Abstract
The long-acting glucagon-like peptide-1 receptor (GLP-1R) agonists, exendin-4 and liraglutide, suppress food intake and body weight. The mediating site(s) of action for the anorectic effects produced by peripheral administration of these GLP-1R agonists are not known. Experiments addressed whether food intake suppression after ip delivery of exendin-4 and liraglutide is mediated exclusively by peripheral GLP-1R or also involves direct central nervous system (CNS) GLP-1R activation. Results showed that CNS delivery [third intracerebroventricular (3rd ICV)] of the GLP-1R antagonist exendin-(9–39) (100 μg), attenuated the intake suppression by ip liraglutide (10 μg) and exendin-4 (3 μg), particularly at 6 h and 24 h. Control experiments show that these findings appear to be based neither on the GLP-1R antagonist acting as a nonspecific competing orexigenic signal nor on blockade of peripheral GLP-1R via efflux of exendin-(9–39) to the periphery. To assess the contribution of GLP-1R expressed on subdiaphragmatic vagal afferents to the anorectic effects of liraglutide and exendin-4, food intake was compared in rats with complete subdiaphragmatic vagal deafferentation and surgical controls after ip delivery of the agonists. Both liraglutide and exendin-4 suppressed food intake at 3 h, 6 h, and 24 h for controls; for subdiaphragmatic vagal deafferentation rats higher doses of the GLP-1R agonists were needed for significant food intake suppression, which was observed at 6 h and 24 h after liraglutide and at 24 h after exendin-4. Conclusion: Food intake suppression after peripheral administration of exendin-4 and liraglutide is mediated by activation of GLP-1R expressed on vagal afferents as well as direct CNS GLP-1R activation.
The prevalence of obesity in the United States has increased by 75% since 1980, with more than one third of adults now classified as obese and another third considered overweight (1, 2). Effective pharmacological treatments for hyperphagia and elevated adiposity are needed to reduce the pathophysiology of obesity and its comorbidities. One promising antiobesity drug target is the hormone, glucagon-like peptide-1 (GLP-1) and its receptor (GLP-1R). GLP-1 is a peptide synthesized and secreted principally from two locations: the L cells in the distal small intestine and proglucagon-expressing neurons in the nucleus tractus solitarius (NTS) of the caudal brain stem (3). Systemic GLP-1 is released in response to nutrient entry into the gastrointestinal (GI) tract (4) and acts on peripheral GLP-1R expressed on vagal afferents and pancreatic β-cells and potentially on central GLP-1R-expressing neurons to engage physiological responses that include enhanced glucose-stimulated insulin secretion, inhibition of gastric emptying, and reduced food intake (see Refs. 5 and 6 for review). Clinical use of the native biologically active forms of the peptide, GLP-1(7–36) NH2 and GLP-1(7–37), for the treatment of type 2 diabetes mellitus (T2DM) and obesity, however, is limited by that fact that endogenous GLP-1 is rapidly degraded by the enzyme dipeptidyl-peptidase-4 and various endopeptidases, thus limiting its half-life to approximately 2 min (7). Pharmacotherapy for the treatment of T2DM has benefited from the development of longer-acting GLP-1R agonists such as exendin-4, a naturally occurring peptide extracted from the saliva of the Gila monster (Heloderma suspectum) that is resistant to rapid enzymatic degradation, and liraglutide, a synthetic GLP-1 agonist in the duration of action of which is enhanced by its binding affinity to albumin and subsequent reduced renal excretion and resistance to enzymatic degradation. In addition to their beneficial effects on glycemic control, recent research shows that both exendin-4 and liraglutide reduce food intake and body weight in human and animal models (e.g. Refs. 8–11), suggesting that these drugs may also be efficacious for the treatment of obesity.
The site(s) of action for the intake suppression triggered by exogenous long-acting GLP-1 analogs and even for endogenous GLP-1 is not clear. For each, the mediating GLP-1R may be limited to those in the periphery or may also involve GLP-1R in the brain. Current debate on this topic is influenced by the fact that GLP-1R are expressed at peripheral and central locations and that proglucagon-expressing cells in the gut and hindbrain endogenously produce GLP-1 (see Refs. 5 and 6 for review). Some evidence supports the notion that endogenously secreted GLP-1 from the GI tract suppresses intake by acting in a local-humoral and/or paracrine-like fashion on adjacent GLP-1R expressed on vagal afferents innervating the hepatoportal bed and GI tract (12–14), respectively, as opposed to acting directly on GLP-1R expressed in the central nervous system (CNS). The extremely short half-life of endogenous GLP-1 makes blood brain barrier (BBB) penetration and subsequent direct CNS action unlikely under normal physiological conditions (6). Further, ip administration of low doses of GLP-1, resembling the physiological profile of action for endogenous GLP-1 released from intestinal L cells, reduces food intake in sham-operated rats, but not in rats with subdiaphragmatic vagal deafferentation (SDA) (13). These findings suggest that the satiating effects of endogeonous GLP-1 are likely mediated by a peripheral site of action that requires mediation by GLP-1R expressed on vagal afferent neurons innervating the GI tract.
The relevant site(s) of action for the intake suppression triggered by exogenous peripheral injection of the long-acting GLP-1 agonists exendin-4 and liraglutide may well differ from that of endogenously released GLP-1. Exendin-4 and liraglutide have dramatically longer durations of action compared with GLP-1, with half-lives approximating 2.5 (15) and 13 h (16), respectively. This, coupled with evidence that exendin-4 (17) and liraglutide (18) penetrate the BBB in rats potentially via passive diffusion, suggests that the energy balance effects of peripheral administration of these GLP-1R agonists may result, in part, from direct activation of GLP-1R expressed in CNS nuclei critical to energy balance regulation. This possibility has yet to be thoroughly examined and is the subject of this report.
