Abstract
Endometriosis is a common estrogen-dependent disorder. Medical treatments currently consist of progestins or GnRH agonists; however, neither is fully effective and both entail significant side effects. Selective estrogen receptor (ER) modulators (SERM) have tissue-selective actions, acting as an ER agonist in some tissues and ER antagonist in others. The SERM bazedoxifene (BZA) effectively antagonizes estrogen-induced uterine endometrial stimulation without countering estrogenic effects in bone or central nervous system. These properties make it an attractive candidate for use in the treatment of endometriosis. Experimental endometriosis was created in reproductive-age CD-1 mice. After 8 wk, 10 animals received ip injections of BZA (3 mg/kg·d) for 8 wk, whereas 10 received vehicle control. Mice were killed, and implant size was assessed. The mean size of the implants after treatment was 60 mm2 in the control group and 21 mm2 in the BZA treatment group (P = 0.03). Quantitative PCR and immunohistochemical analysis were used to determine the effect on endometrial gene expression. PCNA, ERα, and LIF mRNA and protein expression were significantly decreased in endometrium of the treated group. Caspase 3 mRNA expression was increased. Expression of PR and Hoxa10 were not significantly altered by treatment. There was no evidence of ovarian enlargement or cyst formation. Decreased PCNA and ER expression demonstrated that the regression of endometriosis likely involved decreased estrogen-mediated cell proliferation. BZA may be an effective novel agent for the treatment of endometriosis due to greater endometrial-specific estrogen antagonism compared with other SERM.
Endometriosis is a common, estrogen-dependent, chronic gynecological disorder of reproductive-aged women that is associated with pelvic pain, dysmenorrhea, and infertility. It is characterized by the presence of endometrial tissue outside of the uterus. Common sites include the pelvic peritoneum, ovaries, and rectovaginal septum; however, rarely, the pericardium, pleura, and even the central nervous system (CNS) are affected (1). The prevalence of pelvic endometriosis is approximately 10–15% in the reproductive-age female population; in women with pain, infertility, or both, the frequency is 35–50% (2).
The traditional explanation for the existence of endometrium in ectopic locations is based on the common occurrence of retrograde menstruation, where endometrial tissue flows out of the fallopian tubes and into the peritoneal cavity. Implants of endometrium likely persist in some individuals. This theory does not account for the existence of endometriosis in areas far removed from the pelvis; endometriosis is reported to occur in locations that do not communicate with the peritoneal cavity, such as the lung and brain, where retrograde menstruation cannot account for the presence of this tissue. The existence of a circulating source of endometrial progenitor cells suggests an alternative theory for the etiology of endometriosis (3).
Some other classic theories have failed to fully explain the pathogenetic mechanism of endometriosis, and its etiology remains unknown; however, mechanisms related to heredity, impaired immune function, environmental toxins, and stem cells have been implicated (4). As cellular and molecular mechanisms that regulate endometriosis are identified, it has become clear that it is not simply a local disorder; rather, it is a complex, chronic, systemic disease.
Extensive investigation has been performed on the molecular and cellular biology required for establishment and survival of endometrial implants. The mechanisms required include attachment of endometrial cells to the pelvic peritoneum, invasion into the mesothelium, and survival and proliferation of the ectopic endometrial cells. These data have been reviewed elsewhere (5, 6) but ultimately suggest that the development of endometriosis is probably a polygenetic disorder requiring alterations in multiple biological pathways for the establishment and proliferation of the disease state (7). The eutopic endometrium is also affected; altered development of the endometrium contributes to the etiology of infertility in patients with endometriosis (8–10). Ectopic lesions in women with endometriosis are similar to the eutopic endometrium; the lesions typically retain the dependence on estrogens for continued growth and the ability to differentiate in response to progestins. The current medical treatment for endometriosis has focused on the hormonal alteration of the menstrual cycle with the goal of producing pseudo-menopause (hypoestrogenic environment), or pseudo-pregnancy (progestin-dominant environment). Hormonal manipulations are often used as first-line therapy as well as after surgery to prevent recurrence of symptoms associated with endometriosis (11–19).
