Abstract
Fibroblast growth factor (FGF21) plays an important role in regulating hepatic oxidation of fatty acids and gluconeogenesis in response to fasting and during consumption of a ketogenic diet. However, the metabolic pathways through which FGF21 regulates hepatic function are not well defined. To identify the effects of FGF21 on the liver in vivo, we administered FGF21 to mice and analyzed acute effects on signaling and gene expression. We found that FGF21 acts directly on the liver to stimulate phosphorylation of fibroblast growth factor receptor substrate 2 and ERK1/2. Acute FGF21 treatment induced hepatic expression of key regulators of gluconeogenesis, lipid metabolism, and ketogenesis including glucose-6-phosphatase, phosphoenol pyruvate carboxykinase, 3-hydroxybutyrate dehydrogenase type 1, and carnitine palmitoyltransferase 1α. In addition, injection of FGF21 was associated with decreased circulating insulin and free fatty acid levels. FGF21 treatment induced mRNA and protein expression of peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), suggesting that PGC-1α may play a role in regulating FGF21 action. However, studies using mice with liver-specific ablation of PGC-1α revealed the same regulation of gluconeogenic gene expression by FGF21 as seen in wild-type mice, indicating that PGC-1α is not necessary for the effect of FGF21 on glucose metabolism. These data demonstrate that FGF21 acts directly on the liver to modulate hepatic metabolism. The direct effects we examined are not dependent on PGC-1α. In addition, FGF21 treatment is associated with decreased serum insulin levels that my affect hepatic function.
Fatty acid oxidation is an essential component of fasting. During fasting hepatic glycogen stores are depleted and free fatty acids, which are released by the breakdown of triglycerides in white adipose tissue (WAT), become the primary energy source. The end products of fatty acid oxidation are ketone bodies, which are released into the circulation to serve as an energy source for the brain and peripheral tissues. Similarly high rates of fatty acid oxidation and increased circulating ketones are seen when animals consume very low-carbohydrate, high-fat diets. We previously reported that such ketogenic diets, when fed to rodents, result in weight loss and improved glucose tolerance along with dramatic changes in hepatic gene expression (1). These studies led to the identification of FGF21 as a key mediator of the metabolic effects of the ketogenic diet and demonstrate that FGF21 plays a key role in regulating fatty acid oxidation, triglyceride clearance, and ketogenesis in the liver (2).
Previous studies showed that chronic treatment of obese rodents and nonhuman primates with FGF21 leads to beneficial metabolic outcomes including weight loss, reduced serum triglycerides, and improved glucose tolerance (3–5). FGF21 signaling increases insulin-independent glucose uptake in 3T3-L1 adipocytes via elevation of glucose transporter 1 expression (3), suggesting that the beneficial effects of FGF21 in vivo may result in part from its direct effects on adipose tissue. Furthermore, acute FGF21 infusion in mice reverses hepatic insulin resistance (6), whereas acute knockdown of FGF21 in the liver of mice on a ketogenic diet leads to profound changes in hepatic metabolism. Mice lacking FGF21 through genetic deletion of the peptide have an atypical response to ketogenic diets, gaining rather than losing weight and accumulating excess hepatic triglycerides (2). In aggregate, these data suggest that the liver is a major target of FGF21 action. Recently we demonstrated that exogenous FGF21 administration induces MAPK-signaling pathways in the liver and alters hepatic expression of immediate early genes in mice (7). In contrast other reports have failed to find direct effects of FGF21 on downstream signaling targets [ERK1/2 and fibroblast growth factor receptor substrate 2 (FRS2)] in the liver (8, 9). To date, the action of FGF21 on the liver and its role in regulating hepatic metabolism remain controversial. In an effort to clarify this, we have assessed the acute effects of FGF21 on hepatic signaling, gene expression, and transcriptional regulation in vivo.
In this study we established the liver as a target of FGF21 action and confirmed a critical role of β-Klotho as a cofactor in mediating FGF21 action in vivo (10). Furthermore, we found that FGF21 treatment led to an increase in the hepatic expression of peroxisome proliferator-activated receptor-γ coactivator (PGC-1α), a critical mediator of energy metabolism in the liver. Surprisingly, studies using mice with specific hepatic deletion of PGC-1α revealed that this coactivator was not required for the transcriptional effects of FGF21 on a variety of gene targets.
Materials and Methods
Animals
All studies were carried out using male C57Bl/6 mice obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained at 24 C on a 12-h light, 12-h dark cycle. PGC-1α liver-specific knockout (LKO) mice were generated as previously described (11). Briefly, female mice homozygous for the floxed allele were crossed with homozygous floxed male mice carrying a transgene expressing cre-recombinase under control of the albumin promoter. Animals were allowed ad libitum access to food unless otherwise stated. Mice were acclimated to handling for at least 10 d before experimentation. All studies were approved by the Beth Israel Deaconess Medical center IACUC.