Here we employ surgical as well as pharmacological strategies to examine whether the intake inhibitory effects of peripheral (ip) delivery of exendin-4 and liraglutide are mediated exclusively by a peripheral (vagal) site of action, or involve both vagal and CNS mediation. In the first experiment, the GLP1-R antagonist, exendin-(9–39), was injected centrally to evaluate whether blockade of CNS GLP-1R attenuates the intake and body weight-suppressive effects of ip injected exendin-4 and liraglutide. In another experiment the contribution of GLP-1R expressed on subdiaphragmatic vagal afferents was examined by comparing responses from rats with complete SDA (19) to sham-operated controls after ip administration of exendin-4 and liraglutide. Collectively, results from the pharmacological and deafferentation studies were consistent with the hypothesis that these long-acting GLP-1R agonists suppress food intake and body weight through a combined activation of vagal GLP-1R and CNS GLP-1R.
Materials and Methods
Animals and drugs
Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA), housed individually in hanging metal cages under a reverse 12-h light, 12-h dark cycle (lights on 2200 h), had ad libitum access to rodent chow (Purina 5001; Ralston Purina Co., St. Louis, MO) and water except where noted. All procedures conformed to the institutional standards of The University of Pennsylvania Animal Care and Use Committee.
Exendin-4 (American Peptide Co., Sunnyvale, CA), liraglutide (Bachem California, Torrance, CA), and salmon calcitonin (sCT) (Bachem) were dissolved in sterile 0.9% NaCl. Exendin-(9–39) (American Peptide) was dissolved in artificial cerebrospinal fluid (aCSF) (Harvard Apparatus, Holliston, MA).
3rd intracerebroventricular (ICV) cannula implantation
Under ketamine (90 mg/kg), xylazine (2.7 mg/kg), and acepromazine (0.64 mg/kg) anesthesia and analgesia (Metacam, 2 mg/kg), guide cannulae (Plastics One; 26-guage) cemented to the skull using four jewelers screws were implanted at the following coordinates for targeting the third cerebral ventricle (3rd ICV): 2.0 mm caudal to bregma, 7.7 mm below skull surface, on midline. Injectors for 3rd ICV drug administration project 2 mm beyond guide cannulae. After a 7-d surgical recovery period, 3rd ICV cannula placement was assessed by measurement of the sympathoadrenal-mediated hyperglycemic response to the cytoglucopenia induced by 5-thio-d-glucose (210 μg in 2 μl aCSF) (20). A postinjection elevation of at least 100% of baseline glycemia was required for subject inclusion.
SDA surgery
SDA consists of a transection of the left dorsal vagal afferent rootlets and dorsal (left) esophageal vagal trunks, resulting in complete SDA, while sparing approximately half of the abdominal vagal efferents. The SDA surgical procedure has advantages over other vagal afferent ablation approaches. In contrast to neurotoxic lesion of unmyelinated vagal/non-vagal C-fibers by capsaicin, the SDA surgery eliminates all types (both A- and C-fibers) of vagal afferents. Further, unlike complete subdiaphragmatic vagotomy, the SDA surgery leaves intact approximately 50% of the vagal efferents innervating the peritoneal cavity. However, the SDA approach does not eliminate all of the limitations from the capsaicin- or complete-vagotomy approaches, as spinal afferents and approximately 50% of the vagal afferents above the diaphragm remain intact. Further, whereas SDA rats can be easily maintained on solid food, in contrast to complete subdiaphragmatic vagotomy, the eating rate of SDA rats has been reported as reduced relative to controls (13, 21).
The procedures for SDA are described in more detail elsewhere (see Ref. 19). Briefly, in anesthetized rats, the procedure requires exposing and cutting the vagal afferent rootlets unilaterally (left) and then sectioning the contralateral (ventral) subdiaphragmatic vagal trunk. The sham procedure was similar, with the exception that the vagal rootlets and abdominal vagus were exposed, but not manipulated further. Rats were nursed with liquid diets for approximately 1 wk after surgery and were then returned to ad libitum solid chow maintenance.
At the conclusion of all behavioral procedures, SDA surgery was verified functionally and histologically (21). The functional test involved a within-subject repeated measures analysis of cholecystokinin (CCK)-induced food intake suppression, a response that depends on abdominal vagal afferent fibers (22–24). Briefly, after 4-h food deprivation in the beginning of the dark cycle, rats were injected ip with 3 μg/kg CCK-8 (American Peptides) or saline according to a counterbalanced design. CCK-8 (American Peptide) reduced 30-min food intake by 43–72% in sham-operated rats. Using an inclusion criteria used by others, SDA rats were included in the statistical analyses if CCK treatment resulted in a less than 30% reduction of their food intake (13, 21). Histological verification involved examining the presence of retrograde labeling of vagal motor neurons in the dorsal motor nucleus of the vagus (DMV). Rats were injected ip with 2 mg Fluoro-Gold (Fluorochrome) in 1 ml NaCl. Four days later they were anesthetized 4 d later and intracardially perfused with PBS, followed by 4% formalin in PBS. An observer blind to the rats' surgery and behavioral data analyzed Fluoro-Gold-labeling in the DMV on coronal sections. The inclusion criterion for SDA rats were that some retrograde labeling be observed in the left DMV, and that retrograde labeling in the right DMV be less than 3% of the number in the left DMV. For sham rats motor neurons would be labeled bilaterally.
