Abstract
Sexual dimorphism and supplementation studies suggest an important role for estrogens in the amelioration of glucose intolerance and diabetes. Because little is known regarding the signaling mechanisms involved in estradiol-mediated insulin secretion, we investigated the role of the G protein-coupled receptor 30, now designated G protein-coupled estrogen receptor (GPER), in activating signal transduction cascades in β-cells, leading to secretion of insulin. GPER function in estradiol-induced signaling in the pancreatic β-cell line MIN6 was assessed using small interfering RNA and GPER-selective ligands (G-1 and G15) and in islets isolated from wild-type and GPER knockout mice. GPER is expressed in MIN6 cells, where estradiol and the GPER-selective agonist G-1 mediate calcium mobilization and activation of ERK and phosphatidylinositol 3-kinase. Both estradiol and G-1 induced insulin secretion under low- and high-glucose conditions, which was inhibited by pretreatment with GPER antagonist G15 as well as depletion of GPER by small interfering RNA. Insulin secretion in response to estradiol and G-1 was dependent on epidermal growth factor receptor and ERK activation and further modulated by phosphatidylinositol 3-kinase activity. In islets isolated from wild-type mice, the GPER antagonist G15 inhibited insulin secretion induced by estradiol and G-1, both of which failed to induce insulin secretion in islets obtained from GPER knockout mice. Our results indicate that GPER activation of the epidermal growth factor receptor and ERK in response to estradiol treatment plays a critical role in the secretion of insulin from β-cells. The results of this study suggest that the activation of downstream signaling pathways by the GPER-selective ligand G-1 could represent a novel therapeutic strategy in the treatment of diabetes.
17β-Estradiol (E2) and its mimetics have long been known to modulate pancreatic function (1). Pancreatic dysfunction and/or the death of β-cells can give rise to abnormal blood sugar levels, resulting in diabetes and its associated complications. With the escalating number of diabetes cases every year across the globe, there is a renewed interest in the study of estrogenic compounds for their prosurvival and hormone-modulating effects on pancreatic β-cell (2). However, E2 mimetics, including xenoestrogens and phytoestrogens, have also been studied as disruptors of the endocrine effects of physiologic estrogens on the pancreas (3–6). E2 is thought to act predominantly through genomic pathways, regulating gene expression via binding to estrogen receptor (ER)α and ERβ (7). More recently, activation of nongenomic or rapid signaling pathways in response to E2 has received greater attention (8, 9). The G protein-coupled receptor 30 (GPR30), now designated G protein-coupled ER (GPER), has recently been implicated in mediating many of the nongenomic effects of E2 (9, 10). GPER has been shown to activate adenylyl cyclase, ERK, phosphatidylinositol 3-kinases (PI3K), and intracellular calcium mobilization (11–13). Physiological roles for these nongenomic responses via GPER have been implicated in cancer, cardiovascular, immunological, and neurological functions as well as diabetes (14).
The higher prevalence of diabetes in men compared with women and the increased incidence of diabetes in women after menopause highlight the potentially important role of physiologic estrogens in glucose homeostasis (1, 15). A role for E2 in glucose homeostasis is further supported by ERα and aromatase knockout (KO) mice, which develop insulin resistance and are obese (16, 17). The possible significance and mechanisms of GPER action in pancreatic function are poorly understood (18). Recent studies suggest a role for GPER in the survival of pancreatic β-cells as well as secretion of hormones in intact islets (19). Liu et al. (20) have suggested an important role for both extranuclear ERα as well as GPER in islet cell survival, whereas Mårtensson et al. (21) reported that GPER-deficient female mice were hyperglycemic and showed attenuated insulin release in response to supraphysiological concentrations of E2.
Because E2 activates ERα, ERβ, as well as GPER, to distinguish the individual roles of these receptors, we have recently identified both a GPER-selective agonist, G-1 (22), and a GPER-selective antagonist, G15 (23), which can serve as tools to reveal the specificity of GPER action. Furthermore, a majority of E2-related studies on pancreatic function have used intact islets, which consist of a mixed population of cell types, making it difficult to interpret results as a direct consequence on a particular cell type. Although GPER activation has been shown to modulate insulin, glucagon, and somatostatin secretion in pancreatic islets, the specific target and mechanisms of activation remain unknown (19). Hence, we have used the mouse pancreatic β-cell line, MIN6, widely used as a model system for β-cells, to examine the function of GPER in E2-induced insulin secretion by employing GPER-specific ligands as well as islets from wild-type (WT) and GPER KO mice.
In the present study, we show that GPER is expressed in MIN6 cells and that MIN6 cells respond to E2 and G-1 through GPER to activate ERK, serine-threonine kinase (Akt), and mobilize intracellular calcium, with insulin secretion under both low- and high-glucose conditions via an ERK-dependent pathway. Islets from WT mice showed an increase in insulin secretion upon stimulation with E2 or G-1, and this effect was specific to GPER, because it was blocked by the GPER-selective antagonist G15. Furthermore, islets isolated from GPER KO mice failed to secrete insulin in response to E2 or G-1. These results indicate that GPER acts to directly stimulate insulin production in β-cells in response to E2, suggesting a novel target to augment insulin production in diabetes.
Materials and Methods
Reagents
DMEM, phenol red-free DMEM, E2, 4-hydroxytamoxifen (4-HT), fetal bovine serum, normal goat serum (NGS), and newborn calf serum were from Sigma (St. Louis, MO). BSA was from Amresco (Solon, OH). G-1 was synthesized as described (22) and provided by Jeffrey Arterburn (New Mexico State University, Las Cruces, NM). The calcium sensitive dye, Indo-1 AM (Molecular Probes) and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA). Small interfering RNA (siRNA) were purchased from Dharmacon RNAi Technologies (Dharmacon, Lafayette, CO): ON-TARGET plus SMARTpool siRNA for GPER (L-053623-00) and ON- TARGETplus siControl Non-Targeting siRNA (D-001810-02). Protease cocktail was from EMD Chemicals (Gibbstown, NJ).