Recombinant FGF21 protein
Human FGF21 was expressed in Escherichia coli and refolded in vitro as previously described (3).
In vivo FGF-21 signaling
For analysis of acute signaling events in peripheral tissues, FGF-21 was administered via the inferior vena cava (IVC) to anesthetized 10-wk-old mice. In brief, for IVC injection protocol, mice were anesthetized via ip injection of a ketamine/xylazene cocktail. The peritoneal cavity was then exposed and either FGF-21 or saline was injected directly into the IVC in a total volume of 20 μl. After a specified time point, liver, perigonadal adipose tissue, interscapular brown adipose tissue, heart, kidney, and limb muscles were dissected, flash frozen and stored at −80 C. Protein was extracted using radioimmune precipitation assay buffer and assessed using a Western blotting technique. After transfer, blots were probed using antibodies against ERK1/2 (Cell Signaling Technology, Danvers, MA), FRS2α (Cell Signaling).
Immediate early gene response
To assess acute and immediate early gene response, 10-wk-old mice were injected ip with either saline (n = 5) or recombinant FGF21 (n = 5) (Eli Lilly, Indianapolis, IN) (700 ng/g) in a total volume of 200 μl. Mice were then placed back in their home cage without access to food for the remainder of the experiment. After 2, 4, and 6 h mice were euthanized. Tissues were snap frozen in liquid nitrogen before storage at −80 C. Blood was collected by cardiac puncture fractionated via centrifugation at 10,000 rpm for 10 min. Serum was separated and stored at −20 C. Immediate early gene expression was assessed using quantitative RT-PCR.
Isolation of murine hepatocytes
Hepatocytes were isolated from 8- to 10-wk-old male C57BL/6 mice via portal cannulation. The liver was digested by perfusion with type IV collagenase (Worthington Biochemical Corp., Freehold, NJ) in Krebs-Ringer buffer (Sigma Chemical Co., St. Louis, MO). Dissociated cells were plated at 5 × 105 cells per well in rat tail type 1 collagen (BD Biosciences, Palo Alto, CA) coated plates and allowed to adhere for 4 h in Williams' E medium containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Immediately after this, cells were washed and treated with either FGF21 (Lilly Pharmaceuticals) or PBS in Williams' E containing 0.1% BSA for 2 h.
Quantitative RT-PCR
RNA, from flash-frozen tissue, was extracted using an RNAeasy lipid tissue kit (QIAGEN, Chatsworth, CA) according to instructions. A Dnase (QIAGEN) digestion step was included to prevent contamination of genomic DNA. cDNA was generated from 1 μg of RNA, using oligo(dt) primers and Moloney murine leukemia virus reverse transcriptase (advantage RT for PCR; CLONTECH Laboratories, Inc., Palo Alto, CA), and diluted 10-fold to 1 ml. Quantitative PCR was performed using the MX3000 thermal cycler and SYBR Green master mix (Applied Biosystems, Foster City, CA). Expression of each target gene was quantified by transformation against a standard curve and normalized to cyclophilin expression unless otherwise stated. Each reaction was normalized against cyclophillin. Primers were designed using Primer3 online software (27) and obtained from Invitrogen, as detailed in Supplemental Table 1 published on The Endocrine society's Journals Online web site at http://endo.endojournals.org.
Serum analysis
Bloods were collected at the time of euthanasia and serum stored at −80 C. Serum glucose, β-hydroxybutyrate, and triglyceride were analyzed in duplicate using a standard colormetric assay (Stanbio, Boerne, TX). Nonesterified fatty acids (NEFA) were also assessed in the same manner (Wako Chemicals, Richmond, VA). Insulin levels were determined by an ultrasensitive mouse-ELISA (Crystal Chem, Downers Grove, IL).
Immunoblotting
In brief, tissues were homogenized in radioimmune precipitation assay buffer (150 mm NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mm Tris, pH 8.0) supplemented with a Complete Mini Protease Inhibitor Cocktail (Roche) and phosphatase inhibitors. Protein concentrations were determined with the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Protein (30 μg) was analyzed by SDS-PAGE on a 10% Criterion Tris/HCl gel (Bio-Rad Laboratories) and transferred onto nitrocellulose (Protran; Schleicher & Schuell, Keene, NH). Blots were then probed with each specified primary antibody, and the blots were developed with Super Signal West Pico chemiluminescent reagent (Pierce Chemical Co., Rockford, IL).
Statistical analysis
Data are displayed as the mean ± sem. Comparisons between groups were analyzed using ANOVA (version 15, Minitab, Inc., State College, PA) where P < 0.05 was considered statistically significant. Multiple regression analysis was carried out with the use of InStat (GraphPad Software, San Diego, CA).
