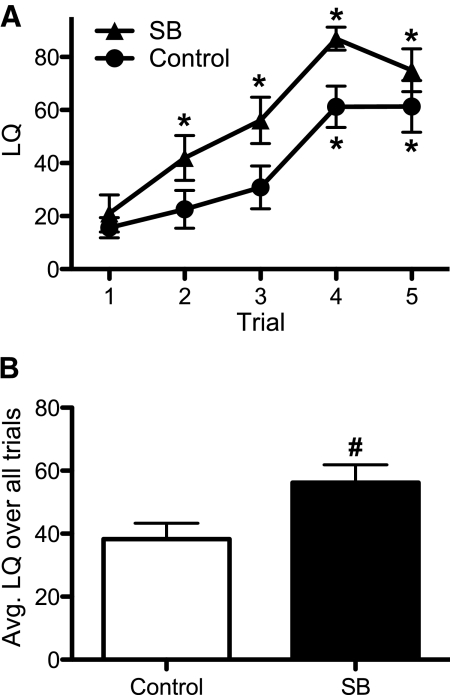
Treatment with the HDAC inhibitor SB hastened and enhanced the acquisition of receptivity in sexually naïve, EB and P replaced, ovariectomized females. Both the SB-treated and control groups started at similar low baseline LQ (LQ lower than 20) in the first trial. An effect of test trial (F4,84 = 32.8, P < 0.0001) illustrated that LQ gradually increased with sex experience in both groups; however, the LQ of SB-treated females significantly increased over trial 1 baseline by trial 2, whereas the LQ of control females did not significantly increase over baseline until trial 4 (Fig. 1A). Interestingly, SB-treated females appeared to plateau at a maximum LQ over 75 in trials 4 and 5, and controls barely reached an LQ of 60 in the same trials (differences were not significant). Therefore, we noted an overall treatment effect on LQ (F1,84 = 5.43, P < 0.03), and SB-treated females had a higher average LQ per trial than controls (Fig. 1B).
Fig. 1.
SB enhances the acquisition of receptivity in C57BL/6J females (mean ± sem). The LQ from OVX hormone-primed mice that either received SB in drinking water (n = 12) or untreated water (n = 11). A, Data for each of the five trails are presented. B, Data are presented as averages over all trials.  , SB-treated females;
, SB-treated females;  , control females. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly greater than control group (P < 0.05).
, control females. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly greater than control group (P < 0.05).
We also found an effect of trial (F4,84 = 3.12, P < 0.02), and a trend for a treatment effect (F1,84 = 3.84, P = 0.063), on the number of mounts the females received from the males. SB-treated females received equivalent numbers of mounts per trial (mean 16.3) as control (mean 18.1) females. The trial effect was caused by a decline in mounts on trial 4. Fewer mounts in the SB-treated group corresponded with more trials (29%) terminating in ejaculation, compared with control females (15% of trials).
Experiment 2: ERα is essential for SB enhancement of lordosis acquisition
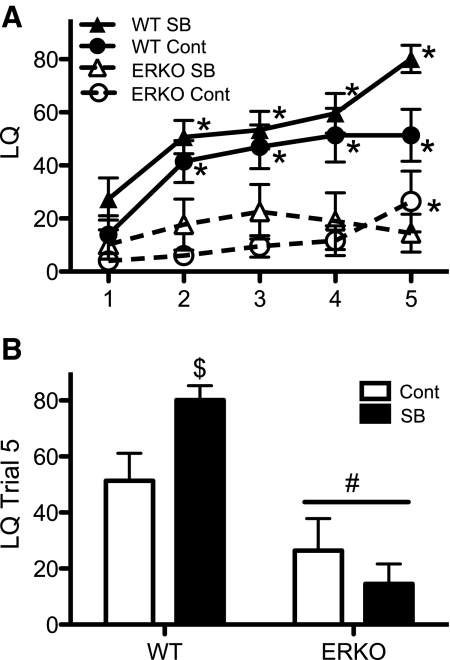
ERαKO female mice are unreceptive (43), and their receptivity deficit was not rescued by SB treatment. An interaction between trial, treatment, and genotype was noted (F4,143 = 2.84, P < 0.03). This interaction revealed that unlike control WT females that reached a LQ maximum of just over 50 in the fourth and fifth trials, SB-treated WT females continued to increase from an LQ of 60 in the fourth trial to an LQ of 80 in the fifth trial (Fig. 2A). SB-treated and control WT females had similar baseline LQ, and with sex experience, WT females in both treatment groups reached significantly higher LQ over their naive baseline levels by the second trial (Fig. 2A). Two-way ANOVA revealed an interaction between genotype and treatment (F1,36 = 5.44, P < 0.03) in the final trial; the SB-treated WT females were significantly higher than all other groups on this trial (Fig. 2B). In contrast to WT females, ERαKO females in both treatment groups never displayed high levels of receptivity (Fig. 2A), and there was a significant effect of genotype on LQ (F1,143 = 32.4, P < 0.0001). Control-treated ERαKO females had a significant increase in their LQ over baseline by the final trial (Fig. 2A); however, ERαKO females still had lower average LQ compared with WT females in this trial (Fig. 2B).