Inhibitors, antibodies, and peptides
Epidermal growth factor receptor (EGFR) inhibitor Tryphostin AG1478, PI3K inhibitor LY294002, and ERK inhibitor PD98059 were from EMD Chemicals. G15 was synthesized as described (23) and provided by Jeffrey Arterburn (New Mexico State University). Polyclonal antibody against the mouse GPER was raised in rabbits using a sequence from the second extracellular loop of mouse GPER (acetyl-FADVREVQWLEVTLGFIC) covalently linked via an additional C-terminal cysteine to KLH. Peptides from the second intracellular loop (ICL2, acetyl-AMRSS/GLFRTKHHARLSC-amide) and second extracellular loop (ECL2, acetyl-FADVREVQWLEVTLGFIC-amide) were used to test antibody specificity by incubating the primary antibody with peptide (2 μg/ml) overnight at 4 C before use. Mouse antiactin antibody was from Millipore (Bedford, MA). Rabbit antiphospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) antibody, rabbit anti-p44/42 MAPK (ERK1/2) antibody, rabbit antiphospho-Akt (Ser473) antibody, and rabbit anti-Akt were from Cell Signaling (Beverly, MA). Donkey antirabbit IgG-horseradish peroxidase (HRP)-conjugated antibody and donkey antimouse IgG-HRP-conjugated antibody were from GE Healthcare (Princeton, NJ). Alexa Fluor 568-conjugated secondary antibodies were from Invitrogen.
Cells and transient transfections
Mouse insulinoma MIN6 cells were a gift from Donald Steiner (University of Chicago, Chicago, IL) with permission from Jun-ichi Miyazaki (University of Osaka, Osaka, Japan) (24) and African green monkey kidney cells, COS7, were purchased from American Type Culture Collection (Manassas, VA). Cells were maintained in DMEM with 25 mm glucose, 2 mm glutamine, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified chamber at 37 C with 95% O2 and 5% CO2. Medium for MIN6 cells was further supplemented with 71 μm β-mercaptoethanol, changed every 2 d, and cells were passaged at 80–90% confluency. For some experiments, MIN6 or COS7 cells were grown on coverslips or six-well plates and transfected with appropriate plasmids/siRNA using Lipofectamine 2000 as per manufacturer's instructions.
Immunofluorescence
Cells, seeded on coverslips and grown for 2–3 d, were washed and fixed in 2% paraformaldehyde 48 h after transfection. For staining GPER, cells were permeabilized and blocked in PBS containing 0.1% Triton X-100 and 3% BSA for 1 h and then incubated with rabbit antimouse GPER antibody (1:1000), using 3% NGS as blocker for 3 h. Cells were washed and incubated with goat antirabbit IgG conjugated to Alexa Fluor 568 in PBS containing 3% NGS for 1 h, and cells were subsequently washed, mounted in Vectashield, and analyzed by confocal microscopy using a Zeiss LSM510 confocal fluorescent microscope (Zeiss, Oberkochen, Germany).
Western blotting
MIN6 cells were grown in six-well plates or 60-mm Petri dishes. For GPER expression analysis, cells were harvested in Nonidet P-40 buffer. For ERK or Akt activation, stimulated serum-starved cells were lysed in RIPA buffer supplemented with sodium fluoride (50 mm), sodium orthovanadate (1 mm), phenylmethylsulfonylfluoride (1 mm), and protease cocktail (1×). Equal amounts of protein were loaded on 4–20% SDS-PAGE gel (Thermo Scientific, Rockford, IL), transferred to polyvinylidene fluoride membrane (Millipore), blocked with 3% new born calf serum in Tris-buffered saline with Tween 20 (50 mm Tris, 150 mm NaCl, and 0.1% Tween 20) before overnight incubation with primary antibodies. Subsequently, blots were incubated with secondary HRP-conjugated antibodies and developed using SuperSignal West Pico Chemiluminescent Substarate (Thermo Scientific). Blots were then scanned and quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
PI3K activation
COS7 cells were grown on coverslips and transfected with mouse GPER-green fluorescent protein (GFP) construct and a reporter plasmid expressing the pleckstrin homology (PH) domain of Akt fused to monomeric red fluorescent protein (RFP) (PH-RFP). Two days after transfection, cells were serum starved overnight and then treated with appropriate ligands in the presence or absence of inhibitors. Cells were then washed, fixed, and mounted for visualization by confocal microscopy.
Intracellular calcium mobilization
MIN6 cells were harvested and incubated at room temperature in Hanks' buffered salt solution containing 2.5 μm indo-1 AM, and 0.05% pluronic acid for 15 min. Cells were resuspended in Hanks' buffered salt solution and kept on ice until assay, performed at a density of 2 × 106 cells/ml. Ca2+ mobilization was determined ratiometrically using λex 340 nm and λem 400/490 nm at 37 C in a spectrofluorometer (QM-2000–2; Photon Technology International, Birmingham, NJ). The relative 490 nm/400 nm ratio was plotted as a function of time.
Animals
Female C57Bl6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). GPER KO mice were provided by Jan Rosenbaum (Proctor & Gamble, Cincinnati, OH) and described previously (25). Mice were backcrossed 10 generations onto C57Bl6 mice and housed at the animal research facility at the University of New Mexico Health Sciences Center. Animals were maintained under a controlled temperature of 22–23 C with a 12-h light, 12-h dark cycle and fed a normal chow ad libitum. Mice were ovariectomized at 10–11 wk of age and allowed to recover for 2 wk before islet isolation. All procedures were approved by and carried out in accordance with the institutional protocols.
Islet isolation
Islets were isolated by ductal injection of ice-cold collagenase as described previously (26). Before injecting the collagenase solution, the common bile duct opening into duodenum was blocked by clamping to maximize solution entry into pancreas. The distended pancreas was then digested at 37 C for about 9–10 min with occasional shaking. The resulting digested slurry was washed and islets purified by density gradient centrifugation using Histopaque. Further purification of islets was achieved by handpicking the islets under a dissection microscope. After isolation, islets were immediately treated with appropriate ligands and assayed for insulin secretion.
Insulin secretion
MIN6 cells (4 × 105) were plated in 24-well plates and grown for 1 wk. One day before treatments, cells were serum starved in phenol red-free DMEM. Cells were then transferred to Krebs-Ringer HEPES bicarbonate buffer containing 0.5% BSA supplemented with 2 mm glucose (with or without inhibitors) for 1 h, stimulated in fresh buffer (with the indicated glucose concentration: 2, 11, or 25 mm) with ligands for 2 h in the presence or absence of inhibitors and supernatants collected for insulin ELISA (Mercodia, Winston Salem, NC). Insulin secretion was expressed as percentage basal with respect to the basal glucose conditions after normalizing for protein content. For insulin measurement in islets, immediately after isolation, islets were incubated in the Krebs-Ringer HEPES bicarbonate buffer with 2 mm glucose and 0.5% BSA for 1 h in the presence or absence of G15 before stimulation with E2 or G-1 in fresh buffer containing 11 mm glucose and 0.5% BSA.
Statistical analysis
Using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA), significance was determined by one-way ANOVA with Dunnett's post hoc test. A value of P < 0.05 was considered significant.
