Abstract
Previously, we reported that 1 nm 17ß-estradiol (E2) induces a rapid action, which is, in part, mediated through the G protein-coupled receptor GPR30 in primate GnRH neurons. Because it has been reported that the diphenylacrylamide compound, STX, causes estrogenic action in the mouse and guinea pig hypothalamus, the present study examined effects of STX in primate GnRH neurons and whether there is an action independent of GPR30. Results are summarized as follows. STX (10 nm) exposure increased 1) the oscillation frequency of intracellular calcium concentration ([Ca2+]i), 2) the percentage of cells stimulated, and 3) the synchronization frequency of [Ca2+]i oscillations. STX (10–100 nm) also stimulated GnRH release. The effects of STX on both [Ca2+]i oscillations and GnRH release were similar to those caused by E2 (1 nm), although with less magnitude. STX (10 nm)-induced changes in [Ca2+]i oscillations were not altered by GPR30 small interfering RNA transfection, indicating that STX-sensitive receptors differ from GPR30. Finally, a higher dose of E2 (10 nm) induced a larger change in [Ca2+]i oscillations than that with a smaller dose of E2 (1 nm), and the effects of 10 nm E2 were reduced but not completely blocked by GPR30 small interfering RNA transfection, indicating that the effects of 10 nm E2 in primate GnRH neurons are mediated by multiple membrane receptors, including GPR30 and STX-sensitive receptors. Collectively, the rapid action of E2 mediated through GPR30 differs from that mediated through STX-sensitive receptors. The molecular structure of the STX-sensitive receptor remains to be identified.
Estrogens play crucial roles in both the homeostatic regulation of GnRH neurons and reproductive behavior (1, 2). At the cellular level, earlier studies indicate that estrogen [17β-estradiol (E2)] causes its effects in the brain through nuclear estrogen receptors (ERα and ERβ) by initiating gene transcription (3). More recent studies, however, suggest that E2 can also initiate nongenomic effects. Rapid and nongenomic actions of E2 were first described in the rat uterus in 1967 by Szego and Davis (4), and subsequently, these E2 effects were demonstrated in neuronal cells, including hypothalamic (5–7), cortical (8, 9), hippocampal (10, 11), and striatal neurons (12).
Despite the firmly established fact that nongenomic, rapid E2 actions occur at the plasma membrane through membrane ERs (mERs), there are considerable differences as to which mER mediate rapid action. First, classical ERs, ERα (13, 14) and ERβ (14), and ERα variants such as ERα36 (15) can mediate rapid E2 action. Second, the G protein-coupled ER, G protein-coupled receptor 30 (GPR30), also has been shown in cancer cells (16), vascular smooth muscle cells (17), and neuronal cells (18), although its membrane localization is currently controversial (19, 20). Third, two mERs, of which molecular structures are unknown, have been reported as well: ER-X in cortical neurons (21) and receptors sensitive to STX (a diphenylacrylamide estrogenic compound) in hypothalamic neurons (22). Interestingly, STX causes E2-like actions, such as reducing core body temperature and maintaining energy homeostasis in female guinea pigs (23–25).
In GnRH neurons as well, several groups have shown that E2 causes direct rapid actions through receptors located at the plasma membrane, although again considerable differences exist between the mER mediating rapid actions in GnRH neurons depending on the models used. In mice, E2 causes rapid changes in GnRH neuronal activity through ERβ (26–29), and mouse and rat GnRH neurons express ERβ (30, 31). Recently, we have found that E2 induces rapid excitatory action in primate GnRH neurons. ERα and ERβ appear not to be involved in this E2 action (32, 33), but rather GPR30 is responsible at least in part for E2-induced actions (34). A similar finding was also recently reported in mouse GnRH neurons (29).
STX, similar to E2, rapidly activates a KATP current in mouse GnRH neurons via a protein kinase signaling pathway in an ICI182,780-dependent manner (35). In mouse and guinea pig proopiomelanocortin (POMC) neurons, STX (and E2) attenuate(s) the GABAB response via a phospholipase C (PLC)/protein kinase C/protein kinase A signaling pathway in an ICI182,780-dependent manner (22, 23). Importantly, the STX-induced signaling is still present in αβERKO mice (mice that do not express ERα or ERβ) (23, 36) and in GPR30 knockout mice (24), suggesting that STX is acting at a receptor distinct from ERα, ERβ, and GPR30. Nonetheless, it is unclear whether STX causes any changes in primate GnRH neurons and, if it does, whether STX action is dependent on GPR30 in this model. Results indicate that STX stimulates oscillations of intracellular calcium concentration ([Ca2+]i) and GnRH release and that the rapid actions caused by STX are not mediated by GPR30 in primate GnRH neurons.
Materials and Methods
Animals
Rhesus monkey embryos (Macaca mulatta) from time-mated pregnancies were delivered by cesarean section under isoflurane anesthesia. A total of 12 fetuses between embryonic d 35 and 38 were used in this study. All experimental procedures were conducted in accordance with the standards outlined in the Principles for the Use of Animals and Guide for the Care and Use of Laboratory Animals. The protocol used in these studies was approved by the Animal Care and Use Committee of the University of Wisconsin-Madison.
Experimental design
Experiment 1a
To address the question of whether STX causes a rapid stimulatory action on GnRH release, similar to E2, cultures were exposed to STX at 10 or 100 nm for 20 min after 60 min of control sampling. Perifusates were continuously collected at 10-min intervals for an additional 60 min. GnRH levels in perifusate samples were assessed by RIA.
Experiment 1b
To determine whether STX causes changes in [Ca2+]i oscillations, similar to those observed with E2, cultured GnRH neurons were exposed to STX at 10 nm for 10 min after at least 20 min of control recording, [Ca2+]i oscillations were continuously recorded for an additional 60 min.
Experiment 2
Because in experiment 1b, STX caused excitatory effects on [Ca2+]i oscillations in GnRH neurons (see Results), we examined whether STX effects were mediated through GPR30. Cultured GnRH neurons were transfected with specific small interfering RNA (siRNA) for human/monkey GPR30 or control siRNA for a 48- to 72-h period, and the effects of STX on [Ca2+]i oscillations were examined, as described for experiment 1b.
