Abstract
Many people believe that sexual orientation (homosexuality vs. heterosexuality) is determined by education and social constraints. There are, however, a large number of studies indicating that prenatal factors have an important influence on this critical feature of human sexuality. Sexual orientation is a sexually differentiated trait (over 90% of men are attracted to women and vice versa). In animals and men, many sexually differentiated characteristics are organized during early life by sex steroids, and one can wonder whether the same mechanism also affects human sexual orientation. Two types of evidence support this notion. First, multiple sexually differentiated behavioral, physiological, or even morphological traits are significantly different in homosexual and heterosexual populations. Because some of these traits are known to be organized by prenatal steroids, including testosterone, these differences suggest that homosexual subjects were, on average, exposed to atypical endocrine conditions during development. Second, clinical conditions associated with significant endocrine changes during embryonic life often result in an increased incidence of homosexuality. It seems therefore that the prenatal endocrine environment has a significant influence on human sexual orientation but a large fraction of the variance in this behavioral characteristic remains unexplained to date. Genetic differences affecting behavior either in a direct manner or by changing embryonic hormone secretion or action may also be involved. How these biological prenatal factors interact with postnatal social factors to determine life-long sexual orientation remains to be determined.
Most people are sexually attracted to individuals of the opposite sex; they are heterosexual. There is, however, a significant minority (3–10% according to many estimates) of men and women who are exclusively attracted to individuals of their own sex; they are homosexual. Intermediate forms of attraction also exist, and as early as in 1948, Kinsey et al. (1) were classifying sexual orientation in seven distinct categories ranging from completely heterosexual to completely homosexual. Sexual orientation (heterosexual vs. homosexual) is a behavioral trait that displays one of the largest degrees of sexual differentiation, given that 90–97% of individuals of one sex display an attraction that is different from that of the other sex.
The mechanisms that determine human sexual orientation have been the subject of heated controversies. These discussions often focused on homosexuality proper, because this orientation is less common and thus sometimes considered wrongly as “abnormal.” It must be noted, however, that trying to understand the origins of homosexuality or heterosexuality essentially represents the same question.
Under the influence of a variety of theories ranging from Freudian psychoanalysis to social constructivism, sexual orientation has been, and often still is, considered as being the result of social experiences during early childhood, in particular improper interaction with one's parents (dominant or possessive mother, distant or absent father). The newborn baby would in this context be sexually neutral; his/her sexual orientation would develop on a tabula rasa and could go either way as a function of postnatal social interactions only. This interpretation ignores, however, a vast corpus of data concerning both animals and humans demonstrating that the prenatal endocrine environment has profound and irreversible effects on a variety of morphological, physiological, and behavioral features of an individual.
Because male and female embryos are exposed to a different hormonal milieu during specific phases of their intrauterine life, male and female newborns are substantially different on the day of birth. I summarize here the main principles that govern sexual differentiation identified by experimental studies in animals. Then I discuss to what extent these principles could also determine sexual differentiation of brain and behavior in humans and finally review evidence derived from epidemiological and clinical studies, suggesting that sexual orientation is a sexually differentiated behavioral feature that is most likely influenced, like other sexually differentiated features, by the prenatal hormonal environment. I do not mean to say that the postnatal social environment has no influence on sexual orientation, but based on currently available data, these social influences seem to play only a minor role, possibly via interaction with prenatal endocrine effects.
Sexual Differentiation of Sex and Courtship Behaviors in Animals
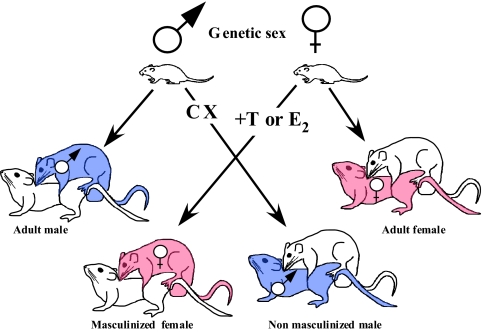
Many behaviors in animals are sexually differentiated and produced preferentially or exclusively by one sex. Estrogens are often unable to activate female-typical behaviors (e.g. receptivity) in males, and vice versa, testosterone does not reliably activate male-typical copulatory behavior in females even after its conversion to estradiol (2). It was originally believed that these sex differences resulted from the presence of different hormones in the two sexes: testosterone in males and estradiol (plus progesterone) in females (3). The seminal work of Young and co-workers (4) demonstrated that, to a large extent, these differences result from the early exposure of embryos to a different endocrine milieu: a high concentration of testosterone for male embryos in mammals and a much lower (lack of?) exposure to sex steroids in females (Fig. 1). These differentiating (organizing) effects usually occur early in life, during the embryonic period or just after birth and are irreversible.
Fig. 1.
In mammals, early exposure to testosterone produces a male phenotype: the behavioral characteristics of the male are strengthened (masculinization) and the ability of males to show behavior typical of females is reduced or lost (defeminization). The female phenotype develops in the apparent absence of hormone action (or in the presence of very low estrogen concentrations). These spontaneous differentiation processes occurring during early development of animals can be entirely reproduced by experimental manipulations (via castration, injections of agonists or antagonists) of steroid concentrations in embryonic or newborn animals. The figure shows that after such treatments, neonatally castrated (CX) males behave like females, whereas females treated early in life with testosterone (T) or its aromatized metabolite estradiol (E2) behave like males. Experimental animals considered are those shaded in blue (genetic males) or red (genetic females). Other subjects represent only the test stimuli.
It is not the type of adult hormone (androgens or estrogens) that determines the behavior that will be expressed (male or female typical), it is the nature of the neural substrate on which this hormone acts (the sex of the animal and associated embryonic exposure to sex steroids). Recent studies also show that genetic mechanisms called “direct,” because they are not mediated through the action of sex hormones, influence some behavioral differences between males and females (5).
These organizing actions of sex steroids on behavior are paralleled by irreversible changes in brain structure. Embryonic sex steroids differentiate the size of several brain structures (see Ref. 6 for a general review on this topic), including the sexually dimorphic nucleus of the preoptic area (SDN-POA). This group of cells is five to six times larger in male rats than in females, and this difference results almost exclusively from the action of testosterone during late embryonic life and the first days of postnatal life. Once acquired, the sex-typical size of the SDN-POA cannot be altered in adulthood by steroid hormones (7, 8).