Results
FGF21 acts directly on the liver in vivo to activate MAPK signaling
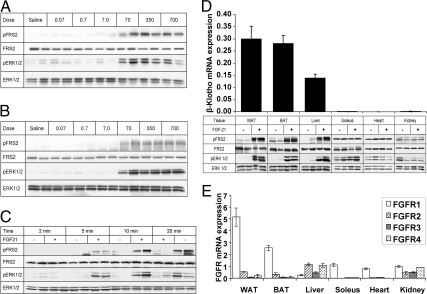
To extend previous work on FGF21 action in the liver (7, 12), we carried out a dose response analysis and assessed the time course of response to exogenous FGF21. We found a robust effect of FGF21 on FGF signaling in the liver (Fig. 1A) and WAT (Fig. 1B), as assessed by significant increases in FRS2 and ERK1/2 phosphorylation, with doses as low as 70 ng/g. Effects on FGF signaling were first noted at the 5-min time point for both FRS2 and ERK1/2 phosphorylation (Fig. 1C). Although a significant increase in FRS2 phosphorylation was observed after 20 min, it appeared that the maximal effect on ERK1/2 occurred 10 min after injection.
Fig. 1.
FGF21 initiates FGF signaling pathways in the liver in vivo. C57Bl6 mice were injected via the IVC with indicated quantities of FGF21. Tissue lysates were immunoblotted for total and phospho-FRS2 and ERK1/2. A, Robust increases in FRS2 and ERK1/2 phosphorylation in the liver in response to FGF21 at doses as low as 70 ng/g. B, Perigonadal WAT used as a positive control showing phosphorylation of FRS2 and ERK1/2 at similar doses to the liver. C, Time course. FGF21 (350 ng/g) was injected IVC and liver harvested at indicated time points. Blot shows increases in phosphorylation at time points as short as 5 min after IVC injection, with a maximal response occurring after 10 min. D, Immunoblots are shown of multiple tissues from mice injected IVC with either 350 ng/g FGF21 or saline. Depicted above is the mRNA expression of β-Klotho in these tissues as assessed by quantitative PCR. These data highlights the fact that FGF21 appears to activate FGF signaling only in tissues that express relatively high levels of β-Klotho. E, FGFR expression in peripheral tissues. Quantitative PCR data are shown normalized to 36B4 expression and given as mean ± sem.
β-Klotho expression determines tissue specificity of FGF21 action
We continued by examining FGF21-mediated activation of FRS2 and ERK1/2 in other peripheral tissues. In addition to the robust FGF21 effect observed in WAT and liver, a substantial increase in phosphorylation of FRS2 and ERK1/2 was observed in brown adipose tissue (BAT). In contrast, there was no signaling response in the kidney, heart, or soleus (skeletal) muscle. Because the presence of β-Klotho is critical to the FGF21 ligand-receptor interaction and consequent signal transduction (10, 13), we evaluated the expression of β-Klotho at the mRNA level using quantitative RT-PCR. In agreement with the observed distribution of FGF21 action, β-Klotho was only detectable in WAT, BAT, and liver (Fig. 1D).
The FGFR1 receptor is suggested to be the cognate receptor for FGF21, with a previous study postulating that low FGFR1 expression in the liver is the limiting factor for FGF21 signaling within this tissue (8). Thus, we evaluated whether FGFR1 receptor mRNA expression was also predictive of FGF21 action in other target tissues. WAT and BAT have comparatively high expression of FGFR1 (Fig. 1E). The liver expressed detectable levels of all four characterized FGF receptors; however, FGFR1 expression in the liver was comparatively low, at approximately 10% the level of expression seen in WAT. In contrast, expression of FGFR2 and FGFR3, alternative receptors shown to mediate the effects of FGF21 in vitro (8, 10, 14, 15), was higher in the liver than that seen in WAT or BAT (Fig. 1A). Importantly, mRNA expression of FGFR1 was also detected in soleus, kidney, and heart, at levels comparable to BAT and higher than in liver. Thus, whereas the expression of β-Klotho was predictive of FGF21-mediated signaling in the tissues surveyed, the levels of FGFR1 did not correlate with FGF21 signaling responses.
FGF21 induces immediate early gene expression in tissues expressing β-Klotho
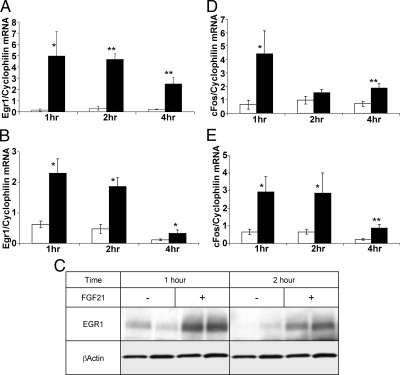
To further elucidate the tissue-specific consequences of FGF21 action in vivo, we examined effects on gene expression. We first evaluated the ability of FGF21 to induce expression of early growth response 1 (Egr1) and cFOS, because both are classical downstream targets of ERK1/2 activity. The capacity of FGF21 to induce expression of Egr1 mRNA was rapid, with a significant increase seen within 1 h after injection in both WAT (Fig. 2A) and liver (Fig. 2B). Enhanced mRNA was concurrent with a significant increase in Egr1 protein expression in the liver at the 1-h and 2-h time points (Fig. 2C). Similarly, cFOS mRNA expression was significantly increased in liver and WAT up to 4 h after FGF21 administration (Fig. 2, D and E). These data are in contrast to a recent report that found Egr1 downstream of FGF21 action in WAT but not the liver (9). We did not find any increase in immediate early gene expression in the kidney (data not shown), which was consistent with the lack of β-Klotho expression.