Experiment 3
The effects of STX were examined in the presence of 100 nm ICI182,780, because the estrogen receptor antagonist ICI182,780 blocked STX effects in mouse hypothalamic neurons (22, 23). 100 nm ICI182,780 infusion was initiated 10 min before 10 nm STX application (10-min period) and continued through 10 min after STX administration. The effects of ICI182,780 and STX on [Ca2+]i oscillations were examined, as described for experiment 1b.
Experiment 4
Because involvement of a PLC-mediated pathway in the STX action in POMC neurons in the guinea pig and mouse hypothalamus has been previously reported (22), we examined whether the PLC inhibitor U73122 could block STX's effects on [Ca2+]i oscillations. After at least 20 min of control recording, 10 μm U73122 (EMD Biosciences, San Diego, CA) was infused for 15 min, during which the 10 nm STX, or vehicle as a control, was simultaneously infused for 10 min. The start of U73122 infusion preceded STX infusion by 5 min.
Experiment 5
It is possible that multiple mechanisms may be involved in rapid E2 action, as the results from experiment 2 indicate that the effects of STX on [Ca2+]i oscillations were still observed in GPR30 siRNA transfected GnRH neurons (see Results). To test this possibility, we examined whether a higher dose (10 nm) of E2 could cause a larger effect and, if it did, whether GPR30 siRNA transfection completely blocked the high dose of E2. After at least 20 min of control recording, cultured GnRH neurons were exposed to either 1 or 10 nm E2 (Schering Co., Bloomfield, NJ) or vehicle for 10 min, and changes in [Ca2+]i oscillations were determined. Subsequently, GnRH neurons were transfected with GPR30 siRNA or control siRNA for 48–72 h, and the effects of 10 nm E2 were examined.
Tissue culture
Culture methods for GnRH neurons derived from the fetal nasal placode region have been described previously (37, 38). Briefly, the nasal placode and the ventral GnRH neuronal migratory pathway (terminal nerve region) were dissected out and cut into small (<0.5 mm3) pieces. For calcium imaging experiments, two to three pieces were plated on each collagen-coated 25-mm round glass coverslip. For perifusion experiments, four to six pieces were plated on each 25-mm round plastic collagen-coated coverslip (Nalge NUNC International, Rochester, NY). This generally resulted in 32 glass coverslips and 24 plastic coverslips per embryo. On the fourth day of culture, cells on glass coverslips were exposed to an antimitotic agent, 5-fluoro-5deoxyuridine (30–40 μm), for 2 d to better visualize GnRH neurons. Cultures were incubated at 37 C, 1.5% CO2 in culture media (Medium 199 plus l-glutamine; Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT), 0.6% glucose, and 50 μg/ml gentamicin (Sigma) for at least 2 wk before experiments. Medium was replaced every 1–3 d as needed. All experiments were carried out during 2–4 wk of age in culture.
Perifusion experiments
Perifusion experiments were performed using Sykes-Moore chambers as described previously (34, 38). During the third to fourth week in culture, two plastic coverslips with cells plated on them were placed face to face with a rubber O-ring separating them. This formed a chamber with a volume of 200 μl. Before sample collection, cells were perifused at 95% O2, 5% CO2, and 37 C for at least 1 h with a modified Krebs-Ringer phosphate buffer (38) with 0.1% glucose (pH 7.4). Samples were collected every 10 min at a flow rate of 30 μl/min for up to 6 h using the ACUSYST perifusion system (Endotronics, Minneapolis, MN). All perifusion cultures were challenged with 56 mm KCl solution at the end of each experiment to verify the viability of the cells. Samples were snap frozen on dry ice and stored at −80 C for GnRH RIA. After each experiment, culture dishes were fixed with 4% paraformaldehyde (pH 7.6) and immunostained for GnRH (see below).
Measurement of [Ca2+]i
As previously detailed (32, 34, 39), [Ca2+]i levels were assessed by loading cultured cells on glass coverslips with 18 μm fura-2 AM (Teflab, Austin, TX) and 6 μl of a mixture of pluronic F-127 (BASF Pharma/Knoll AG, Parsippany, NY) and dimethylsulfoxide for 30 min at 37 C and 1.5% CO2. The coverslip was then placed in a Dvorak-Stotler chamber. Fluorescence imaging of the dye-loaded cells was achieved with an inverted microscope. GnRH neurons were identified and viewed through a ×20 objective lens with a 750 × 750-μm recording field. Cultures were perifused with serum-free M199 medium (pH 7.4, 95% O2, 5% CO2) at a speed of 50 μl/min at room temperature under low light conditions.
[Ca2+]i was determined from the function of the ratio of the 510-nm fura-2 emission excited by illuminations at 340 and 380 nm with a λ-DG-4 light source and filter exchanger (Sutter Instruments, Novato, CA). Fura-2 fluorescence was recorded at 10-sec intervals with a charge-coupled device camera (Photometrics, Tucson, AZ) using Metafluor imaging software (Molecular Devices Corp., Downingtown, PA) and/or NIS Elements AR imaging software (Nikon, Melville, NY). The ratio of the fluorescence intensities (ΔF/F0) from the 340- and 380-nm excitation was used to calculate free [Ca2+]i levels and saved as a text file in Microsoft Excel for analysis. Viable GnRH neurons were assessed with a 56 mm KCl challenge at the end of each [Ca2+]i imaging experiment.
GnRH neurons for [Ca2+]i imaging were identifiable by their unique morphology, size, and migratory pattern: large oval-shaped soma (>10 μm) with neuroprocesses. Because these cultures contain many other cell types including epithelial cells, fibroblasts, and other unidentified cells as well as a small proportion of non-GnRH neurons (37, 39), the identity of each GnRH neuron was confirmed by immunocytochemistry. The immunocytochemistry results were compared with a fluorescent image of the view area and matched with the location on the reference grid of the coverslip (39).