Procedures
Experiment 1a
Rats (n = 8) weighing approximately 400–450 g were given one 3rd ICV injection (volume = 2 μl; aCSF vehicle) followed 15 min later by one ip injection (volume = 1 ml/kg, 0.9% NaCl vehicle). The ip injection occurred immediately before the onset of the dark cycle. The following treatments were administered in a counterbalanced design (ICV/ip): 1) vehicle/vehicle; 2) exendin-(9–39), 100 μg/vehicle; 3) vehicle/liraglutide, 10 μg/kg; and 4) exendin-(9–39), 100 μg/liraglutide, 10 μg/kg; 5) vehicle/exendin-4, 3.0 μg/kg; and 6) exendin-(9–39), 100 μg/exendin-4, 3.0 μg/kg. Subsequent chow intake was recorded at 1 h, 3 h, 6 h, and 24 h (recorded to the nearest 0.1 g, spillage collected and accounted for), and body weights were recorded at 24-h postinjections. Injection treatments were separated by 3–4 d, which allowed sufficient time for body weight recovery. The doses of liraglutide and exendin-4 used were based on pilot work showing that they produced an equivalent magnitude of food intake suppression 6 h after ip administration.
Experiment 1b
To determine whether ICV exendin-(9–39) acts as a nonspecific competing orexigenic signal through blockade of basal CNS GLP-1R signaling (25, 26), we examined whether ICV exendin-(9–39) could attenuate the intake suppression by a non-GLP-1 peripherally administered anorectic agent, the amylin receptor agonist, sCT. Briefly, a separate group of rats (n = 8) weighing approximately 400–450 g was given the following treatments according to a counterbalanced design (ICV/IP): 1) vehicle/vehicle; 2) exendin-(9–39), 100 μg/vehicle; 3) vehicle/sCT, 2.75 μg/kg; and 4) exendin-(9–39), 100 μg/sCT, 2.75 μg/kg. Procedures for injections and for recording food intake and body weight were identical to those described above. The dose of sCT was chosen based on pilot work showing a comparable magnitude of 6-h intake suppression to liraglutide, 10 μg/kg, and exendin-4, 3.0 μg/kg.
Experiment 1c
To assess whether any attenuation of food intake suppression observed with ICV exendin-(9–39) 100 μg cotreatment was based on blockade of peripheral GLP-1R after efflux of ICV exendin-(9–39) into the periphery, a control experiment was conducted in which ip liraglutide (10 μg/kg) and exendin-4 (3.0 μg/kg) were coadministered with peripheral (ip) exendin-(9–39) at 25% (25 μg) and 75% (75 μg) of the ICV dose used in experiment 1a. Although the exact percent of exendin-(9–39) efflux from the CNS in experiment 1a cannot be determined, previous work estimates the amount of exendin-(9–39) found in peripheral circulation after central administration in mice is approximately 17% (27). Thus, the bolus 25 μg and 75 μg (0.5 ml volume) ip doses of exendin-(9–39) employed in experiment 1c are likely to exceed the amount of exendin-(9–39) that may have effluxed after ICV administration in experiment 1a.
A separate group of rats (n = 8) weighing approximately 400–450 g was given two ip injections (separated by 15 min) immediately before dark onset. The following drug treatments were given in counterbalanced fashion: 1) ip vehicle/ip vehicle; 2) ip exendin-(9–39), 25 μg/ip vehicle; 3) ip exendin-(9–39), 75 μg/ip vehicle; 4) ip vehicle/ip liraglutide, 10 μg/kg; 5) IP exendin-(9–39), 25 μg/ip liraglutide, 10 μg/kg; 6) ip exendin-(9–39), 75 μg/ip liraglutide, 10 μg/kg. As with experiment 1a, food intake was recorded at 1 h, 3 h, 6 h, and 24 h after dark onset. A separate group of rats (n = 8, ∼400–500 g) was given identical treatments with the exception that ip exendin-4 treatment (3.0 μg/kg) replaced ip liraglutide (10 μg/kg).
Experiment 2
A separate group of SDA (n = 8; functionally and histologically verified) and sham rats (n = 10) were given the following ip treatments in counterbalanced fashion: 1) vehicle; 2) liraglutide 10 μg/kg; 3) liraglutide, 25 μg/kg; 4) liraglutide, 50 μg/kg; 5) exendin-4, 1.0 μg/kg; and 6) exendin-4, 3.0 μg/kg. Procedures for injections and for recording food intake and body weight were identical to those described above.
Statistical analysis
Food intake and body weight suppression in experiments 1a, 1b, and 2 were evaluated using repeated measures ANOVA. Newman Keuls post hoc analyses were used to compare treatments when significant main effects or interactions were obtained. For experiment 1a, separate analyses were conducted for liraglutide and exendin-4. For experiments 1a and 1b, the a priori criteria for defining attenuation of intake suppression by 3rd ICV exendin-(9–39) were as follows: 1) a significant interaction between ICV drug [exendin-(9–39)] and ip drug (liraglutide or exendin-4 for experiments 1a and 1c; sCT for experiment 1b); and 2) post hoc significant difference between ICV/ip combined drug treatment and ip drug treatment alone. For experiment 2, two-way ANOVA was conducted with Group (SDA/sham) and Drug as variables. Newman Keuls post hoc analyses compared treatments with vehicle treatment when significant main effects or interactions were obtained. Statistical significance was determined by P < 0.05 for all analyses.