Fig. 2.
FGF21 initiates the expression of immediate early genes in the liver. Mice were injected ip with saline (open bars) or 700 ng/g of FGF21 (black bars), and mRNA expression of immediate early genes in both tissues was determined at indicated times. Egr1 expression is shown in perigonadal WAT (panel A) and liver (panel B). Significant increases in Egr1 expression in response to FGF21 treatment in both liver and WAT are shown at all time points. Robust increases in cFOS expression in WAT (panel D) and liver (panel E) are shown at all time points. Data are shown as mean ± sem (*, P < 0.05; **, P < 0.00). C, An immunoblot showing significant increases in EGR1 expression at time points as short as 1 and 2 h in the liver of these mice.
FGF21 activates FGF signaling in isolated hepatocytes
Because the liver is a heterogeneous tissue, we aimed to confirm that the effects of FGF21 on liver reflect effects on hepatocytes; therefore, we treated isolated primary hepatocytes with varying concentrations of FGF21. We found that FGF21 treatment led to a robust increase in ERK1/2 phosphorylation with concentrations as low as 250 ng/ml (Supplemental Fig. 1A). In addition, we found a small, but significant, increase in the expression of Egr1 mRNA (P < 0.04) (Supplemental Fig. 1B).
Acute FGF21 treatment leads to altered expression of genes regulating gluconeogenesis in the liver
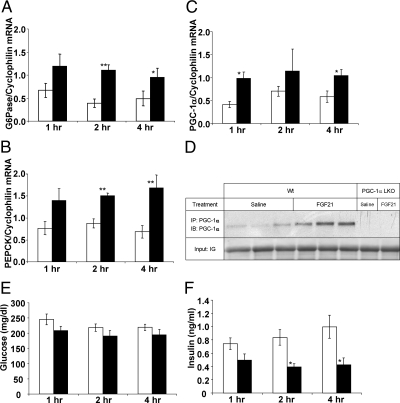
In addition to inducing expression of Egr1 and cFos, ip administration of FGF21 led to rapid induction of glucose-6-phosphatase (G6Pase) and phosphoenol pyruvate carboxykinase (PEPCK) gene expression. mRNA expression for both enzymes was increased within 1 h of injection and reached statistical significance within 2 h (G6Pase, 3-fold, P < 0.00; PEPCK, 1.7-fold, P < 0.00) (Fig. 3, A and B). As previously described (16), mRNA expression of the transcriptional coactivator PGC-1α, a critical regulator of genes involved in gluconeogenesis and energy metabolism, was significantly increased at the 1-h (2.4-fold, P = 0.01) and 4-h (1.8 fold, P = 0.03) time points (Fig. 3C). This was associated with an increase in PGC-1α protein expression (Fig. 3D). The increases in gluconeogenic gene expression did not correspond to changes in serum glucose levels at these time points (Fig. 3E). Of note, the levels of circulating insulin dropped by 53% within 2 h of FGF21 injection (Fig. 3F).
Fig. 3.
Acute FGF21 treatment increases gluconeogenic gene expression in the liver. Data are shown 1, 2, and 4 h after ip injection of saline (open bars) or FGF21 (black bars). A, Bar graph showing a significant increase in G6Pase mRNA expression 2 and 4 h after FGF21 injection. B, PEPCK mRNA expression is also significantly increased at these time points. C, PGC-1α expression is significantly increased 1 h after injection. D, Immunoprecipitation showed increased expression of PGC-1α protein under FGF21 treatment. Blot also highlights absence of PGC-1α protein in the liver of PGC-1α LKO mice. Circulating glucose (E) and insulin (F) levels are shown throughout the time course. Data are shown as mean ± sem (*, P < 0.05; **, P < 0.00). Wt, Wild type.