Partner Preference and Its Control in Animals
These organizing effects of embryonic sex steroids specifically concern the type of behaviors that will be displayed by adults. More recent studies demonstrate that similar principles also contribute to determine the sex of the partner that will be the target of these behaviors. Unlike other aspects of human sexuality that have no equivalent in animals (e.g. gender identity), sexual orientation can be studied in nonhuman animals by offering them a choice between a male or female sexual partner. Recording the time spent with each partner and the type of behavior displayed toward them will provide a measure of a behavioral phenotype (sexual partner preference) that represents a reasonable (although imperfect) model of human sexual orientation.
The sexual preference of a male for a female is controlled, like the expression of male-typical sexual behavior, by the medial part of the POA. Experimental lesion of this brain region causes a reversal of the males' preference in rats and ferrets: after surgery, they prefer to spend time with other males rather than with sexually receptive females (9, 10).
This preference for a partner of the same or the opposite sex is also determined by prenatal hormones and can be reversed by hormonal treatments during early development (weeks preceding or immediately after birth depending on the species) (11–15). The preference determined at that time subsequently appears to be a stable characteristic of the individual. Like the type (male or female typical) of sexual behavior displayed in adulthood, sexual partner preference seems to be determined by sex steroids during embryonic or early postnatal life. Exposure to testosterone (or its metabolite estradiol) induces male-typical partner preference (preference for a female over a male sex partner), whereas in the absence of high concentrations of these steroids, a female pattern of mate preference will develop (preference for male partner).
Sexual interactions between same sex partners (male mounting another male or female mounting another female) are observed quite frequently in a broad variety of animal species (16, 17). Often, these behaviors are expressed only when a suitable partner of the opposite sex is not available due to captivity (zoo or other captive populations), or when a skewed sex ratio in the population, or the presence of dominant males is preventing access to females. These behaviors do not represent a true same sex preference but serve as an outlet for sexual motivation in the absence of suitable partners of the opposite sex.
A case of spontaneous same sex preference has, however, been described and studied in detail. It concerns a sheep population in the western part of the United States. A significant fraction of rams in this population (8%) mate exclusively with other males when given a choice between a male or female partner. Various factors that could explain this male-directed sexual behavior, such as rearing in single sex groups, which is common in sheep, were ruled out (see Ref. 18 for review), and studies then focused on endocrine and neural aspects of this preference for males.
The SDN of the ovine POA (oSDN), a structure that is approximately three times larger in males than in females and contains about four times more neurons, was shown to be significantly smaller in male-oriented rams (MoR) than in female-oriented rams (FoR). The oSDN also contained fewer neurons and expressed aromatase at reduced levels in MoR compared with FoR (19). The features of this nucleus therefore correlate with sexual partner preference: subjects attracted to males (females and MoR) are similar and distinguished from subjects attracted to females (FoR).
Roselli et al. (20) demonstrated that the volume of the oSDN is already larger in males than in females around the end of embryonic life (around d 135) and differentiates under the influence of testosterone in males. Embryonic treatment of females with testosterone between 30 and 90 d of gestation results in a masculinized oSDN in females (18, 20). Furthermore, the size of this nucleus is no longer modified in adulthood by castration or treatment with testosterone (21). If the small size of oSDN in MoR is determined like in females by a relative lack of early exposure to testosterone, it might represent (one of) the cause(s), and not a consequence, of their atypical sexual attraction. This nucleus is indeed located in the center of the POA, a region involved in the control of sexual behavior and male-typical partner preferences, and it would in this scenario differentiate before subjects had an opportunity to express their sexual partner preference. This unfortunately remains impossible to prove with the current technology, because it is not possible to measure the volume of the oSDN in a male embryo and to know at the same time what would have been his later partner preference.
In conclusion, these studies demonstrate that sexual orientation in animals is a sexually differentiated feature like other sexually differentiated behaviors or morphological characteristics. Male-typical sexual orientation is controlled at least in part by the POA (like sexual behavior), and it differentiates under the influence of pre-/perinatal sex steroids.
Many Sex Differences in Humans Are Organized by Embryonic Sex Steroids
Do these endocrine mechanisms demonstrated in animals have any significance in humans? The answer to this question should be considered in two steps. 1) Do we have any evidence that sex steroids are, in humans like in animals, implicated in the sexual differentiation of morphology (e.g. genital structures) but also of brain (e.g. SDN-POA) and sexual behavior? And 2) are there any data indicating that embryonic sex steroids have, like in animals, organizational effects on sexual orientation in humans? The answer to the first of these questions is clearly yes, and there is probably no need to elaborate on the arguments supporting this conclusion especially in an endocrine journal. To just briefly restate the obvious:
Sex steroids (testosterone, estradiol, progesterone) are present in the human plasma in concentrations similar to those observed in other mammals.
Receptors for these steroids are present in humans, and their brain distribution is similar and even nearly identical to the general pattern observed in vertebrates.
Testosterone action during embryonic life clearly controls the differentiation of male-typical external and internal genital structures.
Sex differences in brain structures have been identified, although their control by embryonic steroids is usually not established at this time.
Physiological or behavioral differences between men and women are too numerous to be summarized here (22). These differences are complex in nature, and their origin is more difficult to determine than for differences in genital morphology. Learning, education, and expectations of society clearly play an important role in the genesis of behavioral and even sometimes physiological differences. Nevertheless, quite often, these environmental factors build on and amplify smaller, sometimes minor, differences caused by biological factors that were already present at birth. Many physiological and behavioral differences are thus rooted in biology. This is quite obviously the case for many sexually differentiated diseases related to brain function (e.g. anorexia nervosa affects 93 women for every seven men; Gilles de la Tourette syndrome affects 90 men for every 10 women) (see Refs. 22–24 for an extensive list of such differences). How would education or society induce such differences? But many behavioral differences also probably depend to some extent on biological mechanisms often already acting during prenatal life (e.g. increased aggressivity and greater interest in male-typical activities in girls prenatally exposed to high androgen concentration due to congenital adrenal hyperplasia (CAH) (see Refs. 25, 26).
A Hormonal Theory of Homosexuality
The second question (do embryonic sex steroids affect sexual orientation in human?) is obviously more difficult to answer for a variety of reasons, such as the intrinsic difficulties in assessing in a reliable manner the sexual orientation of a subject, the long latency between embryonic endocrine events potentially controlling sexual orientation and its overt manifestation in adulthood, and finally, the complete impossibility for ethical reasons of manipulating the process.