Selective estrogen receptor (ER) modulators (SERM) have actions that are tissue selective, often acting as predominantly ER agonists in the skeleton, on serum lipid metabolism, and on a number of coagulation factors but as ER antagonists in the breast and uterus (20). The SERM raloxifene has been approved for the prevention and treatment of postmenopausal osteoporosis. It binds to the ER with an affinity similar to 17β-estradiol (21). In ovariectomized rats, raloxifene does not stimulate the endometrium and modestly inhibits estrogen-induced endometrial proliferation (22).
Raloxifene use as a potential treatment of endometriosis has been examined in animal models. One study evaluated the effects of raloxifene on the growth of surgically transplanted uterine tissue in ovariectomized estrogen-treated rats. Daily oral administration of 10 mg/kg raloxifene for 7 or 14 d decreased the weight of the explants by approximately 70% on d 7 and 68% on d 14 compared with controls (23). One clinical trial evaluated treatment with raloxifene in women with chronic pelvic pain due to endometriosis. Subjects with biopsy-proven endometriosis were randomly allocated to 6 months of daily treatment with raloxifene (180 mg) or placebo. This study was terminated when the raloxifene group experienced greater pain and had a second surgery significantly sooner than the placebo group (24).
Another SERM, bazedoxifene (BZA), is currently under evaluation for the treatment of osteoporosis and, in combination with conjugated estrogens, for menopausal symptoms. BZA does not stimulate the endometrium in post menopausal women (25, 26) and also effectively antagonizes conjugated estrogen-induced uterine stimulation (27). A comparison of the effect of BZA, raloxifene, and lasofoxifene, alone or in combination with 17β-estradiol in the murine uterus, demonstrated that BZA has the greatest antagonistic and the least agonistic effect (28). These data suggest that BZA may be superior to other SERM in antagonizing the effect of 17β-estradiol or other ER agonists on endometriosis as well. Here we determine the efficacy of BZA in a mouse model of endometriosis.
Materials and Methods
Animal care and treatment
Eight-week-old CD1 female mice were obtained from Charles River Laboratories (Wilmington, MA) and kept under controlled conditions (a 12-h light, 12-h dark cycle and 22 C). All mice were allowed to have at least 1 wk of acclimation to this environment before surgery. Laparotomy was performed by midline incision under anesthesia using xylazine (Lloyd Laboratories, Quezon, Philippines) and ketamine (Fort Dodge Animal Health, Overland Park, KS). Whole uterus was removed from donor mice, washed in PBS, and divided into two horns. The lumen of each horn was opened longitudinally and transversely divided into two pieces. Two pieces of each uterine horn were sutured to the parietal peritoneum of each recipient mouse to create experimental endometriosis. Finally, the abdominal wall was closed using 4-0 vicryl sutures. Experimental endometriosis was created in a total of 20 mice. After 8 wk, mice were divided in two groups, Ten received ip dimethylsulfoxide (10%) plus sesame oil (90%), and 10 received ip injections of 3 mg/kg BZA (Pfizer, Collegeville, PA) in dimethylsulfoxide (10%) plus sesame oil (90%) five times a week for a total of 8 wk. BZA used in this study was provided by Wyeth (now Pfizer). The dose of BZA was determined based on previously published animal studies (28, 29). Mice were subsequently euthanized, and the implant from each side of the abdominal wall, when it existed, was removed and measured (length and width); one lesion was placed in formalin and the other in RNALater (Invitrogen Technologies, Carlsbad, CA) and frozen immediately at −80 C for later use. Uteri were removed and weighed, and one horn was fixed in 10% formalin and embedded in paraffin for histological and immunohistochemical analysis. The other was placed in RNALater and frozen immediately at −80 C for RNA extraction. This study was approved by Institutional Animal Care and Use Committee, Yale University, conforming to the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
Real-time PCR
Quantitative real-time RT-PCR was performed using iScript cDNA Synthesis Kit and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). RNA was reverse transcribed for 30 min at 42 C. PCR was performed for 45 cycles at 95 C for 15 sec, 61 C for 20 sec, and 72 C for 25 sec. Hoxa10, ER, progesterone receptor (PR), and leukemia inhibitory factor (LIF) and ß-actin primers were used as previously described (30, 31). Proliferating cell nuclear antigen (PCNA) primers were 5′-CCATCAGCACCTATAGCAGCAT-3′ (forward) and 5′-GCACGGCTCCTTCCATTTCT-3′ (reverse); caspase 3 primers were 5′-ACAGTGGCTGTGGGAAAGTC-3′ (forward) and 5′-AACTGCTTTTCCCCAGTGTG-3′ (reverse). Expression was normalized to the expression of ß-actin from the same sample. Melting curve analysis was conducted to determine the specificity of the amplified products and to ensure the absence of primer-dimer formation. All products obtained yielded the predicted melting temperature. Analysis of relative gene expression data used 2−ΔCT method. Group means were evaluated for statistical significance using a two-tailed t test. Differences of P ≤0.05 were considered significant.
