Abstract
RT-PCR shows that mouse skeletal muscle contains type-2 iodothyronine deiodinase (D2) mRNA. However, the D2 activity has been hard to measure. Except for newborn mice, muscle homogenates have no detectable activity. However, we have reported D2 activity in mouse muscle microsomes. As the mRNA, activity is higher in slow- than in fast-twitch muscle. We addressed here the major problems in measuring D2 activity in muscle by: homogenizing muscle in high salt to improve yield of membranous structures; separating postmitochondrial supernatant between 38 and 50% sucrose, to eliminate lighter membranes lacking D2; washing these with 0.1 m Na2CO3 to eliminate additional contaminating proteins; pretreating all buffers with Chelex, to eliminate catalytic metals; and eliminating the EDTA from the assay, as this can bind iron that enhances dithiothreitol oxidation and promotes peroxidation reactions. Maximum velocity of T3 generation by postgradient microsomes from red muscles was approximately 1100 fmol/(h · mg) protein with a Michaelis-Menten constant for T4 of 1.5 nm. D2-specific activity of Na2CO3-washed microsomes was 6–10 times higher. The enrichment in D2 activity increased in parallel with the capacity of microsomes to load (sarco/endoplasmic reticulum Ca2+-ATPase) and bind Ca2+ (calsequestrin), indicating that D2 resides in the inner sarcoplasmic reticulum, close to the nuclei. The presence of D3 in the sarcolemma suggests that the most of D2-generated T3 acts locally. Estimates from maximum velocity, Michaelis-Menten constant, and muscle T4 content suggest that mouse red, type-1, aerobic mouse muscle fibers can generate physiologically relevant amounts of T3 and, further, that muscle D2 plays an important role in thyroid hormone-dependent muscle thermogenesis.
The main product of thyroid secretion is T4, which has weak hormonal activity. The enzyme-catalyzed removal of the 5′ iodine of T4 generates T3, which has at least 10 times more activity and a much faster effect, being considered the active form of the hormone, ultimately responsible for virtually all the effect of the thyroidal secretion. There are two 5′iodothyronine deiodinases, type-1 iodothyronine deiodinase and type-2 iodothyronine deiodinase (D2). A third deiodinase, called type-3 iodothyronine deiodinase (D3) removes the 5 iodine, from the inner ring of T4 or T3, generating, respectively, rT3 and 3,3′-T2, both devoid of biological activity. These three enzymes have not only an important role in modulating circulating T3 levels, but D2 and D3 are particularly important in regulating the intracellular concentration of T3 in a tissue-specific manner (see Refs. 1, 2 for reviews). This provides an additional and physiologically critical level of regulation that allows tissues to vary the level of intracellular T3 according to specific needs, for example, in stages of central nervous system development (3), regenerating liver (4) and skeletal muscle (5), regionally in the ischemic or hypertrophied myocardium (6), and in human skeletal muscle, which in addition may significantly contribute to circulating T3 (7).
In our quest to understand the thermogenic effect of thyroid hormone, we undertook the study of mice deficient in all known products of the thyroid hormone receptor α gene (Thra), the Thra-0/0 mice (8). As other transgenic models lacking the thyroid hormone receptor α1, Thra-0/0 mice have lower body temperature and bradycardia as conspicuous phenotypic features. Thra-0/0 are cold intolerant due the failure of brown adipose tissue (BAT) to produce heat, even though BAT expresses all known essential genes involved in its thermogenic function (9). Surprisingly, these mice look normal and seem in no distress at room temperature, 20–22 C, which is 8–10 C below the thermoneutrality (TN) temperature (30 C for mice) (10). At TN, obligatory thermogenesis suffices to keep core body temperature without the participation of other temperature homeostasis mechanisms (10), but below TN, BAT contributes progressively to thermogenesis, so that at room temperature, it accounts for around 30% of total heat production in rats (11) and probably 40% or more in mice (9). However, in spite of having a dysfunctional BAT, Thra-0/0 mice living at room temperature have increased, not reduced, energy expenditure and are hyperphagic but leaner and less sensitive to high-fat diets (12), This phenotype is exaggerated in colder environments and negated at TN, indicating the lack of BAT thermogenesis is substituted by an alternate form of facultative thermogenesis (12).
A distinct finding of Thra-0/0 mice is 2- to 3-fold increase in D2 mRNA in skeletal muscle. As in WT mice, the mRNA is about 10 times more abundant in type-I (red, slow, or aerobic) muscle than in type-II fibers (white, fast, or glycolytic muscle) (12). The relative abundance of D2 mRNA is lower than in BAT (9, 12), and the presence of significant activity has been questioned (13). However, others have been able to demonstrate activity in muscle of several species (7, 14, 15), particularly in the newborn mouse (5), and we recently established there is D2 activity in the skeletal muscle of adult mice (16). As with the mRNA, D2 activity was higher in aerobic than glycolytic muscle. However, the D2-specific activity in conventionally prepared microsomes from adult mice was low and required prolonged incubation to obtain significant amounts of product (16). Not only our work on Thra-0/0 mice (9, 12), but also that of Kozak's lab on uncoupling protein 1 knockout mice (Ucp1−/−) (17, 18), demonstrate that, in the absence of BAT thermogenesis, muscle and other tissues could contribute to facultative thermogenesis, and data from both models implicate D2 as playing a major role. To test this possibility is necessary to demonstrate in a reliable and consistent manner the presence of relevant levels of D2 activity in muscle.
There are many possible reasons why D2 activity in skeletal muscle could be underestimated. Briefly, these include the complex structure of muscle causing difficulties in extracting membranous structures deeply located in the cells (19–21), the presence of high concentration of iron, and its contribution to oxidations, peroxidation, and generation of reactive oxygen species that ultimately lead to oxidation of sulfhydryl groups and specifically dithiothreitol (DTT) (22), essential for the demonstration of D2 activity in vitro (23). Having established the presence of significant activity in mouse skeletal muscle (16), we decided to address those problems inherent to muscle to obtain a more accurate estimation of the amount and specific activity of D2 in mouse muscle. Because this article (16) was just published, in the studies presented here, we only refer to it and did not repeat those studies, because this would have made these studies prohibitive in terms of work and number of mice.