Acute treatment with FGF21 led to altered expression of peroxisome proliferator-activated receptor (PPAR)α- and FoxO1-regulated genes
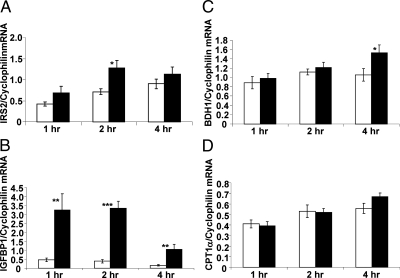
Chronic treatment with FGF21, or loss of function in mice, results in altered expression of genes encoding enzymes regulating the processes of fatty acid oxidation and ketogenesis (2, 4, 17). Many of these genes, including G6Pase and PEPCK, are downstream targets of the nuclear receptor PPARα and the fork head transcription factor FoxO1, suggesting that the physiological actions of FGF21 on liver may be mediated by these transcription factors. Consistent with this hypothesis, FoxO1 targets insulin receptor substrate 2 (IRS2) and, more strikingly, IGF-binding protein 1 (IGFBP1) were acutely regulated by FGF21 administration (Fig. 4, A and B) with increasing expression observed within 1 h (IRS2, 1.6-fold, P = 0.097; IGFBP1, 6.9-fold, P = 0.009), and reaching significance within 2 h (IRS2, 1.8-fold, P = 0.012; IGFBP1, 8.5-fold, P < 0.000). Additionally, there was a significant increase in expression of 3-hydroxybutyrate dehydrogenase type 1 (BDH1), an enzyme involved in the interconversion of ketone bodies (Fig. 4C) 4 h after FGF21 administration (1.45 fold, P = 0.037). The expression of carnitine palmitoyltransferase (CPT)1α, a PPARα-regulated gene, was also increased after 4 h; however, this did not reach significance (Fig. 4D). However, other genes regulating lipid oxidation downstream of PPARα were not changed within this time frame (data not shown), suggesting that the effects of FGF21 on PPARα target genes may only occur under chronic treatment.
Fig. 4.
FGF21 acutely induces FoxO1 target gene expression in the liver. FGF21 significantly increases IRS2 (panel A) and IGFBP1 (panel B) mRNA expression in the liver. mRNA expression of 3-hydroxybutyrate dehydrogenase type 1 (BDH1) (panel C) and CPT1a (panel D) are also shown. Data are shown as mean ± sem (*, P < 0.05; **, P < 0.00; ***, P < 0.000).
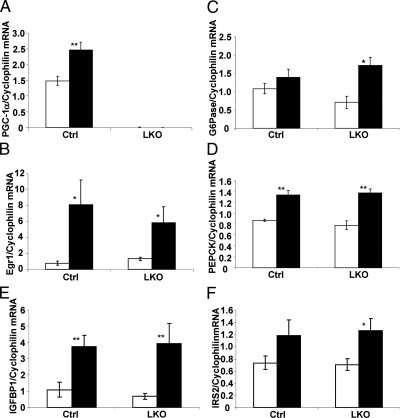
PGC-1α is not required for FGF21-mediated acute regulation of genes in the liver
PGC-1α acts as a potent co-activator of the transcriptional activity of PPARα and FoxO1, making it a clear candidate as a downstream regulator of FGF21 action. To test this hypothesis, hepatic gene expression in PGC-1α LKO mice was compared with littermate controls after injection of FGF21. As expected, exogenous FGF21 induced PGC-1α expression in control mice, whereas PGC-1α expression was undetectable in the LKO (Figs. 5A and 3D). Mice with intact PGC-1α (flox/flox control mice) (Ctrl) and mice with liver-specific deletion of PGC-1α (flox/flox, alb-cre) responded normally to FGF21 with respect to immediate early gene expression (Fig. 5B). The levels of gluconeogenic expression at baseline in LKO mice trended lower than control mice, consistent with PGC-1α being a key positive regulator of these genes (Fig. 5, C and D). Importantly, FGF21 significantly induced G6Pase (1.8-fold, P = 0.04) and PEPCK (1.8-fold, P < 0.00) expression in the PGC-1α LKO mice (Fig. 5, C and D). In fact, the fold induction of G6Pase in LKO mice was greater than that observed at a similar time point in wild-type littermate controls; however, absolute fasting-induced expression levels were similar between groups. Furthermore, loss of PGC-1α from the liver did not impair FGF21-induced expression of IGFBP1 and IRS2 (Fig. 5, E and F). FGF21 had no effect on circulating levels of glucose, triglycerides, or β-hydroxybutyrate within this time frame, but there was a significant reduction in circulating insulin and NEFA to the same extent in both groups of mice (Table 1).
Fig. 5.
Liver PGC-1α is not required for the induction of gluconeogenic expression in the liver. LoxP control and PGC-1α LKO mice were injected with 700 ng/g of FGF21 and gene expression was analyzed 4 h after injection. As a positive control the response in PGC-1α expression (panel A) and EGR1 (panel B) are shown. Significant increases in hepatic G6Pase (panel C), PEPCK (panel D), IGFBP1 (panel E), and IRS2 (panel F) expression in the PGC-1α LKO mice are shown. Data are shown as mean ± sem (*, P < 0.05; **, P < 0.00). Ctrl, Control.
Table 1.