Many studies have analyzed the potential influence of steroids on human sexual orientation. They have clearly established that sexual orientation is not affected by activational effects of steroids in adulthood. Gonadectomy does not influence orientation nor does adult treatment with androgens and estrogens. Furthermore, numerous studies have clearly established that plasma concentrations of sex steroids are perfectly “normal” (typical of the gonadal sex) in both gay men and lesbians (27).
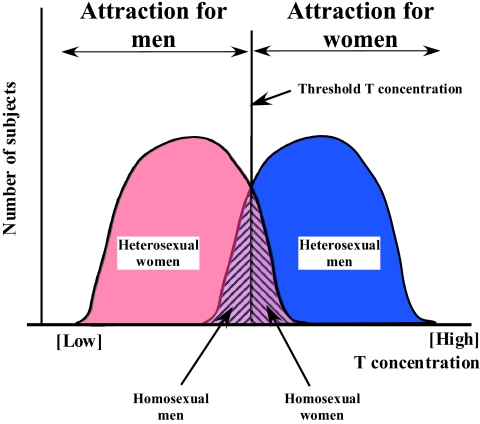
Organizational effects of steroids are by contrast more likely to be implicated. Sexual orientation is a sexually differentiated function that might depend, like many other behavioral characteristics, on variations in the early (fetal) exposure to sex steroids (androgens and also possibly their estrogenic metabolites). Exposure to a high concentration of testosterone during a critical phase of development would lead to a male-typical orientation (attraction to women), whereas a lower embryonic exposure to steroids would lead to a female-typical orientation (attraction to men). There would be a critical concentration of testosterone required to masculinize this feature like other aspects of behavior in animals and humans (see Fig. 2).
Fig. 2.
Theoretical model illustrating how fluctuations around an average concentration of testosterone (T) during embryonic life could lead to a homosexual or heterosexual orientation.
On average, male embryos are exposed to higher concentrations of testosterone than female embryos, but these concentrations vary around a mean value for various reasons (environmental, genetic, etc.). Male subjects at the lower end of this sex-specific distribution could thus acquire a female-typical orientation (and be gay), whereas females at the high end of the concentration curve would acquire a male-typical sexual attraction and be lesbian. Even if they are not attracted by the same specific individuals, females and gay men share an attraction for men, whereas males and lesbians share an attraction for women.
It has been argued that such a theory is impossible, because it would imply that homosexual men have feminized (or at least less masculine) genital structures whereas lesbians should have experienced some masculinization of genital structures similar to what is observed for example in CAH girls. Although detailed attention has been given to this possibility, no data supporting this idea have been collected, and if anything, penises of gay men are possibly larger than in heterosexual men, suggesting that, contrary to the theory in its simplest form, they would have been hypermasculinized (these data are, however, based on self measurements, and it has been argued that homosexuals may have been excited more than heterosexuals by the view/manipulation of their own penis, thus biasing these measurements).
Recall, however, that genital structures and the brain (supporting behavior) presumably differentiate at different times during embryonic life (see Ref. 28 for supporting evidence in rhesus monkeys). It is therefore possible that the hormonal imprinting that takes place at these two time periods is substantially different. A male embryo could for example be exposed to high (male typical) concentrations of testosterone during the first three months of gestation (and have perfectly masculine genitalia) but then experience a (temporary?) drop in testosterone concentration during the final maturation of the brain and thus fail to develop a male-typical sexual attraction.
Alternatively, the differentiation of sexual orientation could diverge from genital morphology, because steroid action in the brain may be affected while remaining normal in the genital skin. Remember that to produce its effects, testosterone must often be metabolized in its target structures (aromatized in the brain, 5α-reduced in the genital area), bind to specific receptors, and then activate complex intracellular signaling cascades, eventually leading to changes in protein synthesis and/or neural activity. Any aspect of this complex suite of actions could be deficient in the brain but not in the periphery, leading also to a discrepancy between somatic sex differentiation and sexual orientation.
Although this theory remains speculative (and is likely to remain unproven due to the logistic difficulties mentioned before), two types of evidence suggest that it contributes substantially to the control of sexual orientation in humans.
Atypical Sexually Differentiated Characteristics in Gays and Lesbians
Practical reasons make it nearly impossible to determine the hormonal milieu to which an individual was exposed during his/her embryonic life. We must therefore rely on indirect evidence. A number of sexually differentiated morphological, physiological, and behavioral characteristics seem to be irreversibly influenced by embryonic hormones in humans like in animals. Many studies have quantified these features comparatively in homosexual and heterosexual populations to research whether homosexual subjects had been exposed to atypical hormonal conditions during their development. Positive results were obtained in a number of these studies.
The sexually differentiated characteristics that have been studied in this context include variables that could be secondarily affected by homosexuality [e.g. performance in cognitive tests (see Ref. 29) or physiological responses to the smell of androgenic or estrogenic steroids produced by males or females (30, 31)]. These traits were shown to be significantly different in homosexual and heterosexual men and/or women, but we shall not review them here in detail, because they do not represent conclusive evidence for exposure to an atypical endocrine milieu during embryonic life (see Refs. 32–34 for detail).
In contrast, other morphological and physiological features are clearly influenced by prenatal testosterone, and it is difficult to conceive how they could possibly be affected secondarily by adult sexual orientation. We briefly discuss three of these traits that are significantly modified in gays or lesbians.
The ratio of the lengths of the second (index) to the fourth (ring) fingers (2D:4D)
The 2D:4D is significantly smaller in men than in women. This ratio was shown by two out of three studies to be masculinized in CAH women exposed to an excess of androgens in utero and also masculinized in females of a variety of mammalian and even avian species by injection of androgens during embryonic life. This ratio has therefore been used as a biomarker for embryonic exposure to testosterone in the human fetus (35), although a number of studies have questioned its reliability (e.g. Ref. 36). Multiple studies in humans have shown that this ratio is masculinized (smaller) in lesbians compared with heterosexual women. Although there have been occasional failures to replicate this effect, and its significance has been questioned (e.g. Is the effect size meaningful? Does it reflect differences in bone length or in fat accumulation) (37), it has been confirmed by several meta-analyses of available data (e.g. Refs. 35, 38), suggesting that lesbians have, on average, been exposed to higher than typical concentrations of androgens during development. Interestingly, most studies have failed to detect a corresponding feminization of this feature in gay men, and surprisingly, some studies have even reported a lower (hypermasculinized) 2D:4D ratio in some gay men (39). A modification of the length of long bones (arms and legs), a feature also supposed to be influenced by early exposure to sex steroids, has been reported in gay men (40).