We show here that the enzyme is present in muscle of C57Bl/6 mice, residing in or by the sarcoplasmic reticulum and that by addressing the methodological challenges mentioned above, we recover substantially more activity and find high specific activities, much greater than that of conventionally prepared (high-speed centrifugation of postmitochondrial supernatant from 0.32 m sucrose homogenates) BAT microsomes of mice kept at room temperature. In an accompanying article (54), we demonstrate the regulation of D2 activity by cold, via the sympathetic nervous system, and the role of the increased D2 activity for the temperature homeostasis and hypermetabolism of Thra-0/0 mice.
Materials and Methods
Animals
Studies were done in C57Bl/6 mice obtained from The Jackson Laboratory (Bar Harbor, ME) or from homozygous wild-type genotype obtained from our own colony. Most of experiments were done in 2- to 4-month-old mice, males or females. We have found no significant differences between genders. Mice were maintained at 21 C, with water and food ad libitum, and 12-h light, 12-h dark cycle. Unless specified otherwise, the assay protocol was using preparations of red muscles of the hind limb (24), most frequently soleus and quadratus femoris, for accessibility and D2 content (see below).
D2 assays
Assays were performed in whole homogenates, total microsomes, and microsomal fractions as indicated in the text. Substrate was 5′-125I-labeled rT3 or 5′-125I-labeled T4 at 1–2 nm, as indicated. Assay buffer was 0.1 m NaPO4 (pH 7.0) ± 1 mm Na EDTA, 20 mm DTT, and 1 mm propylthiouracil. Assays were run for variable times as indicated. When 125I-rT3 was the substrate, assay volume was 300 μl, and the product measured was 125I(−). Reaction was stopped with 200 μl of horse serum, followed by 100 μl ice-cold 50% trichloroacetic acid and 50 μl of an approximately 30% suspension of Dowex-50Wx2 200-400 mesh in 2 m acetic acid. Tubes were centrifuged at 2000 × g and approximately 4 C for 10 min. An aliquot of 400 μl of supernatant was counted. Reaction was quantified as femtomoles of 125I(−) released per hour per milligram of protein or simply as 125I(−) net cpm over blanks. To calculate fmol/(h · mg), the net 125I(−) cpm was multiplied by 2 and by the total concentration of substrate and normalized to 1 h and 1 mg of protein. When 125I-T4 was the substrate, reaction volume was 100 μl, and the reaction was stopped with 100 μl of ethanol: 2 n NH4OH, 9:1, at room temperature, vortexed, and the ethanol extract was collected by centrifugation at 10,000 × g for 10 min. The extract was applied to Whatman 3MM filter paper, and relevant compounds were resolved by chromatography in hexane: tert-amyl alcohol:NH4OH as described (25). Sufficient unlabeled iodide, T4, T3, and rT3 or 3,3′-T2 was added to the extracts to identify the relevant products by chemical staining. After running for 24–36 h, strips were allowed to dry in a fume hood and stained with a spray of 4-aminoantipyrine followed by potassium ferricyanide and PdCl2 to localize the iodothyronines and iodide, respectively. Location of products was marked, and each strip was cut in 0.5-cm segments, which were then counted in a γ counter. Net 125I-T3 produced was obtained by subtracting the amount present in blank strips, expressed as fraction of the initial 125-I-T4, multiplied by 2 (for 50% reduction in the specific activity of the product) and the total concentration of T4. Activity was also expressed as fmol/(h · mg) protein. Occasionally, where indicated, products were separated by HPLC as described previously (16).
Because we found small amounts of D3 in some of our preparations, we added 25–100 nm unlabeled T3 to protect T4 and T3 from 5-deiodination. Blanks were the assay buffer either at time zero or at the end of the reaction or skeletal muscle microsomes obtained from D2-knockout mice (Dio2−/−). These latter did not significantly differ from the buffer blanks on the amount of 125I-T3 but generated slightly more iodide and some 125I-rT3, probably from the presence of small amounts of D3. In most recent experiments, we routinely used microsomes from Dio2−/− mice treated identically to the test microsomes, because the products formed by Dio2−/− microsomes represent D2-independent effects of the microsomes on the substrate. When using these, net 125I-T3 was expressed as a fraction of the total counts recovered from the strip, to correct of the small amounts of 125I-T4 that could have been metabolized or degraded by microsomal material.
Subcellular fractionation
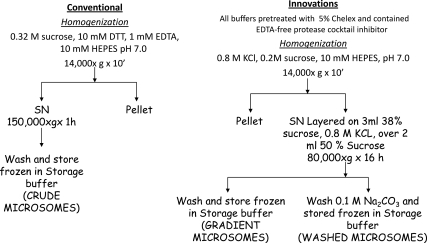
Because crude homogenates of skeletal muscle consistently had low or undetectable activity, we resorted to microsomes. This were prepared initially as described (26), but still activities were low; there was significant nonspecific deiodination of the substrate, most notably from 125-I-rT3, which is likely a result of peroxidation (16, 27), and small but significant amounts of D3. Therefore, we undertook a systematic approach to interfering factors, which ultimately led to the method summarized below, resulting in improved yield, higher specific activity and insignificant nonspecific deiodination. Figure 1 shows the comparison of the two subcellular fractionation protocols, the conventional protocol (Fig. 1, left) of microsomal preparation, used repeatedly by us previously for measuring D2 activity in other tissues (26), the product of which we termed crude microsomes. Except where indicated, crude microsomes in the present study were obtained for convenience from high-salt homogenates postmitochondrial supernatant (see below). From the modified method, we obtained highly enriched microsomal fractions in the interface of 38–50% sucrose (gradient microsomes) or 0.1 m Na2CO3-washed gradient microsomes (washed microsomes). Briefly, the changes introduced were aimed at eliminating catalytic metals, largely Fe, the oxidation-reduction of which results in substantial oxidation of DTT (22, 28, 29); we eliminated EDTA, which, by chelating Fe2+, favors its oxidation to Fe3+ (29, 28); and added EDTA-free proteases (Roche, Indianapolis, IN) (16). EDTA may protect from metallo-proteases, but in muscle, this mechanism is offset by the presence of Fe. The final adopted protocol (Fig. 1) included the treatment of all buffers with 5% Chelex (Sigma, St. Louis, MO) in a rotator at 4 C overnight to remove catalytic metals.