Fig. 2.
SB does not rescue the LQ acquisition deficit in ERαknockout (ERKO) females (mean ± sem). The LQ data from hormone-primed ERKO females and their WT littermates that were either SB treated (SB) or not treated (Cont) are shown. Four groups with 10 females in each were formed and tested for receptivity. A, Data over five trials are shown. B, Data from the final trial are presented.  , WT SB;
, WT SB;  , WT Cont;
, WT Cont; 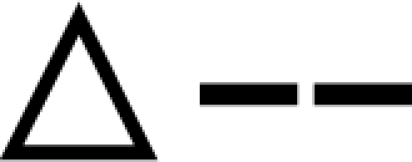 , ERKO SB; ○--, ERKO Cont. *, Significantly different from own trial 1 baseline (P < 0.05); $, significantly different from all other groups (P < 0.05). #, significantly different from WT females (P < 0.05).
, ERKO SB; ○--, ERKO Cont. *, Significantly different from own trial 1 baseline (P < 0.05); $, significantly different from all other groups (P < 0.05). #, significantly different from WT females (P < 0.05).
Neither genotype nor treatment (F1,144 = 1.03, 0.23, respectively) affected the number of mount attempts directed at the females. An effect of trial (F4,144 = 2.42, P = 0.051) reflected a gradual decline in mount attempts over trials. No interactions between trial and genotype or treatment were noted for the number of mounts received. Importantly, there were no effects of treatment or genotype, nor was there an interaction (F1,36 ≤ 0.25) on the number of mounts received in the last trial. Therefore, the high average LQ of SB-treated WT females in trial 5 is not due to a systemic difference in mounts received on this trial.
Experiment 3: SB enhancement of lordosis acquisition is hormone dependent
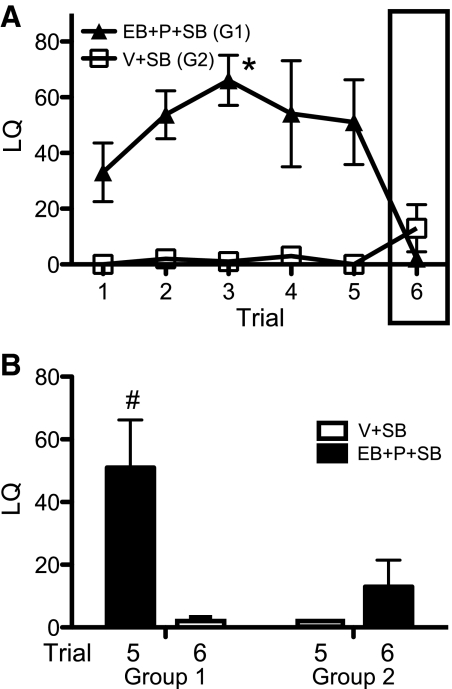
SB alone does not activate lordosis. SB-treated females were either hormone replaced (EB + P + SB) or given vehicle (V + SB). For trials 1–5, hormone replacement had an effect on LQ (F1,40 = 24.0, P < 0.002). By trial 3, the EB + P + SB-treated females of group 1 (G1) had a significantly higher LQ score over trial 1 baseline (Fig. 3A). The oil vehicle (V + SB)-treated females in group 2 (G2) never displayed LQ scores significantly higher than a baseline of essentially 0 in any trial (Fig. 3A). There were no differences in the numbers of mounts received by females in the two treatment groups. After the fifth trial described above, females were switched from EB + P to vehicle injections (G1), and vice versa (G2), for a sixth trial; both groups were maintained on SB (Fig. 3). Examining only the data from trials 5 and 6 (experience with males in trials 1–4), there was an effect of hormone treatment on LQ (F1,8 = 12.5, P < 0.01). When females received EB + P, their LQ was higher than when treated with vehicle (Fig. 3B; black bars > white bars). There was also an effect of group on LQ (F1,8 = 5.31, P = 0.05). Comparing both groups under hormone replacement (black bars only), females that had received several prior hormone treatments had significantly higher LQs than females given their first experience with EB + P treatments (Fig. 3B). Thus, only group 1 females that were hormone replaced in trials 1–5 acquired high receptivity scores with sex experience, whereas group 2 females that were not hormone replaced in trials 1–5 did not acquire high receptivity (Fig. 3B). This result reinforces the fact that SB is not able to substitute for ovarian hormones to induce behavioral estrus but may facilitate a learning aspect of receptivity or cause an increase in maximal LQ receptivity.
Fig. 3.
