Abstract
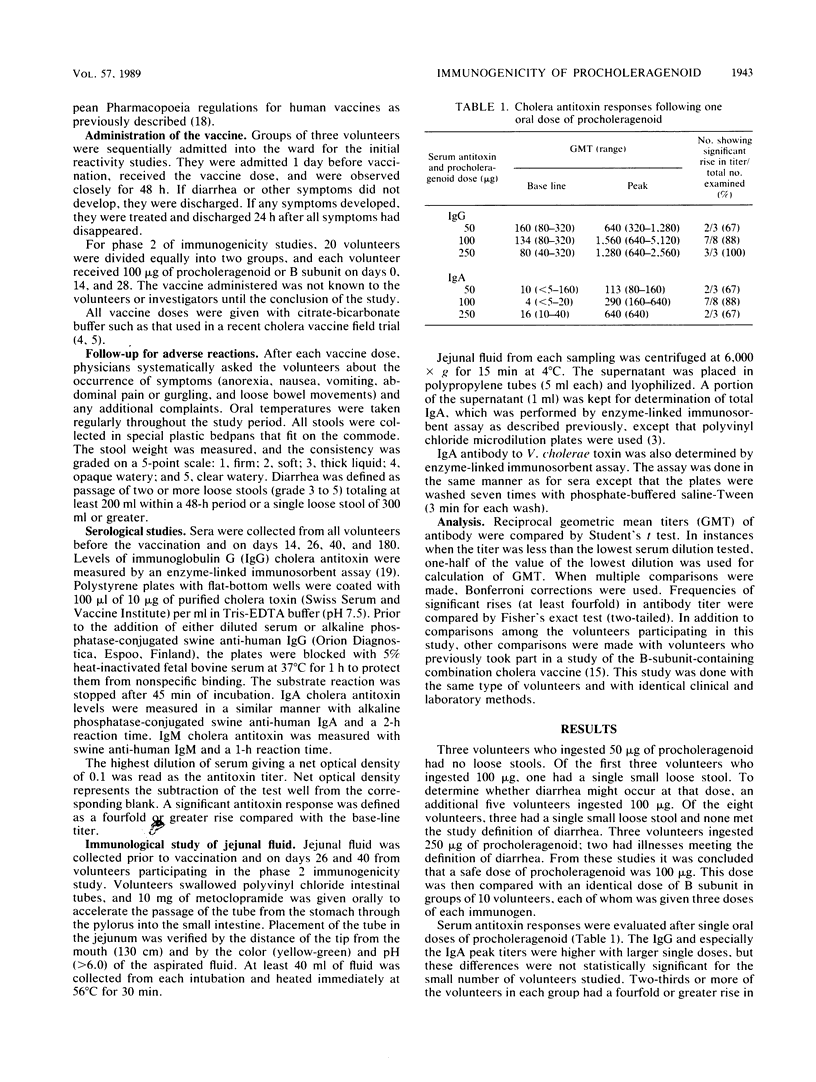
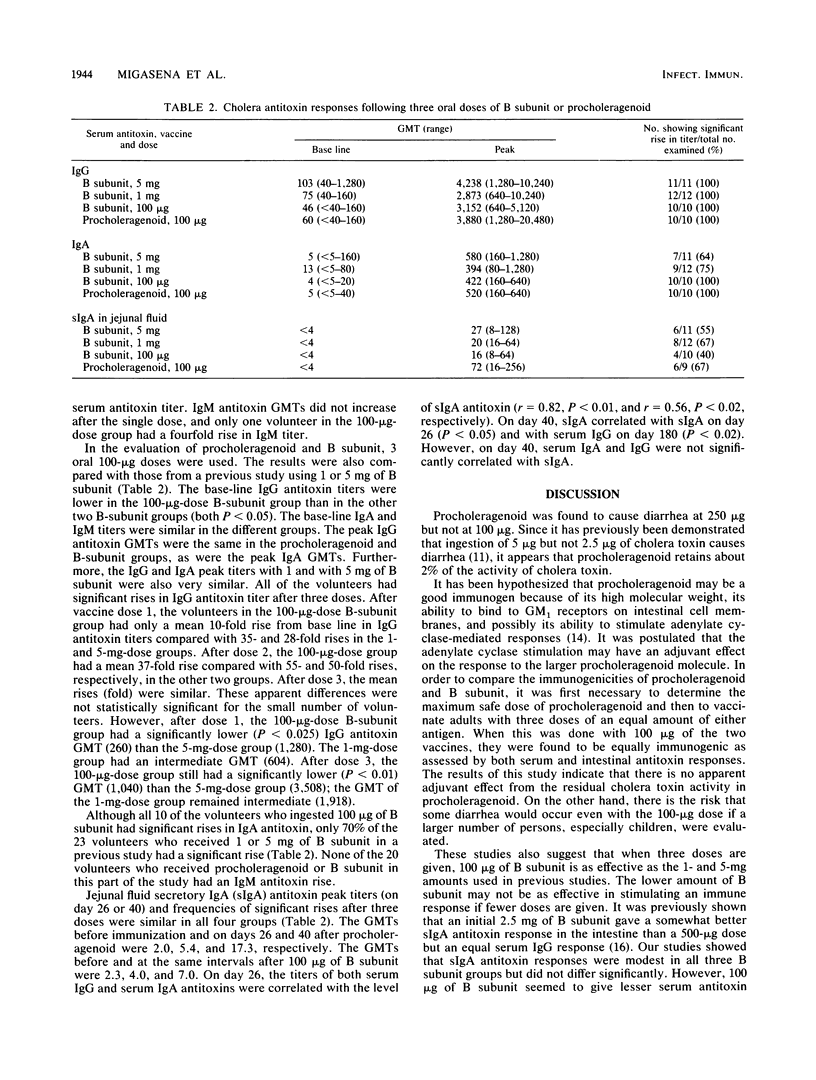
Procholeragenoid, a stable high-molecular-weight aggregate of cholera toxin derived by heat treatment, was evaluated for reactivity and immunogenicity in adult Thai volunteers. Since procholeragenoid is known to retain some residual activity of cholera toxin, increasing amounts were ingested until diarrhea occurred; 250 micrograms induced diarrhea, but 100 micrograms did not. Procholeragenoid and cholera toxin B subunit, both in 100-micrograms amounts, were then compared for systemic and intestinal antitoxin responses. When three peroral doses were given, these immunogens gave comparable responses. The secretory immunoglobulin A antitoxin responses to three doses of 100 micrograms of B subunit did not differ significantly from responses found in previous studies of Thai adults given 1 or 5 mg of B subunit, but serum antitoxin responses were less after 1 or 2 doses of 100 micrograms than after doses of 1 or 5 mg. Serum antitoxin levels were similar after 3 doses of B subunit. Procholeragenoid in the maximum safe dose of 100 micrograms does not offer any immunologic advantage over B subunit, although it may be less expensive and easier to produce. However, these studies suggest that higher amounts of B subunit are more immunogenic and may be preferable, if found to be sufficiently cost effective, when added to oral killed whole Vibrio cholerae vaccines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black R. E., Levine M. M., Clements M. L., Young C. R., Svennerholm A. M., Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987 May;55(5):1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J. D., Harris J. R., Sack D. A., Chakraborty J., Ahmed F., Stanton B. F., Khan M. U., Kay B. A., Huda N., Khan M. R. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis. 1988 Jul;158(1):60–69. doi: 10.1093/infdis/158.1.60. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Stanton B. F., Kay B. A., Khan M. U., Yunus M., Atkinson W. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986 Jul 19;2(8499):124–127. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Stanton B. F., Chakraborty J., Sack D. A., Khan M. R., Huda S., Ahmed F., Harris J. R., Yunus M., Khan M. U. B subunit-whole cell and whole cell-only oral vaccines against cholera: studies on reactogenicity and immunogenicity. J Infect Dis. 1987 Jan;155(1):79–85. doi: 10.1093/infdis/155.1.79. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Fujita K., LoSpalluto J. J. Procholeragenoid: an aggregated intermediate in the formation of choleragenoid. J Immunol. 