Fig. 1.
Schematic representation of the subcellular fractionation methods to obtain microsomal membrane preparations maximally enriched in D2 activity. Left panel, Classical method used previously by us and other laboratories to obtain microsomes enriched in D2 from tissues, such as BAT and brain. Right panel, Method developed to obtain high specific activities and reduce or eliminate factors that could reduce the D2 activity or interfere with its stability in skeletal muscle. SN, Supernatant.
The extraction of inner membrane subcellular fractions from muscle is difficult because of its structure and the “stickiness” of the abundant myosin-actin complex. We first increased the sucrose concentration to 0.4 m to increase the buoyancy of microsomes. Because the positive effect on D2 recovery was limited, we then performed the initial homogenization in 0.8 m KCl and 0.2 m sucrose (to keep the same specific gravity) buffered with 10 mm HEPES buffer (pH 7.0) (21, 30, 31). This resulted in a 2- to 3-fold increase in the yield of microsomes but no significant increase in specific activity. To enrich membranes in D2, we used discontinuous sucrose gradients previously described to separate various microsomal fractions (31). We found negligible D2 activity in the fractions retained by less than 38% sucrose, with most of the activity being at the interface between 38 and 45% sucrose, although still there was a significant fraction in the pellet sedimenting through the 45% sucrose. Based on these observations, we layered the postmitochondrial (14,000 × g supernatant) on 38% sucrose layered in turn on a cushion of 50% sucrose. The membranes retained by this cushion contained over 95% of the activity. To test whether gradient microsomes still entrapped proteins devoid of D2, we washed them 0.1 m Na2CO3, to open the vesicles and release proteins presumably devoid of D2 activity (32). The wash consisted of 1 ml of ice-cold 0.1 m Na2CO3 (pH 11.5) for 30 min, after which the particulate material was pelleted at 250,000 × g for 1 h and resuspended in 1 ml of 0.32 m sucrose, 0.1 m phosphate buffer, and 10 mm DTT (pH 7.0) (“storage buffer”). The resulting pellet was washed once more in this buffer, repelleted at 100,000 × g for 1 h, and stored in small aliquots at approximately 1 μg of protein per microliter. This approach resulted in a remarkable increase in D2-specific activity but with a low protein yield (≈7–15 μg/100 mg wet weight muscle) (see Table 1).
Table 1.
Protein recovery in different cellular fractions of red and white muscle (mg of protein/g of wet muscle weight)
| mg/g tissue (% total homogenate) |
||||
|---|---|---|---|---|
| Red muscle | White muscle | |||
| Homogenate | 96.33 (100) | 97.93 (100) | ||
| 1000 × g pellet | 19.75 (20.50) | 7.07 (7.22) | ||
| 14,000 × g pellet | 3.71 (3.85) | 1.67 (1.71) | ||
| 14,000 × g supernatant | 11.29 (11.72) | 16.74 (17.09) | ||
| Conventional | Sucrose layers | Conventional | Sucrose layers | |
|---|---|---|---|---|
| Cytosol | 3.51 (3.64) | 3.99 (4.07) | ||
| Crude microsomes | 7.78 (8.08) | 12.77 (13.04) | ||
| Gradient microsomes | 4.25 (4.41) | 3.62 (3.70) | ||
| Washed microsomes | 0.16 (0.17) | 0.08 (0.08) |
Mixed red muscle (soleus and quadratus femoris) or white muscle (VL) were homogenized as described in Materials and Methods using high salt sucrose buffered. Homogenate was fractionated by differential centrifugation. The first centrifugation at 1000 × g for 10 min generated a pellet containing largely nuclei, cell debris, and insoluble material. The 1000 × g supernatant was centrifuged at 14,000 × g for 15 min to collect mitochondria. This supernatant, from where we would obtain microsomes, was divided in 1/5 for obtaining crude microsomes (see Fig. 1) and 4/5 that were used to obtain highly purified microsomes through two layers of sucrose (gradient microsomes), part of which were further purified by a wash with 0.1 m Na2CO3 to obtain the washed microsomes, as depicted in Fig. 1. Results are presented as milligrams of protein per gram of wet tissue or, in parenthesis, as percentage of the soluble protein of the homogenate. Appropriate corrections were made for the size of the aliquots.
Subcellular localization of D2 in red skeletal muscle
To determine whether D2 resided in sarcoplasmic reticulum, we measured simultaneously sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) activity and calsequestrin (CSQ) using well-established methods (20, 33). Studies were performed on subcellular fractions of soleus and quadratus femoris, unless indicated otherwise. Because D2 in other tissues localizes to endoplasmic reticulum (2), and we find D2 activity enriched in heavy microsomes, we expected it to copurify with SERCA activity and CSQ, two markers of heavy microsomes, which are located around myofibrils and muscle cell nuclei (31, 34–36). Instead of measuring SERCA activity as rate of ATP-dependent Ca2+ transport, which is challenging due to variables as ryanodine channels status, leaking vesicles or variable intravesicular binding capacity, we measured Ca loading or uptake, using potassium oxalate to retain the Ca2+ transported within the microsomal vesicles (34). In contrast, in the absence of oxalate, the binding of Ca2+ by the microsomal vesicles is an accurate estimate of CSQ the major binding protein of the heavy sarcoplasmic reticulum (35, 37). For both Ca2+ loading and binding, we followed Hemmings methods (20) with some modifications. Briefly, 50 μg of the microsomal preparation was incubated in a 500 μl reaction mixture containing 50 mm Tris-HCl (pH 6.8), 0.25 m sucrose, 0.2 mm EGTA, 10 mm MgCl2, 10 mm ATP, 20 mm sodium azide, and 0.2 mm 45CaCl2, with or without 5 mm potassium oxalate. Whether with or without oxalate, samples were incubated for 20 min at 37 C, which is sufficient to obtain maximum Ca2+ binding or SERCA-mediated Ca2+ loading. An aliquot of 450 μl was then filtered through a 0.45-μm pore cellulose acetate filters (Type HATF; Millipore, Bedford, MA) presoaked for 30–90 min in 0.25 m KCl. The filters were then washed with 0.25 m sucrose, 20 mm NaCl, dried, placed in vials with scintillation fluid, and counted in a PerkinElmer (Waltham, MA) liquid scintillation counter. Blanks filters without microsomes were run in parallel to correct for the 45Ca adsorbed to the filters, which was minimal, yet subtracted from the total 45Ca retained in the presence of microsomes.