Ovarian hormone replacement is essential for lordosis and SB enhancement of LQ (mean ± sem). The LQ in females treated with SB in their drinking water and either hormone primed for trials 1–5 (EB + P + SB) and injected with vehicle on trial 6 (G1, n = 5) or injected with vehicle for trials 1–5 (V + SB) and hormone primed only on trial 6 (G2, n = 5) is shown. Box around trial 6 in A indicates the hormone treatment shift. A, LQ data are given over all trials.  , EB + P + SB in trials 1–5 and no hormone in trial 6 (G1);
, EB + P + SB in trials 1–5 and no hormone in trial 6 (G1);  , V + SB trials 1–5 and hormone for trial 6 (G2). B, The LQ in trials 5 and 6 (black histogram, EB+P; white histogram, vehicle) are presented. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly higher than all the other values (P = 0.05).
, V + SB trials 1–5 and hormone for trial 6 (G2). B, The LQ in trials 5 and 6 (black histogram, EB+P; white histogram, vehicle) are presented. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly higher than all the other values (P = 0.05).
Experiment 4: SB enhancement of lordosis requires P
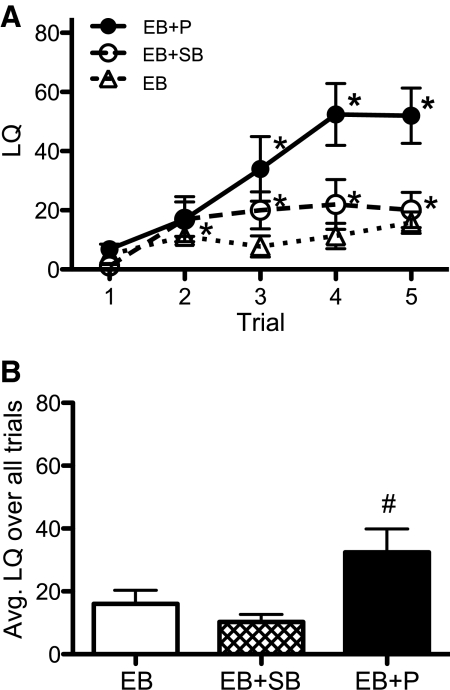
P replacement is necessary for receptivity in mice, and SB treatment does not activate receptivity in its absence. Females drinking normal water were injected with either EB and P (EB + P) or EB and vehicle (EB); a final group consumed SB drinking water and hormone treatment with only EB and vehicle (EB + SB). We found main effects of trial (F4,108 = 16.9, P < 0.0001) and treatment (F2,108 = 4.93, P < 0.02) as well as an interaction (F8,108 = 4.86, P < 0.0001). By trial 3, EB + P females had significantly higher LQ over trial 1 baseline and reached an average LQ of just over 50 by trials 4 and 5 (Fig. 4A). The EB females had increased LQ over baseline by trial 2, whereas EB + SB females never had an increase in LQ over baseline. Neither group with only EB replacement reached an average LQ higher than 20 on any trial (Fig. 4A). In trials 3, 4, and 5, EB + P females had significantly higher LQ than EB-only females on normal or SB-treated water (Fig. 4A). Overall, the average LQ per trial for EB + P females was higher than the other groups (Fig. 4B).
Fig. 4.
Estradiol alone without P replacement is insufficient for lordosis and SB enhancement of LQ (mean ± sem). The LQ for the three groups of 10 females each is shown. One group was hormone primed with both estradiol and P (EB + P). Two other groups were primed with EB alone: one was SB treated (EB + SB), and the other was not (EB). A, LQ data over five trials are shown. B, The average LQ for each group is given over all five trials.  , EB + P;
, EB + P;  , EB;
, EB; 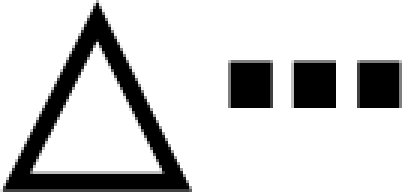 , SB. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly different from other groups (P < 0.05).
, SB. *, Significantly different from own trial 1 baseline (P < 0.05); #, significantly different from other groups (P < 0.05).
SB treatment does not affect elevated plus maze behavior
It has been reported that SB, and other HDAC inhibitors, affect behaviors that model anxiety and depression. We did not note any treatment effects in the EPM for time spent in the open (t21 = 0.337) or closed (t21 = 1.42) arms (Table 1). Control females spent significantly more time in the center of the EPM compared with SB-treated females (t21 = 2.15, P < 0.05). Locomotor activity, measured by the total number of entries into open and closed arms, was no different between treatment groups (t21 = 0.377). No treatment or genotype effects on EPM or locomotor behaviors were observed in the ERαKO mice (experiment 2).
Table 1.