Immunohistochemistry
Tissue was embedded in paraffin, cut into 5-μm sections, and mounted onto slides. Immunohistochemical analysis of Hoxa10, ER, PR, PCNA, and LIF expression were performed as previously described (30–32). Briefly, slides were deparaffinized and dehydrated through a series of xylene and ethanol washes, followed by permeabilization in 95% cold ethanol. After a 5-min rinse in distilled water, slides were steamed in 0.01 m sodium citrate buffer for 20 min and cooled for 20 min. Slides were rinsed for 5 min in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 min followed by a 5-min PBST wash. Nonspecific binding was blocked with 1.5% normal horse serum in PBST for 1 h at room temperature. The primary antibodies used were Hoxa10 (sc-17159; 1:3000), PR H-190 (sc-7208; 1:500), PCNA (Fl-2b1; 1:400), LIF (sc-1336; 1:500), ERα (1:600) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Slides were incubated with the primary antibody overnight at 4 C. Normal goat IgG (Santa Cruz) was used as a negative control. Horse α-goat biotinylated secondary antibodies were used for Hoxa 10, PCNA, LIF, and ERα and goat α-rabbit was used for PR (Vector Laboratories, Burlingame, CA) and applied for 1 h at 4 C. Slides were washed in 1× PBST, incubated in ABC Elite (Vector) for 15 min at room temperature, washed in 1× PBST, and incubated for 5 min in diaminobenzidine (Vector). A 20-sec exposure to hematoxylin was used as a counterstain. All slides were processed simultaneously for each primary antibody. Slides were rehydrated through 3-min ethanol and xylene washes and mounted with Permount (Fisher). Three hundred consecutive cells in each of 15 nonadjacent microscopic fields were evaluated from each animal to quantify the expression of each cell-type-specific marker.
An H-score for immunohistochemical staining or labeling index for PCNA nuclear staining was determined separately for the glandular and stromal cells on each slide. Each slide was scored by three different observers who were blinded to the diagnosis. The H-score was calculated with the following equation: H-score = ΣPi(i + 1), where i is the intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively), and Pi is the percentage of stained cells for each intensity, varying from 0–100%. The average score from each of the observers was calculated, and the Mann-Whitney U rank sum test was used to compare H-scores. A P value ≤ 0.05 was considered significant. For PCNA labeling index, nuclei from more than 1000 cells were counted from at least 10 random fields in each sample. The PCNA labeling index was calculated as the percentage of positive cell nuclei. The student's t test was used to compare labeling index between BZA-treated animals and controls.