Serum hormone and metabolite levels
| Wt |
LKO |
|||
|---|---|---|---|---|
| Saline | FGF21 | Saline | FGF21 | |
| Glucose (mg/dl) | 200 ± 7.26 | 173 ± 15.02 | 181 ± 14.9 | 188 ± 19.2 |
| Triglycerides (mg/dl) | 45.2 ± 4.1 | 46.2 ± 4.2 | 51.7 ± 5.4 | 39.7 ± 3.29 |
| NEFA (mEq/liter) | 0.55 ± 0.087 | 0.35 ± 0.018a | 0.624 ± 0.063 | 0.390 ± 0.085a |
| β-Hydroxybutyrate (mg/dl) | 1.91 ± 0.373 | 1.78 ± 0.166 | 2.22 ± 0.231 | 1.77 ± 0.141 |
| Insulin (ng/ml) | 0.43 ± 0.012 | 0.25 ± 0.022a | 0.43 ± 0.050 | 0.27 ± 0.052a |
Table details serum metabolite and insulin levels of wild-type and PGC-1α LKO mice treated with either saline or FGF21 for 4 h.
P < 0.05.
FGF21 regulates hepatic PGC-1α expression but not PEPCK or G6Pase
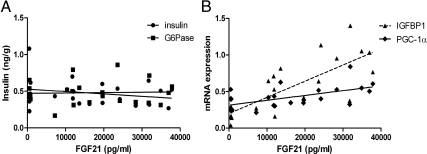
Acute FGF21 administration at pharmacological doses lowered circulating insulin levels (Fig. 3F and Table 1), which confounds interpretation of the effect of FGF21 infusion on PGC-1α, PEPCK, and G6Pase. To address the contribution of reduced circulating insulin levels to changes in hepatic gene expression, we carried out a dose-response curve using several low doses of FGF21 treatment. These low doses of FGF21 had no effect on circulating insulin concentrations 2 h after injection, and there was no correlation between the administered FGF21 levels and insulin (r2 = 0.054; P = 0.29) (Fig. 6A). In addition, hepatic G6Pase expression did not correlate with the FGF21 serum levels at this time point (r2 = 0.0015; P = 0.86). In contrast, FGF21 levels positively correlated with the expression of PGC-1α (r2 = 0.32; P = 0.0048), IGFBP1 (r2 = 0.59; P < 0.0001) (Fig. 6B) and negatively with circulating NEFA [r2 = 0.2631; P = 0.012 (data not shown)]. Multiple regression analysis demonstrated that whereas circulating levels of FGF21, insulin, and NEFA predicted 41% of the variance in hepatic PGC-1α expression, FGF21 was the only significant contributory factor (t = 2.31; P = 0.0325). Using a similar model, we found that FGF21 levels also predicted the variance in expression of Egr1 (34.36%; P = 0.0226) and IGFBP1 (50.6%; P = 0.0009), independent of insulin.
Fig. 6.
Exogenous FGF21 correlates with PGC-1α but not gluconeogenic gene expression. Mice were injected ip with either saline or FGF21 (50 ng/g, 100 ng/g, or 200 ng/g) and serum insulin, exogenous FGF21 and hepatic gene expression analyzed 2 h after injection. Figure shows regression analysis between serum insulin, hepatic g6pase expression, and exogenous levels of FGF21 (panel A) and hepatic expression of IGFBP1 and PGC-1α (panel B).
Discussion
In the liver FGF21 expression is physiologically regulated, increasing with fasting (2, 16, 18) and in response to consumption of a ketogenic diet; furthermore, FGF21 is required for a normal response to both these states. However, the potential action of FGF21 on the liver remains unclear; indeed some reports indicate that the liver is not a target of FGF21 action (8, 16, 19). In agreement with previous studies (7, 12), we found that FGF21 induces rapid phosphorylation of the downstream signaling proteins FRS2 and ERK1/2 in the liver to an extent similar to that observed in WAT. In addition to the previously demonstrated induction of immediate early gene expression in the liver (7), FGF21 also induces IGFBP1 and PGC-1α and had significant effects on circulating concentrations of insulin and NEFA.
Analysis of FGF21 signaling can be problematic because FGF21 interacts with multiple FGFR isoforms. Whereas hepatic expression of FGFR1 is comparatively low, FGF21 action may be mediated through the other FGF receptors expressed in this tissue. We found that hepatic expression of other FGF receptors, particularly FGFR2 and FGFR3, was relatively high and similar to levels of FGFR4, the receptor believed to mediate FGF19 signaling in this tissue (8). More importantly, the action of FGF21 in vivo paralleled the tissue-specific expression of β-Klotho, and not of the FGF receptor subtypes. We were able to detect increased ERK1/2 phosphorylation and Egr1 expression in primary hepatocytes; however, expression of β-Klotho declined rapidly, making cultured hepatocytes unsuitable for assessing longer-term actions of FGF21. This may part explain, in part, the failure of others to demonstrate FGF21 action in theses cells. In aggregate, our data on signaling and gene expression indicate the liver is indeed a direct target of FGF21 action. We propose that it is not FGFR1 expression, per se, which is required for tissue-specific FGF21 signaling, but rather the presence or absence of β-Klotho (10). Consistent with this, the heart, kidney, and soleus muscle, which do not express β-Klotho, fail to respond to FGF21 with either signaling events or changes in gene expression.