Results
Experiment 1a: food intake and body weight suppression after ip liraglutide or exendin-4 combined with 3rd ICV exendin-(9–39)
Food intake
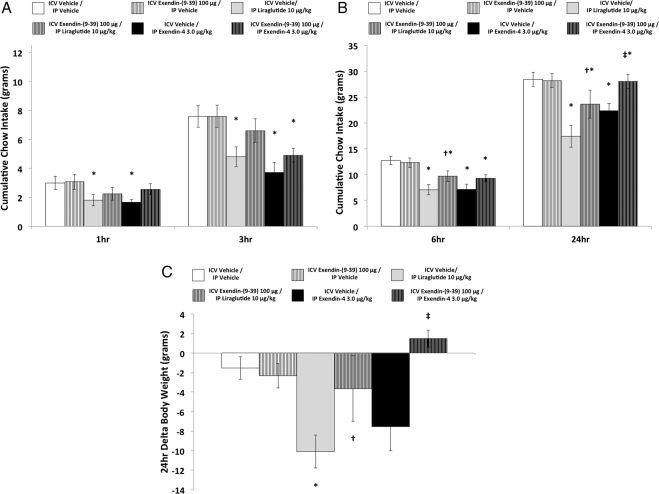
ICV exendin-(9–39) pretreatment significantly attenuated intake suppression produced by ip liraglutide at 6 h and 24 h (Fig. 1B). A moderate, but nonsignificant level of attenuation of ip liraglutide-induced food intake suppression was observed with ICV exendin-(9–39) cotreatment at 1 h and 3 h (Fig. 1A). Statistical support for these conclusions is provided by a significant interaction between liraglutide and exendin-(9–39) at 6 h [F(1,8) = 7.77, P < 0.05] and 24 h [F(1,8) = 12.27, P < 0.01]. At 1 h and 3 h, significant main effects were obtained for liraglutide [Fs(1,18) > 7.3, ps < 0.05], but the interaction with exendin-(9–39) did not reach significance at these time points (results from treatment comparisons at all time points are indicated in Fig. 1).
Fig. 1.
Cumulative chow intake at 1 h and 3 h (A), at 6 h and 24 h (B), and δ body weight at 24 h (C) after ICV (vehicle or exendin-(9–39), 100 μg) and ip (vehicle, liraglutide, 10 μg/kg; or exendin-4, 3.0 μg/kg) drug coadministration (* denotes significant difference compared with ICV vehicle/ip vehicle treatment; † denotes significant difference compared with ICV vehicle/ip liraglutide treatment; ‡ denotes significant difference compared with ICV vehicle/ip exendin-4 treatment). P < 0.05 = significant difference; data are mean ± sem.
ICV exendin-(9–39) pretreatment significantly attenuated the suppression of food intake produced by ip exendin-4 at 24 h (Fig. 1b). Moderate, but nonsignificant, attenuation of food intake suppression with exendin-(9–39) cotreatment was observed at 1, 3, and 6 h (Fig. 1, A and B). The interaction between exendin-4 and exendin-(9–39) was significant only at the 24-h time point [F(1,8) = 13.58; P < 0.01]. Significant main effects of exendin-4 were obtained at 1, 3, and 6 h [Fs(1,8) > 6.8, ps < 0.05], but the drug interactions at these time points were not significant.
Body weight change
As seen in Fig. 1c, body weight was significantly suppressed 24 h after ip liraglutide administration, and the magnitude of this suppression was significantly attenuated by ICV exendin-(9–39) pretreatment. This was supported by a significant drug interaction [F(1,8) = 7.58; P < 0.05].
Body weight was significantly suppressed after IP exendin-4 alone compared with combined ICV exendin-(9–39)/ip exendin-4 treatment (P < 0.05), but not compared with vehicle/vehicle treatment (Fig. 1C). This pattern of results yielded a significant drug interaction [F(1,8) = 6.29; P < 0.05].
Experiment 1b: food intake and body weight suppression after ip salmon calcitonin combined with 3rd ICV exendin-(9–39)
ICV exendin-(9–39) pretreatment did not attenuate the suppression of food intake produced by ip administration of the amylin receptor agonist sCT at 3 h and 6 h. This conclusion is statistically supported by nonsignificant drug interactions at all time points. Significant main effects of sCT were obtained at 3 h and 6 h [Fs(1,7) > 14.68; ps < 0.01], but not at 1 h and 24 h. The mean ± sem food intake values are as follows at 3 h and 6 h after treatment: vehicle/vehicle = 7.55 ± 1.00 and 11.88 ± 1.42; ICV vehicle/ip sCT = 3.46 ± 0.61 and 5.98 ± 1.06; ICV exendin-(9–39)/ip vehicle = 5.51 ± 0.76 and 10.35 ± 0.79; exendin-(9–39)/ip sCT = 4.24 ± 0.84 and 7.13 ± 1.11.
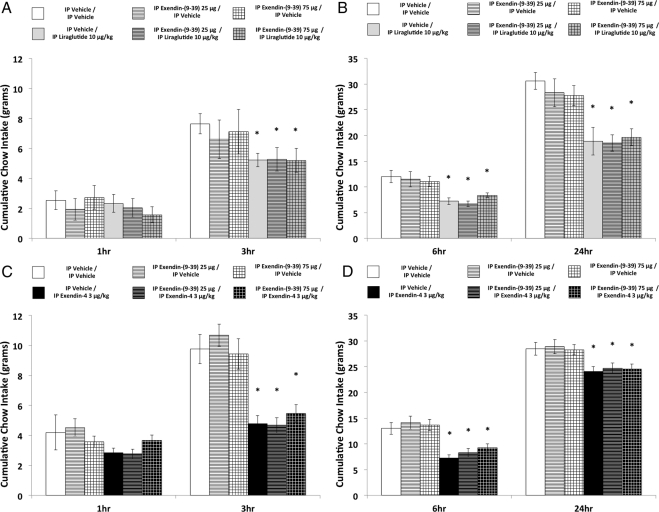
Experiment 1c: food intake and body weight suppression after ip liraglutide or exendin-4 combined with ip exendin-(9–39)
Food intake suppression produced by ip liraglutide was not significantly attenuated with ip exendin-(9–39) 25 μg or 75 μg coadministration at any time point (Fig. 2, A and B). This conclusion was supported by ANOVA. Significant main effects were obtained for liraglutide at 3 h, 6 h, and 24 h [Fs(1,7) > 10.9; ps < 0.05], whereas the liraglutide × exendin-(9–39) interaction was not significant at any time point [largest F at 6 h, F(2,14) = 2.86; P = 0.09] (results from treatment comparisons at all time points are indicated in Fig. 2).
Fig. 2.