Results
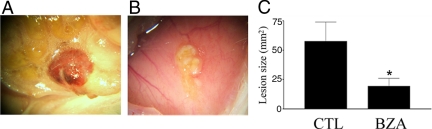
After 8 wk of treatment with BZA or vehicle control, all animals appeared healthy with no obvious adverse effects. Estrous cyclicity was confirmed using vaginal cytology. The BZA-treated animals consistently demonstrated decreased lesion size compared with vehicle-treated animals. Macroscopic analysis showed that there were no active endometriotic implants in BZA-treated mice (Fig. 1). Endometriotic lesions appeared smaller, blanched, and dormant in the BZA-treated group, whereas those in the control group typically appeared red and friable. The surface area (square millimeters) of each endometriotic lesion (active or inactive) was calculated by multiplying length (millimeters) by width (millimeters). The mean surface area of endometriotic lesions was significantly lower in BZA-treated mice than the placebo group, 21 and 60 mm2 in the BZA treatment and control groups, respectively (P = 0.03) (Fig. 1). Treatment was not initiated until establishment of lesions (8 wk). The total number of identifiable lesions was not different between groups, consistent with generation of a similar number of initial lesions. All lesions were identified at the sites of initial placement (n = 20 animals and a total of 40 sites). There was no evidence of ovarian enlargement or cysts in the treatment group after 8 wk of BZA treatment.
Fig. 1.
Endometriotic implants appearance 8 wk after treatment. Panel A is representative of the control group and B the BZA treatment group. C, Mean lesion size; n = 40 lesions (20 lesions per group in 10 animals). CTL, Control. Scale bar, 100 μm. *, P < 0.03.
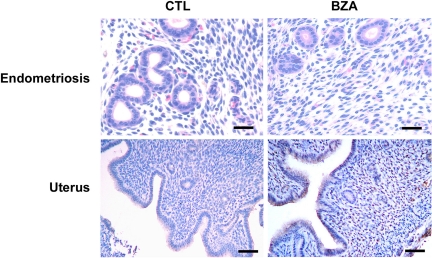
Uterine wet weight was significantly decreased by BZA treatment (Table 1). Histology demonstrated a significant reduction in gland size and number in the BZA-treated mouse uterus compared with control treated animals in both the eutopic and ectopic tissue (Fig. 2). The diminished number of glands and a decrease in luminal cell height in the uterus as a consequence of BZA treatment is quantified in Table 1. The epithelial cell height in the uteri of BZA-treated mice was significantly decreased compared with controls. Similarly, the lesions of endometriosis demonstrated large numbers of crowded and distended glands in the controls as opposed to rare collapsed glands with predominance of stroma in the BZA-treated animals. The glands pictured in Fig. 1 were uncommon and typically small, and the complete absence of glands was seen in most tissue sections.
Table 1.
Mean uterine weight, gland number, and epithelial height
| Control | BZA | P value | |
|---|---|---|---|
| Uterine wet weight (mg) | 28 ± 3 | 19 ± 4 | <0.01 |
| Gland number per field | 19 ± 8 | 5 ± 3 | <0.05 |
| Epithelial height (μm) | 43.7 ± 7.8 | 21.1 ± 5.3 | <0.001 |
Fig. 2.
Photomicrographs of endometriosis lesions and the mouse uterus stained with hematoxylin and eosin. Top, Endometriosis demonstrating crowded glands in the controls as opposed to rare collapsed glands with predominance of stroma in the BZA treatment group. Scale bar, 50 μm. Bottom, Representative photomicrographs of the uterus in the control and BZA treatment groups. Note the significant reduction in gland size and number in the uteri of BZA-treated mice. Scale bar, 150 μm.
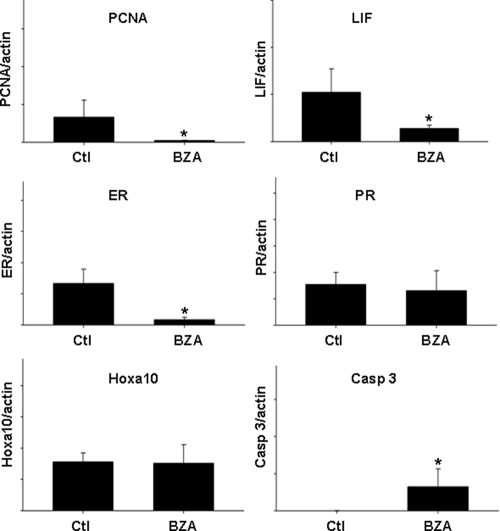
PCNA was used as a marker of cell proliferation. PCNA mRNA expression was significantly decreased in the uterus of the treated group (P = 0.016). The mean expression of PCNA normalized to β-actin was decreased by more than 10-fold in the treated group compared with the control group (Fig. 3). Similarly, expression of the ER was significantly altered by treatment. ER mRNA expression decreased by more than 10-fold (P = 0.003). As a representative estrogen-responsive gene in the endometrium, we also examined LIF expression, which was decreased by nearly 5-fold (P = 0.006). Hoxa10 and PR were used as markers of endometrial differentiation/decidualization; expression of these two genes was not significantly altered by treatment. Finally, caspase 3 was used as a marker of apoptosis. Caspase 3 mRNA was induced after treatment with BZA (P < 0.001), although there was no evidence of an increase in apoptosis seen in the histological examination of the treated tissues above, suggesting that an increase in activated caspase 3-mediated apoptosis unlikely.