Data presentation and statistical analysis
Unless indicated otherwise, data are presented as mean ± sem. Because of the small size of mouse muscles, most of the assay preparations were obtained from pools from 4–10 mice. The means are from triplicates or quadruplicates of these pools. Thus, the variability of the data does not show variability among mice. Statistical significance of differences between or among results was tested by Student's t test or ANOVA, as appropriate, followed by the appropriate post hoc test to compare individual treatments, usually Bonferroni's test for selected pairs of experimental interventions.
Results
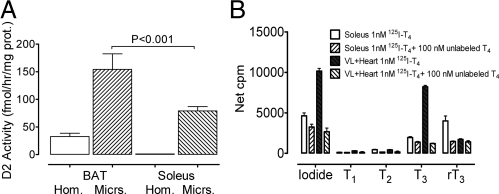
D2 is located in the endoplasmic reticulum, which in muscle (sarcoplasmic reticulum) is intertwined with muscle fibers, rich in actin and myosin. Muscle also has abundant myoglobin and iron, and very active oxidative and reductive reactions, all of which may negatively affect the activity of D2 in crude preparations. Results of an early experiment are shown in Fig. 2. Homogenates and microsomes from BAT and soleus of mice reared at room temperature, approximately 22 C, were prepared following the conventional method, in 0.32 m sucrose, as summarized in Fig. 1. We assayed about 100 μg of microsomal protein for 1.5 h. The activities detected in BAT homogenate and microsomes were within what others, and we have found previously in mice acclimated at room temperature (9). As expected, we were not able to demonstrate significant D2 activity in muscle homogenates, even from soleus, a red muscle with 5–10 more D2 mRNA than fast twitch muscle, but as reported earlier (38), we consistently found activity in crude muscle microsomes (Fig. 2A). The D2 activity of soleus microsomes was about double that of BAT homogenates and less than half that of BAT microsomes. We then compared crude microsomes of soleus and vastus lateralis (VL) under similar conditions but using approximately 1 nm125I-T4 as substrate (Fig. 2B). Because fast twitch VL of quadriceps has very low D2 activity, we spiked these microsomes with approximately 1 μg of heart microsomes of mice overexpressing D2 (39). Products were separated by HPLC as described elsewhere (16). The D2-enriched VL microsomes had substantial activity, with a ratio of 125-I− to 125-I-T3 close to one. Soleus microsomes produced more iodide than T3 but generated, in contrast to the D2-enriched VL microsomes, a substantial amount of 125-I-rT3. All three products, as well as the 125I(−) and 125I-T3 produced by the D2-enriched VL microsomes were blocked by an excess of unlabeled T4 (100 nm) (Fig. 2B). This effect of unlabeled T4 suggests soleus crude microsomes contain a significant amount of D3, and further that the excess 125I(−) is not the result of nonspecific, nonenzymatic deiodination of 125I-T4 but probably from 5′ deiodination of 125I-rT3 generated by D3. For some reason, this seems more available to D2 than the 125I-T4 added as substrate, perhaps because this is more soluble in, and hence entrapped by, membrane phospholipids than rT3 (40). We have found D3 mRNA and activity in muscle, and it is likely that the D3 of these microsomes come from contamination with cell membrane, where D3 resides (2). Either VL has much less D3 or the contamination with sarcolemma is less than in crude microsomes of soleus. The separation of microsomes between two concentrations of sucrose nearly eliminates (see Fig. 4C and description below) these D3-containing membranes, as tested with 125I-T3.
Fig. 2.
A, Comparison of BAT and soleus D2 activities measured by iodide release from 2 nm 125-I-rT3 in whole tissue homogenates (Hom.) and crude microsomes (Micrs.) (prepared the conventional way in 0.32 m sucrose). Mice were kept previously at approximately 22 C. BAT homogenate and microsomes level of activity was not different from values previously found by us and others (9). Conventionally prepared microsomes of soleus had significant activity but less than half that of BAT microsomes. B, Comparison of conventionally prepared microsomes (0.32 m sucrose) of soleus and VL microsomes, where these were spiked with about 1 μg of heart microsomes of transgenic mice overexpressing D2 (39). Substrate was approximately 1 nm 125-I-T4, and the products were separated by HPLC as described elsewhere (16).
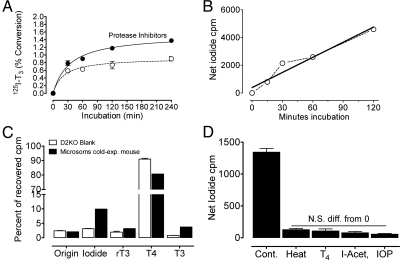
Fig. 4.
Time course of D2 activity measured by (A) gradient microsomes and the production of 125-I-T3 ± EDTA-free protease inhibitors or (B) by 0.1 m Na2CO3-washed microsomes and the production 125-I− from approximately 1 nm125I-T4 in the presence of 25 nm unlabeled T3 and EDTA-free protease inhibitors. C, Results of paper chromatography from the products of Dio2−/− microsomes (blanks for D2) and from soleus gradient microsomes from mice exposed for 4 h to 4 C. D, 125I(−) produced from 125I-rT3 by 0.1 m Na2CO3-washed microsomes in the absence and the presence of 100 nm unlabeled T4, 1 m iodo-acetate, or 100 μm iopanoic acid (I-Acet), or after heating the microsomes at 65 C for 10 min. The release of 125I(−) after these treatments was not significantly different (ANOVA) from blanks nor were these affected by the treatments. IOP, Iopanoic acid; Cont., control; N.S. diff., non-significant difference.