Transfections
As previously described (34), GnRH neurons were transferred to serum-free media and transiently transfected with human/monkey-specific GPR30 siRNA using the Fugene HD Transfection reagent (Roche Diagnostics, Indianapolis, IN) at 37 C following the manufacturer's instructions for 48–72 h before initiation of experiments. Dharmacon GPR30 siRNA SMARTpool (Dharmacon, Lafayette, CO) contained four distinct sequences (GGAUGAGCUUCGACCGCUA, GACGAGGCCUGCUUCUGUU, UAGGAAACCUCACGACUGG, and GGGUGAAGCGCCUCAGUUA). All four siRNA sequences match 100% with human GPR30 mRNA (NM_001039966), and two of the four siRNA match completely or nearly 100% with the rhesus monkey GPR30 mRNA sequence (accession no. XM_001084531). However, we were not able to confirm the alignment of the remaining two siRNA sequences because available rhesus monkey GPR30 mRNA sequence (XM_001084531) does not include the corresponding 5′ and 3′ untranslated region in human GPR30 mRNA.
Protein isolation and Western blot analyses
Olfactory placode cells were homogenized in lysis buffer (1% Triton X-100, 100 mm NaCl, 50 mm HEPES, 10 mm EDTA, 2 mm Na3VO4, 1 mm phenylmethylsulfonyl fluoride, 10 mm NaF, 4 mm Na4P2O7, 5 μg/ml leupeptin, and 5 μg/ml aprotinin) at a ratio of 1 g tissue per 5 ml buffer. Cellular debris was cleared by centrifugation (12,000 × g for 15 min at 4 C). The protein concentration of the supernatant was determined using a Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) with BSA as the standard.
Proteins were denatured in Laemmli, separated on 10% SDS-PAGE gels, and transferred to Immobilon-P polyvinylidene difluoride membranes (Sigma). Blots were blocked in 5% nonfat milk/Tris-buffered saline with 0.1% Tween 20 (TBST) at room temperature for 1 h, incubated with either an affinity-purified polyclonal human GPR30 antibody raised in a rabbit (1:200 dilution, described in Ref. 16) or β-actin (1:1000 dilution) in 5% nonfat milk/TBST overnight at room temperature, rinsed three times for 10 min each with TBST at room temperature, incubated with goat antirabbit IgG horseradish peroxidase conjugate (1:5000 dilution of 1 mg/ml stock; Jackson ImmunoResearch Labs, West Grove, PA) or sheep antimouse IgG horseradish peroxidase conjugate (1:5000 dilution of 1 mg/ml stock; Amersham Biosciences, Piscataway, NJ), and then rinsed three times for 10 min each with TBST. Immunoreactive proteins were detected using enhanced chemiluminescence (ECL Plus Western blotting detection reagents; Amersham Biosciences) according to the manufacturer's recommendations. Bands were imaged using a Molecular Dynamics (Sunnyvale, CA) Storm model 840 phosphoimager. Loading control was assessed by reprobing the same blot with an antibody against the β-actin. Protein expression was quantitated using ImageQuant software (Molecular Dynamics) and standardized for loading control.
Immunocytochemistry
Culture dishes used for perifusion and [Ca2+]i imaging experiments were stained using standard immunocytochemical procedures with an antisera cocktail of GF-6 and LR-1 [gifts from Dr. N. M. Sherwood (University of British Columbia, Victoria, Canada; 1:9000 dilution) and Dr. R. A. Benoit (University of Montreal, Montreal, Canada; 1:15,000)] for 40–42 h, the Vectastain ABC peroxidase system (Vector Laboratories, Burlingame, CA), and 3,3′-diaminobenzidine (Sigma) as the chromogen. GnRH-positive cells were matched up with a digitized fluorescent image from the [Ca2+]i imaging experiments. Fibroblasts, epithelial cells, and other GnRH-negative neurons were excluded from all analysis.
GnRH Assay
GnRH concentrations in perifusate samples were measured in 200-μl aliquots by RIA using antiserum R-1245 (gift from Dr. T. Nett, Colorado State University, Fort Collins, CO) as detailed previously (34, 38, 40). Synthetic GnRH (Richelieu Laboratory, Inc., Montreal, Canada) was used as the trace and for the standard curves. The sensitivity of the assay at 95% binding was 0.05 pg/tube. Intra- and interassay coefficients of variation were 8.3 and 11.4%.
Data analysis
Effects of treatment (E2/STX/U73122/ICI182,780 plus STX vs. vehicle, E2/STX in the presence of GPR30 siRNA, and STX in the presence of U73122 vs. respective controls) were examined using two-way ANOVA repeated measure followed by Bonferroni analysis.
In perifusion experiments, we excluded cultures from the data analysis when no GnRH neurons were found or cells did not respond to 56 mm KCl. When GnRH levels were detectable, approximately 40–350 GnRH neurons were found in each culture.
Peaks in [Ca2+]i oscillations were determined by Pulsar algorithm (41) as described previously (32). After [Ca2+]i peaks were established, the average amplitude, interpeak interval, and number of peaks were counted in each neuron in 20-min blocks: −20–0, 0–20, 20–40, and 40–60 with time 0 corresponding to the initiation of treatment. Mean values for all neurons in a culture dish (n = 15–62) were calculated from these parameters. For statistical comparison of each treatment, overall mean values were obtained by averaging the mean values of each culture. For the purpose of graphical representation, the frequency was presented as a percentage relative to the −20–0 min as 100%. All statistical analysis was conducted using raw data.
Synchronization of [Ca2+]i peaks in each culture was determined as previously described (32). First, the average number of peaks was determined for the entire experimental time period. Then the mean number of peaks was calculated in consecutive 50-sec periods for the entire experiment. A synchronization was considered to occur if the mean rate in two consecutive 50-sec periods was greater than the total mean + 3 sd, based on previous observations (32, 39). The total number of synchronizations in the 60-min period during and after treatments was expressed as the synchronization frequency. The effects of treatments were analyzed against their respective controls, and statistics were performed using Student's t test (unpaired, two tailed). All groups consisted of six cultures per group. Data are presented as means ± sem. Significance was established at P < 0.05.