EPM behavior
| Experiment 1 |
Experiment 2 |
|||||
|---|---|---|---|---|---|---|
| Cont (n = 11) | SB (n = 12) | WT Cont (n = 8) | WT SB (n = 6) | ERαKO Cont (n = 6) | ERαKO SB (n = 4) | |
| Open arm time (sec) | 21.2 ± 4.9 | 23.4 ± 4.4 | 15.9 ± 4.0 | 24.8 ± 10.0 | 26.6 ± 13.1 | 15.3 ± 6.8 |
| Closed arm time (sec) | 178 ± 10.7 | 199 ± 9.6 | 199 ± 5.4 | 186 ± 23.4 | 173 ± 27.5 | 170 ± 18.6 |
| Center time (sec) | 100 ± 8.7 | 77.8 ± 6.2a | 84.9 ± 3.3 | 89.3 ± 17.8 | 100 ± 19.0 | 115 ± 15.9 |
| Total arm entries | 18.2 ± 1.4 | 18.9 ± 1.3 | 17.6 ± 0.6 | 14.8 ± 3.7 | 15.7 ± 2.0 | 15.5 ± 2.7 |
Mean ± sem behavioral data collected the day after the last sex behavior trial, females from experiments 1 and 2. Females were tested in the EPM for 5 min. In experiment 1, SB-treated females were compared with controls. In experiment 2, WT and ERαKO females both treated (SB) and not treated (Cont) were compared (four groups: WT Cont, WT SB, ERαKO Cont, and ERαKO SB). Total arm entries represent locomotor activity (open arm + closed arm entries). n, Number of females in each treatment group. Cont, Control.
Significantly different from controls (P < 0.05).
Effects of SB on histone H3 acetylation (AcH3) in brain
Choice of oral administration of SB in our study was consistent with other HDAC inhibitor treatment studies that have demonstrated an increase of histone acetylation in the brain (25, 28, 30, 44). Whole-brain histone extracts, from hormone-primed females in experiment 2, were used to detect hyperacetylation caused by SB treatment. In a two-way ANOVA with SB treatment and genotype as factors, there was a trend for a main effect of SB treatment on AcH3 in WT and ERαKO females (Fig. 5: F1,8 = 4.40, P = 0.069). No difference in AcH3 was detected between WT and ERαKO genotypes (F1,8 = 0.201). When genotypes were pooled by treatment, we noted a significant effect of SB on AcH3 (t10 = 2.31, P < 0.05; Fig. 5B). Western blots of whole-tissue hypothalamic lysates from experiment 4 confirmed a significant (t8 = 2.69, P < 0.05) difference in AcH3 in females receiving SB drinking water compared with the control group (Fig. 5C). The relatively low fold changes we noted reflect the fact that histone acetylation is a dynamic and important process. Larger changes in acetylation may produce strong behavioral effects, whereas the types of behavioral changes we examined were very subtle.
Fig. 5.
Acetylation levels of histones in brain tissue from SB treated females. A, A representative Western blot of AcH3 subunit and total H4 subunit loading control from whole-brain histone extractions of WT and ERα knockout (ERKO) littermate females both treated with SB and untreated (Cont). For each group, three individuals are shown. B, Mean ± sem level of AcH3 normalized to H4 in whole-brain histone and expressed as a fraction of the mean WT Cont levels (n = 6 per treatment group). C, Mean ± sem level of AcH3 normalized to total H3 of hypothalamic tissue from EB (Cont) and EB + SB (SB) (n = 5 in each group). *, Significantly larger than Cont groups (P < 0.05).
Discussion
Female C57BL/6J mice treated with SB acquired a higher average LQ per trial compared with control females (experiment 1). When ERαKO females were given the same SB treatment, they did not respond with increased receptivity (experiment 2). Furthermore, SB enhancement of receptivity was completely hormone dependent; SB treatment and interactions with males did not increase receptivity (experiments 3 and 4). It is unlikely that the behavior of the males was responsible for these effects. None of the data support any differences in attractivity between SB-treated and control females; there were no differences between the groups in the number of mounts received. Because both SB (28) and estradiol (33, 36) treatments reduce rodent behaviors that model anxiety and depression, one possible explanation for higher receptivity in SB-treated females might be enhanced proceptivity due to decreased anxiety. In fact, SB and estradiol treatments alone did not affect rat behavior in the forced swim test but significantly decreased immobility when used in combination (37). Here no significant differences between groups in the amount of time spent in the open and/or closed arms of the EPM were found. We did note that SB-treated C57BL/6J females in experiment 1 spent significantly less time in the center of the elevated plus maze. This difference is hard to interpret, although some have suggested that it reflects indecisive behavior (45); however, this finding was not replicated in experiment 2.