Cumulative chow intake at 1 h, 3 h, 6 h, and 24 h in rats that received ip exendin-(9–39) (0, 25, and 75 μg) combined with ip liraglutide (10 μg/kg) coadministration (panels A and B) and in rats that received ip exendin-(9–39) (0, 25, and 75 μg) combined with ip exendin-4 (3 μg/kg) coadministration (panels C and D) (* denotes significant difference compared with ICV vehicle/ip vehicle treatment; † denotes significant difference compared with ICV vehicle/ip liraglutide treatment; ‡ denotes significant difference compared with ICV vehicle/ip exendin-4 treatment). P < 0.05 = significant difference; data are mean ± sem.
Similarly, food intake suppression produced by ip exendin-4 was not significantly attenuated with ip exendin-(9–39) 25 μg or 75 μg coadministration at any time point (Fig. 2, C and D). ANOVA revealed significant main effects for exendin-4 at 3 h, 6 h, and 24 h [Fs(1,7) > 23.83; ps < 0.01] and nonsignificant exendin-4 × exendin-(9–39) interactions at these time points [Fs(2,14) < 1.0].
Experiment 2: food intake and body weight suppression by ip liraglutide and exendin-4 in rats with subdiaphragmatic vagal deafferentation and sham-operated control rats
Food intake
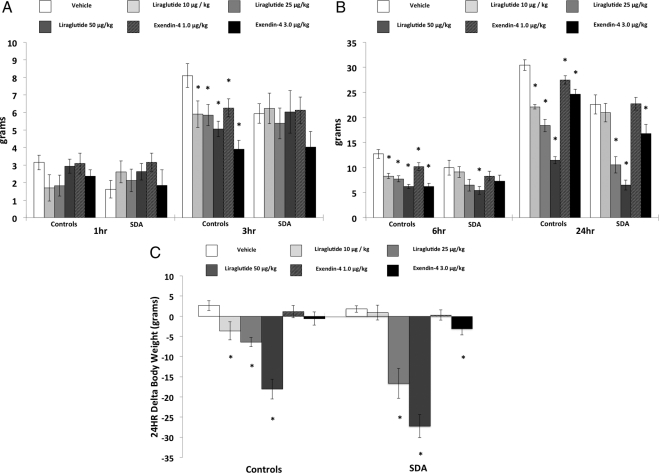
Liraglutide suppressed 3 h intake in sham-operated, but not in SDA rats (Fig. 3A). At 6 h and 24 h, food intake was suppressed after ip liraglutide in both sham-operated and SDA rats (Fig. 3B). The effective dose range differed between the two groups at 6 h and 24 h after ip liraglutide, because all three doses produced significant intake suppression relative to vehicle for sham rats, whereas only the higher two doses were effective for SDA rats. Statistical support for these conclusions was based on a significant Group × Drug interactions at 3 h and 24 h [Fs(3,48) > 2.9; ps < 0.05], whereas this interaction did not achieve significance at 6 h [F(3,48) = 1.6] (individual dose comparisons to vehicle treatment are indicated in Fig. 3, A and B).
Fig. 3.
Cumulative chow intake in rats with SDA and control rats at 1 h and 3 h (A), at 6 h, and 24 h (B), and δ body weight at 24 h (C) after vehicle, ip liraglutide (10, 25, or 50 μg/kg), or exendin-4 (1.0, or 3.0 μg/kg) treatment (* denotes significant difference compared with vehicle treatment). P < 0.05 = significant difference; data are mean ± sem.
At 3 h and 6 h, ip exendin-4 treatment suppressed food intake relative to vehicle treatment for sham but not for SDA rats. Food intake at 24 h was suppressed by ip exendin-4 in both SDA and sham rats (Fig. 3); however, both doses of exendin-4 were effective in significantly suppressing 24-h food intake relative to vehicle for sham rats whereas only the high dose (3.0 μg/kg) was effective for SDA rats. These conclusions were supported by a significant Group × Drug interaction at 3 h [F(2,32) = 3.76, P < 0.05]; however, the Group × Drug interactions did not achieve significance at 6 h and 24 h [Fs(2,32) > 1.3].
Body weight change
Body weight was significantly suppressed at 24 h after ip liraglutide administration in both sham and SDA rats (Fig. 3C); all three doses were effective in suppressing body weight relative to vehicle for sham rats, whereas only the higher two doses were effective in SDA rats. Statistical support for these conclusions was provided by a significant Group × Drug interaction [F(3,48) = 5.96; P < 0.01] (individual dose comparisons to vehicle treatment are indicated in Fig. 3C).
The highest dose of exendin-4 (3.0 μg/kg) significantly suppressed body weight for SDA rats, whereas significant body weight suppression was not observed after exendin-4 treatment for sham rats (Fig. 3). However, evidence for a Group difference for exendin-4-induced body weight loss was not supported by a significant Group × Drug interaction [F(2,32) < 1.0].
Discussion
The long-acting GLP-1R agonists, exendin-4 and liraglutide, are efficacious in the treatment of T2DM and may hold promise for the treatment of obesity (5, 6, 28). Both ligands reduce food intake and body weight in humans and in animal models (5, 29–34). Given that GLP-1R are expressed on terminals of vagal afferent fibers innervating the GI tract and hepatoportal bed, as well as on CNS neurons associated with energy balance regulation, the current experiments examined whether the relevant site of action for the food intake-suppressive effects of these ligands is vagal afferent mediated, CNS mediated, or results from the combined action on peripheral and central GLP-1R populations. Results from two separate experimental strategies were consistent and showed that food intake suppression after ip delivery of either liraglutide or exendin-4 involves vagal afferent mediation and direct activation of GLP1-R in the CNS. Coadministration of a GLP1-R antagonist [exendin-(9–39)] to the brain (3rd ventricle) attenuated the intake-suppressive effects of ip liraglutide and exendin-4, particularly at 6 h and 24 h after administration. Food intake was suppressed in rats with subdiaphragmatic vagal afferent ablation (SDA rats) 24 h after ip injections of the GLP-1R agonists; however, higher doses were needed to suppress intake in SDA compared with control rats. Together, these findings support the hypothesis that the intake-suppressive effects of systemically administered liraglutide and exendin-4 are mediated by combined action on vagal afferent and CNS GLP-1R populations.