Results
STX stimulated GnRH release
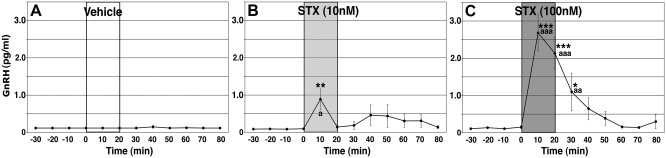
As seen in Fig. 1B, 10 nm STX infusion resulted in a significant increase in GnRH release at 10 min when compared with the level before the treatment (P < 0.01, n = 7). Similarly, 100 nm STX (Fig. 1C) caused an increase in GnRH release at 10 min (P < 0.001; n = 16), which remained elevated for an additional 20 min. Vehicle (Fig. 1A) alone did not change GnRH levels (n = 9). When compared with vehicle control, STX-induced GnRH elevations at 10 min were significantly higher after both 10 nm (P < 0.05) and 100 nm (P < 0.001) treatments. Additionally, the elevated GnRH levels with 100 nm STX at 20 and 30 min were also significantly higher than those in vehicle control (P < 0.001 and P < 0.01, respectively).
Fig. 1.
STX stimulated GnRH release in cultured primate GnRH neurons. A–C, Vehicle (A) or STX at 10 nm (B) or 100 nm (C) was infused for 20 min, whereas perifusate samples were collected at 10-min intervals. Note that STX (100 nm) resulted in an increase in GnRH release within 10 min, similar to E2, and effects lasted for an additional 10 min after infusion was terminated, shorter than the E2 effect (34). Vehicle had no effect. The interaction between challenges was significantly different (P < 0.001). *, P < 0.05 vs. before STX; **, P < 0.01 vs. before STX; ***, P < 0.001 vs. before STX; a, P < 0.05 vs. vehicle; aa, P < 0.01 vs. vehicle; aaa, P < 0.001 vs. vehicle.
STX caused stimulatory effects in [Ca2+]i oscillations
To further characterize the stimulatory action of STX, we examined the effect of 10 nm STX on [Ca2+]i oscillations in GnRH neurons. As seen in the example (Fig. 2A), 10 nm STX caused an increase in the frequency of [Ca2+]i oscillations in individual cells and increased the synchronization frequency among GnRH neurons. Vehicle treatment did not change the pattern of [Ca2+]i oscillations or the synchronization frequency in GnRH neurons (Fig. 2B). Statistical analysis indicated that 10 nm STX significantly increased the frequency of [Ca2+]i oscillations (P < 0.001, Fig. 3A) as well as the percentage of stimulated cells (P < 0.05, Fig. 3B) from the baseline level (n = 263 neurons), whereas the vehicle treatment did not cause any changes (n = 327 neurons, Fig. 3). The STX-induced increases in both the frequency of [Ca2+]i oscillations (P < 0.001, Fig. 3A) and the percentage of cells stimulated by treatment (P < 0.05, Fig. 3B) were also significantly different from vehicle controls. In addition, STX significantly increased the synchronization frequency when compared with vehicle controls (P < 0.01, Table 1).
Fig. 2.
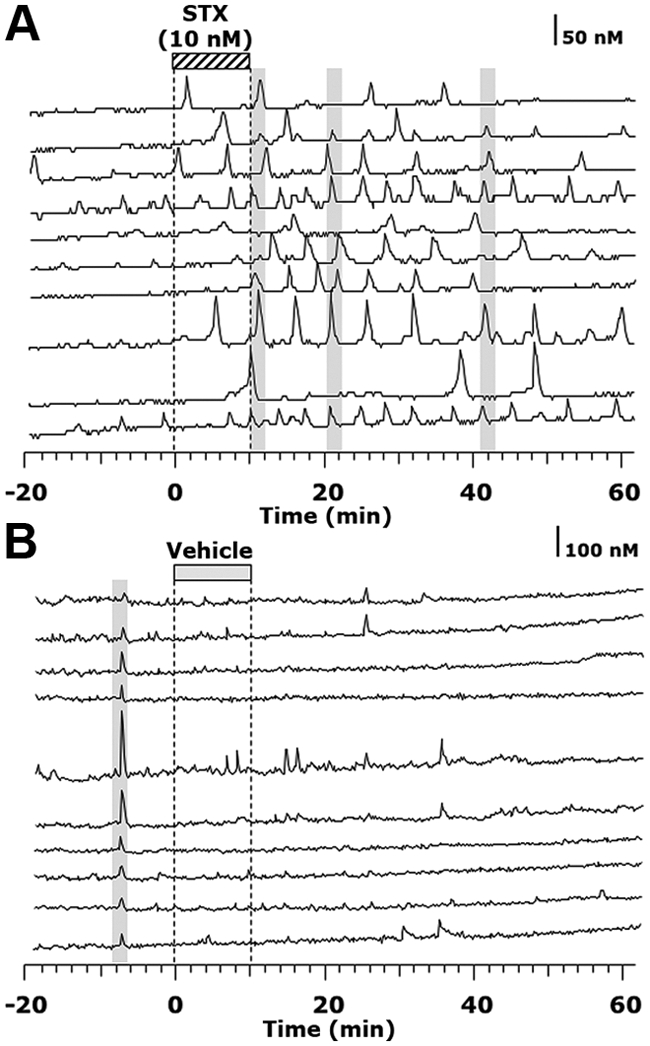
STX elicited an increase in the frequency and the synchronizations (gray bars) of [Ca2+]i oscillations. A and B, Single-cell traces of [Ca2+]i oscillations in a STX-treated culture (A) and a control culture (B).
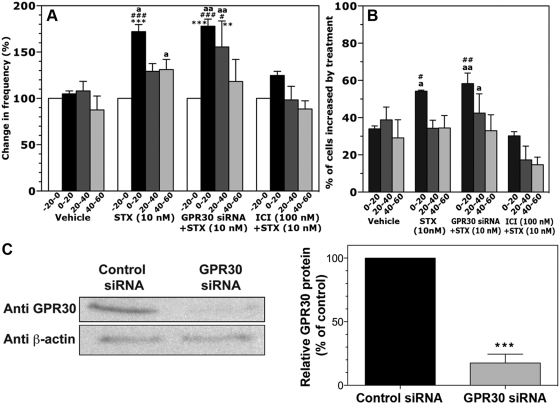
Fig. 3.