To avoid these confounders and have a better estimate of the amount of D2 in red muscle, we had to purify the D2-containing microsomes further. Nonetheless, in the meantime, using negative and positive controls, such as microsomes from D2 knockout mice (41) and heart microsomes of mice selectively overexpressing D2 in the heart (39), we demonstrated the presence of D2 activity in skeletal muscle, its greater abundance in slow- than in fast-twitch muscle, and the stimulation of its activity in hypothyroidism (16), a unique characteristic of D2. With the methodology used (crude microsomes from 0.32 m sucrose homogenates), however, the specific activity was low, and long incubations were necessary to obtain sufficient product accumulation (16). Hence, we continued our efforts to improve the assay and to demonstrate the identity of muscle D2 with that of other tissues.
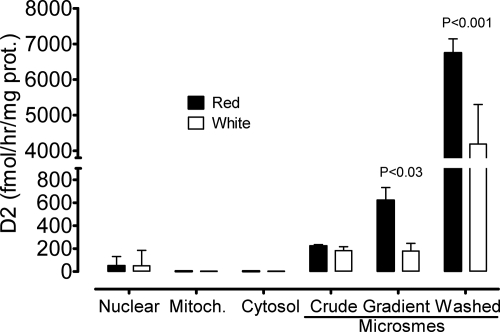
Table 1 and Fig. 3 show results of a representative experiment comparing predominantly white muscle (quadriceps VL) and red muscle (soleus, quadratus femoris). Muscles were obtained from eight mice, homogenized in high-salt sucrose buffer and submitted to differential centrifugation to obtain a 1,000 × g pellet (nuclei, cell debris, and insoluble fibrous material), and the supernatant was centrifuged at 14,000 × g for 10 min to obtain the mitochondrial pellet. The 14,000 × g supernatant was expected to contain, and did, most of D2 activity. We took about 20% of this supernatant and centrifuged it at 150,000 × g for 1 h to obtain microsomes in the conventional way (crude microsomes) and sedimented the remainder through 38% sucrose over a cushion of 50% sucrose (gradient microsomes). A large fraction of these was then washed with 0.1 m Na2CO3 to obtain washed microsomes (Fig. 1). The protein recovery is shown in Table 1. Protein concentrations were appropriately corrected for the size of the aliquots and expressed as milligrams per gram wet muscle weight or as percent of the protein recovered in the crude homogenate normalized to 1 g of wet muscle. There were differences in the protein recovery of some fractions. The 1000 × g pellet of red muscle had about 3-fold more protein, possibly reflecting a higher proportion of cell debris and nuclei than in white muscle. As expected from the higher mitochondrial content of red muscle, the 14,000 × g pellet of this muscle had more protein than that of white muscle. The crude microsomes, obtained by the conventional method, contained more protein than the red muscle possibly, reflecting the richer inner membranes of fast-twitch muscle. Most of these membranes were lost through the sucrose and with the Na2CO3-washed microsomes, because the protein content of red muscle gradient and washed microsomes were more abundant than in fast-twitch muscle.
Fig. 3.
Enrichment in D2 activity with purification of microsomal fractions of red and white skeletal muscle. Muscles were obtained from eight mice and fractionated following the improved method schematically shown on the right panel of Fig. 1. Table 1 shows the recovery of protein from the original material. Fractions were assayed in triplicate with approximately 1.5 nm 125-I-rT3. Reaction time was shortened to 15 min based on time-course studies described in the text and illustrated in Fig. 4.
Figure 3 shows the distribution of D2 activity among those various fractions. The substrate was 1.5 nm 125I-rT3. There was detectable activity in the 1000 × g pellet, probably from larger sheets of membranes of incompletely broken cells or part of the nuclear envelope. There was no detectable activity in the mitochondrial pellet or in the cytosol. The 14,000 × g supernatant, containing most of the D2 activity, was submitted to further fractionation in parallel, following the conventional and the new approach. With both methods, the activity was higher in microsomal fractions of red than of white muscle. Most importantly, in red muscle, the centrifugation through 38% sucrose resulted in greater than 3-fold gain in specific activity in red muscle compared with the crude microsomes. The wash with sodium carbonate of the fraction of the gradient microsomes resulted in an impressive 10-fold increase in D2-specific activity in both muscle types. Although there was a large loss of D2 activity, because the protein recovered was much less than 1/10 of that of gradient microsomes, the D2-specific activity reached extremely high values in washed microsomes, with net production of T3 usually over 4% from 10 μg of protein in 30 min (data not shown in Fig. 3).
The accurate quantification of enzyme reaction velocity requires measuring the initial rate of substrate consumption or product formation, before the linearity with time is lost. In crude microsomes using HPLC to separate products, we allowed the reaction to go for 16 h, and although it was not linear with time, there was a substantial increase in net product accumulation (iodide or T3) between 4 and 16 h (16). Subsequent studies (data not shown) indicate that the linearity of the reaction with time of these crude microsomes was lost in the first 1–2 h. Thus, even with crude muscle microsomes, there is a time-dependent loss of enzyme activity. The time course of 5′deiodination with more purified preparations is shown in Fig. 4. In gradient microsomes, using 125I-T4 as substrate and measuring 125I-T3 as product (Fig. 4A), the reaction is linear for barely 30 min. Product accumulation improved with EDTA-free protease inhibitors, but still there was a substantial reduction in velocity during the 4-h observation. With washed microsomes (Fig. 4B), the reaction was linear for 2 h, but then it also declined (data not shown).
Fig. 4C depicts the major products generated by gradient microsomes from 1.5 nm125I-T4. Raw data, expressed as percent of total recovered counts in the chromatogram, are depicted, comparing Dio2−/− microsomes with those from soleus with high D2 activity (exposed for 4 h to 4 C) (38). Even after subtracting the 125I-T3 and 125I(−) counts from the Dio2−/− microsomes from the test Dio2+/+ microsomes, there is still a slight excess of 125I(−) over 125I-T3 counts. On the other hand, the loss of 125I-T4 was significantly greater than formation of 125I-T3 even after correcting this by the halving of radioactive-specific activity resulting from the random loss of one 125I(−). The small excess loss of 125I-T4 in the test microsomes is probably accounted for by some residual D3, because these microsomes generated a small amount of 125I-rT3. As mentioned before, the 125I-rT3 generated in the assay is apparently more accessible to D2 than 125I-T4 and accounts for the small excess of 125I(−) over 125I-T3. The corollary of this analysis is that there is no gross nonspecific 125I(−) generation by gradient microsomes, and as shown below, it is most likely product of D2 activity and can be used in purified microsome preparations as a reliable indicator of D2 activity. Thus, in Fig. 4D, we show that the 125I(−) production from 125I-rT3 is totally blocked by 100 nm unlabeled T4; by iopanoic acid, a competitive inhibitor of D2; as well as a nonspecific inhibitor of sulfhydryl groups (iodo-acetate) and by simply heating the microsomes before the assay. Note that none of these treatments affected the blanks (ANOVA) (data not shown). These results indicate that in highly purified microsomes, the nonenzymatic, nonspecific production of 125I(−) is virtually eliminated.