The GLP-1R-expressing CNS neurons directly activated by ip liraglutide and exendin-4 that mediate food intake suppression are likely distributed anatomically (35). Previous research shows that the NTS in the caudal brain stem is a critical CNS site of action for endogenous GLP-1R-mediated control of food intake (36). It is clear, however, that the NTS is not the only CNS GLP1-R-expressing nucleus relevant to energy balance control. Indeed, direct activation of GLP-1R in the paraventricular hypothalamus suppresses food intake in rats (37–39). Other potential targets of GLP-1R activation after ip administration of liraglutide and exendin-4 include several nuclei the contributions of which to food intake control are less well characterized (e.g. ventral tegmental area, hippocampus, central nucleus of the amygdala) (3, 39). Because exendin-(9–39) was delivered to the 3rd ventricle and provided GLP-1R antagonist ligand access to multiple CNS targets, further testing is required to determine which GLP-1R-expressing nuclei contribute to the intake suppression produced by ip administration of liraglutide and exendin-4. Also, given that regionally distinct CNS GLP-1R populations have been implicated in food intake control and visceral illness (39), it is unclear whether the attenuation of intake suppression observed with ICV exendin-(9–39) cotreatment is based on amelioration of the satiating, nauseating, or combined effects of the GLP-1R agonists.
Two pieces of evidence suggest that vagal afferent GLP-1R activation also contributes to food intake suppression produced by ip liraglutide and exendin-4. First, all doses of liraglutide and exendin-4 suppressed 3-h and 6-h food intake in control rats, whereas exendin-4 did not suppress food intake in SDA rats at these time points, and only the highest dose of liraglutide suppressed food intake at 6 h for SDA rats. Second, higher doses of exendin-4 and liraglutide were needed to suppress 24-h food intake in SDA rats compared with controls. GLP-1R-mediated vagal afferent stimulation results in a polysynaptic activation of neurons in the central visceral projection pathway (e.g. NTS, parabrachial nucleus, area postrema, hypothalamic paraventricular nucleus, and dorsal medial hypothalamus) (40–43). It is interesting to note that these CNS structures also express GLP-1R (3). For these reasons we think that it is worth considering the hypothesis that the suppression of food intake after ip injection of exendin-4 and liraglutide involves a combination of direct CNS GLP-1R activation via ligand penetrance through the BBB and polysynaptic neuronal activation that originates with GLP-1R-driven vagal afferent stimulation. Support for this hypothesis comes from results of the lower doses of liraglutide (10 μg/kg) and exendin-4 (1 μg/kg) that reduced food intake at 24 h in control but not SDA rats, whereas higher doses of each ligand inhibited intake in both SDA and control rats. These findings are interpreted to suggest that the intake suppression triggered by the low doses of liraglutide and exendin-4 in control rats is attributable to the combined signaling that results from vagal afferent activation, vagus-driven polysynaptic CNS activation, and by direct CNS GLP-1R activation. In the absence of vagal afferent-mediated effects (i.e. SDA rats), significant intake suppression by the lower doses was not observed. Further, given that the GLP-1R agonists suppressed food intake in SDA rats, it is unlikely that the attenuation of food intake suppression observed with ICV exendin-(9–39) cotreatment in experiment 1a is based entirely on blockade of endogenous GLP-1 released by vagal afferent-driven polysynaptic CNS activation. Taken together, results from experiments 1 and 2 support the hypothesis that intake suppression by ip liraglutide and exendin-4 involves combined activation of CNS GLP-1R by direct ligand penetrance and by vagal afferent-driven polysynaptic activation.
Previous research addresses the site(s) and mechanism of action by which systemic GLP-1(7–36) (12–14, 44–47) and long-acting GLP-1R agonists (29, 30, 48–50) inhibit food intake. Williams et al. (14) show that ICV administration of the GLP-1R antagonist exendin-(9–39) fails to attenuate 30-min intake suppression by ip GLP-1(7–36). Rüttimann et al. (13) demonstrate that vagal afferent signaling mediates the suppression of intake during the first spontaneous meal after ip delivery of GLP-1(7–36). Similarly, others also show that ip administration of GLP-1 does not suppress short-term intake after total subdiaphragmatic vagotomy (12), and ip exendin-4 does not suppress intake up to 8 h after administration in capsaicin-treated mice (50). These findings are consistent with the present data, because ip exendin-4 and liraglutide did not significantly suppress intake in SDA rats until 24 h and 6 h, respectively, an outcome that suggests that direct CNS GLP-1R activation by the GLP-1R agonists occurs primarily within this timeframe. We note that exendin-4 and liraglutide penetrance into the brain and subsequent CNS GLP-1R activation may also occur at earlier time points after ip administration, a notion consistent with the moderate attenuation of intake suppression observed at 1 h and 3 h with ICV exendin-(9–39) cotreatment in experiment 1a. Furthermore, the lack of significant intake suppression by the GLP-1R agonists in SDA rats at 3 h could potentially be based on a floor effect given the low level of baseline intake in this group compared with controls. Data from experiments 1 and 2, together with previous findings (50), however, support a larger contribution of vagal afferent vs. CNS GLP-1R signaling at short- (1–3 h) compared with long-term (6–24 h) periods after ip administration of long-acting GLP-1R agonists.
