Abstract
A panel of monoclonal antibodies with specificity for a wound isolate of Proteus mirabilis was established. Of nine antibodies studied in detail, three were broadly reactive with various Proteus isolates, while six reacted in a serotype-specific fashion with the strain used for immunization. Five of the six serotype-specific antibodies were reactive with lipopolysaccharide. The sixth serotype-specific antibody, 4-F (immunoglobulin G1 [IgG1]), was potently protective in a burn wound sepsis model and recognized a protein antigen. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot (immunoblot) analysis were used to determine that 4-F was reactive with flagellar protein. Approximately 1.3 micrograms of the antibody was sufficient to provide protection against 8 50% lethal doses of wound isolate, and approximately 26 micrograms provided full protection against challenge with 333 50% lethal doses. In vitro test results indicated that 4-F inhibited the motility of the wound isolate, and in vivo testing showed that it inhibited dissemination of the inoculum from the burn site to the liver and spleen. Whereas the antibody was highly effective in preventing the death of mice subsequent to challenge at a burn site, no protection was seen following an intraperitoneal challenge. These results may therefore indicate that the protection observed in the burn model is solely a reflection of the capacity of 4-F to neutralize bacterial motility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Babu J. P., Giampapa C. S., Hasty D. L., Simpson W. A., Beachey E. H. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985 Jun;48(3):625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. R., Montie T. C. Opsonophagocytosis of Pseudomonas aeruginosa treated with antiflagellar serum. Infect Immun. 1987 Dec;55(12):3204–3206. doi: 10.1128/iai.55.12.3204-3206.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong K. T., Huston M. Implications of endotoxin contamination in the evaluation of antibodies to lipopolysaccharides in a murine model of gram-negative sepsis. J Infect Dis. 1987 Nov;156(5):713–719. doi: 10.1093/infdis/156.5.713. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Anti-Pseudomonas aeruginosa activity of an intravenous human IgG preparation in burned mice. J Trauma. 1983 Jun;23(6):530–534. doi: 10.1097/00005373-198306000-00015. [DOI] [PubMed] [Google Scholar]
- Coughlin R. T., Bogard W. C., Jr Immunoprotective murine monoclonal antibodies specific for the outer-core polysaccharide and for the O-antigen of Escherichia coli 0111:B4 lipopolysaccharide (LPS). J Immunol. 1987 Jul 15;139(2):557–561. [PubMed] [Google Scholar]
- Cross A. S., Zollinger W., Mandrell R., Gemski P., Sadoff J. Evaluation of immunotherapeutic approaches for the potential treatment of infections caused by K1-positive Escherichia coli. J Infect Dis. 1983 Jan;147(1):68–76. doi: 10.1093/infdis/147.1.68. [DOI] [PubMed] [Google Scholar]
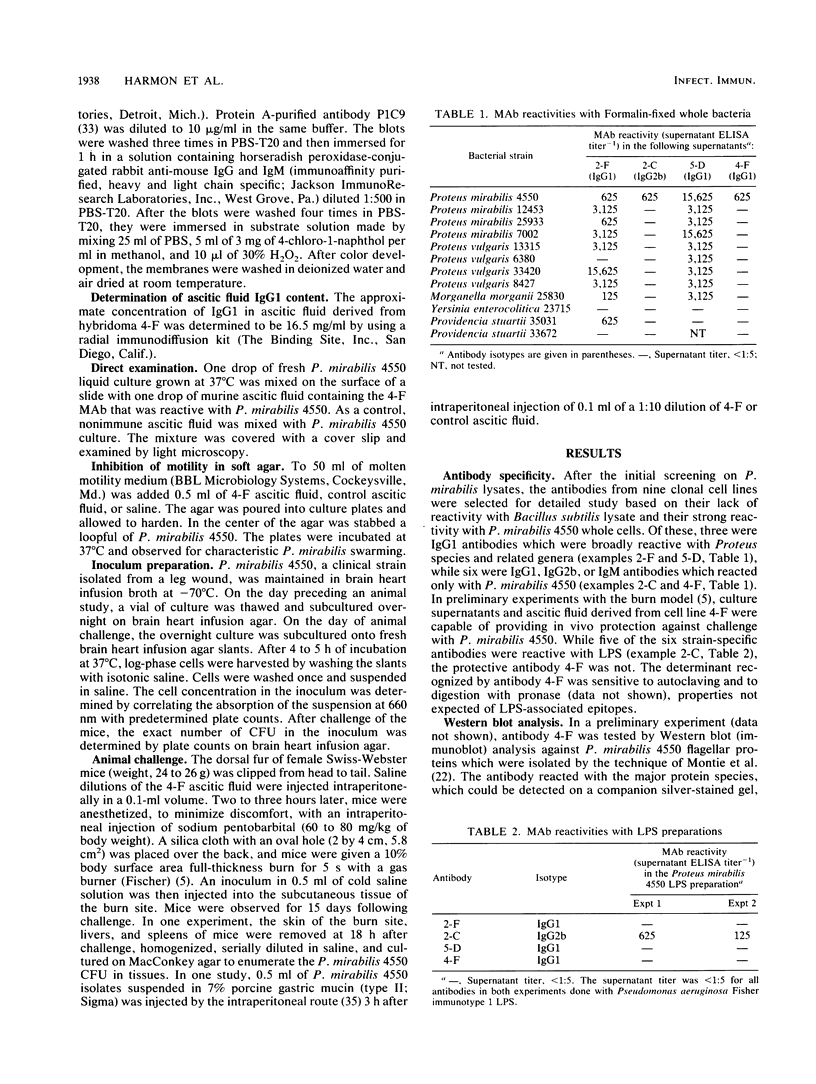
- Driver K., Lambert P. A. Surface antigens of Proteus mirabilis revealed by electroblotting from sodium dodecyl sulphate-polyacrylamide gels. Microbios. 1984;41(160):87–98. [PubMed] [Google Scholar]
- Duncan A. R., Woof J. M., Partridge L. J., Burton D. R., Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988 Apr 7;332(6164):563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Bogard W. C., Jr, Cerra F. B. Efficacy of type-specific and cross-reactive murine monoclonal antibodies directed against endotoxin during experimental sepsis. Surgery. 1985 Aug;98(2):283–290. [PubMed] [Google Scholar]
- Ey P. L., Russell-Jones G. J., Jenkin C. R. Isotypes of mouse IgG--I. Evidence for 'non-complement-fixing' IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol Immunol. 1980 Jun;17(6):699–710. doi: 10.1016/0161-5890(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Insel R. A. Protection from infection with Haemophilus influenzae type b by monoclonal antibody to the capsule. J Infect Dis. 1982 Aug;146(2):249–254. doi: 10.1093/infdis/146.2.249. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Mouat E. C. Immunotherapeutic potential of monoclonal antibodies against Pseudomonas aeruginosa protein F. Eur J Clin Microbiol. 1985 Apr;4(2):224–227. doi: 10.1007/BF02013602. [DOI] [PubMed] [Google Scholar]