Our results support the hypothesis that SB treatment increases the potential to acquire a high level of receptivity, and this idea is illustrated by comparing the change in LQ over repeated trials between treatment groups (experiment 1). The higher average LQ per trial in the SB-treated females is not a consequence of higher baseline receptivity because treated and untreated females had nearly identical LQs in the first test trials. Sex experience increased LQ over baseline in both treatment groups, but the SB-treated females showed a faster increase. By the second trial, SB-treated females had higher LQ over trial 1 (baseline), whereas the control group LQ did not increase over baseline until the fourth trial. In experiment 2, SB-treated WT females did not show a more rapid increase in LQ over their baseline compared with WT control females; however, LQ in SB-treated females was higher than the control females in every trial. Similar to experiment 1, SB-treated WT females in experiment 2 reached a significantly higher maximum LQ than control females by their final trial. In the WT females, differences between the LQ curve profiles for experience-acquired receptivity in experiments 1 and 2 may be due to one or more differences between ERαKO and normal C57BL/6J mice. For example, WT females in the ERαKO line are reared with breeders that are heterozygous for the ERα mutation and with siblings that carry the mutation. Moreover, the uterine environment in the heterozygous dams may provide important nutritional, hormonal, or other differences. Overall, the nearly identical sexually naive baseline LQ in the first trial for SB-treated and control females, compared with the higher average LQ in subsequent trials for SB-treated females, suggests that SB acts on a learning or long-term memory mechanism of experience-acquired receptivity. Several studies have shown that both estradiol and HDAC inhibitors improve aspects of learning and memory in rodents (30, 32, 34, 46).
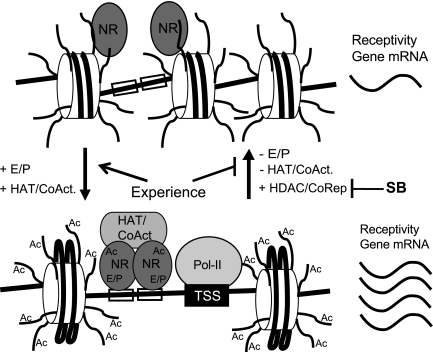
Potentiation of nuclear steroid receptor activity is one potential mechanism by which SB enhances the acquisition of lordosis receptivity. Gene knockout, antisense RNA, and agonist studies demonstrate that ERα and progesterone receptor (PR) activation in the brain are essential for female sex behavior in rodents (12, 43, 47–51). Ligand-activated nuclear steroid receptors recruit coactivators of gene transcription to the promoters of steroid-responsive genes (19, 52, 53), and steroid receptor coactivator (SRC)-1, SRC2, and possibly cAMP response element-binding protein, are important for lordosis in rats (54, 55). Some of these coactivators, including cAMP response element-binding protein/p300 and SRC1, are chromatin-modifying enzymes with endogenous histone acetyltransferase (HAT) activity (17, 56). Histone acetylation at the promoter by HAT has been implicated in ERα and PR activation of estradiol- (18, 19) and P (57, 58)-responsive genes, respectively. It is possible that blocking the dynamic removal of acetyl groups from histones with SB treatment could increase the expression of estradiol- and P-responsive genes important for experience acquired receptivity by increasing transcription or delaying silencing by corepressors (59, 60) (Fig. 6).
Fig. 6.
Model for potential mechanism of estradiol and P signaling and histone deacetylase inhibition on receptivity. In the top panel, nuclear steroid receptors (NR) are not bound to ligand and do not occupy the DNA (straight black lines) promoters of estrogen- and P-responsive genes. In this transcriptionally inactive state, the chromatin is hypoacetylated, the RNA polymerase-II (Pol-II) is not recruited to the transcriptional start site, and the receptivity-promoting gene mRNA is expressed at low levels. In the bottom panel, ligand-activated (E/P) nuclear receptors dimerize and bind to estrogen and P response elements (open boxes), recruit coactivators (CoAct) including HAT to the promoter, cause acetylation (Ac) of histone (cylinders) tails and/or NR (AcNR), and recruit the basal transcription machinery and Pol-II to the transcription start site (TSS) to increase the expression of receptivity-promoting genes. Loss of ligand binding returns the genes to an inactive state in part by HDAC corepressor activity. HDAC inhibitors like SB impede the dynamic removal of acetyl groups leading to hyperacetylation that allows for increased and/or prolonged transcription of genes in the active state.
Unlike WT females, ERαKO mice do not acquire receptivity with sex experience, and SB treatment was not able to rescue the lordosis deficit in the knockouts (experiment 2), proving that the actions of this HDAC inhibitor do not circumvent the need for a functional ERα. ERα can be activated either ligand dependently or ligand independently through growth factor-mediated posttranslation modification of the activating factor-1 domain (61). In addition, the HAT coactivator p300 acetylates lysines within a motif of the ERα hinge domain that increases ligand-dependent, but not ligand-independent, transcriptional transactivation of a reporter gene in cell culture (22). Importantly, the same ERα lysine residues of the hinge region were also essential for induction of transcription in this system by the HDAC inhibitors SB and trichostatin A. Thus, acetylation of these ERα residues turns off an attenuation mechanism of ligand-dependent activation, perhaps by blocking the receptors association with HDAC corepressors (22). PR can also be acetylated in the hinge region on agonist stimulation with consequences on PR phosphorylation, nuclear localization, and transactivation of gene transcription (20). In addition, the orphan receptor estrogen-related receptor-α interacts with HAT and HDAC to modify transcription, and the deacetylated state of estrogen-related receptor-α DNA-binding domain directly correlates with an increased ability to bind DNA and activate transcription (62). Therefore, SB treatment may cause an increase in posttranslational acetylation of ERα, PR, or similar receptors in the brain to modulate their activities in a ligand-dependent or ligand-independent manner (21).