Two control experiments were conducted to help elucidate potential mechanisms that may mediate the effects observed in experiment 1a that intake suppression by ip GLP-1R agonist was attenuated by ICV GLP-1R antagonist coadministration. First we addressed whether ICV delivery of the GLP-1R antagonist exendin-(9–39) could act as a nonspecific, competing orexigenic signal (see Refs. 51–53 for discussion) through blockade of basal CNS GLP-1R signaling (25, 26). Results showed that ICV delivery of exendin-(9–39) did not affect the suppression of intake at 6 h by ip administration of the amylin receptor agonist sCT, a result that contrasts with the attenuating effect of the antagonist on intake suppression by ip liraglutide. Second, we addressed whether the results of experiment 1a were based on blockade of peripheral GLP-1R after efflux of exendin-(9–39) out of the CNS into the periphery. Results showed that exendin-(9–39) delivered ip at two doses (25% and 75% of the dose administered ICV) that exceed the amount estimated to efflux into peripheral circulation (27) did not significantly attenuate intake suppression by ip delivery of liraglutide or exendin-4. Thus, the results of these control experiments support the interpretation that ICV exendin-(9–39) acted as a competitive antagonist for peripherally administered liraglutide and exendin-4 that accessed the brain and acted directly on CNS GLP-1R.
In conclusion, results demonstrate that the intake-inhibitory effects of the GLP-1R agonists, exendin-4 and liraglutide, are mediated by activation of GLP-1R expressed on subdiaphragmatic vagal afferents, as well as in the brain. The use of different methods yields data that are consistent with the hypothesis that vagal afferent action contributes to both short- and longer-term intake inhibition by these GLP-1R ligands, whereas direct CNS action related to ligand BBB penetration in the absence of vagal afferent signaling may be sufficient for intake inhibition at 24 h after administration. The multisite action of exendin-4 and liraglutide described in this work provides an additional rationale for the use of these GLP-1R agonists for human obesity treatment.
Acknowledgments
We thank the following for their contributions to this work: Dr. Wolfgang Langhans, Dr. Robert Phillips, Dr. Timothy Moran, Amber Alhadeff, Laura Rupprecht. Andrea Spaeth, and Shiru Zhaou.
This work was supported by National Institutes of Health grants DK21397 (to H.J.G.), DK085435 (to M.R.H.), and DK089752 (to S.E.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- BBB
- blood brain barrier
- CCK
- cholecystokinin
- CNS
- central nervous system
- DMV
- dorsal motor nucleus of the vagus
- GI
- gastrointestinal
- GLP-1R
- glucagon-like peptide-1 receptor
- ICV
- intracerebroventricular
- NTS
- nucleus tractus solitarius
- sCT
- salmon calcitonin
- SDA
- subdiaphragmatic vagal deafferentation
- T2DM
- type 2 diabetes mellitus.
References
- 1. Center for Disease Control and Prevention 2009. Overweight and obesity: data and statistics. Center for Disease Control and Prevention. www.cdc.gov/obesity/data/trends.html
- 2. Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. 2007. The epidemiology of obesity. Gastroenterology 132:2087–2102 [DOI] [PubMed] [Google Scholar]
- 3. Merchenthaler I, Lane M, Shughrue P. 1999. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403:261–280 [DOI] [PubMed] [Google Scholar]
- 4. Mojsov S, Kopczynski MG, Habener JF. 1990. Both amidated and nonamidated forms of glucagon-like peptide I are synthesized in the rat intestine and the pancreas. J Biol Chem 265:8001–8008 [PubMed] [Google Scholar]
- 5. Hayes MR, De Jonghe BC, Kanoski SE. 2010. Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100:503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holst JJ. 2007. The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439 [DOI] [PubMed] [Google Scholar]
- 7. Holst JJ, Deacon CF. 2005. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48:612–615 [DOI] [PubMed] [Google Scholar]
- 8. Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. 2009. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374:1606–1616 [DOI] [PubMed] [Google Scholar]
- 9. Bradley DP, Kulstad R, Schoeller DA. 2010. Exenatide and weight loss. Nutrition 26:243–249 [DOI] [PubMed] [Google Scholar]
- 10. Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, Levin BE, Larsen PJ, Knudsen LB, Fosgerau K, Vrang N. 2010. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol 206:287–296 [DOI] [PubMed] [Google Scholar]
- 11. Thum T, Anker SD. 2010. Liraglutide for weight loss in obese people. Lancet 375:551–552; author reply 552–553 [DOI] [PubMed] [Google Scholar]
- 12. Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. 2005. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044:127–131 [DOI] [PubMed] [Google Scholar]
- 13. Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. 2009. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams DL, Baskin DG, Schwartz MW. 2009. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150:1680–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abu-Hamdah R, Rabiee A, Meneilly GS, Shannon RP, Andersen DK, Elahi D. 2009. Clinical review: The extrapancreatic effects of glucagon-like peptide-1 and related peptides. J Clin Endocrinol Metab 94:1843–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agerso H, Jensen LB, Elbrond B, Rolan P, Zdravkovic M. 2002. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia 45:195–202 [DOI] [PubMed] [Google Scholar]
- 17. Kastin AJ, Akerstrom V. 2003. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27:313–318 [DOI] [PubMed] [Google Scholar]
- 18. McClean P, Kung K, McCurtin R, Gault V, Holscher C, 2009. Novel GLP-1 analogues cross the blood brain barrier: a link between diabetes and Alzheimer's disease. The 39th Proc Annual Meeting of the Society for Neuroscience, Chicago, 2009 [Google Scholar]
- 19. Norgren R, Smith GP. 1994. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267:R1136–R1141 [DOI] [PubMed] [Google Scholar]
- 20. Ritter RC, Slusser PG, Stone S. 1981. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213:451–452 [DOI] [PubMed] [Google Scholar]