Results
Expression of mouse GPER in MIN6 cells
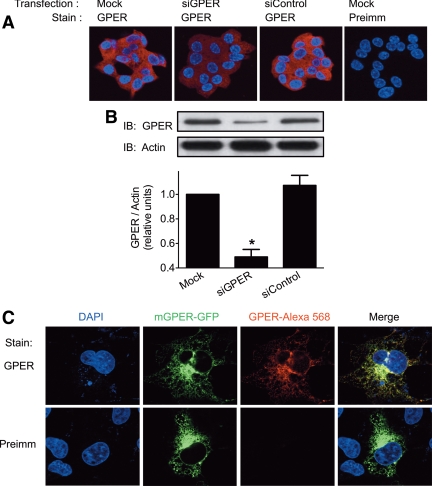
MIN6 cells have been widely used as a model system to study mechanisms of insulin secretion from pancreatic β-cells. Although MIN6 cells have been shown to be responsive to E2 (27) and genistein (28), a phytoestrogen, the receptor mediating these responses is unclear although presumed to be ERα. We therefore sought to determine whether MIN6 cells express GPER, a potential mediator of E2 responsiveness. MIN6 cells demonstrated GPER expression as determined by both immunofluorescence staining and Western blotting using a polyclonal antibody generated against residues in the second extracellular loop of mouse GPER (Fig. 1). Immunofluorescence staining with this antibody revealed an intracellular pattern for GPER in MIN6 cells that decreased significantly in intensity upon transfection with siRNA targeting GPER (siGPER) (Fig. 1A). This staining was also blocked by the peptide against which the antibody was raised but not by another peptide from mouse GPER (Supplemental Fig. 1A, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Western blots with the anti-GPER antibody revealed the presence of a major specific band for GPER in MIN6 cells (MW ∼ 40 kDa) (Fig. 1B). Antibody specificity in Western blots was similarly confirmed using siGPER (Fig. 1B) and peptide blocking (Supplemental Fig. 1B). Additional bands of higher and lower molecular weight (Supplemental Fig. 1B) were also diminished by siGPER, suggesting the presence of oligomers and degradation products as recently described (29). The localization of mouse GPER and the specificity of the polyclonal antibody for the receptor were further evaluated in COS7 cells transfected with mouse GPER-GFP, which showed a reticular GFP pattern that upon staining with the polyclonal anti-GPER antibody revealed extensive colocalization (Fig. 1C).
Fig. 1.
Expression and localization of mouse GPER. A, Immunofluorescence of MIN6 cells stained for GPER (red) in the presence or absence of siGPER. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). B, Western blot analysis of MIN6 cells showing GPER expression in the presence or absence of siRNA for GPER. Blot is representative of three experiments, which were quantified and normalized to actin levels (lower panel); *, P < 0.01 vs. mock treatment. C, COS7 cells transfected with mouse GPER-GFP (mGPER-GFP) showing intracellular localization of GPER and colocalization of staining with antibody raised against mouse GPER. Preimm, Preimmune serum. IB, Immunoblot.
MIN6 cells mobilize intracellular calcium upon stimulation with E2 and G-1
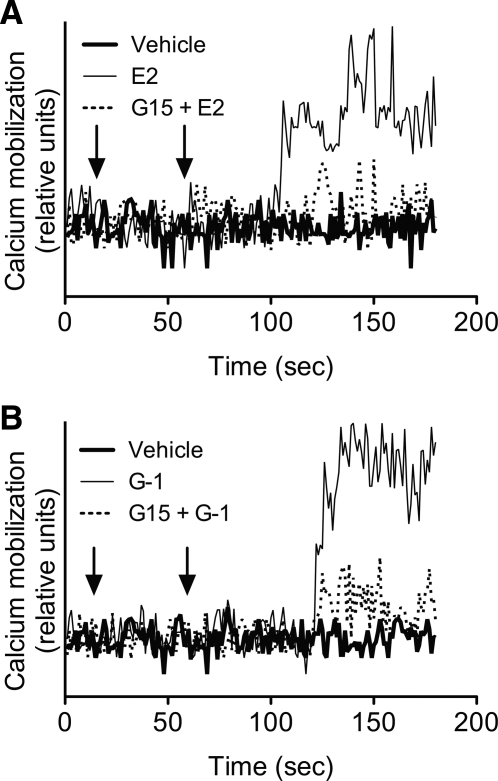
Intracellular calcium elevation in pancreatic β-cells is known to be a critical step in the secretion of insulin (30). To determine whether GPER is capable of modulating calcium levels, we stimulated indo-1 AM-loaded MIN6 cells with E2 or G-1, a GPER-selective agonist. Both agonists demonstrated a delayed (40–60 sec) elevation of intracellular calcium (Fig. 2) compared with previous observations in cancer cell lines that show an almost immediate initiation of calcium elevation using the same technique (13). This effect was reversed by the GPER-selective antagonist G15, suggesting that increases in calcium mobilization upon E2 or G-1 stimulation are mediated by GPER.
Fig. 2.
Intracellular calcium mobilization in MIN6 cells via GPER. MIN6 cells treated with E2 (A) or G-1 (B), both 10 nm, show intracellular calcium mobilization that can be blocked by the GPER antagonist G15 (200 nm). G15 or vehicle was added at the first arrow; E2, G-1, or vehicle was added at the second arrow. Representative of five experiments.
E2 and G-1 induce activation of ERK and PI3K/Akt in MIN6 cells
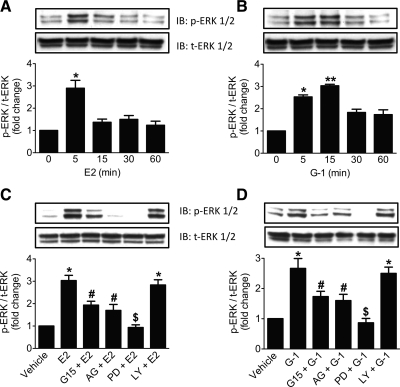
Because GPER is known to activate ERK and Akt in various cancer cell lines (13, 31) and ERK (32) as well as Akt (33) are activated in MIN6 cells by glucose, we sought to determine whether GPER stimulation can activate ERK and/or Akt in MIN6 cells. After treatment of MIN6 cells with E2 or G-1, Western blots were probed for phospho-ERK (p-ERK), which showed activation in a time-dependent manner (Fig. 3). For E2-treated cells, a significant E2-induced peak in ERK phosphorylation was observed with a 5-min stimulation, (Fig. 3A), whereas G-1 induced a more sustained p-ERK response, declining only after 15 min (Fig. 3B).
Fig. 3.