In mice, FGF21 treatment induces rapid expression of the transcriptional coactivator PGC-1α in the liver, as well as PEPCK and G6Pase expression (16). Induction of gluconeogenic genes is consistent with increased PGC-1α activity (20). The role of PGC-1α to regulate genes important in the transition from glycolytic to oxidative metabolism is consistent with the program of events that we now associate with the function of FGF21 in regulating the fasting response. Indeed, we initially thought PGC-1α to be a plausible candidate as a mediator of FGF21 activity in liver. Surprisingly, we found no abnormality in the ability of FGF21 to acutely induce gluconeogenic gene expression in the PGC-1α LKO mice. It is important to note that circulating insulin, a potent suppressor of PGC-1α, G6Pase, and PEPCK expression in liver, was also reduced in response to acute FGF21 injection. Thus, the complex interrelationship between FGF21 and insulin makes it difficult to isolate the role of FGF21 from insulin on hepatic gene expression.
Our data contrast those reported in a recent study examining the effect of FGF21-induced gluconeogenic gene expression in a whole body PGC-1α KO mice (16). A crucial difference between these studies is the use of different models of PGC-1α ablation, a total body KO mouse (16) vs. a liver-specific KO. These differences suggest that extrahepatic PGC-1α expression may be required for FGF21-induced gluconeogenic gene expression in vivo. Whereas one group (16) suggests that this might be mediated indirectly through the central nervous system (21), our data demonstrate that the FGF21-induced reduction in circulating insulin more closely predicts the changes in gluconeogenic gene expression. It has been shown that pancreatic PGC-1α inhibits insulin secretion (22). Thus, it is possible that dependence on pancreatic PGC-1α for FGF21-mediated suppression of insulin secretion may explain the discrepancies between mouse models. To eliminate the confounding effects of altered insulin levels, we correlated changes in hepatic gene expression with exogenously administered FGF21. At the low doses administered, FGF21 had little effect on circulating insulin concentrations. With this model, we conclude that exogenous FGF21 had direct effects on PGC-1α, IGFBP1, and immediate early gene expression in liver, but not on G6Pase or PEPCK. However, the fact that acute constant FGF21 infusion lowers circulating glucose levels and reduces hepatic glucose production within a similar time frame (4–6 h) (6) makes the physiological relevance of increased PEPCK and G6Pase expression uncertain.
It should be noted that to minimize the effects of endogenous FGF21, which is elevated during fasting, all studies thus far have been performed in fed mice, a state in which insulin levels are normally high. FGF21 may act to potentiate the fasting response by reducing insulin levels, relieving the insulin-mediated suppression of gluconeogenic genes within liver. Importantly, whereas PGC-1α itself can be regulated by insulin action in liver, the acute effects of FGF21 on PGC-1α expression were not dependent on insulin concentration. Although PGC-1α was not required for FGF21-mediated induction of hepatic gluconeogenic gene expression, its importance in regulating FGF21 action in the milieu of a fasted mouse is still unknown. It remains possible that PGC-1α is a critical factor in the ability of FGF21 to regulate genes responsible for fatty acid oxidation and ketosis during the fasting response. In addition, two recent studies have highlighted additional roles for PGC-1α in the biology of FGF21. Although PGC-1α is a transcriptional coactivator associated with the action of PPARα, hepatic FGF21 expression is paradoxically high in the PGC-1α LKO mouse (23). This occurs through PGC-1α acting to enhance heme biosynthesis leading to activation of the transcriptional corepressor Rev-Erbα. This process represents a novel negative feedback loop in which PGC-1α is able to suppress the expression of FGF21. An additional study has found that PGC-1α appears to be critical to the role of FGF21 in increasing the oxidative capacity of adipocytes in vitro through enhanced activation of AMP kinase (24). The mechanisms described in this study may represent, in part, some of the processes that regulate fatty acid oxidation downstream of FGF21. In addition, it has recently been found that thyroid hormone positively regulates FGF21 expression in the liver of mice (25). Because many of the hepatic effects of FGF21 are synergistic with the ability of thyroid hormone to influence mitochondrial function (26), this may represent a mechanism for the role of FGF21 downstream of T3. Nevertheless, the complex relationship between insulin, FGF21 action, and PGC-1α in the fasted state will be addressed in future studies.