Oto-acoustic emissions
One set of studies also investigated the physiology of the inner ear and more specifically the small noises produced in the cochlea (presumably) by movements of the tympanic membrane, the so-called oto-acoustic emissions (OAE). OAE are produced either spontaneously or in response to short noises in the environment (e.g. clicks). These OAE are more frequent in women than in men as well as in females compared with males in a variety of animal species. In animals, OAE are masculinized (decrease in frequency) after embryonic treatment of females with androgens. OAE were shown to be significantly less frequent in lesbians compared with heterosexual women, again suggesting that these lesbians were exposed to higher concentrations of androgens than usual during early life. Similar studies assessing other aspects of acoustic physiology (e.g. auditory evoked potentials) that are also sexually differentiated confirmed a masculinization of these traits in lesbians (41). Interestingly, feminization of these features was never observed in gay men, and some studies even reported hypermasculinization of these traits.
Brain structures
Several brain structures have also been shown to be different between homosexual and heterosexual subjects, and in this case, studies focused almost exclusively on males. The first of these differences concerns the suprachiasmatic nucleus, the central clock of the organism, that was shown to be significantly larger in gay men than in heterosexual subjects (42). However, this nucleus is not sexually differentiated in a control heterosexual population, and its links to reproduction are only indirect. The significance of this difference and potential relation with sexual orientation are thus difficult to assess.
The size of the anterior commissure, measured in the midsagital plane, was reported to be larger in gay men than in heterosexual control subjects (43). This difference is more interesting for the purpose of the present discussion, because the size of this commissure is known to be larger in women than in men. However the size of this commissure has no obvious relationship to sexual orientation (it could relate to functional lateralization), so the meaning of the difference between homosexual and heterosexual men remains difficult to interpret.
Finally, researchers from the laboratory of Roger Gorski (who had identified the SDN-POA of rats) at the University of California, Los Angeles (Los Angeles, CA) discovered in the human POA a nucleus they called interstitial nucleus of the anterior hypothalamus number 3 (INAH3) (a member of four cellular condensations of the POA) that was significantly larger in men than in women (44). Subsequent studies showed that INAH3 is significantly smaller in homosexual men than heterosexual men, so that its size is essentially equal to what is observed in women (45). An independent study based on different brains confirmed the reduced size of INAH3 in male homosexuals compared with heterosexuals, although the magnitude of the difference observed in this replication was lower than in the original study and not statistically significant (46). In this study, homosexual men also had a greater cell density (more cells per unit volume) but a similar total number of neurons in INAH3 than heterosexual men: neurons were more densely packed, potentially because they formed fewer synapses (during development?).
The mechanisms that control the development of this nucleus in humans are unknown, but INAH3 volume does not seem to depend significantly on hormonal status in adulthood (47). In rats and sheep, the size of a potentially homologous nucleus located in the same part of the POA (SDN-POA in rat, oSDN in sheep) is irreversibly determined by embryonic sex steroids. Moreover, lesions of this nucleus in adult male rats or ferrets modifies sexual partner preference, an animal model of sexual orientation. If the same mechanisms control the development of INAH3 in humans, the smaller INAH3 of gay men could then be a marker of deficient exposure to androgens during ontogeny and even be a cause of the modified sexual orientation. Alternatively, the small INAH3 of gay men could also be a consequence of their sexual orientation.
Clinical Studies
A potential implication of the embryonic hormonal environment in the control of sexual orientation is also supported by studies of various clinical disorders that affect the endocrine system during fetal life. In some cases, these early endocrine disruptions lead to a complete sex reversal, so that, postnatally, subjects are raised assuming a sex (gender) that is opposite to their genetic sex. For example XY subjects with complete androgen insensitivity syndrome are born with female genitalia and are typically raised as girls at least until puberty, when the absence of menstruation leads to medical examination and diagnosis. These subjects usually have a female gender identity and a female-typical sexual orientation (they are sexually attracted to men) (48). These cases demonstrate that sexual orientation is not necessarily associated with the genetic sex but tell us little about the role of prenatal hormones vs. postnatal environment, because both concur to produce a female-typical orientation.
The same confusion is potentially associated with the 5α-reductase deficiency affecting XY men who are born with genital structures that are not masculinized and are often raised as daughters. The rise in plasma testosterone associated with puberty later masculinizes (at least in part) the genital structures, and these individuals usually conform to a male gender and male-typical sexual orientation. It has been argued that the relative ease with which these subjects apparently change gender and (presumably) sexual orientation at puberty despite having been raised as females was related to their exposure to androgens during embryonic life (testosterone secretion is apparently normal in these subjects, it is only its 5α-reduction that is deficient) (49, 50). However, the condition of these subjects is usually known from birth, so that their sex of rearing was not necessarily unequivocally female. Furthermore the obvious social advantages related to adopting a male gender identity in the societies where this 5α-reductase deficiency frequently occurs and was studied raise additional questions about the reasons underlying the rapid and easy postpubertal change in gender orientation, identity, and role.
In other clinical conditions, however, the prenatal endocrine environment may push sexual orientation in a direction opposite to the effects of postnatal social environment. These cases therefore provide a more useful test of the role of either type of factor. Three such clinical conditions are important to mention here, because they are associated with a significantly increased incidence of homosexual orientation.
Congenital adrenal hyperplasia
Girls exposed in utero to abnormally high levels of androgens not only show masculinization of genital structures and of a variety of behavioral traits (e.g. aggressive play), but they also display a markedly increased probability of interest or participation in homosexual relationships in comparison with control females or to unaffected sisters (51–53). Some studies reported up to 30–40% of CAH girls having some form of homosexual attraction compared with 10% or less in control populations. Because the endocrine defect was corrected soon after birth, genital structures were surgically feminized, and these girls were presumably raised as girls, these data therefore suggest that prenatal androgens are involved in the determination of sexual orientation in women. This effect of prenatal androgens could be mediated through a direct action on the brain as well as through an indirect action on the genitalia that would secondarily induce an overall reduction of sexual activity and interest in sex in general, or more specifically of heterosexual activity. Although genitalia are surgically feminized at birth, they still do not have an ideal structure in some women and therefore allow little or no penetrative heterosexual relationships. These modified genitalia could also induce a general aversion toward sexual activity (see Ref. 26 for a more detailed discussion of this issue).