A and B, Effects of STX on the [Ca2+]i oscillation frequency (A) and the percentage of stimulated cells (B). STX elicited an increase in the frequency of [Ca2+]i oscillations and the percentage of stimulated cells, similar to those reported with E2. To examine the mechanism of STX actions, the effects of STX were tested in cells transfected with GPR30 siRNA as well as in the presence of ICI182,780 (ICI). Note that the STX-induced changes in [Ca2+]i oscillations were not blocked in cells transfected with GPR30 siRNA, whereas ICI blocked the STX-induced changes in [Ca2+]i oscillations. **, P < 0.01 vs. −20–0; ***, P < 0.001 vs. −20–0 (within treatment); #, P < 0.05 vs. vehicle; ##, P < 0.01 vs. vehicle; ###, P < 0.001 vs. vehicle at corresponding time (between treatments); a, P < 0.05, and aa, P < 0.01 vs. ICI (100 nm) plus STX (10 nm) at corresponding time (between treatments). C, Transfection of cultures with human/monkey GPR30 siRNA reduced GPR30 protein expression. Immunoblotting of membrane protein with antibodies against GPR30 or β-actin, from control siRNA (lane 1) and GPR30 siRNA (lane 2) transfected cultures, is shown on the left. Densitometry analysis, performed with ImageQuant software using results from three separate experiments, is shown on the right. Note that transfection with GPR30 siRNA significantly (P < 0.001) reduced GPR30 expression in olfactory placode cultures. ***, P < 0.001 vs. control siRNA.
Table 1.
Effects of treatments on the synchronization of calcium oscillations in primate GnRH neurons
| Treatments | Synchronizations in the 60-min period after initiation of treatment (n) |
|---|---|
| Vehicle | 1.00 ± 0.29 |
| STX (10 nm) | 2.50 ± 0.43a |
| GPR30 siRNA + STX (10 nm) | 2.33 ± 0.21a |
| ICI182,780 (100 nm) + STX (10 nm) | 0.40 ± 0.24 |
| U73122 (10 μm) | 0.67 ± 0.42 |
| U73122(10 μm) + STX (10 nm) | 0.50 ± 0.34 |
| E2 (1 nm) | 2.50 ± 0.67a |
| E2 (10 nm) | 2.00 ± 0.51a |
| GPR30 siRNA + E2 (10 nm) | 2.17 ± 0.54a |
In all treatment groups, n = 6.
P < 0.01 vs. vehicle control.
Stimulatory effects of STX were not mediated through GPR30
It has been shown that although E2 action through GPR30 is mediated by Gαs (42), STX-sensitive receptors activate Gαq (22). However, because GPCRs have been demonstrated to bind multiple Gα proteins (43, 44), and because there is a potential for receptor cross-talk, we examined whether the stimulatory action of STX occurred in GnRH neurons in which cellular GPR30 was knocked down. As seen in Fig. 3A, application of 10 nm STX in GnRH neurons treated with siRNA for human/monkey GPR30 caused an increase in the frequency of [Ca2+]i oscillations from basal levels (P < 0.001; n = 231). Comparison between groups indicated that STX treatment in the presence of GPR30 siRNA caused significant increases in the frequency of [Ca2+]i oscillations (P < 0.001) as well as the percentage of cells stimulated (P < 0.01, Fig. 3B) compared with the vehicle control or scrambled sequence control siRNA transfected cells (data not shown, see Ref. 34). Furthermore, GPR30 siRNA treatment did not block the STX-induced synchronizations of [Ca2+]i oscillations (P < 0.01, Table 1). In all parameters, the STX-induced effects did not differ in the presence or absence of GPR30 siRNA treatment. Protein levels (17.6 ± 6.9%) in GPR30 siRNA transfected cultures were significantly (P < 0.001) reduced relative to those (100%) in control siRNA transfected cultures (n = 3, Fig. 3C).
Stimulatory effects of STX were blocked by ICI182,780
Because in mouse hypothalamic neurons, ICI182,780 significantly blocked STX effects (22, 23), we examined the effects of STX in the presence of ICI182,780. As seen in Fig. 3A, application of 10 nm STX in GnRH neurons treated with 100 nm ICI182,780 blocked the STX-induced increase in the frequency of [Ca2+]i oscillations from basal levels (n = 177 neurons). Although there was a slight trend of increase during the first 20 min of STX application, this increase was not significant. Comparison between groups indicated that the frequency increase of [Ca2+]i oscillations with STX treatment in the presence of ICI182,780 was significantly different from STX alone (P < 0.05, Fig. 3A) but not from vehicle. Similarly, percentage of cells stimulated by STX in the presence of ICI182,780 was also significantly different from STX alone (P < 0.05, Fig. 3B) but not from vehicle. Moreover, ICI182,780 treatment blocked the STX-induced increase in the synchronization frequency (Table 1).
The PLC inhibitor U73122 blocked STX-induced effects on [Ca2+]i oscillations
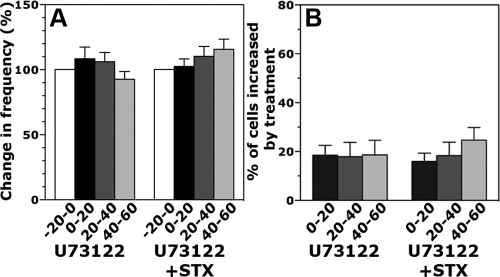
If STX-sensitive receptors are present in primate GnRH neurons, blocking a PLC mechanism should abrogate the STX effects, as was shown in guinea pig and mouse POMC neurons (22). Treatment with 10 μm U73122 completely blocked STX-induced increases in the frequency of [Ca2+]i oscillations (Fig. 4A), the percentage of stimulated cells (n = 263 neurons, Fig. 4B), and the synchronization frequency (Table 1). U73122 itself caused no significant effects (n = 251 neurons).
Fig. 4.