Finally, the evidence presented demonstrates that the liver is a direct target of FGF21 action. β-Klotho is an essential component of FGF21-mediated signaling and characterizes immediate early gene activation as a biomarker for hepatic FGF21 action in vivo as well as WAT (9). FGF21 acutely induces PGC-1α in liver; however, this coactivator of transcription is not required for FGF21-mediated induction of hepatic gluconeogenic gene expression. Moreover, at high doses, FGF21 can also affect hepatic gene expression indirectly by decreasing circulating the insulin levels. In aggregate, we show that FGF21 acts as both a paracrine and endocrine factor, activating signaling and transcriptional events within the liver as well as peripheral tissues and highlighting the liver as a key site of action for the metabolic effects of FGF21. Understanding the full range of hepatic actions of FGF21 will be the focus of future studies.
Acknowledgments
This work was supported in part by National Institutes of Health Grant DK028082 as well a Picower Foundation grant and a Young Investigator award from the Obesity Society.
Disclosure Summary: H.A.B. and A.K. are employed by Eli Lilly and Company; E.M.F. has consulted once for Ambrex Pharma.
Footnotes
- BAT
- Brown adipose tissue
- CPT
- carnitine palmitoyltransferase
- G6Pase
- glucose-6-phosphatase
- FGF
- fibroblast growth factor
- FGFR
- FGF receptor
- FRS2
- fibroblast growth factor receptor substrate 2
- IGFBP
- IGF-binding protein
- IRS
- insulin receptor substrate
- IVC
- inferior vena cava
- KO
- knockout
- LKO
- liver-specific knockout
- NEFA
- nonesterified free fatty acids
- PEPCK
- phosphoenol pyruvate carboxykinase
- PGC
- PPARγ coactivator
- PPAR
- peroxisome proliferator-activated receptor
- WAT
- white adipose tissue.
References
- 1. Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. 2007. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 292:E1724–1739 [DOI] [PubMed] [Google Scholar]
- 2. Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. 2007. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5:426–437 [DOI] [PubMed] [Google Scholar]
- 3. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. 2005. FGF-21 as a novel metabolic regulator. J Clin Invest 115:1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A. 2008. FGF21 corrects obesity in mice. Endocrinology 149:6018–6027 [DOI] [PubMed] [Google Scholar]
- 5. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. 2007. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148:774–781 [DOI] [PubMed] [Google Scholar]
- 6. Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. 2009. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150:4084–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. 2010. Obesity is an FGF21 resistant state. Diabetes 59:2781–2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. 2007. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 282:26687–26695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tomiyama K, Maeda R, Urakawa I, Yamazaki Y, Tanaka T, Ito S, Nabeshima Y, Tomita T, Odori S, Hosoda K, Nakao K, Imura A, Nabeshima Y. 2010. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc Natl Acad Sci USA 107:1666–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kharitonenkov A, Dunbar JD, Bina HA, Bright S, Moyers JS, Zhang C, Ding L, Micanovic R, Mehrbod SF, Knierman MD, Hale JE, Coskun T, Shanafelt AB. 2008. FGF-21/FGF-21 receptor interaction and activation is determined by βKlotho. J Cell Physiol 215:1–7 [DOI] [PubMed] [Google Scholar]
- 11. Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. 2009. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58:1499–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, Ge H, Weiszmann J, Lu SC, Graham M, Busby J, Hecht R, Li YS, Li Y, Lindberg RA, Veniant MM. 2009. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models—-Association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab 297:E1105–E1114 [DOI] [PubMed] [Google Scholar]
- 13. Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. 2007. βKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104:7432–7437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moyers JS, Shiyanova TL, Mehrbod F, Dunbar JD, Noblitt TW, Otto KA, Reifel-Miller A, Kharitonenkov A. 2007. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARγ signaling. J Cell Physiol 210:1–6 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T. 2008. βKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 22:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. 2009. FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106:10853–10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. 2009. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150:4931–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. 2007. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab 5:415–425 [DOI] [PubMed] [Google Scholar]
- 19. Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. 2008. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab 8:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. 2003. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- 21. Sarruf DA, Thaler JP, Morton GJ, German J, Fischer JD, Ogimoto K, Schwartz MW. 2010. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes 59:1817–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonner-Weir S, Weir G, Spiegelman BM. 2003. Suppression of β cell energy metabolism and insulin release by PGC-1α. Dev Cell 5:73–83 [DOI] [PubMed] [Google Scholar]
- 23. Estall JL, Ruas JL, Choi CS, Laznik D, Badman M, Maratos-Flier E, Shulman GI, Spiegelman BM. 2009. PGC-1α negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erbα axis. Proc Natl Acad Sci USA 106:22510–22515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chau MD, Gao J, Yang Q, Wu Z, Gromada J. 2010. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1α pathway. Proc Natl Acad Sci USA 107:12553–12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams AC, Astapova I, Fisher FM, Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. 2010. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARα-dependent manner. J Biol Chem 285:14078–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weitzel JM, Iwen KA, Seitz HJ. 2003. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol 88:121–128 [DOI] [PubMed] [Google Scholar]
- 27. Rozen S, Skaletsky HJ. 2000. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S. eds. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 365–386 [DOI] [PubMed] [Google Scholar]