Treatment of pregnant mothers with diethylstilbestrol (DES)
Between 1939 and 1960, about 2 million pregnant women were treated with DES in Europe and the United States to prevent spontaneous miscarriages. This treatment turned out to be ineffective and also to have detrimental long-term consequences, but one of the unexpected outcomes was that girls born from these treated mothers showed a significant increase in nonheterosexual (bisexual or homosexual) fantasies or sexual activity, whereas the socialization of these subjects was fundamentally consistent with their genetic female sex (54, 55). The reproducibility of this effect has been questioned, but if the effect of DES is real, which will be difficult to confirm given that this treatment has been abandoned for a long time, it would indicate that estrogens as well as androgens (testosterone) are able to masculinize sexual orientation. This would fit with rodent data, where many effects of testosterone on sexual differentiation are produced after conversion into estradiol by aromatase in the brain, but would be in conflict with other data from humans that assign a prominent role to androgens in sexual differentiation. Note, however, that in rhesus monkeys, fetal exposure to DES was shown to increase adult mounting behavior although not to the male-typical level (56).
Cloacal exstrophy
Complex genito-urinary malformations occasionally occur during embryonic development resulting in the birth of XY males who, in addition to various malformations of the pelvis, have no penis. These subjects have normal testes and were thus presumably exposed to a male-typical pattern of androgen secretions before birth. Typically, in the past, these subjects were assigned a female sex, submitted to vaginoplasty, and raised as girls. Follow-up studies have demonstrated that in a significant number of cases (about half), when adults, these subjects chose to adopt a male identity, gender role, and male-typical sexual orientation that presumably relate to their embryonic exposure to androgens (57, 58).
Altogether, these clinical cases are consistent with the idea that embryonic hormones play a substantial role in determining adult sexual orientation. It should be noted, however, that these changes in sexual orientation as a result of embryonic endocrine disruption always only concern a fraction of affected individuals (usually a maximum of 30–40%), so that at least 60–70% of subjects in these conditions retain the heterosexual orientation consistent with their gender assignment at birth.
Genetic and Immunological Factors?
If embryonic hormones affect adult sexual orientation, then what is the cause of the endocrine changes that result in an atypical orientation in some subjects? Based on retrospective analysis of men born in Berlin during World War II (with all the problems that are potentially associated with such an approach), it has been suggested that exposure to chronic stress might be a critical determinant (59–61), but to our knowledge, these data have never been replicated and are considered unreliable by some authors.
Individual genetic differences could affect the synthesis of steroid hormones or their activity in the brain of the embryo, although to date, no evidence for such a mechanism has been obtained despite active research (62–64). Alternatively, studies in mice indicate that genes located on the sex chromosomes contribute in a direct manner to the sexual differentiation of brain and behavior (5). Various arguments suggest a significant genetic contribution to sexual orientation. Whether this partial genetic control is mediated by alterations of steroid action or more directly by a sexually differentiated expression of specific genes has not been determined.
Multiple epidemiological studies have demonstrated a correlation between concordance of sexual orientation and genetic relatedness. For example, if a boy is gay, between 20 and 25% of his brothers will share this sexual orientation, compared with 4–6% in a control population. Similarly, lesbian women have a greater probability than heterosexual women of having a homosexual sister.
Twins studies indicate that this correspondence in sexual orientation probably does not reflect a communality of postnatal experiences (psychosocial factors) but rather genetic similarity. Several studies indeed demonstrated that there is a better agreement of sexual orientation in monozygotic (identical) twins than in dizygotic twins (fraternal twins conceived from different ova and sperm) (65). If a dizygotic gay twin has a brother, there is on average a 15% probability that the brother will also be homosexual, but this probability rises to 65% in monozygotic twins (66). Overall, these studies suggest that in social conditions typical of Western societies, 50–60% of the variance in sexual orientation in humans has a genetic origin.
Although this genetic contribution was identified many years ago, the responsible gene(s) remain(s) unknown. Sexual orientation in men tends to be transmitted through the matriarchal lineage: a gay man has a higher probability of having gay men among his ancestors on the maternal side (uncles, cousins), than on the paternal side. This was originally interpreted as a sign of inheritance through gene(s) located on the X chromosome, and one study identified a linkage with markers located in the subtelomeric region of the long arm of the X chromosome, a region called Xq28 (67). This association with Xq28 was replicated in one subsequent study (68) and in another set of data that were not published in a peer-reviewed journal (see Ref. 69) but not in a fourth one (70). A meta-analysis of all these data strongly supports (P < 0.0001) the existence of this linkage (71). More recent studies have suggested that the differential heritage through the matriarchal lineage could also be the result of epigenetic modifications of the expression of genes located on several other chromosomes (71, 72).
In summary, the existence of a genetic contribution to the control of sexual orientation is now firmly established, but the specific gene(s) that are implicated in this process have not been identified so far. Whether or not this (these) gene(s) affect sexual orientation by modifying steroid secretion or action during ontogeny has also not been determined.
Finally, to complete the picture of biological factors affecting sexual orientation, it should be noted that the factor most reliably associated with homosexuality in males is the presence in the family of older brothers born of the same mother. The incidence of homosexuality increases by 33% for each older brother and is accompanied by a small but statistically significant decrease in weight at birth. These effects do not appear to be explained by differences in education or family background and may be the result of accumulation of antibodies in the mother during successive pregnancies against one or more proteins expressed specifically by the male brain. This interpretation currently remains an untested hypothesis and the specific antigenic proteins underlying this phenomenon have not been identified. Candidate proteins have, however, been suggested as potential target(s) for this immune reaction based on their distribution and properties (73). Whether this effect involves hormone actions is unknown.
Conclusions
There is thus substantial evidence suggesting that sexual orientation, and homosexuality in particular, is influenced before birth by a set of biological mechanisms. These mechanisms include genes that affect sexual orientation by currently unidentified mechanisms and hormonal actions classically mediating sexual differentiation. Our current understanding of these prenatal factors admittedly suffers many limitations. For example, all embryonic endocrine disorders that have been associated with an increased incidence of homosexuality have a limited effect size and never affect more than 30–40% of subjects. Furthermore, all identified correlates of homosexuality that suggest exposure to an atypical endocrine environment during ontogeny in gays and/or lesbians are only weakly associated with sexual orientation and often are modified in a reliable manner in one sex only (2D:4D ratio, OAE in women) or have been studied only in one sex (INAH3 volume in men). They are statistically correlated with sexual orientation but are unable to predict it accurately due to the large variance in this relationship.