Based on these results, we adopted as routine assay conditions the use of gradient microsomes, a 30-min assay with approximately 1.5 nm125I-T4 as substrate, in the presence of 25 nm T3, measuring the reaction by the production of 125-I-T3 (identified by paper chromatography). Even though the amount of 125I-T3 recovered was not different whether we used buffer blanks or Dio2−/− microsomes, we adopted these latter as routine blanks.
As mentioned, D2 resides in the endoplasmic reticulum as shown by subcellular fractionation and fluorescence of tagged D2 expressed in transfected cells (2). “Microsomes” from muscle are heterogeneous in their buoyant properties and content of SERCA and Ca-binding proteins (31, 34, 35). To obtain insight on the fraction of sarcoplasmic reticulum present in crude microsomes of muscles, we compared the enrichment of D2 activity with two markers of inner cell sarcoplasmic reticulum, namely SERCA, by its capacity to maximally load Ca in the microsomes in the presence of oxalate, and Ca binding, largely an expression of CSQ, as detailed in Materials and Methods. Fig. 5 shows one such experiment. D2 was measured 125I-T3 production from 1.5 nm125I-T4 and in the presence of 25 nm T3. Tissue was homogenized in high-salt buffer. The postmitochondrial supernatant (14,000 × g) contained the three proteins, but as expected from the dilution, the activity or concentration was low. A fraction of this supernatant was purified the conventional way to obtain crude microsomes and the rest in the discontinuous sucrose gradient, as in Fig. 1, to obtain gradient microsomes. To facilitate the comparison of the parallelism between the enrichment of the three proteins are plotted in separate panels with “y”-axes of equal height. There is a striking parallelism between D2-specific activity and Ca2+ loading and Ca2+ binding. Thus, our gradient microsomes correspond to inner part of the sarcoplasmic reticulum, located around the myofibrils and D2 resides, as in other cells, in close proximity to nuclei, which in muscle are on the surface of the myocytes. Because it is not possible to measure Ca2+ loading or binding in Na2CO3-washed microsomes, we only measured D2-specific activity in washed microsomes, and it was higher by a factor of 6 (data not shown).
Fig. 5.
Specific activity of D2 (A), maximal Ca binding (B), and maximal Ca loading by crude microsomes and gradient microsomes (C) from soleus and quadratus femoris from normal C57Bl/6 mice. Tissue was homogenized in high-salt buffer as detailed in Fig. 1. Crude microsomes were obtained otherwise in the conventional manner, whereas gradient and washed microsomes were obtained in the innovated manner, as indicated in Fig. 1. Ca binding and Ca loading with oxalate were measured as described in Materials and Methods, based on published methods (20). D2 activity was measured as 125I-T3 produced in 30 min from approximately 1.5 nm 125I-T4 in the presence of 25 nm unlabeled T3. Because data on Ca loading and binding could not be obtained in washed microsomes, data are not shown. D2-specific activity was six times higher in these microsomes (see text). PM-SN, Post-mitochondrial supernatant.
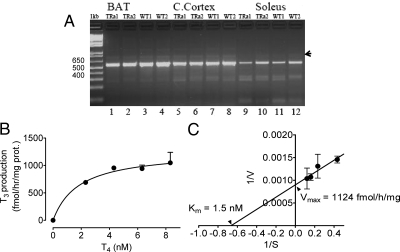
Further characterization of muscle D2 included mRNA structure and size and kinetic properties. Conceivably, some apparent peculiarities of D2 in muscle could be due to a different mRNA structure. Two small open reading frames (108 and 134 bp) have been described in the large intron of Dio2, the D2 gene, one of them, the most upstream, D2b, containing a possible selenocysteine (42). These two additional exons are detected in brain, heart, and kidney but have not been looked for in other tissues, including BAT and skeletal muscle. In the tissues found, D2b is expressed more than D2c, although always the D2a mRNA is vastly more abundant (42). To investigate the presence of these mRNA variants in muscle, we designed primers spanning from position 155 5′-ATG GGA CTC CTC AGC GTA GA-3′ in exon 1 to 722 in exon 2, 5′-CAC ATC GGT CCT CTT GGT TC-3′, which in the absence of the putative exons would generate 568-bp long cDNA. If the short exons (108 and 134) were expressed to a significant extent, they should give products 108, 134, or 242 bp longer, if both were transcribed. Such products are readily resolved in gel electrophoresis. As shown in Fig. 6A, BAT, cerebral cortex, and soleus express predominantly the 568-bp product, although a faint band close to 700 bp is present in cortex and BAT that might represent the presence of some D2c. This band is faint in soleus D2 mRNA, but this may be more apparent than real, because we had less soleus amplicon. Therefore, muscle D2 mRNA is not different from that of BAT and cerebral cortex, neither in wild-type mice nor in Thra-0/0 mice that have higher D2 activity, as reported in the accompanying article.
Fig. 6.