Activation of ERK in MIN6 cells by GPER. A and B, Western blot analyses of a time course of MIN6 cells stimulated with E2 or G-1 (A and B, respectively), both at 10 nm, showing p-ERK and total-ERK (t-ERK) levels. C and D, Western blot analyses for p-ERK and t-ERK levels in MIN6 cells upon stimulation with E2 and G-1, respectively (both 10 nm, 5 min for E2 and 15 min for G-1) in the presence or absence of G15 (200 nm, 1 h), AG1478 (200 nm, 1 h), PD98059 (25 μm, 1 h), or LY294002 (25 μm, 1 h). Blots are representative of three different experiments, and activation is expressed as the ratio of p-ERK/t-ERK. Results are expressed as mean ± sem; *, P < 0.05; **, P < 0.01 vs. 0 time point or vehicle; #, P < 0.05; $, P < 0.01 vs. E2 (C) and G-1 (D), respectively. IB, Immunoblot.
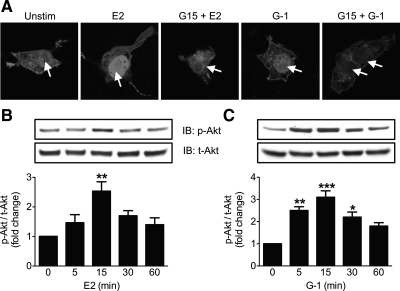
To determine the ability of recombinant mouse GPER to activate PI3K, we used COS7 cells cotransfected with mouse GPER-GFP and PH-RFP, a PIP3-binding reporter construct that contains the PH domain of Akt fused to RFP. Upon PI3K activation, the resulting PIP3 is localized on membranes via accumulation of the PH-RFP reporter. Stimulation with either E2 or G-1 demonstrated nuclear translocation of the reporter only in GPER-expressing cells, a response that was inhibited by pretreatment with the GPER antagonist G15 (Fig. 4A and Supplemental Fig. 2). This pattern has been previously observed using human GPER (13) and suggests that the expression of mouse GPER is sufficient to elicit E2-mediated PI3K/Akt activation. E2-induced Akt phosphorylation in MIN6 cells peaked at about 15 min and then decreased, whereas G-1-treated cells again showed a more sustained Akt activation (Fig. 4, B and C).
Fig. 4.
Activation of PI3K/Akt pathway by GPER. A, PI3K activation in response to E2 or G-1 in COS7 cells is blocked by GPER antagonist G15. COS7 cells cotransfected with mouse GPER-GFP and PIP3 reporter plasmid PH-RFP were stimulated with E2 or G-1 (both at 10 nm for 15 min) in the presence or absence of GPER antagonist G15 (200 nm, 1 h), and PI3K activation was determined as the translocation of the reporter to the nucleus (arrows). B and C, Activation of Akt [measured as ratio of phospho-Akt (p-Akt)/total-Akt (t-Akt) levels] in MIN6 cells treated with E2 (B) or G-1 (C) over a time course shown by Western blot analyses. Blots are a representative of three independent experiments; *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. 0 time point. Unstim, Unstimulated; IB, immunoblot.
Mechanism of E2- and G-1-induced ERK activation
The mechanism and specificity of GPER-mediated ERK activation were investigated using signaling inhibitors. Pretreatment with the GPER-selective antagonist G15 significantly blocked ERK activation by both E2 and G-1 (Fig. 3, C and D). Because GPER is known to transactivate EGFR in a number of cancer cell lines, we tested the ability of the EGFR inhibitor AG1478 to inhibit ERK phosphorylation. In addition, we tested the PI3K inhibitor LY294002 and as a positive control, the MAPK kinase (i.e. ERK) inhibitor PD98059. Incubation of MIN6 cells with PD98059 and AG1478 before stimulation with E2 or G-1 significantly blocked ERK activation by both agonists, whereas the PI3K inhibitor LY294002 was without effect (Fig. 3, C and D), suggesting that PI3K/Akt activation is downstream or independent of EGFR and ERK activation.
E2-induced insulin release is mediated by GPER via ERK and PI3K
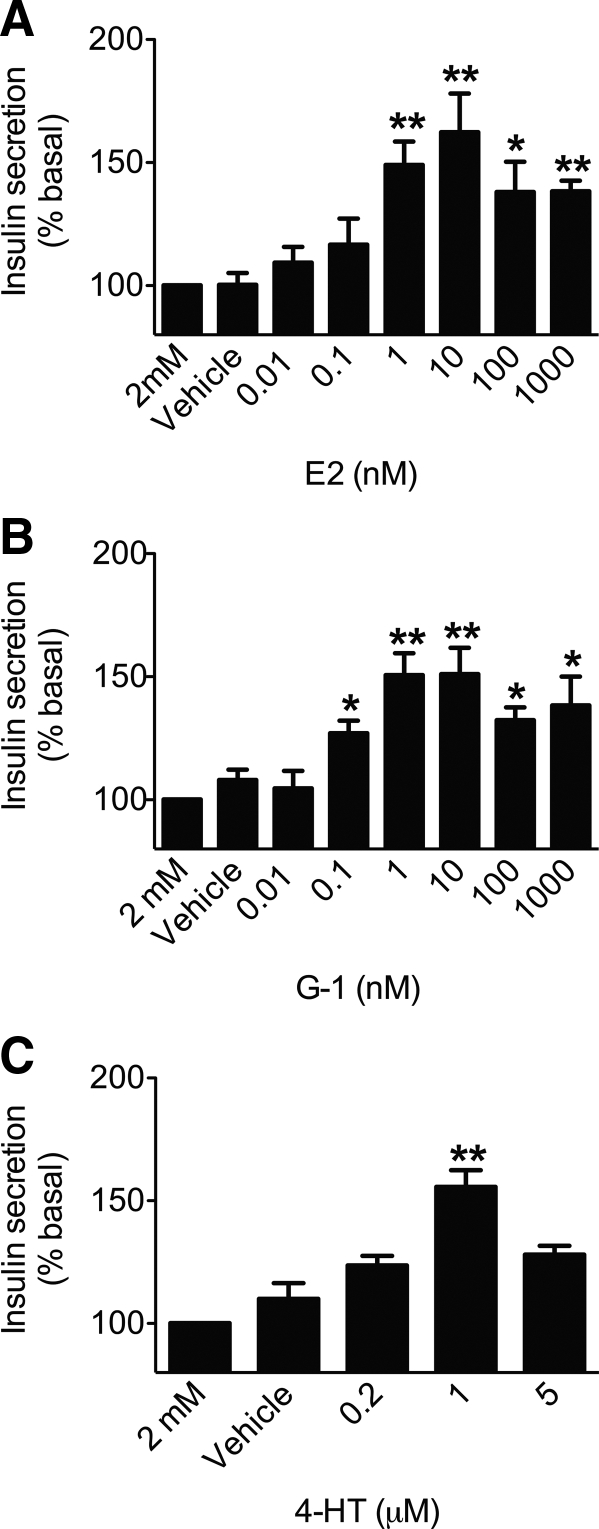
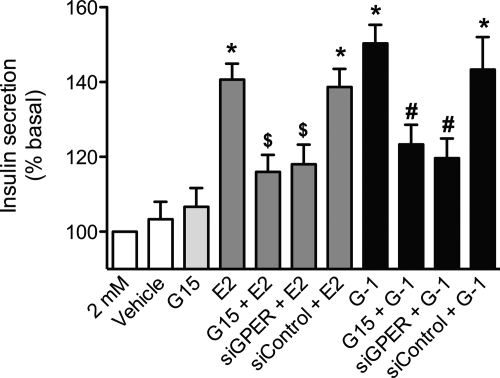