The limitations of the results probably relate not only to the complexity of the behavioral trait under consideration but also to methodological difficulties specific to their study, such as the long latency between putative hormone actions and their effects, the absence for ethical reasons of truly experimental studies, and the taboos associated with human sexuality. One should also consider that gays and lesbians probably do not constitute homogeneous populations. In addition to the obvious gradation between heterosexuality and homosexuality that was already recognized by Kinsey et al. (1, 74), some lesbians display obvious male characteristics (“butch”), whereas others do not (“femme”), and the same dichotomy exists in gay men. These differences are unfortunately rarely taken into account in experimental studies.
Despite these limitations, I believe that biological studies suggest a significant contribution of genetic and hormonal factors in the control of sexual orientation. In contrast, alternative explanations based on features of the postnatal environment, such as relationships with parents, social interactions, or early sexual experiences, although they are widely accepted in the public, are not usually supported by quantitative experimental studies. It is clear, however, that none of the biological factors identified so far is able to explain by itself the incidence of homosexuality in all individuals. Three possibilities can be contemplated to explain this failure.
Either there are different types of homosexuality. Some forms could be determined by genetic effects, others by hormones, and yet others by the older brothers effect and the associated immunological modifications.
Or the effects of different biological factors interact to varying degrees in each individual, and it is only when several of these predisposing factors are combined that an homosexual orientation is observed.
Or finally, all biological factors that have been associated with homosexuality only become effective in conjunction with exposure to a given (as yet unspecified) psychosocial postnatal environment. The postnatal environment would in this scenario play an important permissive role, but it is then surprising that no quantitative study has been able so far to formally identify aspects of this environment that play this limiting role in the control of sexual orientation. It has, however, been suggested that embryonic hormones may directly affect aspects of juvenile behavior (e.g. play behavior) and that this in turn could condition the development of sexual orientation (75, 76).
Current knowledge does not allow discriminating between these interpretations (see Ref. 34 for a more detailed discussion). It is clear, however, that biological factors acting during prenatal life play a significant role in the control of sexual orientation and that homosexuality is not, for most people, the result only of postnatal experiences or a free choice. It is often an awareness that presents itself to the individual during their adolescence or early adult life. The acceptance of a nonheterosexual orientation in a minority of subjects is often the cause of significant psychological distress and social isolation. In contrast, heterosexual orientation emerges with the individual often being unaware of the underlying process. There is no question of choice here. Data presented in this review strongly suggest that most human beings do not choose to be heterosexual or homosexual. What they choose is to assume or not their orientation and eventually reveal it openly. Sexual orientation represents a highly complex behavioral trait under multifactorial control that includes genetic, hormonal, and presumably immunological determinants potentially acting in concert with the social postnatal environment. More interdisciplinary research is needed to better understand this fascinating aspect of human behavior.
Acknowledgments
This work was supported by the National Institutes of Health Grant MH50388 and by the Belgian Fonds de la Recherche Fondamentale Collective 2.4537.09.
Disclosure Summary: The author has nothing to disclose.
Footnotes
- CAH
- Congenital adrenal hyperplasia
- 2D:4D
- ratio of the lengths of the second (index) to the fourth (ring) fingers
- DES
- diethylstilbestrol
- FoR
- female-oriented ram
- INAH3
- interstitial nucleus of the anterior hypothalamus number 3
- MoR
- male-oriented ram
- OAE
- oto-acoustic emission
- oSDN
- sexually dimorphic nucleus of the ovine preoptic area
- SDN-POA
- sexually dimorphic nucleus of the preoptic area.
References
- 1. Kinsey AC, Pomeroy WR, Martin CE. 1948. Sexual behavior in the human male. Philadelphia: W.B. Saunders Co; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goy RW, McEwen BS. 1980. Sexual differentiation of the brain. Cambridge, MA: The MIT Press [Google Scholar]
- 3. Beach FA. 1948. Hormones and behavior. New York: Paul B. Hoeber, Inc [Google Scholar]
- 4. Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizational action of prenatally administered testosterone propionate on the tissues mediating behavior in the female guinea pig. Endocrinology 65:369–382 [DOI] [PubMed] [Google Scholar]
- 5. Arnold AP, Chen X. 2009. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McCarthy MM, De Vries GJ, Forger NG. 2009. Sexual diferentiation of the brain: mode, mechanisms, and meaning. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT. eds. Hormones, brain and behavior. San Diego: Academic Press; 1708–1744 [Google Scholar]
- 7. Jacobson CD, Csernus VJ, Shryne JE, Gorski RA. 1981. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci 1:1142–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobson CD, Shryne JE, Shapiro F, Gorski RA. 1980. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol 193:541–548 [DOI] [PubMed] [Google Scholar]
- 9. Paredes RG, Baum MJ. 1995. Altered sexual partner preference in male ferrets given excitotoxic lesions of the preoptic area anterior hypothalamus. J Neurosci 15:6619–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paredes RG, Tzschentke T, Nakach N. 1998. Lesions of the medial preoptic area anterior hypothalamus (MPOA/AH) modify partner preference in male rats. Brain Res 813:1–8 [DOI] [PubMed] [Google Scholar]