Fig. 3.
Results of quantitative real-time RT-PCR demonstrate level of gene expression in the uterus of vehicle control and BZA-treated mice normalized to expression of β-actin. Expression of PCNA, ER, and LIF were significantly decreased after BZA treatment (*, P = 0.016, P = 0.003, and P = 0.006, respectively). Caspase 3 expression was increased (*, P < 0.001), whereas Hoxa10 and PR expression were unchanged.
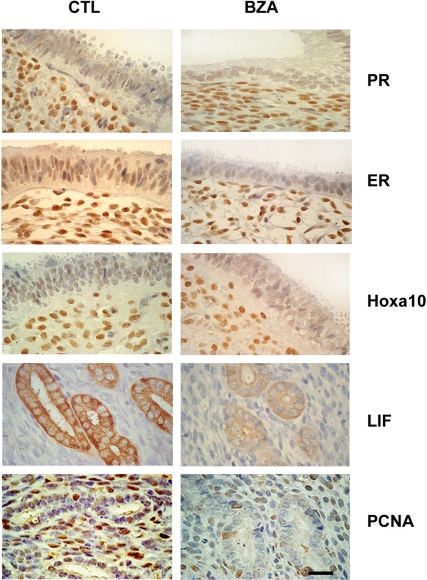
To confirm that the changes in mRNA expression identified above accurately reflected protein expression we carried out IHC on five of the genes assayed by quantitative PCR (Fig. 4). Consistent with the changes identified above, PR and Hoxa10 protein expression did not appear altered by BZA treatment. ER, PCNA, and LIF protein expression were significantly decreased in the endometrium of the treated group. A labeling index was used to assess the number of cells expressing PCNA. The labeling index decreased from 41 and 32% to 4 and 5% in the stroma and glandular tissue, respectively, after BZA treatment (P < 0.001 and P < 0.001, respectively). ER and LIF were assessed by H-score. The mean ER H-score in controls was 1.9 and 3.1 in the glands and stroma, respectively. The mean H-score for ER in the treated group was 1.2 and 2.6 in the glands and stroma, respectively (P < 0.03 and P < 0.01, respectively, in control vs. treatment groups). LIF expression was observed in the glandular epithelium and not in the stroma. The mean LIF H-score in the control group was 3.3 and 2.1 in the group treated with BZA (P < 0.001).
Fig. 4.
Immunohistochemistry identified protein expression in endometrial stroma and glands. Representative photomicrographs demonstrating PR, ERα, Hoxa10, LIF, and PCNA expression in control and BZA-treated endometrium. BZA treatment led to a reduction of both glandular and stromal expression of ER and PCNA. LIF was expressed exclusively in the glands and was decreased by BZA treatment. There was no change in PR or Hoxa10 expression after treatment. Scale bar, 30 μm.
Discussion
As a class of compounds, SERM display a continuum of ER agonist and antagonist activity, depending both on the compound and the target tissue. BZA has perhaps the greatest ER antagonist activity on the endometrium of any SERM. The present study shows that in this murine model, BZA is an effective treatment of endometriosis as measured by a decrease in the size of endometriotic implants as well as a marker of endometrial proliferation. The beneficial effect occurred without evidence of a progestin-like differentiating effect. The regression of endometriotic lesions appears to have occurred primarily by suppression of estrogen-induced proliferation. This mechanism likely involves both suppression of ER expression as well as direct ER antagonism by this SERM. This is in contrast to the differentiation/decidualization as is seen with progestin treatment and would have had an effect on PR and Hoxa10 expression.