Based on the ability to mobilize intracellular calcium and the multiple signaling pathways activated by E2 and G-1 via GPER in MIN6 cells, we tested whether activation of GPER leads to a stimulation of insulin secretion. Dose-response analyses for E2, G-1, and another GPER agonist 4-HT in MIN6 cells produced bell-shaped curves (Fig. 5). The responses for both E2 and G-1 peaked between 1 and 10 nm and then gradually declined (at 100 nm and 1 μm), although at these latter concentrations, the responses remained significantly higher than vehicle (Fig. 5, A and B), whereas for 4-HT, the response peaked at 1 μm (Fig. 5C). To determine the contribution of GPER to E2- and G-1-mediated insulin secretion, MIN6 cells were treated with a low dose of E2 or G-1 (1 nm) under varying glucose concentrations with G15 or siRNA. Both E2- and G-1-stimulated cells exhibited significantly higher insulin secretion under low-glucose (2 mm) and high-glucose (25 mm) conditions, which was inhibited by either G15 pretreatment or knockdown of GPER using siRNA (Fig. 6 and Supplemental Fig. 3).
Fig. 5.
Dose-dependent insulin secretion in MIN6 cells by E2, G-1, or 4-HT. MIN6 cells were incubated for 2 h with the indicated doses of E2 (A), G-1 (B), and 4-HT (C). Insulin was assayed in the medium by ELISA and normalized to cellular protein content. Results are expressed as the mean ± sem (three independent experiments performed in triplicate) of percentage insulin secretion vs. 2 mm glucose; *, P < 0.05; **, P < 0.01 vs. basal glucose (2 mm glucose only), defined as 100%.
Fig. 6.
Insulin secretion in MIN6 cells via GPER activation. MIN6 cells were stimulated with E2 or G-1 (both at 1 nm, 2 h) in the presence of 2 mm glucose and/or GPER antagonist G15 (200 nm, 1 h pretreatment + 2 h during stimulation) or after transfection with control or GPER-targeted siRNA 2 d before stimulation as above. Results are expressed as mean ± sem (three independent experiments performed in triplicate) of percentage insulin secretion vs. 2 mm glucose (defined as 100%); *, P < 0.001 vs. 2 mm; $, P < 0.05 vs. E2; #, P < 0.01 vs. G-1.
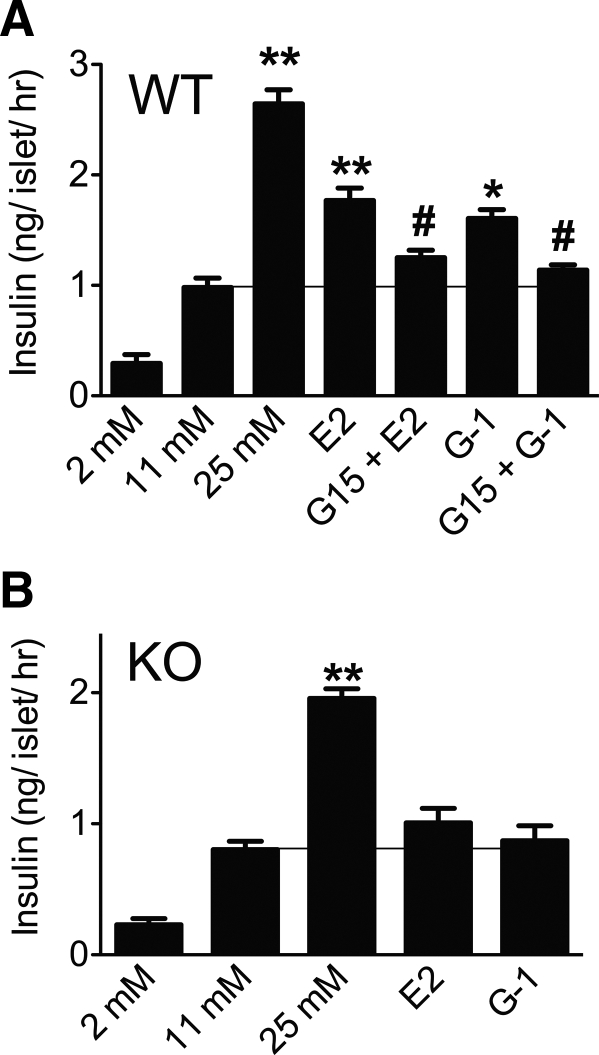
We next examined the role of E2 and G-1 in intact islets. Islets from WT mice responded to E2 or G-1 (in the presence of 11 mm glucose) with significantly higher insulin secretion (∼50% the secretion observed with 25 mm alone) that was effectively reversed by pretreatment with G15 (Fig. 7A). Conversely, islets from GPER KO mice failed to respond to either E2 or G-1 (Fig. 7B). Because islets from KO mice were not stimulated by E2 or G-1 to secrete insulin, KO islets were incubated with high glucose (25 mm) to determine whether the absence of GPER results in a nonspecific effect. Islets from the GPER KO mice still exhibited an increase in insulin secretion upon treatment with 25 mm glucose (Fig. 7B), which was however marginally lower than that observed in the islets from WT mice (Fig. 7A).
Fig. 7.
G-1 stimulates insulin secretion from mouse islets via GPER. Islets isolated from the WT (A) or GPER KO (B) mice were treated with E2 and G-1 (both 100 nm, 1 h) in buffer containing 11 mm glucose in the presence or absence of GPER antagonist, G15 (1 μm, 1-h pretreatment + 1 h during stimulation). Insulin secretion was measured using ELISA and expressed as ng/islet·h. To assess physiological functioning of the islets, insulin secretion was also measured from the islets in buffer containing 2 and 25 mm glucose. Results are expressed as mean ± sem from three experiments; *, P < 0.05; **, P < 0.01 vs. 11 mm glucose; #, P < 0.05 vs. respective E2 or G-1 treatment.