- 11. Bakker J, Brand T, van Ophemert J, Slob AK. 1993. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci 107:480–487 [DOI] [PubMed] [Google Scholar]
- 12. Bakker J, van Ophemert J, Slob AK. 1993. Organization of partner preference and sexual behavior and its nocturnal rhythmicity in male rats. Behav Neurosci 107:1049–1058 [DOI] [PubMed] [Google Scholar]
- 13. Bakker J, Van Ophemert J, Slob AK. 1996. Sexual differentiation of odor and partner preference in the rat. Physiol Behav 60:489–494 [DOI] [PubMed] [Google Scholar]
- 14. Henley CL, Nunez AA, Clemens LG. 2009. Estrogen treatment during development alters adult partner preference and reproductive behavior in female laboratory rats. Horm Behav 55:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henley CL, Nunez AA, Clemens LG. 2011. Hormones of choice: the neuroendocrinology of sexual orientation in animals. Front Neuroendocrinol 32:146–154 [DOI] [PubMed] [Google Scholar]
- 16. Bagemihl B. 1999. Biological exuberance. Animal homosexuality and natural diversity. New York: St. Martin's Press [Google Scholar]
- 17. Poianni A. 2010. Animal homosexuality. A biological perspective. Cambridge, UK: Cambridge University Press [Google Scholar]
- 18. Roselli CE, Reddy RC, Kaufman KR. 2011. The development of male-oriented behavior in rams. Front Neuroendocrinol 32:164–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. 2004. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology 145:478–483 [DOI] [PubMed] [Google Scholar]
- 20. Roselli CE, Stadelman H, Reeve R, Bishop CV, Stormshak F. 2007. The ovine sexually dimorphic nucleus of the medial preoptic area is organized prenatally by testosterone. Endocrinology 148:4450–4457 [DOI] [PubMed] [Google Scholar]
- 21. Roselli CE, Estill CT, Stadelman HL, Stormshak F. 2009. The volume of the ovine sexually dimorphic nucleus of the preoptic area is independent of adult testosterone concentrations. Brain Res 1249:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis L, Hershberger S, Field E, Wersinger S, Pelis S, Geary D, Palmer C, Hoyenga K, Hetsroni A, Karadi K. 2008. Sex differences: summarizing more than a century of scientific research. New York: Psychology Press [Google Scholar]
- 23. Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA. 2008. Sex differences in the brain. From Genes to behavior. Oxford: Oxford University Press [Google Scholar]
- 24. Bao AM, Swaab DF. 2011. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front Neuroendocrinol 32:214–226 [DOI] [PubMed] [Google Scholar]
- 25. Hines M. 2004. Brain gender. Oxford: Oxford University Press [Google Scholar]
- 26. Hines M. 2011. Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Front Neuroendocrinol 32:170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meyer-Bahlburg HF. 1984. Psychoendocrine research on sexual orientation. Current status and future options. Prog Brain Res 61:375–398 [DOI] [PubMed] [Google Scholar]
- 28. Goy RW, Bercovitch FB, McBrair MC. 1988. Behavioral masculinization is independent of genital masculinization in prenatally androgenized female rhesus macaques. Horm Behav 22:552–571 [DOI] [PubMed] [Google Scholar]
- 29. Baron-Cohen S. 2004. The essential difference: men, women and the extreme male brain. London: Penguin Press Science [Google Scholar]
- 30. Savic I, Berglund H, Lindström P. 2005. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci USA 102:7356–7361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berglund H, Lindström P, Savic I. 2006. Brain response to putative pheromones in lesbian women. Proc Natl Acad Sci USA 103:8269–8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. LeVay S, Valente SM. 2006. Human sexuality. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 33. LeVay S. 2010. Gay, straight, and the reason why. The science of sexual orientation. New York: Oxford University Press [Google Scholar]
- 34. Balthazart J. 2011. Biology of homosexuality. New York: Oxford University Press, in press [Google Scholar]
- 35. Breedlove SM. 2010. Minireview: organizational hypothesis: instances of the fingerpost. Endocrinology 151:4116–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berenbaum SA, Bryk KK, Nowak N, Quigley CA, Moffat S. 2009. Fingers as a marker of prenatal androgen exposure. Endocrinology 150:5119–5124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallen K. 2009. Does finger fat produce sex differences in second to fourth digit ratios? Endocrinology 150:4819–4822 [DOI] [PubMed] [Google Scholar]
- 38. Grimbos T, Dawood K, Burriss RP, Zucker KJ, Puts DA. 2010. Sexual orientation and the second to fourth finger length ratio: a meta-analysis in men and women. Behav Neurosci 124:278–287 [DOI] [PubMed] [Google Scholar]
- 39. Williams TJ, Pepitone ME, Christensen SE, Cooke BM, Huberman AD, Breedlove NJ, Breedlove TJ, Jordan CL, Breedlove SM. 2000. Finger-length ratios and sexual orientation. Nature 404:455–456 [DOI] [PubMed] [Google Scholar]
- 40. Martin JT, Nguyen DH. 2004. Anthropometric analysis of homosexuals and heterosexuals: implications for early hormone exposure. Horm Behav 45:31–39 [DOI] [PubMed] [Google Scholar]
- 41. McFadden D. 2011. Sexual orientation and the auditory system. Front Neuroendocrinol 32:201–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swaab DF, Hofman MA. 1990. An enlarged suprachiasmatic nucleus in homosexual men. Brain Res 537:141–148 [DOI] [PubMed] [Google Scholar]
- 43. Allen LS, Gorski RA. 1992. Sexual orientation and the size of the anterior commissure in the human brain. Proc Natl Acad Sci USA 89:7199–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen LS, Hines M, Shryne JE, Gorski RA. 1989. Two sexually dimorphic cell groups in the human brain. J Neurosci 9:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. LeVay S. 1991. A difference in hypothalamic structure between heterosexual and homosexual men. Science 253:1034–1037 [DOI] [PubMed] [Google Scholar]