No medical treatment for endometriosis is universally effective. Medical treatments for endometriosis are usually aimed at reducing the production of endogenous estrogens or inducing endometrial differentiation with progestins. Commonly used medical therapies used in the treatment of endometriosis include GnRH agonists, oral contraceptives, progestins, danazol, and recently, aromatase inhibitors. These agents represent standard therapies for endometriosis and are associated with side effects that impact on long-term use and adherence. For instance, GnRH agonist, a widely prescribed medical treatment for severe endometriosis, is accepted as highly efficacious; however, it is also associated with symptoms of estrogen deficiency (including vasomotor symptoms, vaginal dryness, and sleep disturbance) as well as bone demineralization. The bone loss limits treatment to 6 months unless norithindrone or other hormone therapy is concomitantly administered (33, 34). Progestins show long-term efficacy in endometriosis, but these agents are associated with weight gain and altered mood in some women when administered at doses required for efficacy (35). Depot medroxyprogesterone acetate has demonstrated efficacy; however, long-term use of depot medroxyprogesterone acetate preparations has been shown to decrease bone mineral density and to delay resumption of ovulation that may follow its discontinuation (36, 37). Danazol is characterized by adverse changes in lipid metabolism and adverse androgenic effects, which lead to low patient acceptance and compliance (38). Although medical treatments have resulted in about a 50% reduction of symptoms in women with moderate and severe endometriosis, the recurrence rate for pelvic pain is as high as 75% at 5-yr follow-up (39). There are ongoing investigations regarding new medications for the treatment of endometriosis. More efficacious medical treatments with acceptable side effects are needed. Among 15 registered and completed phase II/III clinical trials on endometriosis evaluating various compounds, a majority of them have not released their outcome in the public domain, and the three that did so reported results that are much less exciting than that seen in preclinical studies (40).
BZA is a novel treatment alternative for endometriosis. Here we demonstrate that endometriotic implants completely regressed in BZA-treated mice. Caution is warranted before proceeding to clinical trial given the discordant outcomes of animal experiments and human trials using raloxifene as an endometriosis treatment; however, given the greater ER antagonist activity of BZA in the uterus, there is rationale to expect it to have advantages over the previously tested SERM.
There are several explanations for the failure of the rodent raloxifene model to translate into clinical efficacy. Duration of treatment, the specific SERM used as well as dose are all critically important. This study was longer in duration than the raloxifene rodent study, giving assurance that steady state was reached. We also used animals with intact ovaries, whereas rats undergoing raloxifene treatment were ovariectomized and treated with estrogen. SERM can block the estrogen-mediated hypothalamic and pituitary inhibition of gonadotropin secretion, leading to ovarian stimulation and increased estrogen production. This in turn may increase pain. An indirect effect on the ovaries would not have been apparent in the ovariectomized model and only manifest in the clinical trial where subjects had intact ovaries. The longer-term suppression used in this study takes into account not only the direct SERM effect on the endometrium but also secondary responses that result from the effect of SERM on the CNS.
Furthermore, the dose used in the raloxifene clinical trial was three times the dose approved for use in menopausal women yet still did not equal the weight-adjusted effective dose used in the rat model; doses similar to those used in the human trial failed to cause lesion regression in rats. The doses used clinically were necessary to inhibit 17β-estradiol effect on the endometrium; however, they also likely completely suppressed the estrogenic effect on the CNS and led to increased gonadotropin production. BZA is a more selective SERM than raloxifene, having a greater ER antagonist effect on the endometrium and lower antagonist effect in the CNS. Thus, we expect that, compared with raloxifene, relatively low doses of BZA will more effectively inhibit the endometrium, while not fully blocking the inhibitory effect of 17β-estradiol on the CNS.