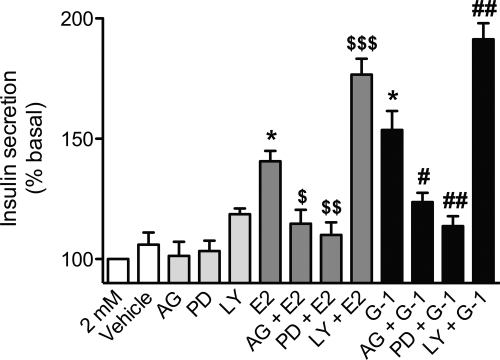
Having shown that loss of GPER in MIN6 cells or in islets reduces E2- or G-1-mediated increases in insulin secretion, we next examined the importance of the activated signaling pathways involved in mediating insulin secretion. In MIN6 cells, the EGFR inhibitor AG1478 and the ERK inhibitor PD98059 resulted in a significant reduction in E2- and G-1-induced insulin secretion at 2 mm glucose (Fig. 8) as well as at 11 and 25 mm glucose (Supplemental Fig. 4). Interestingly, ERK inhibition in the absence of E2/G-1 stimulation also reduced glucose-stimulated insulin secretion at 11 and 25 mm glucose (Supplemental Fig. 4) but had no effect on basal insulin secretion at 2 mm glucose (Fig. 8). In contrast, treatment with the PI3K inhibitor LY294002 alone increased insulin secretion only at stimulatory glucose concentrations (11 and 25 mm) (Supplemental Fig. 4), whereas subsequent E2/G-1 stimulation further enhanced insulin secretion at all glucose concentrations (Fig. 8 and Supplemental Fig. 4). Together, these results indicate that E2 and G-1 act through GPER in pancreatic β-cells to bring about insulin secretion in an EGFR- and ERK-dependent manner.
Fig. 8.
Dependence of insulin secretion in MIN6 cells on GPER-mediated signal transduction pathways. MIN6 cells were treated with E2 or G-1 (both 1 nm, 2 h) in the presence or absence of signal transduction inhibitors (1-h pretreatment + 2 h during stimulation): AG1478 (200 nm), PD98059 (25 μm), and LY294002 (25 μm). Results are expressed as mean ± sem (three independent experiments performed in triplicate) of percentage insulin secretion vs. 2 mm glucose; *, P < 0.001 vs. 2 mm glucose; $, P < 0.05 vs. E2; $$, P < 0.01 vs. E2; $$$, P < 0.001 vs. E2; #, P < 0.01 vs. G-1; ##, P < 0.001 vs. G-1.
Discussion
E2 is an important regulator of glucose homeostasis and islet physiology, especially in females. Physiologic estrogens protect against glucose intolerance, insulin resistance, development of diabetes, and control fat deposition in females (34). Beyond a normal physiological range, an increase or decrease in E2 levels can interfere with normal metabolism and may lead to insulin resistance and diabetes (35). The protective nature of E2 and its mimetics on islets and particularly β-cells has been investigated over the past decade (18). E2 elicits protective effects in a mouse model of diabetes in males, whereas ovariectomy in females worsens the disease (36, 37). Initially, ERα was considered to be the only receptor mediating these effects, because ERα KO mice are obese and develop insulin resistance that is associated with impaired oxidative metabolism and inflammation (16, 38). Nevertheless, it has been speculated for some time that there exists a membrane receptor that mediates the rapid nongenomic effects of E2 on islets (39). However, evidence for such a receptor has only begun to accumulate in the past few years (40). GPER, the newly identified membrane ER, is emerging as an important component of the complex system that regulates the survival as well as function of pancreatic islets (19–21).
Immunofluorescence staining of GPER in MIN6 cells revealed a predominantly intracellular localization, consistent with many previous reports, although rapid recycling (and an association with intermediate filaments), which leads to predominantly intracellular localization at steady state, has recently been reported (29). This is largely in agreement with our previous studies, where staining for GPER colocalizes predominantly with an intracellular marker for endoplasmic reticulum (13), although other groups have reported GPER to be localized to the plasma membrane (10, 41). A recent study has also revealed the presence of GPER (colocalized with intracellular hormones) in various cell types in mouse islets as well as in MIN6 cells, although at lower levels in the latter (19).
Recent studies have highlighted the role of GPER in pancreatic islet survival and insulin secretion (19–21). Here, MIN6 cells showed an increase in insulin secretion both at low- and high-glucose conditions in the presence of E2 or G-1. Previous studies have shown that E2 or its mimetics can increase insulin content and release (3, 42), although contradictions exist. Nadal's group reported a significant increase in insulin content and secretion in islets cultured in 11 mm glucose after treatment with low doses of E2 or bisphenol A (1 or 10 nm) for 48 h (42). They attributed this increase in insulin content to ERα and detected insulin release only at stimulatory glucose concentrations (i.e. 7 and 16 mm glucose). On the other hand, Mårtensson et al. (21) demonstrated that in islets from mice of both sexes treated with supraphysiological dose of 5 μm E2, an increase in insulin secretion along with a concomitant decrease in glucagon secretion occurred under nonstimulatory (1 mm glucose) as well as stimulatory (20 mm glucose) conditions. These E2-mediated effects were absent in islets isolated from GPER KO mice. Besides E2, insulin secretion in response to hormones (e.g. ACTH) under nonstimulatory conditions has also been reported (43).
Dose-response analyses for E2 and G-1 in MIN6 cells exhibited a bell-shaped curve with moderate physiological doses being more effective at increasing insulin secretion than higher doses. An inverted U-shape response for insulin content was previously demonstrated in islets exposed to E2 or bisphenol A for 48 h (42). A recent study in islets, however, demonstrated a sigmoid curve for insulin secretion (at 12 mm glucose) with a decline in glucagon secretion (at 1 mm glucose), in response to both E2 and G-1 treatment for 1 h (19). Such differences could arise from the type of cells used (islets vs. MIN6 cells), type or time of treatments, and culture conditions.