Epidermal growth factor and IGF-I can both induce lordosis in OVX rats. It has been reported that ovarian hormone replacement and the PR are not needed for extracellular growth factor induced lordosis; instead, these factors require ligand-independent activation of ERα by membrane-bound receptor pathways (48, 63). Furthermore, dopamine has been indicated to increase lordosis through ligand-independent phosphorylation and activation of PR (51). In experiments 3 and 4, ovarian hormone replacement was necessary for lordosis, regardless of SB treatment. SB was neither effective at inducing experience acquired lordosis in sexually naive females without estradiol and progesterone nor was it effective at maintaining receptivity in sexually experienced females in the absence of hormone replacement. These results suggest that SB treatment does not activate ERα or PR to enhance receptivity and lordosis acquisition and that the receptors must be activated by other means, in this case by their cognate ligands. This is consistent with SB affecting the described ligand-dependent acetylation of the ERα and PR hinge regions (20, 22), rather than posttranslation acetylation alone activating the receptor(s).
Our findings provide support for SB affecting lordosis by blocking the dynamic removal of either posttranslational acetylation of ERα and PR or histone acetylation laid down by the activated receptors at estradiol- and P-responsive genes (Fig. 6); however, there are other possible interpretations. Hyperacetylation of histones at the promoter of the Esr1 or Pgr genes themselves may influence expression of the receptors (64, 65). SB could increase the expression of non-estradiol- and P-responsive genes that interact with the receptors or their downstream transactivation gene products to promote lordosis (23, 66). Indeed, SB treatment causes hyperacetylation of total brain histones globally throughout the genome and not just at estrogen- and P-responsive genes. In addition, the absence of ERα in the knockouts throughout their whole life could have altered brain development that is obviously not reversible by SB treatment in adulthood (67). Furthermore, inhibiting HDAC (that have general lysine deacetylase activity) by SB could also cause increased acetylation of proteins other than histones and nuclear hormone receptors, such as nuclear receptor coactivators and corepressors, thus causing acetylation-mediated modification of their activities (52, 68). Clearly there are many potential targets to be investigated by which SB influences lordosis, including acetylated transcription factors and coregulators and acetylated histones associated with downstream gene targets. Future experiments will address these molecular mechanisms and how they integrate endocrine-mediated acquisition of sexual behaviors, which are facilitated by experience.
Acknowledgments
We thank Dr. Pierre Chambon for providing us with ERαKO mice to start our colony. We acknowledge Savera Shetty, Michelle Edwards, and Aileen Wills for their technical assistance.
This work was supported by National Institutes of Health Grant R01 MH057759 and the Biotechnology Training Program at the University of Virginia Grant T32 GM08715.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- AcH3
- Histone H3 acetylation
- EB
- estradiol benzoate
- EPM
- elevated plus maze
- ERα
- estrogen receptor-α
- ERαKO
- ERα gene knockout
- HAT
- histone acetyltransferase
- HDAC
- histone deacetylase
- LQ
- lordosis quotient
- OVX
- ovariectomy
- P
- progesterone
- PR
- P receptor
- SB
- sodium butyrate
- SRC
- steroid receptor coactivator
- TBST
- Tris-buffered saline (Tris-HCl; NaCl) with Tween 20
- V
- vehicle
- WT
- wild type.