Taken together, these results suggest that BZA is more likely to demonstrate clinical efficacy than raloxifene. BZA is predicted to have superior clinical efficacy and present a novel agent for the treatment of endometriosis. Further experiments using animal models as well as clinical trials will be helpful in determining the utility of this compound in women with endometriosis.
Acknowledgments
This work was supported by Pfizer and National Institutes of Health Grant U54HD052668 and a scholarship from CAPES (the Brazilian Federal Agency for Support and Evaluation of Postgraduate Education) to J.K.
Current address for J.K.: Department of Obstetrics and Gynecology, Federal University of Parana, and University of Sao Paulo, Ribeirao Preto, Brazil.
Disclosure Summary: J.K. and C.F. have nothing to declare. B.K. is an employee of Pfizer. H.T. has received grant support from Pfizer.
Footnotes
- BZA
- Bazedoxifene
- CNS
- central nervous system
- ER
- estrogen receptor
- LIF
- leukemia inhibitory factor
- PBST
- PBS with 0.1% Tween 20
- PCNA
- proliferating cell nuclear antigen
- PR
- progesterone receptor
- SERM
- selective estrogen receptor modulator.
References
- 1. Sarma D, Iyengar P, Marotta TR, terBrugge KG, Gentili F, Halliday W. 2004. Cerebellar endometriosis. AJR Am J Roentgenol 182:1543–1546 [DOI] [PubMed] [Google Scholar]
- 2. Giudice LC, Kao LC. 2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 3. Du H, Taylor HS. 2007. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25:2082–2086 [DOI] [PubMed] [Google Scholar]
- 4. Gupta S, Goldberg JM, Aziz N, Goldberg E, Krajcir N, Agarwal A. 2008. Pathogenic mechanisms in endometriosis-associated infertility. Fertil Steril 90:247–257 [DOI] [PubMed] [Google Scholar]
- 5. Sasson IE, Taylor HS. 2008. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci 1127:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seli E, Berkkanoglu M, Arici A. 2003. Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 30:41–61 [DOI] [PubMed] [Google Scholar]
- 7. Bischoff F, Simpson JL. 2004. Genetic basis of endometriosis. Ann NY Acad Sci 1034:284–299 [DOI] [PubMed] [Google Scholar]
- 8. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. 1999. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- 9. Penna I, Du H, Ferriani R, Taylor HS. 2008. Calpain5 expression is decreased in endometriosis and regulated by HOXA10 in human endometrial cells. Mol Hum Reprod 14:613–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Penna IA, Hongling Du, Kallen AN, Taylor HS. 2010. Endothelin type A receptor (ETA) expression is regulated by HOXA10 in human endometrial stromal cells. Reprod Sci 17:471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen C, Hopewell S, Prentice A, Gregory D. 2009. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 2:CD004753. [DOI] [PubMed] [Google Scholar]
- 12. Al Kadri H, Hassan S, Al-Fozan HM, Hajeer A. 2009. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst Rev 1:CD005997. [DOI] [PubMed] [Google Scholar]
- 13. Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. 2008. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG 115:818–822 [DOI] [PubMed] [Google Scholar]
- 14. Rodgers AK, Falcone T. 2008. Treatment strategies for endometriosis. Expert Opin Pharmacother 9:243–255 [DOI] [PubMed] [Google Scholar]
- 15. Davis L, Kennedy SS, Moore J, Prentice A. 2007. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev 3:CD001019. [DOI] [PubMed] [Google Scholar]
- 16. Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. 2007. Ovulation suppression for endometriosis. Cochrane Database Syst Rev 3:CD000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abou-Setta AM, Al-Inany HG, Farquhar CM. 2006. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst Rev 4:CD005072. [DOI] [PubMed] [Google Scholar]
- 18. Appleyard TL, Mann CH, Khan KS. 2006. Guidelines for the management of pelvic pain associated with endometriosis: a systematic appraisal of their quality. BJOG 113:749–757 [DOI] [PubMed] [Google Scholar]
- 19. Allen C, Hopewell S, Prentice A. 2005. Non-steroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev 4:CD004753. [DOI] [PubMed] [Google Scholar]