A and B, Treatment of GnRH primary cultures with 10 μm of the broad-spectrum PLC inhibitor U73122 for 10 min abrogated the STX-induced increase in the frequency of [Ca2+]i oscillations (A) and the percentage of cells stimulated by treatment (B). This is similar to U73122 effects on STX-sensitive receptor signal transduction pathway in guinea pig POMC neurons (22).
Effects of GPR30 siRNA on changes induced by a higher dose of E2 (10 nm) on [Ca2+]i oscillations
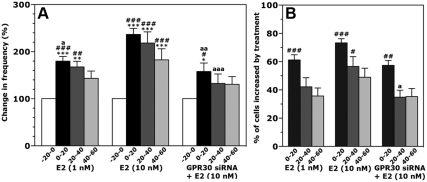
Although in a previous study (34), GPR30 siRNA treatment of GnRH neurons blocked the E2 (1 nm) effect, in the present study, the same procedure failed to block the effects of STX (10 nm), indicating the presence of multiple mechanisms for rapid E2 action. To further examine this possibility, we tested whether the effects of a higher dose (10 nm) of E2 could be blocked by GPR30 siRNA transfection. A 10-min application of E2 at both 1 nm (n = 183 neurons) and 10 nm (n = 285 neurons) significantly increased the frequency of [Ca2+]i oscillations (Fig. 5A) as well as the percentage of cells stimulated by treatment (Fig. 5B) (for both, P < 0.001). In addition, the effects of 10 nm E2 were larger and longer than those with 1 nm (P < 0.05). Both 1 and 10 nm E2 similarly increased the synchronization frequency among GnRH neurons (P < 0.01 for both vs. vehicle, Table 1).
Fig. 5.
A and B, E2 (1 and 10 nm) elicited an increase in the frequency (A) and the percentage of stimulated cells (B) in a dose-responsive manner. Although the effect of E2 at 1 nm was completely blocked by transfection with GPR30 siRNA (see Ref. 34), the effect of E2 at 10 nm reduced, but did not completely block, the frequency of [Ca2+]i oscillations and the percentage of stimulated cells in cultures transfected with GPR30 siRNA. *, P < 0.05 vs. −20–0; **, P < 0.01 vs. −20–0; ***, P < 0.001 vs. −20–0 (within treatment); #, P < 0.05 vs. vehicle; ##, P < 0.01 vs. vehicle; ###, P < 0.001 vs. vehicle at the corresponding time (between treatments; vehicle from experiment 2, see Fig. 3); a, P < 0.05 vs. E2 (10 nm); aa, P < 0.01 vs. E2 (10 nm); aaa, P < 0.001 vs. E2 (10 nm) at the corresponding time (between treatments).
Examination of 10 nm E2 effects in GPR30 siRNA transfected GnRH neurons indicated that GPR30 siRNA partially blocked the E2-induced changes in the frequency of [Ca2+]i oscillations and percentage of cells stimulated. In fact, 10 nm E2 with GPR30 siRNA transfection (n = 215 neurons) caused an increase in the frequency of [Ca2+]i oscillations (P < 0.05) and the number of stimulated cells (P < 0.01 vs. vehicle from Fig. 3). However, these 10 nm E2-induced changes in GPR30 siRNA-treated GnRH neurons were significantly reduced compared with 10 nm E2 alone (P < 0.01, Fig. 5, A and B). GPR30 siRNA transfection was not able to block the 10 nm E2 increase in the synchronization frequency (P < 0.01 vs. vehicle, Table 1).
Discussion
In the present study, the effects of STX on the activity of GnRH neurons were investigated. The results indicate that STX induces stimulatory actions, similar to E2 actions, on primate GnRH neurons. The results further indicate that the STX actions were not mediated through GPR30. Collectively, STX-sensitive receptors appear to differ from GPR30, and therefore, E2 appears to cause rapid actions through multiple membrane receptors in primate GnRH neurons.
It has been shown that STX, a nonsteroidal compound, which does not bind ERα or ERβ, induces estrogenic action in hypothalamic neurons. Administration of STX in female ovariectomized guinea pigs significantly decreased the core body temperature, food intake, and peripheral fat accumulation and increased cancellous tibial bone density (25). Electrophysiological studies also indicate that STX attenuates the GABAB response in POMC neurons through membrane receptors activating a Gαq-PLC-protein kinase C and protein kinase A pathway, which is similar to that with estradiol (22, 23). In addition, STX causes its estrogen-like action in αERKO, βERKO, αβERKO, and GPR30-null mice (23, 24). Although the structure of STX-sensitive receptors is currently unknown, our observations in the present study suggest that STX-sensitive receptors are a novel mER in primate GnRH neurons and that STX causes its action via a PLC pathway.
Previously, we have reported in primate GnRH neurons that E2 stimulates firing rate within a minute (45) and the frequency of [Ca2+]i oscillations and their synchronization (32) and release of the decapeptide within 10 min (34). Moreover, we have found that the rapid action of 1 nm E2 in GnRH neurons is, in part, mediated by GPR30, because the E2-induced rapid stimulation of [Ca2+]i oscillations is attenuated by GPR30-specific siRNA transfection, and G1 induces effects similar to those with E2 (34). The results of the present study further show that a STX-sensitive mechanism appears to also be involved in the rapid E2 action in GnRH neurons, because STX increased the frequency of [Ca2+]i oscillations and their synchronization and GnRH release. Nonetheless, it is important to point out that increased [Ca2+]i oscillations and GnRH release are consistently induced not only by STX, E2 (34), and G1 (34) (Kenealy, B., and E. Terasawa, unpublished observations) but also by ATP (46) and depolarization stimuli with high K+ (38) in primate GnRH neurons. The mechanism of STX action, which leads to an increased frequency of [Ca2+]i oscillations and their synchronization and subsequent GnRH neurosecretion is yet to be investigated.