References
- 1. Boling JL, Blandau RJ. 1939. The estrogen-progesterone induction of mating responses in the spayed female rat. Endocrinology 25:359–364 [Google Scholar]
- 2. McGill TE. 1962. Sexual behavior in three inbred strains of mice. Behaviour 19:341–350 [Google Scholar]
- 3. Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. 2010. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol 31:341–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards DA. 1970. Induction of estrus in female mice: estrogen-progesterone interactions. Horm Behav 1:299–304 [Google Scholar]
- 5. Whalen RE, Hardy DF. 1970. Induction of receptivity in female rats and cats with estrogen and testosterone. Physiol Behav 5:529–533 [DOI] [PubMed] [Google Scholar]
- 6. Hardy DF, DeBold JF. 1971. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav 2:287–297 [Google Scholar]
- 7. Davidson JM, Rodgers CH, Smith ER, Bloch GJ. 1968. Stimulation of female sex behavior in adrenalectomized rats with estrogen alone. Endocrinology 82:193–195 [DOI] [PubMed] [Google Scholar]
- 8. Edwards DA, Whalen RE, Nadler RD. 1968. Induction of estrus: estrogen-progesterone interactions. Physiol Behav 3:29–33 [Google Scholar]
- 9. Hardy DF, DeBold JF. 1973. Effects of repeated testing on sexual behavior of the female rat. J Comp Physiol Psychol 85:195–202 [DOI] [PubMed] [Google Scholar]
- 10. Gerall AA. 1967. Effects of early postnatal androgen and estrogen injections on the estrous activity cycles and mating behavior of rats. Anat Rec 157:97–104 [DOI] [PubMed] [Google Scholar]
- 11. Thompson M, Edwards DA. 1971. Experiential and strain determinants of the estrongen-progesterone induction of sexual receptivity in spayed female mice. Horm Behav 2:299–305 [Google Scholar]
- 12. Kudwa AE, Rissman EF. 2003. Double oestrogen receptor α and β knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J Neuroendocrinol 15:978–983 [DOI] [PubMed] [Google Scholar]
- 13. Kudwa AE, Boon WC, Simpson ER, Handa RJ, Rissman EF. 2007. Dietary phytoestrogens dampen female sexual behavior in mice with a disrupted aromatase enzyme gene. Behav Neurosci 121:356–361 [DOI] [PubMed] [Google Scholar]
- 14. Bakker J, Honda S, Harada N, Balthazart J. 2002. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci 22:9104–9112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manning ET, Ikehara T, Ito T, Kadonaga JT, Kraus WL. 2001. p300 forms a stable, template-committed complex with chromatin: role for the bromodomain. Mol Cell Biol 21:3876–3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 17. Dilworth FJ, Chambon P. 2001. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene 20:3047–3054 [DOI] [PubMed] [Google Scholar]
- 18. Kim MY, Hsiao SJ, Kraus WL. 2001. A role for coactivators and histone acetylation in estrogen receptor α-mediated transcription initiation. EMBO J 20:6084–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daniel AR, Gaviglio AL, Czaplicki LM, Hillard CJ, Housa D, Lange CA. 2010. The progesterone receptor hinge region regulates the kinetics of transcriptional responses through acetylation, phosphorylation, and nuclear retention. Mol Endocrinol 24:2126–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faus H, Haendler B. 2006. Post-translational modifications of steroid receptors. Biomed Pharmacother 60:520–528 [DOI] [PubMed] [Google Scholar]
- 22. Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SA, Lopez GN, Kushner PJ, Pestell RG. 2001. Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem 276:18375–18383 [DOI] [PubMed] [Google Scholar]
- 23. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. 2003. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370:737–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsankova N, Renthal W, Kumar A, Nestler EJ. 2007. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 8:355–367 [DOI] [PubMed] [Google Scholar]
- 25. Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, Fass DM, Renthal W, Rush AJ, 3rd, Wu EY, Ghose S, Krishnan V, Russo SJ, Tamminga C, Haggarty SJ, Nestler EJ. 2009. Antidepressant actions of histone deacetylase inhibitors. J Neurosci 29:11451–11460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abel T, Zukin RS. 2008. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol 8:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. 2009. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature 459:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schroeder FA, Lin CL, Crusio WE, Akbarian S. 2007. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62:55–64 [DOI] [PubMed] [Google Scholar]
- 29. Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. 2006. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9:519–525 [DOI] [PubMed] [Google Scholar]
- 30. Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. 2007. Recovery of learning and memory is associated with chromatin remodelling. Nature 447:178–182 [DOI] [PubMed] [Google Scholar]
- 31. Davie JR. 2003. Inhibition of histone deacetylase activity by butyrate. J Nutr 133:2485S–2493S [DOI] [PubMed] [Google Scholar]
- 32. Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. 2009. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA 106:9447–9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. 1998. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci USA 95:13941–13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. 2004. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA 101:2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takuma K, Matsuo A, Himeno Y, Hoshina Y, Ohno Y, Funatsu Y, Arai S, Kamei H, Mizoguchi H, Nagai T, Koike K, Inoue M, Yamada K. 2007. 17β-estradiol attenuates hippocampal neuronal loss and cognitive dysfunction induced by chronic restraint stress in ovariectomized rats. Neuroscience 146:60–68 [DOI] [PubMed] [Google Scholar]
- 36. Walf AA, Frye CA. 2005. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology 30:1288–1301 [DOI] [PubMed] [Google Scholar]