- Harmon R. C., Stein N., Frelinger J. A. Monoclonal antibodies reactive with H-2 determinants. Immunogenetics. 1983;18(5):541–545. doi: 10.1007/BF00364395. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Naglich J. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: immunization using divalent flagella preparations. J Trauma. 1986 Feb;26(2):118–122. doi: 10.1097/00005373-198602000-00003. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- McManus A. T., McLeod C. G., Jr, Mason A. D., Jr Experimental Proteus mirabilis burn surface infection. Arch Surg. 1982 Feb;117(2):187–191. doi: 10.1001/archsurg.1982.01380260057010. [DOI] [PubMed] [Google Scholar]
- Montie T. C., Craven R. C., Holder I. A. Flagellar preparations from Pseudomonas aeruginosa: isolation and characterization. Infect Immun. 1982 Jan;35(1):281–288. doi: 10.1128/iai.35.1.281-288.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie T. C., Doyle-Huntzinger D., Craven R. C., Holder I. A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982 Dec;38(3):1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazin G. J., Braude A. I. Immobilizing antibodies in urine. II. Prevention of ascending spread of Proteus mirabilis. Invest Urol. 1974 Sep;12(2):129–133. [PubMed] [Google Scholar]
- Pennington J. E., Small G. J., Lostrom M. E., Pier G. B. Polyclonal and monoclonal antibody therapy for experimental Pseudomonas aeruginosa pneumonia. Infect Immun. 1986 Oct;54(1):239–244. doi: 10.1128/iai.54.1.239-244.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S., Kawamura T., Masuho Y., Tomibe K. Characterization of a human monoclonal antibody to lipopolysaccharides of Pseudomonas aeruginosa serotype 5: a possible candidate as an immunotherapeutic agent for infections with P. aeruginosa. J Infect Dis. 1985 Nov;152(5):965–970. doi: 10.1093/infdis/152.5.965. [DOI] [PubMed] [Google Scholar]
- Sawada S., Suzuki M., Kawamura T., Fujinaga S., Masuho Y., Tomibe K. Protection against infection with Pseudomonas aeruginosa by passive transfer of monoclonal antibodies to lipopolysaccharides and outer membrane proteins. J Infect Dis. 1984 Oct;150(4):570–576. doi: 10.1093/infdis/150.4.570. [DOI] [PubMed] [Google Scholar]
- Sherman D. M., Acres S. D., Sadowski P. L., Springer J. A., Bray B., Raybould T. J., Muscoplat C. C. Protection of calves against fatal enteric colibacillosis by orally administered Escherichia coli K99-specific monoclonal antibody. Infect Immun. 1983 Nov;42(2):653–658. doi: 10.1128/iai.42.2.653-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Sidberry H., Kaufman B., Wright D. C., Sadoff J. Immunoenzymatic analysis by monoclonal antibodies of bacterial lipopolysaccharides after transfer to nitrocellulose. J Immunol Methods. 1985 Feb 11;76(2):299–305. doi: 10.1016/0022-1759(85)90307-2. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B. J., Pollack M., Young L. S., Koles N., Gascon R., Pier G. B. Functionally active monoclonal antibody that recognizes an epitope on the O side chain of Pseudomonas aeruginosa immunotype-1 lipopolysaccharide. Infect Immun. 1986 Sep;53(3):656–662. doi: 10.1128/iai.53.3.656-662.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Hirayama N., Tamura Y. Production, characterization, and protective effect of monoclonal antibodies to Clostridium chauvoei flagella. Infect Immun. 1987 Aug;55(8):1779–1783. doi: 10.1128/iai.55.8.1779-1783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. P., Black J. R., Barritt D. S., Connell T. D., Cannon J. G. Resistance to meningococcemia apparently conferred by anti-H.8 monoclonal antibody is due to contaminating endotoxin and not to specific immunoprotection. Infect Immun. 1987 Aug;55(8):1927–1928. doi: 10.1128/iai.55.8.1927-1928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]