- 20. Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. 1997. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647 [DOI] [PubMed] [Google Scholar]
- 21. Fuchs-Young R, Glasebrook AL, Short LL, Draper MW, Rippy MK, Cole HW, Magee DE, Termine JD, Bryant HU. 1995. Raloxifene is a tissue-selective agonist/antagonist that functions through the estrogen receptor. Ann NY Acad Sci 761:355–360 [DOI] [PubMed] [Google Scholar]
- 22. Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR, et al. 1994. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yao Z, Shen X, Capodanno I, Donnelly M, Fenyk-Melody J, Hausamann J, Nunes C, Strauss J, Vakerich K. 2005. Validation of rat endometriosis model by using raloxifene as a positive control for the evaluation of novel SERM compounds. J Invest Surg 18:177–183 [DOI] [PubMed] [Google Scholar]
- 24. Stratton P, Sinaii N, Segars J, Koziol D, Wesley R, Zimmer C, Winkel C, Nieman LK. 2008. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol 111:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pinkerton JV, Archer DF, Utian WH, Menegoci JC, Levine AB, Chines AA, Constantine GD. 2009. Bazedoxifene effects on the reproductive tract in postmenopausal women at risk for osteoporosis. Menopause 16:1102–1108 [DOI] [PubMed] [Google Scholar]
- 26. Archer DF, Pinkerton JV, Utian WH, Menegoci JC, de Villiers TJ, Yuen CK, Levine AB, Chines AA, Constantine GD. 2009. Bazedoxifene, a selective estrogen receptor modulator: effects on the endometrium, ovaries, and breast from a randomized controlled trial in osteoporotic postmenopausal women. Menopause 16:1109–1115 [DOI] [PubMed] [Google Scholar]
- 27. Kharode Y, Bodine PV, Miller CP, Lyttle CR, Komm BS. 2008. The pairing of a selective estrogen receptor modulator, bazedoxifene, with conjugated estrogens as a new paradigm for the treatment of menopausal symptoms and osteoporosis prevention. Endocrinology 149:6084–6091 [DOI] [PubMed] [Google Scholar]
- 28. Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. 2008. Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol 287:40–46 [DOI] [PubMed] [Google Scholar]
- 29. Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. 2009. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 150:1897–1903 [DOI] [PubMed] [Google Scholar]
- 30. Rackow BW, Taylor HS. 2010. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril 93:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee B, Du H, Taylor HS. 2009. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 80:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor HS, Fei X. 2005. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol 19:2839–2846 [DOI] [PubMed] [Google Scholar]
- 33. Prentice A, Deary AJ, Goldbeck-Wood S, Farquhar C, Smith SK. 2000. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev 2:CD000346. [DOI] [PubMed] [Google Scholar]
- 34. Mounsey AL, Wilgus A, Slawson DC. 2006. Diagnosis and management of endometriosis. Am Fam Physician 74:594–600 [PubMed] [Google Scholar]
- 35. Mahutte NG, Arici A. 2003. Medical management of endometriosis-associated pain. Obstet Gynecol Clin North Am 30:133–150 [DOI] [PubMed] [Google Scholar]
- 36. Vercellini P, Fedele L, Pietropaolo G, Frontino G, Somigliana E, Crosignani PG. 2003. Progestogens for endometriosis: forward to the past. Hum Reprod Update 9:387–396 [DOI] [PubMed] [Google Scholar]
- 37. Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. 2006. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril 85:314–325 [DOI] [PubMed] [Google Scholar]
- 38. Winkel CA, Scialli AR. 2001. Medical and surgical therapies for pain associated with endometriosis. J Womens Health Gend Based Med 10:137–162 [DOI] [PubMed] [Google Scholar]
- 39. Surrey ES, Hornstein MD. 2002. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: long-term follow-up. Obstet Gynecol 99:709–719 [DOI] [PubMed] [Google Scholar]
- 40. Guo SW, Hummelshoj L, Olive DL, Bulun SE, D'Hooghe TM, Evers JL. 2009. A call for more transparency of registered clinical trials on endometriosis. Hum Reprod 24:1247–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]