Identity of D2 present in skeletal muscle of normal and thyroid hormone receptor (TR)α-deficient mice. A, Primers complementary to the 5′ and 3′ coding sequences of both D2 exons were used to amplify cDNA from RT of mRNA from the indicated tissues of individual mice. Amplified cDNA was resolved in 1% agarose gel. Size of markers in the vicinity of major band is indicated on the left. It is evident that size of the major band has the expected size of 578 bp (see text). Arrowhead on the right indicates tenuous band of about 700 bp, which may be mRNA containing the transcribed two small open reading frames identified in the intron of Dio2 (see text) (42). B and C, Kinetics of T4 5′ deiodination by gradient microsomes. Reaction was carried out for 30 min with 10 μg of gradient microsomesprotein and the indicated total concentrations of T4 in the presence of 25 nm T3. In B, the data were fit to a simple hyperbole, and in C, data were transformed to obtain a Lineweaver-Burk plot. Calculated Vmax and Km are shown. TR, Thyroid hormone receptor; C. Cortex, cerebral cortex; WT, wild type.
In addition to the similar transcription products of Dio2, the kinetic analysis shows that gradient microsomes from soleus D2 have saturable activity fitting a simple hyperbola. The assay conditions were those adopted as described above. In this case, we used 10 μg of gradient microsomes protein, run the reaction for 30 min in a total volume of 100 μl. The linearized data in a Lineweaver-Burk plot gave demonstrated a Michaelis-Menten constant (Km) virtually identical to that reported brain or BAT (2, 23) and an impressive maximal velocity (Vmax) of more than 1100 fmol/h · mg protein. Therefore, we can conclude that the D2 present in skeletal muscle is not different from that found in cerebral cortex or BAT and that its specific enzyme activity is very high in purified sarcoplasmic reticulum preparation, being enriched in parallel with SERCA and CSQ.
Discussion
Northern blot screening of rat tissues after the cloning of D2 showed no detectable D2 mRNA in skeletal muscle (43), and we know of no reports of Northern blot detection in mouse skeletal muscle either. In contrast, the mRNA was readily demonstrable in human skeletal muscle, where there was activity in fresh tissue (44), which has been later estimated to generate a significant fraction of circulating T3 (7). Later, with the sensitivity of RT-PCR, D2 mRNA has been readily found in the skeletal muscle of mouse (see Refs. 12, 13, 15 among others), but demonstrating D2 activity convincingly has been a challenge, except in the newborn mouse (5), to the point that some researchers estimate it unlikely that D2 plays a significant role as a local or systemic source of T3 (13) in this species. However, skeletal muscle has been long known as a thyroid hormone-responsive tissue (45), and it participates in shivering (46), as well as in nonshivering, thermogenesis (47). This latter function is thought to result largely from stimulation by T3 of Ca2+ turnover via stimulation of SERCA activity and an enlarged sarcoplasmic Ca pool. The SERCA1 gene is stimulated by T3 particularly in red muscle, so that the inherent fastest activity of this enzyme isoform combined with the higher, tonic activity of red muscle in sustaining posture increases ATP turnover and is considered an important factor in thyroid hormone thermogenesis (47).
We find that Thra-0/0 compensate a BAT deficiency with an alternate form of facultative thermogenesis, which is associated with increased muscle D2 mRNA (12) and, as reported in abstract form (38, 48) and in the accompanying article (54), a substantial cold-dependent, adrenergically mediated stimulation of D2 activity. We have just reported activity in crude microsomes of mouse muscle (16), but the levels of activity are low. We have considered that there are multiple reasons to underestimate D2 in muscle, largely derived from the structure and biochemistry of this tissue, as discussed in preceding sections, all of which are likely to affect negatively the enzyme extraction, activity, and oxidize DTT, which is essential to measure D2 activity in vitro (22, 27). We presented here our results of dealing with these factors. Our results support our view that D2 skeletal muscle activity is underestimated, and we have been able to demonstrate unprecedented high specific activities in the same range of cold-stimulated BAT.
We have here demonstrated that D2 mRNA is not different from other tissues and that the enzyme in purified microsomes exhibits the same kinetics of brain microsomes, where it has been best characterized (23) with a virtually identical Km for T4 of 1.5 nm and a Vmax in partially purified microsomes (gradient microsomes) of over 1100 fmol/h · mg protein. This is about eight times higher than the activity of crude microsomes BAT microsomes from mice acclimated at room temperature (Fig. 2) and comparable with that of BAT microsomes from cold exposed mice.
Due to factors present in skeletal muscle, we found that measuring D2 by iodide release from 125I-T4 or 125I-rT3 in whole homogenates was difficult, in part the result of metals, particularly iron via the generation of H2O2 (27). As recently reported by us, spurious generation of iodide was a problem with conventionally prepared muscle microsomes (16). We found also that crude microsomes of red muscle had significant amounts of D3 (Fig. 2B) as shown by the generation of 125I-rT3 from 125I-T4. Because this was ameliorated by an excess cold T4, we propose that even though the amount of 125I-rT3 is less than the substrate, 125I-T4, somehow 125I-rT3 is more available to D2 than 125I-T4 contributing to the apparent excess 125I(−). Thus, the small excess 125I(−) over 125I-T3 in gradient or washed microsomes is not nonenzymatic but also the result of D2 activity. This is supported by the elimination of this excess by adding either an excess of cold T4 (Figs. 2B and 4C) or cold T3 (16). Although with maximal purification of microsomes (washed microsomes) we eliminated the D3, gradient microsomes still could contain some as shown in Fig. 4C, for which we routinely include 25–100 nm unlabeled T3 when assaying gradient microsomes.
Fluorophore-tagged D2 expressed in cells is found, as expected from old kinetic studies, around the nucleus, whereas type-1 iodothyronine deiodinase and D3 reside in the cell membrane (2). This inner membrane system forms part of the endoplasmic reticulum, and this is connected to the perinuclear membrane vesicles and the nuclear envelope (49, 50). If this holds true for skeletal muscle, the enzyme should copurify with markers of the sarcoplasmic reticulum. The results shown in Fig. 5 confirm this impression, because there was a remarkable parallelism between D2-specific activity and SERCA-mediated Ca2+ loading and Ca2+ binding by microsomes, this latter largely expression of CSQ (37). D3 is present in muscle as shown here and its presence in crude microsomes containing sarcolemma suggests that, as in other tissues, D3 resides in the outer cell membrane. These considerations indicate that muscle D2, as in other tissues, it is a major local source of T3, because it is generated close the cell nuclei.