The mechanism of rapid E2 action in the brain appears to be more complex than previously thought (47). Rapid E2 actions can be mediated through ERα and ERβ in mouse GnRH neurons (26–29, 48); GPR30 in monkey GnRH neurons (34); STX-sensitive receptors in mouse POMC, dopamine, and GnRH neurons (22, 35, 49); and ER-X in mouse cortical neurons (21). Among the mER that mediate rapid E2 action in neurons, GPR30 (34) and STX-sensitive receptors (22) are both G protein-coupled receptors. Moreover, rapid E2 actions through ERα and ERβ in rat hippocampal and striatal neurons are associated with the G protein-coupled, metabotropic glutamate type I and II receptors (mGluR1 and mGluR2) (50–52). Finally, rapid increases in [Ca2+]i induced by the ERα agonist propyl pyrazole triol (PPT), the ERβ agonist diaryl propionitrile (DPN), and STX in mouse hypothalamic astrocytes, were blocked by a mGluR1 inhibitor, indicating that E2 action mediated by ERα, ERβ, and STX-sensitive receptors may signal via a similar pathway with mGluR1 (53). G1 also induced increases in [Ca2+]i in astrocytes, but this was not blocked by the mGluR1 inhibitor, indicating that G1 signals differently from E2 and STX in astrocytes (53). Recently, neuroprotective effects by the GPR30 agonist, G1, and STX have also been reported in rat hippocampal CA1 neurons after an ischemic insult (54). Therefore, in general, the involvement of G protein-coupled receptors appears to be important and unique for mediating rapid E2 action in neuronal cells and astrocytes. In fact, observations in this study as well as a previous study in mice (24) showing that STX action in neuronal cells is not mediated by GPR30, clearly differ from a recent report that STX activates GPR30 in endometrial cancer cells (55). Perhaps the different interaction between STX and GPR30 in endometrial cancer cells may be related to the growth transformation in these cancer cells, because STX has no proliferative effects in normal uterine cells (22, 23, 25).
In the present study, the effects of STX on [Ca2+]i oscillations were neither blocked nor attenuated in GnRH neurons with GPR30 knockdown. One might argue that STX-induced [Ca2+]i oscillations in knockdown cells are due to residual (15–18%) GPR30 (34) (this study). This, however, is unlikely because 1) if there is any interaction between the two receptors, we should have seen some reduction in STX effects in GnRH neurons with GPR30 knockdown, and 2) STX-sensitive receptors and GPR30 have significantly different properties. STX effects are mediated through Gαq activation (22), are observed in GPR30-null mice (24), and are blocked by ICI182,780 (22), whereas GPR30 effects are mediated through a Gαs pathway with epidermal growth factor receptor activation in cancer cells (16) but are not blocked by 100 nm ICI182,780 and are enhanced by a higher dose (1 μm) of ICI182,780 (34).
The observations in the previous (34) and present studies suggest the presence of two mER in primate GnRH neurons. First, STX effects are not blocked by cellular deletion of GPR30. Second, a 10 nm dose is required for STX- or G1-induced (34) changes in [Ca2+]i oscillations similar to those observed with 1 nm E2, indicating that both may be required for full E2 effects, although we cannot rule out that the synthetic compounds G1 and STX may not be as potent as the natural hormone E2 in eliciting effects in monkey GnRH neurons. Third, the effects of STX are blocked by ICI182,780, whereas G1 effects are not blocked by ICI182,780 (56). Although we cannot exclude the possibility that 10 nm E2 may activate residual GPR30 in GPR30 siRNA transfected cells, it is tempting to speculate that a full response induced by E2 requires a summation of multireceptor activation. This will be further investigated in future experiments.
Previously, we reported two puzzling observations in primate GnRH neurons. In an earlier study, the E2-induced increase in firing activity was blocked by ICI182,780 (45), whereas in later studies, the E2-induced increase in [Ca2+]i oscillations was not blocked by ICI182,780 (32, 34). The relationship between firing activity and [Ca2+]i oscillations in primate GnRH neurons is currently unknown. However, the findings of the present study, indicating that the actions of E2 appear to be mediated through two different mER (i.e. STX-sensitive receptors and GPR30), both of which resulted in increased [Ca2+]i oscillations, provides a possible explanation for these puzzling observations. That is, an increase in firing activity by E2 may be a consequence of STX-sensitive receptor activation, which is also sensitive to ICI182,780, whereas an increase in [Ca2+]i oscillations by E2 is due to convergence of STX-sensitive receptor and GPR30-mediated pathways. The data in which ICI182,780 blocked the STX-induced stimulation of [Ca2+]i oscillations further suggest that [Ca2+]i oscillations may be a convergence point for multiple mER action. Moreover, our collateral study (33) indicates that ERα and ERβ are not involved in the E2-induced increase in [Ca2+]i oscillations, because transfection with neither ERα nor ERβ-specific siRNA blocked the E2-induced increase in [Ca2+]i oscillations in primate GnRH neurons. Therefore, because E2-induced [Ca2+]i oscillations through STX-sensitive receptors and GPR30 are independent mechanisms, inactivation of STX-sensitive receptors by ICI182,780 can be overridden by E2 activation through GPR30.
In conclusion, we report that STX causes a stimulatory action in primate GnRH neurons by a GPR30-independent mechanism. This provides evidence for multiple mechanisms by at least two different mER of rapid action caused by E2 in GnRH neurons. The implications of current findings from embryonic GnRH neurons to adult reproductive function may require some caution. Nonetheless, having more than one mechanism of action may provide flexibility for modulation of GnRH neuronal activity by different external signals, which is important for successful reproductive function. The role of STX-sensitive receptors and GPR30 mediating E2 action in primate GnRH neurons in vivo remains to be investigated.
Acknowledgments
We thank Mr. Nicholas Shiel (University of Wisconsin-Madison) for technical assistance.
This work was supported by National Institutes of Health (NIH) Grants R01HD15433 and R01HD11355 for E.T. and R01NS 43330 for O.K.R. and was made possible to perform by NIH support (P51RR000167, RR15459, and RR020141) to the Wisconsin National Primate Research Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- [Ca2+]i
- Intracellular calcium concentration
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GPR30
- G protein-coupled receptor 30
- mER
- membrane ER
- PLC
- phospholipase C
- POMC
- proopiomelanocortin
- siRNA
- small interfering RNA
- STX
- diphenylacrylamide estrogenic compound
- TBST
- Tris-buffered saline with 0.1% Tween 20.