To establish the specificity of GPER for E2-mediated insulin release, we employed both G15 and siRNA in MIN6 cells as well as islets from GPER KO mice. Our results are supported by previous studies wherein islets from GPER-deficient male and female mice showed a loss of 5 μm E2-induced insulin secretion and a loss of the decrease in glucagon secretion, although insulin secretion remained responsive to glucose, tolbutamide, and high K+ (21). In this study, Mårtensson et al. also showed that deletion of GPER resulted in increased plasma glucose levels in females and compromised glucose tolerance. In our studies, islets from GPER KO mice that failed to respond to E2 or G-1 remained responsive to high glucose, suggesting that loss of GPER did not substantially affect basic β-cell function. Interestingly, and consistent with an earlier report (21), levels of insulin secretion were modestly lower in islets from KO mice compared with WT mice at 11 mm as well as 25 mm glucose, whereas at 2 mm glucose, no apparent differences were observed. Taken together from these results, we speculate that loss of GPER may result in a modest compromise in the synthesis/secretion of insulin.
Virtually nothing is known regarding the mechanism(s) whereby E2 or G-1 mediate insulin secretion in β-cells. Our results also revealed that both E2 and G-1 induce activation of ERK and Akt in MIN6 cells. In many cell types (predominantly cancer cell lines), ERK activation by GPER occurs via transactivation of EGFR (44). Consistent with these observations, treatment with the EGFR inhibitor, AG1478 suppressed ERK activation, whereas pretreatment with the PI3K inhibitor, LY294002, did not, suggesting that ERK is either upstream or independent of PI3K activation. Immunostaining of MIN6 cells with p-ERK antibody showed that ERK is activated within 5 min of exposure to E2 or G-1, but phosphorylated ERK was not translocated to the nucleus (our unpublished data) as is observed in response to activation by glucose (32). This is in agreement with previous reports using β-cells dispersed from islets in which 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT) an E2 mimetic, produced phosphorylated ERK that remained in the cytoplasm, suggesting that estrogenic ERK activation in β-cells does not follow the paradigm of nuclear translocation (42). Recently, quercetin (a GPER agonist) (45) but not two other antioxidants (that are likely not GPER agonists) was shown to stimulate insulin secretion in INS-1 cells in an ERK-dependent manner, suggesting that quercetin may have been acting in part through GPER (46).
Although ERK and PI3K both modulate insulin release in pancreatic β-cells, ERK is a positive effector (47), whereas PI3K is a negative effector (48), and little is known of the role of the EGFR (49), although EGF has been reported to stimulate insulin secretion (50). Inhibition of EGFR or ERK substantially blocked the E2- or G-1-induced increase in insulin secretion. Thus, GPER mediates insulin release through transactivation of the EGFR that subsequently activates ERK. In contrast, inhibition of PI3K led to an increase in insulin release compared with that of E2 or G-1 alone. This suggests that the ERK and PI3K pathways are activated independently and oppose each other. Thus, in the absence of any inhibitors, the E2- or G-1-mediated increase in insulin release is the net result of interactions of both pathways with ERK-mediated stimulation exceeding PI3K-mediated inhibition of insulin secretion. Treatment with AG1478 or PD89059 reduces insulin secretion, because the positive effect of ERK activation is lost. On the contrary, treatment with LY294002 abolishes the inhibitory effect of PI3K on MIN6 cells, resulting in a further increase in insulin secretion. The effects of ERK and PI3K inhibition were also observed under basal high-glucose conditions (11 and 25 mm) but not at 2 mm glucose, suggesting a contributing role for ERK and an inhibitory function for PI3K in glucose-stimulated insulin secretion, as previously observed by others (46, 51).
A recent study by Liu et al. (20) suggested an interaction of an extranuclear ERα as well as GPER in maintaining the integrity/viability of pancreatic islets. They demonstrated that both E2 and G-1 protect against streptozotcin induced damage in MIN6 cells as well as in islets from mice and humans. In another study, Balhuizen et al. (19) demonstrated that, in addition to modulating hormone secretion, GPER reduced apoptosis in islets of female mice treated with proinflammatory cytokines. Similarly, genistein, a plant-based isoflavone that mimics E2, besides inducing insulin secretion also counteracts streptozotocin induced damage to β-cells in mice (5) and has been shown to reduce hyperglycemia in alloxan-treated rats (52). Because binding and functional studies have shown that genistein activates GPER (45), it is tempting to speculate that genistein may act through GPER in β-cells.
In this study, we show for the first time that GPER-selective agonists and antagonists regulate insulin release in pancreatic β-cells via the ERK and PI3K/Akt pathways. Given the ability of GPER to modulate hormone secretion and survival in pancreatic β-cells, the GPER agonist G-1 could serve as an important potential small molecule for therapeutic intervention in diabetes.
Acknowledgments
We thank Dr. Helen Hathaway and Dr. Chelin Hu from University of New Mexico for assistance with animal studies and Philip Pratt from University of Colorado (Denver, CO) for guidance in harvesting islets.
This work was supported by the National Institutes of Health Grant CA127731. Confocal images in this study were generated in the Fluorescence Microscopy Facilities, which received support from the University of New Mexico Health Sciences Center and the University of New Mexico Cancer Center.
Disclosure Summary: E.R.P. is an inventor on United States Patent Number 7,875,721. G.S. has nothing to disclose.
Footnotes
- Akt
- Serine-threonine kinase
- E2
- 17β-estradiol
- EGFR
- epidermal growth factor receptor
- ER
- estrogen receptor
- GFP
- green fluorescent protein
- GPER
- G protein-coupled ER
- HRP
- horseradish peroxidase
- 4-HT
- 4-hydroxytamoxifen
- KO
- knockout
- NGS
- normal goat serum
- p-ERK
- phospho-ERK
- PH
- pleckstrin homology
- PI3K
- phosphatidylinositol 3-kinase
- RFP
- red fluorescent protein
- siGPER
- siRNA targeting GPER
- siRNA
- small interfering RNA
- WT
- wild type.