- 21. Arnold M, Mura A, Langhans W, Geary N. 2006. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 26:11052–11060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. 1997. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol Regul Integr Comp Physiol 272:R1245–R1251 [DOI] [PubMed] [Google Scholar]
- 23. Smith GP, Jerome C, Norgren R. 1985. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol 249:R638–R641 [DOI] [PubMed] [Google Scholar]
- 24. Walls EK, Phillips RJ, Wang FB, Holst MC, Powley TL. 1995. Suppression of meal size by intestinal nutrients is eliminated by celiac vagal deafferentation. Am J Physiol Regul Integr Comp Physiol 269:R1410–R1419 [DOI] [PubMed] [Google Scholar]
- 25. Schepp W, Schmidtler J, Riedel T, Dehne K, Schusdziarra V, Holst JJ, Eng J, Raufman JP, Classen M. 1994. Exendin-4 and exendin-(9–39)NH2: agonist and antagonist, respectively, at the rat parietal cell receptor for glucagon-like peptide-1-(7–36)NH2. Eur J Pharmacol 269:183–191 [DOI] [PubMed] [Google Scholar]
- 26. Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. 1998. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J Clin Invest 101:1421–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banks WA, During MJ, Niehoff ML. 2004. Brain uptake of the glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther 309:469–475 [DOI] [PubMed] [Google Scholar]
- 28. Knudsen LB. 2010. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 64:4–11 [DOI] [PubMed] [Google Scholar]
- 29. Hayes MR, Skibicka KP, Grill HJ. 2008. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149:4059–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott KA, Moran TH. 2007. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293:R983–R987 [DOI] [PubMed] [Google Scholar]
- 31. Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. 2007. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 56:8–15 [DOI] [PubMed] [Google Scholar]
- 32. Raun K, von Voss P, Knudsen LB. 2007. Liraglutide, a once-daily human glucagon-like peptide-1 analog, minimizes food intake in severely obese minipigs. Obesity (Silver Spring) 15:1710–1716 [DOI] [PubMed] [Google Scholar]
- 33. Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, Verhoeven R, Bugánová I, Madsbad S. 2007. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care 30:1608–1610 [DOI] [PubMed] [Google Scholar]
- 34. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. 2006. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8:436–447 [DOI] [PubMed] [Google Scholar]
- 35. Grill HJ. 2006. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14(Suppl 5):216S–221S [DOI] [PubMed] [Google Scholar]
- 36. Hayes MR, Bradley L, Grill HJ. 2009. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150:2654–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMahon LR, Wellman PJ. 1997. Decreased intake of a liquid diet in nonfood-deprived rats following intra-PVN injections of GLP-1 (7–36) amide. Pharmacol Biochem Behav 58:673–677 [DOI] [PubMed] [Google Scholar]
- 38. McMahon LR, Wellman PJ. 1998. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol Regul Integr Comp Physiol 274:R23–R29 [DOI] [PubMed] [Google Scholar]
- 39. Kinzig KP, D'Alessio DA, Seeley RJ. 2002. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22:10470–10476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. 2007. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res 1162:76–84 [DOI] [PubMed] [Google Scholar]
- 41. Viltart O, Sartor DM, Verberne AJ. 2006. Chemical stimulation of visceral afferents activates medullary neurones projecting to the central amygdala and periaqueductal grey. Brain Res Bull 71:51–59 [DOI] [PubMed] [Google Scholar]
- 42. Hayes MR, Covasa M. 2006. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 1088:120–130 [DOI] [PubMed] [Google Scholar]
- 43. Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. 2007. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 1149:118–126 [DOI] [PubMed] [Google Scholar]
- 44. Chelikani PK, Haver AC, Reidelberger RD. 2005. Intravenous infusion of glucagon-like peptide-1 potently inhibits food intake, sham feeding, and gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 288:R1695–R1706 [DOI] [PubMed] [Google Scholar]
- 45. Baumgartner I, Pacheco-Lopez G, Ruttimann EB, Arnold M, Asarian L, Langhans W, Geary N, Hillebrand JJ. 2010. Hepatic-portal vein infusions of GLP-1 reduce meal size and increase c-Fos expression in the NTS, AP, and CeA in rats. J Neuroendocrinol 22:557–563 [DOI] [PubMed] [Google Scholar]
- 46. Bucinskaite V, Tolessa T, Pedersen J, Rydqvist B, Zerihun L, Holst JJ, Hellström PM. 2009. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 21:978–e78 [DOI] [PubMed] [Google Scholar]
- 47. Imeryüz N, Yeğen BC, Bozkurt A, Coşkun T, Villanueva-Peñacarrillo ML, Ulusoy NB. 1997. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol 273:G920–G927 [DOI] [PubMed] [Google Scholar]
- 48. Baraboi ED, Smith P, Ferguson AV, Richard D. 2010. Lesions of area postrema and subfornical organ alter exendin-4-induced brain activation without preventing the hypophagic effect of the Glp-1 receptor agonist. Am J Physiol Regul Integr Comp Physiol 298:R1098–R1110 [DOI] [PubMed] [Google Scholar]
- 49. Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. 1995. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7:2294–2300 [DOI] [PubMed] [Google Scholar]
- 50. Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. 2005. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146:3748–3756 [DOI] [PubMed] [Google Scholar]
- 51. Reidelberger RD, Haver AC, Apenteng BA, Anders KL, Steenson SM. 2011. Effects of exendin-4 alone and with peptide YY(3–36) on food intake and body weight in diet-induced obese rats. Obesity (Silver Spring) 19:121–127 [DOI] [PubMed] [Google Scholar]
- 52. Morley JE, Flood JF. 1987. An investigation of tolerance to the actions of leptogenic and anorexigenic drugs in mice. Life Sci 41:2157–2165 [DOI] [PubMed] [Google Scholar]
- 53. Grady EF, Böhm SK, Bunnett NW. 1997. Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am J Physiol 273:G586–G601 [DOI] [PubMed] [Google Scholar]