References
- 1. Herbison AE. 1998. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- 2. Pfaff D. 2005. Hormone-driven mechanisms in the central nervous system facilitate the analysis of mammalian behaviours. J Endocrinol 184:447–453 [DOI] [PubMed] [Google Scholar]
- 3. Vasudevan N, Pfaff DW. 2008. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol 29:238–257 [DOI] [PubMed] [Google Scholar]
- 4. Szego CM, Davis JS. 1967. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA 58:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yagi K. 1973. Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res 53:343–352 [DOI] [PubMed] [Google Scholar]
- 6. Kelly MJ, Moss RL, Dudley CA. 1976. Differential sensitivity of preoptic-septal neurons to microelectrophoresed estrogen during the estrous cycle. Brain Res 114:152–157 [DOI] [PubMed] [Google Scholar]
- 7. Lagrange AH, Rønnekleiv OK, Kelly MJ. 1995. Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- 8. Singh M, Sétáló G, Jr, Guan X, Warren M, Toran-Allerand CD. 1999. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci 19:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. 1999. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J Neurosci 19:2455–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu Q, Korach KS, Moss RL. 1999. Rapid action of 17β-estradiol on kainite induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology 140:660–666 [DOI] [PubMed] [Google Scholar]
- 11. Wong M, Moss RL. 1992. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci 12:3217–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gajjar TM, Anderson LI, Dluzen DE. 2003. Acute effects of estrogen upon methamphetamine induced neurotoxicity of the nigrostriatal dopaminergic system. J Neural Transm 110:1215–1224 [DOI] [PubMed] [Google Scholar]
- 13. Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. 2007. Direct interactions with GαI and Gβγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol 21:1370–1380 [DOI] [PubMed] [Google Scholar]
- 14. Razandi M, Pedram A, Greene GL, Levin ER. 1999. Cell membrane and nuclear receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- 15. Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. 2010. Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 24:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 17. Haas E, Bhattacharya I, Brailoiu E, Damjanoviæ M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. 2009. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104:288–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammond R, Nelson D, Gibbs RB. 2011. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology 36:182–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. 2007. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- 20. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 21. Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. 2002. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci 22:8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. 2003. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci 23:9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. 2006. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci 26:5649–5655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu J, Rønnekleiv OK, Kelly MJ. 2008. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids 73:985–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. 2010. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151:4926–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Temple JL, Laing E, Sunder A, Wray S. 2004. Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. 2003. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 23:5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chu Z, Andrade J, Shupnik MA, Moenter SM. 2009. Differential regulation of gonadotropin releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29:5616–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun J, Chu Z, Moenter SM. 2010. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 30:3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharifi N, Reuss AE, Wray S. 2002. Prenatal LHRH neurons in nasal explant cultures express estrogen receptor β transcript. Endocrinology 143:2503–2507 [DOI] [PubMed] [Google Scholar]
- 31. Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. 2001. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- 32. Abe H, Keen KL, Terasawa E. 2008. Rapid action of estrogens on intracellular calcium oscillations in primate LHRH-1neurons. Endocrinology 149:1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kenealy BP, Keen KL, Terasawa E. 25 February 2011. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor α and estrogen receptor β. Steroids 10.1016/j.steroids.2011.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. 2009. Involvement of G-protein couple receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Kelly MJ, Rønnekleiv OK. 2010. 17β-Estradiol rapidly increases KATP activity in GnRH via a protein kinase signaling pathway. Endocrinology 151:4477–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- 37. Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. 1993. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- 38. Terasawa E, Keen KL, Mogi K, Claude P. 1999. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- 39. Richter TA, Keen KL, Terasawa E. 2002. Synchronization of Ca2+ oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol 88:1559–1567 [DOI] [PubMed] [Google Scholar]
- 40. Gearing M, Terasawa E. 1988. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull 21:117–121 [DOI] [PubMed] [Google Scholar]
- 41. Merriam GR, Wachter KW. 1982. Algorithms for the study of episodic hormone secretion. Am J Physiol 243:E310–E318 [DOI] [PubMed] [Google Scholar]
- 42. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. 2002. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- 43. Krsmanovic LZ, Hu L, Leung PK, Feng H, Catt KJ. 2010. Pulsatile GnRH secretion: roles of G protein-coupled receptors, second messengers and ion channels. Mol Cell Endocrinol 314:158–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krsmanovic LZ, Mores N, Navarro CE, Arora KK, Catt KJ. 2003. An agonist-induced switch in G protein coupling of the gonadotropin-releasing hormone receptor regulates pulsatile neuropeptide secretion. Proc Natl Acad Sci USA 100:2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abe H, Terasawa E. 2005. Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terasawa E, Keen KL, Grendell RL, Golos TG. 2005. Possible role of 5′adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons Mol Endocrinol 19:2736–2747 [DOI] [PubMed] [Google Scholar]
- 47. Kelly MJ, Rønnekleiv OK. 2008. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol 290:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Romanò N, Lee K, Abrahám IM, Jasoni CL, Herbison AE. 2008. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology 149:5335–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 2009. 17β-Estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci 29:10552–10562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. 2005. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci 25:5066–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boulware MI, Kordasiewicz H, Mermelstein PG. 2007. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci 27:9941–9950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grove-Strawser D, Boulware MI, Mermelstein PG. 2010. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience 170:1045–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. 2010. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. J Neurosci 30:12950–12957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. 2010. Acute administration of non-classical estrogen receptor agonists attenuates ischemia induced hippocampal neuron loss in middle-aged female rats. PLoS One 5:e8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA. 2009. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res 69:5415–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chimento A, Sirianni R, Delalande C, Silandre D, Bois C, Andò S, Maggiolini M, Carreau S, Pezzi V. 2010. 17β-Estradiol activates rapid signaling pathways involved in rat pachytene spermatocytes apoptosis through GPR30 and ERα. Mol Cell Endocrinol 320:136–144 [DOI] [PubMed] [Google Scholar]