References
- 1. Liu S, Mauvais-Jarvis F. 2010. Minireview: estrogenic protection of β-cell failure in metabolic diseases. Endocrinology 151:859–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mauvais-Jarvis F. 2011. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab 22:24–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. 2006. The estrogenic effect of bisphenol A disrupts pancreatic β-cell function in vivo and induces insulin resistance. Environ Health Perspect 114:106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73:135–162 [DOI] [PubMed] [Google Scholar]
- 5. Szkudelska K, Nogowski L. 2007. Genistein—a dietary compound inducing hormonal and metabolic changes. J Steroid Biochem Mol Biol 105:37–45 [DOI] [PubMed] [Google Scholar]
- 6. Watson CS, Jeng YJ, Kochukov MY. 2010. Nongenomic signaling pathways of estrogen toxicity. Toxicol Sci 115:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hewitt SC, Harrell JC, Korach KS. 2005. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol 67:285–308 [DOI] [PubMed] [Google Scholar]
- 8. Levin ER. 2008. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol 295:R1425–R1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, Hathaway HJ. 2008. Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu Rev Physiol 70:165–190 [DOI] [PubMed] [Google Scholar]
- 10. Filardo E, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. 2007. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148:3236–3245 [DOI] [PubMed] [Google Scholar]
- 11. Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M. 2007. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 [DOI] [PubMed] [Google Scholar]
- 12. Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. 2002. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- 13. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 14. Mizukami Y. 2010. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J 57:101–107 [DOI] [PubMed] [Google Scholar]
- 15. Schneider JG, Tompkins C, Blumenthal RS, Mora S. 2006. The metabolic syndrome in women. Cardiol Rev 14:286–291 [DOI] [PubMed] [Google Scholar]
- 16. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. 2000. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nadal A, Alonso-Magdalena P, Soriano S, Ripoll C, Fuentes E, Quesada I, Ropero AB. 2011. Role of estrogen receptors α, β and GPER1/GPR30 in pancreatic β-cells. Front Biosci 16:251–260 [DOI] [PubMed] [Google Scholar]
- 19. Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. 2010. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol Cell Endocrinol 320:16–24 [DOI] [PubMed] [Google Scholar]
- 20. Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, Mauvais-Jarvis F. 2009. Importance of extranuclear estrogen receptor-α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58:2292–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. 2009. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–698 [DOI] [PubMed] [Google Scholar]
- 22. Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. 2006. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2:207–212 [DOI] [PubMed] [Google Scholar]
- 23. Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. 2009. In vivo effects of a GPR30 antagonist. Nat Chem Biol 5:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. 1990. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- 25. Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. 2008. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22:636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. 2009. A practical guide to rodent islet isolation and assessment. Biol Proced Online 11:3–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Majed HT, Squires PE, Persaud SJ, Huang GC, Amiel S, Whitehouse BJ, Jones PM. 2005. Effect of 17β-estradiol on insulin secretion and cytosolic calcium in Min6 mouse insulinoma cells and human islets of Langerhans. Pancreas 30:307–313 [DOI] [PubMed] [Google Scholar]
- 28. Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. 2006. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes 55:1043–1050 [DOI] [PubMed] [Google Scholar]
- 29. Sandén C, Broselid S, Cornmark L, Andersson K, Daszkiewicz-Nilsson J, Mårtensson UE, Olde B, Leeb-Lundberg LM. 2011. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol Pharmacol 79:400–410 [DOI] [PubMed] [Google Scholar]
- 30. Kasai H, Hatakeyama H, Ohno M, Takahashi N. 2010. Exocytosis in islet β-cells. Adv Exp Med Biol 654:305–338 [DOI] [PubMed] [Google Scholar]
- 31. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 32. Benes C, Roisin MP, Van Tan H, Creuzet C, Miyazaki J, Fagard R. 1998. Rapid activation and nuclear translocation of mitogen-activated protein kinases in response to physiological concentration of glucose in the MIN6 pancreatic β cell line. J Biol Chem 273:15507–15513 [DOI] [PubMed] [Google Scholar]
- 33. Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. 2002. Glucose promotes pancreatic islet β-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab 283:E784–E793 [DOI] [PubMed] [Google Scholar]
- 34. Brown LM, Clegg DJ. 2010. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 122:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. 2009. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol 587:5031–5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leiter EH. 1982. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci USA 79:630–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamabe N, Kang KS, Zhu BT. 2010. Beneficial effect of 17β-estradiol on hyperglycemia and islet β-cell functions in a streptozotocin-induced diabetic rat model. Toxicol Appl Pharmacol 249:76–85 [DOI] [PubMed] [Google Scholar]
- 38. Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. 2010. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab 298:E304–E319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nadal A, Rovira JM, Laribi O, Leon-quinto T, Andreu E, Ripoll C, Soria B. 1998. Rapid insulinotropic effect of 17β-estradiol via a plasma membrane receptor. FASEB J 12:1341–1348 [DOI] [PubMed] [Google Scholar]
- 40. Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. 2000. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor α and estrogen receptor β. Proc Natl Acad Sci USA 97:11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. 2006. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochem Biophys Res Commun 346:904–910 [DOI] [PubMed] [Google Scholar]
- 42. Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. 2008. A Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One 3:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Al-Majed HT, Jones PM, Persaud SJ, Sugden D, Huang GC, Amiel S, Whitehouse BJ. 2004. ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans. J Endocrinol 180:155–166 [DOI] [PubMed] [Google Scholar]
- 44. Filardo EJ. 2002. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol 80:231–238 [DOI] [PubMed] [Google Scholar]
- 45. Maggiolini M, Vivacqua A, Fasanella G, Recchia AG, Sisci D, Pezzi V, Montanaro D, Musti AM, Picard D, Andò S. 2004. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem 279:27008–27016 [DOI] [PubMed] [Google Scholar]
- 46. Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, Richard S, Quignard JF, Gross R, Petit P, Bataille D, Oiry C. 2010. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol 161:799–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Longuet C, Broca C, Costes S, Hani EH, Bataille D, Dalle S. 2005. Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 β-cell line and islets of Langerhans. Endocrinology 146:643–654 [DOI] [PubMed] [Google Scholar]
- 48. Hagiwara S, Sakurai T, Tashiro F, Hashimoto Y, Matsuda Y, Nonomura Y, Miyazaki J. 1995. An inhibitory role for phosphatidylinositol 3-kinase in insulin secretion from pancreatic B cell line MIN6. Biochem Biophys Res Commun 214:51–59 [DOI] [PubMed] [Google Scholar]
- 49. Miettinen P, Ormio P, Hakonen E, Banerjee M, Otonkoski T. 2008. EGF receptor in pancreatic β-cell mass regulation. Biochem Soc Trans 36:280–285 [DOI] [PubMed] [Google Scholar]
- 50. Lee HY, Yea K, Kim J, Lee BD, Chae YC, Kim HS, Lee DW, Kim SH, Cho JH, Jin CJ, Koh DS, Park KS, Suh PG, Ryu SH. 2008. Epidermal growth factor increases insulin secretion and lowers blood glucose in diabetic mice. J Cell Mol Med 12:1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Collier JJ, White SM, Dick GM, Scott DK. 2004. Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochem Biophys Res Commun 324:1018–1023 [DOI] [PubMed] [Google Scholar]
- 52. Yang W, Wang S, Li L, Liang Z, Wang L. 2011. Genistein reduces hyperglycemia and islet cell loss in a high-dosage manner in rats with alloxan-induced pancreatic damage. Pancreas 40:396–402 [DOI] [PubMed] [Google Scholar]