- 46. Byne W, Tobet S, Mattiace LA, Lasco MS, Kemether E, Edgar MA, Morgello S, Buchsbaum MS, Jones LB. 2001. The interstitial nuclei of the human anterior hypothalamus: an investigation of variation with sex, sexual orientation, and HIV status. Horm Behav 40:86–92 [DOI] [PubMed] [Google Scholar]
- 47. Garcia-Falgueras A, Swaab DF. 2008. A sex difference in the hypothalamic uncinate nucleus: relationship to gender identity. Brain 131:3132–3146 [DOI] [PubMed] [Google Scholar]
- 48. Wisniewski AB, Migeon CJ, Meyer-Bahlburg HF, Gearhart JP, Berkovitz GD, Brown TR, Money J. 2000. Complete androgen insensitivity syndrome: long-term medical, surgical, and psychosexual outcome. J Clin Endocrinol Metab 85:2664–2669 [DOI] [PubMed] [Google Scholar]
- 49. Imperato-McGinley J, Miller M, Wilson JD, Peterson RE, Shackleton C, Gajdusek DC. 1991. A cluster of male pseudohermaphrodites with 5α-reductase deficiency in Papua New Guinea. Clin Endocrinol 34:293–298 [DOI] [PubMed] [Google Scholar]
- 50. Imperato-McGinley J, Zhu YS. 2002. Androgens and male physiology the syndrome of 5α-reductase-2 deficiency. Mol Cell Endocrinol 198:51–59 [DOI] [PubMed] [Google Scholar]
- 51. Money J, Schwartz M, Lewis VG. 1984. Adult erotosexual status and fetal hormonal masculinization and demasculinization: 46, XX congenital virilizing adrenal hyperplasia and 46, XY androgen-insensitivity syndrome compared. Psychoneuroendocrinology 9:405–414 [DOI] [PubMed] [Google Scholar]
- 52. Dittmann RW, Kappes ME, Kappes MH. 1992. Sexual behavior in adolescent and adult females with congenital adrenal hyperplasia. Psychoneuroendocrinology 17:153–170 [DOI] [PubMed] [Google Scholar]
- 53. Zucker KJ, Bradley SJ, Oliver G, Blake J, Fleming S, Hood J. 1996. Psychosexual development of women with congenital adrenal hyperplasia. Horm Behav 30:300–318 [DOI] [PubMed] [Google Scholar]
- 54. Ehrhardt AA, Meyer-Bahlburg HF, Rosen LR, Feldman JF, Veridiano NP, Zimmerman I, McEwen BS. 1985. Sexual orientation after prenatal exposure to exogenous estrogen. Arch Sex Behav 14:57–77 [DOI] [PubMed] [Google Scholar]
- 55. Meyer-Bahlburg HF, Ehrhardt AA, Rosen LR. 1995. Prenatal estrogens and the development of homosexual orientation. Dev Psychol 31:12–21 [Google Scholar]
- 56. Goy RW, Deputte BL. 1996. The effects of diethylstilbestrol (DES) before birth on the development of masculine behavior in juvenile female rhesus monkeys. Horm Behav 30:379–386 [DOI] [PubMed] [Google Scholar]
- 57. Reiner WG, Gearhart JP. 2004. Discordant sexual identity in some genetic males with cloacal exstrophy assigned to female sex at birth. N Engl J Med 350:333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Meyer-Bahlburg HF. 2005. Gender identity outcome in female-raised 46, XY persons with penile agenesis, cloacal exstrophy of the bladder, or penile ablation. Arch Sex Behav 34:423–438 [DOI] [PubMed] [Google Scholar]
- 59. Dörner G, Geier T, Ahrens L, Krell L, Münx G, Sieler H, Kittner E, Müller H. 1980. Prenatal stress as possible aetiogenetic factor of homosexuality in human males. Endokrinologie 75:365–368 [PubMed] [Google Scholar]
- 60. Dörner G, Schenk B, Schmiedel B, Ahrens L. 1983. Stressful events in prenatal life of bi- and homosexual men. Exp Clin Endocrinol 81:83–87 [DOI] [PubMed] [Google Scholar]
- 61. Dörner G. 1980. Sexual differentiation of the brain. In: Vitamins and hormones. New York: Academic Press, Inc.; 325–381 [DOI] [PubMed] [Google Scholar]
- 62. Macke JP, Hu N, Hu S, Bailey M, King VL, Brown T, Hamer D, Nathans J. 1993. Sequence variation in the androgen receptor gene is not a common determinant of male sexual orientation. Am J Hum Genet 53:844–852 [PMC free article] [PubMed] [Google Scholar]
- 63. DuPree MG, Mustanski BS, Bocklandt S, Nievergelt C, Hamer DH. 2004. A candidate gene study of CYP19 (aromatase) and male sexual orientation. Behav Genet 34:243–250 [DOI] [PubMed] [Google Scholar]
- 64. Mustanski BS, Dupree MG, Nievergelt CM, Bocklandt S, Schork NJ, Hamer DH. 2005. A genomewide scan of male sexual orientation. Hum Genet 116:272–278 [DOI] [PubMed] [Google Scholar]
- 65. Bailey JM, Pillard RC, Dawood K, Miller MB, Farrer LA, Trivedi S, Murphy RL. 1999. A family history study of male sexual orientation using three independent samples. Behav Genet 29:79–86 [DOI] [PubMed] [Google Scholar]
- 66. Diamond M. 1993. Some genetic considerations in the development of sexual orientation. In: Haug M, Whalen RE, Aron C, Olsen KL. eds. The development of sex differences and similarities in behavior. Dordrecht, The Netherlands: Kluwer Academic Publishers; 291–309 [Google Scholar]
- 67. Hamer DH, Hu S, Magnuson VL, Hu N, Pattatucci AML. 1993. A linkage between DNA markers on the X chromosome and male sexual orientation. Science 261:321–327 [DOI] [PubMed] [Google Scholar]
- 68. Hu S, Pattatucci AM, Patterson C, Li L, Fulker DW, Cherny SS, Kruglyak L, Hamer DH. 1995. Linkage between sexual orientation and chromosome Xq28 in males but not in females. Nat Genet 11:248–256 [DOI] [PubMed] [Google Scholar]
- 69. Sanders AR, Dawood K. 2003. Nature encyclopedia of life sciences. London: Nature Publishing Group [Google Scholar]
- 70. Rice G, Anderson C, Risch N, Ebers G. 1999. Male homosexuality: absence of linkage to microsatellite markers at Xq28. Science 284:665–667 [DOI] [PubMed] [Google Scholar]
- 71. Bocklandt S, Vilain E. 2007. Sex differences in brain and behavior: hormones versus genes. Adv Genet 59:245–266 [DOI] [PubMed] [Google Scholar]
- 72. Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E. 2011. The genetics of sex differences in brain and behavior. Front Neuroendocrinol 32:227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bogaert AF, Skorska M. 2011. Sexual orientation, fraternal birth order, and the maternal immune hypothesis: a review. Front Neuroendocrinol 32:247–254 [DOI] [PubMed] [Google Scholar]
- 74. Kinsey AC, Pomeroy WR, Martin CE, Gebhard PH. 1953. Sexual behavior in the human female. Philadelphia: Saunders [Google Scholar]
- 75. Bem DJ. 1996. Exotic becomes erotic: a developmental theory of sexual orientation. Psychol Rev 103:320–335 [Google Scholar]
- 76. Bem DJ. 2000. Exotic becomes erotic: interpreting the biological correlates of sexual orientation. Arch Sex Behav 29:531–548 [DOI] [PubMed] [Google Scholar]