1971 Oct;107(4):1043–1051. [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Fürer E., Cryz S. J., Jr, Dorner F., Nicolet J., Wanner M., Germanier R. Protection against colibacillosis in neonatal piglets by immunization of dams with procholeragenoid. Infect Immun. 1982 Mar;35(3):887–894. doi: 10.1128/iai.35.3.887-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Antigenicity of cholera toxoid in humans. J Infect Dis. 1977 Apr;135(4):512–516. doi: 10.1093/infdis/135.4.512. [DOI] [PubMed] [Google Scholar]
- Germanier R., Fürer E., Varallyay S., Inderbitzin T. M. Preparation of a purified antigenic cholera toxoid. Infect Immun. 1976 Jun;13(6):1692–1698. doi: 10.1128/iai.13.6.1692-1698.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migasena S., Desakorn V., Suntharasamai P., Pitisuttitham P., Prayurahong B., Supanaranond W., Black R. E. Immunogenicity of two formulations of oral cholera vaccine comprised of killed whole vibrios and the B subunit of cholera toxin. Infect Immun. 1989 Jan;57(1):117–120. doi: 10.1128/iai.57.1.117-120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr, Craig J. P., Germanier R., Fürer E. Procholeragenoid: a safe and effective antigen for oral immunization against experimental cholera. Infect Immun. 1983 Jun;40(3):1112–1118. doi: 10.1128/iai.40.3.1112-1118.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr Oral immunization of dogs with purified cholera toxin, crude cholera toxin, or B subunit: evidence for synergistic protection by antitoxic and antibacterial mechanisms. Infect Immun. 1982 Aug;37(2):687–694. doi: 10.1128/iai.37.2.687-694.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Sack D. A., Holmgren J., Bardhan P. K. Intestinal antibody responses after immunisation with cholera B subunit. Lancet. 1982 Feb 6;1(8267):305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- Tayot J. L., Holmgren J., Svennerholm L., Lindblad M., Tardy M. Receptor-specific large-scale purification of cholera toxin on silica beads derivatized with lysoGM1 ganglioside. Eur J Biochem. 1981 Jan;113(2):249–258. doi: 10.1111/j.1432-1033.1981.tb05060.x. [DOI] [PubMed] [Google Scholar]
- Young C. R., Levine M. M., Craig J. P., Robins-Browne R. Microtiter enzyme-linked immunosorbent assay for immunoglobulin G cholera antitoxin in humans: method and correlation with rabbit skin vascular permeability factor technique. Infect Immun. 1980 Feb;27(2):492–496. doi: 10.1128/iai.27.2.492-496.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