- 37. Zhu H, Huang Q, Xu H, Niu L, Zhou JN. 2009. Antidepressant-like effects of sodium butyrate in combination with estrogen in rat forced swimming test: involvement of 5-HT(1A) receptors. Behav Brain Res 196:200–206 [DOI] [PubMed] [Google Scholar]
- 38. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- 39. Minamiyama M, Katsuno M, Adachi H, Waza M, Sang C, Kobayashi Y, Tanaka F, Doyu M, Inukai A, Sobue G. 2004. Sodium butyrate ameliorates phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet 13:1183–1192 [DOI] [PubMed] [Google Scholar]
- 40. Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. 2001. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA 98:9808–9813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JH, Bonthuis P, Ding A, Rais S, Rissman EF. 2009. Androgen- and estrogen-independent regulation of copulatory behavior following castration in male B6D2F1 mice. Horm Behav 56:254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsai HW, Grant PA, Rissman EF. 2009. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. 1997. Estrogen receptors are essential for female sexual receptivity. Endocrinology 138:507–510 [DOI] [PubMed] [Google Scholar]
- 44. Murray EK, Hien A, de Vries GJ, Forger NG. 2009. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150:4241–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Holmes A, Rodgers RJ. 1998. Responses of Swiss-Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav 60:473–488 [DOI] [PubMed] [Google Scholar]
- 46. Zhao Z, Fan L, Frick KM. 2010. Epigenetic alterations regulate estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA 107:5605–5610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mazzucco CA, Walker HA, Pawluski JL, Lieblich SE, Galea LA. 2008. ERα, but not ERβ, mediates the expression of sexual behavior in the female rat. Behav Brain Res 191:111–117 [DOI] [PubMed] [Google Scholar]
- 48. Apostolakis EM, Garai J, Lohmann JE, Clark JH, O'Malley BW. 2000. Epidermal growth factor activates reproductive behavior independent of ovarian steroids in female rodents. Mol Endocrinol 14:1086–1098 [DOI] [PubMed] [Google Scholar]
- 49. Walf AA, Ciriza I, Garcia-Segura LM, Frye CA. 2008. Antisense oligodeoxynucleotides for estrogen receptor-β and α attenuate estradiol's modulation of affective and sexual behavior, respectively. Neuropsychopharmacology 33:431–440 [DOI] [PubMed] [Google Scholar]
- 50. Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH. 1994. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology 135:1409–1414 [DOI] [PubMed] [Google Scholar]
- 51. Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely O, O'Malley BW. 1996. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol 10:1728–1737 [DOI] [PubMed] [Google Scholar]
- 52. Leader JE, Wang C, Fu M, Pestell RG. 2006. Epigenetic regulation of nuclear steroid receptors. Biochem Pharmacol 72:1589–1596 [DOI] [PubMed] [Google Scholar]
- 53. Kraus WL, Kadonaga JT. 1998. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev 12:331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Apostolakis EM, Ramamurphy M, Zhou D, Oñate S, O'Malley BW. 2002. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endocrinol 16:1511–1523 [DOI] [PubMed] [Google Scholar]
- 55. Molenda-Figueira HA, Williams CA, Griffin AL, Rutledge EM, Blaustein JD, Tetel MJ. 2006. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav 50:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kraus WL, Manning ET, Kadonaga JT. 1999. Biochemical analysis of distinct activation functions in p300 that enhance transcription initiation with chromatin templates. Mol Cell Biol 19:8123–8135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 1999. Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. Proc Natl Acad Sci USA 96:9485–9490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aoyagi S, Archer TK. 2007. Dynamic histone acetylation/deacetylation with progesterone receptor-mediated transcription. Mol Endocrinol 21:843–856 [DOI] [PubMed] [Google Scholar]
- 59. Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M, Ngo S, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW. 1998. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci USA 95:2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu L, Glass CK, Rosenfeld MG. 1999. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev 9:140–147 [DOI] [PubMed] [Google Scholar]
- 61. Hall JM, Couse JF, Korach KS. 2001. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- 62. Wilson BJ, Tremblay AM, Deblois G, Sylvain-Drolet G, Giguère V. 2010. An acetylation switch modulates the transcriptional activity of estrogen-related receptor α. Mol Endocrinol 24:1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Etgen AM, González-Flores O, Todd BJ. 2006. The role of insulin-like growth factor-I and growth factor-associated signal transduction pathways in estradiol and progesterone facilitation of female reproductive behaviors. Front Neuroendocrinol 27:363–375 [DOI] [PubMed] [Google Scholar]
- 64. Yang X, Phillips DL, Ferguson AT, Nelson WG, Herman JG, Davidson NE. 2001. Synergistic activation of functional estrogen receptor (ER)-α by DNA methyltransferase and histone deacetylase inhibition in human ER-α-negative breast cancer cells. Cancer Res 61:7025–7029 [PubMed] [Google Scholar]
- 65. Duong V, Licznar A, Margueron R, Boulle N, Busson M, Lacroix M, Katzenellenbogen BS, Cavaillès V, Lazennec G. 2006. ERα and ERβ expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene 25:1799–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. 2003. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington's disease mice. J Neurosci 23:9418–9427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. 1997. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav 31:232–243 [DOI] [PubMed] [Google Scholar]
- 68. Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. 1999. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell 98:675–686 [DOI] [PubMed] [Google Scholar]