A measure of the physiological relevance of muscle D2 activity is that the specific activity is higher than that found in BAT microsomes of mice acclimated at room temperature, when BAT contributes to 30–40% of oxygen consumption (9, 11). We show in the accompanying article that D2 activity in Thra-null mice is largely responsible for their hypermetabolism. Unfortunately, we do not have all the information for an accurate quantitative estimate of the contribution of muscle D2 to the production of T3 in the mouse, and most of the information necessary for such an estimate is from rats or rabbits. Nonetheless, our data and the ensuing analysis help in providing a quantitative perspective. Thus, as shown in Fig. 6, the Vmax of gradient microsomes from soleus and quadratus femoris is 1124 fmol T3/h · mg protein and the Km is 1.5 nm. Rat muscle has been reported to contain approximately 0.89–1.16 ng T4/g of wet tissue, on average approximately 1 ng/g (51). With an estimated water content of 60%, the T4 concentration is around 1.6 ng/ml of muscle water or 2.1 nm. If these values are similar in the mouse, we can calculate from the Michaelis-Menten equation, V = Vmax [S]/(Km + [S]), where V is the velocity of the reaction and [S] the substrate concentration, a maximal T3 production of 653 fmol or 0.425 ng T3/h · mg of gradient microsomal protein. Because we recovered 4.25 mg of gradient microsomal protein per gram of muscle (soleus and quadratus femoris) (Table 1), the total production per gram of tissue could be as high as 1.8 ng T3/h or 43 ng T3/d. Obviously, one has to consider that a significant fraction of muscle T4 may be bound to proteins or not immediately accessible to D2, so the steady-state production is probably lower. Thus, for 1% of T4 being immediately available to the enzyme (0.021 nm), the corresponding values would be 0.01 ng/h or 0.24 ng/(d · g) of muscle, which is not a large amount and can be consider close to the low boundary, but this is without taking into account the loss of D2 during the subcellular fractionation, which is likely to be substantial. As a reference, the recovery of Ca binding capacity, based on our value of 14 nmol/mg of microsomal protein (Fig. 5) and data reported by Meissner (34), of about 220 nmol/mg protein, turns to be around 6%. If the recovery of D2 were the same, even the lowest estimated boundary would still result in several nanograms of T3/(d · g) of intact muscle. Even though both soleus and quadratus femoris in an average size adult C57Bl/6 mouse weigh not more than 40 mg (our data), it is likely that the total amount of type-I fibers exceeds by far 1 g per mouse (52). Lastly, total T3 daily production in the mouse has been calculated to be around 500 ng/100 body weight (53), which fits with the daily dose we need to normalize thyroid hormone-dependent enzyme in liver in hypothyroid mice (data not shown). Therefore, D2 of red muscle alone is likely to produce several nanograms of T3 per day and make a significant contribution to total daily T3 production. However, our data reported in an accompanying article and the presence of D3 in the sarcolemma suggest that most of this T3 acts locally, regulating muscle thermogenesis and metabolism, with probably a small fraction making mixing with circulating T3.
In summary, our work has led us to develop a more specific, sensitive, and robust method to measure D2-mediated T4 to T3 conversion in skeletal muscle by addressing factors that result in an underestimation of the real D2 activity. Thus, we enriched muscle extract in D2 by increasing the recovery of intracellular membranes with the use of high-salt homogenizing buffer; we eliminated extraneous protein and other potentially interfering factors, by concentrating D2-rich microsomal fractions by centrifugation through a discontinuous sucrose gradient; we removed metals catalyzing peroxidation and oxidation of DTT; and we partially protected the enzyme with protease inhibitors. Lastly, using classical paper chromatography, we cleanly recovered the most relevant product of D2 action on T4, the active form of thyroid hormone, T3. With this improved methodology, we have demonstrated that muscle D2 produces physiologically relevant amounts of T3 that can play an important role in muscle thermogenesis and metabolic regulation, as shown in an accompanying article and in another study under revision elsewhere (Marsili A., C. Aguayo-Mazzucato, T. Chen, A. Kumar, M. Chung, E. P. Lunsford, J. W. Harney, T.-V. Van-Tran, E. Gianetti, W. Ramadan, C. Chou, S. Bonner-Weir, P. R. Larsen, J. E. Silva, and A. M. Zavacki, submitted for publication).
Acknowledgments
We thank the valuable technical contributions of Paula Pelletier and Cyril Chou.
This work was supported by the Department of Medicine and the Academic Affairs Office of Baystate Medical Center, by internal grants from the Combined Biomedical Research Grants of Baystate Health and University of Massachusetts, and partially by the National Institutes of Health Grant DK 44128 (to P.R.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- Brown adipose tissue
- CSQ
- calsequestrin
- D2
- type-2 iodothyronine deiodinase
- D3
- type-3 iodothyronine deiodinase
- DTT
- dithiothreitol
- Km
- Michaelis-Menten constant
- SERCA
- sarco/endoplasmic reticulum Ca2+-ATPase
- Thra
- thyroid hormone receptor α gene
- TN
- thermoneutrality
- VL
- vastus lateralis
- Vmax
- maximal velocity.
References
- 1. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 2. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan MM, Yaskoski KA. 1981. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest 67:1208–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kester MH, Toussaint MJ, Punt CA, Matondo R, Aarnio AM, Darras VM, Everts ME, de Bruin A, Visser TJ. 2009. Large induction of type III deiodinase expression after partial hepatectomy in the regenerating mouse and rat liver. Endocrinology 150:540–545 [DOI] [PubMed] [Google Scholar]
- 5. Dentice M, Marsili A, Ambrosio R, Guardiola O, Sibilio A, Paik JH, Minchiotti G, DePinho RA, Fenzi G, Larsen PR, Salvatore D. 2010. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. J Clin Invest 120:4021–4030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J, Engel FB, Obregon MJ, Larsen PR, Bianco AC, Huang SA. 2008. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. 2005. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 115:2524–2533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gauthier K, Plateroti M, Harvey CB, Williams GR, Weiss RE, Refetoff S, Willott JF, Sundin V, Roux JP, Malaval L, Hara M, Samarut J, Chassande O. 2001. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol Cell Biol 21:4748–4760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marrif H, Schifman A, Stepanyan Z, Gillis MA, Calderone A, Weiss RE, Samarut J, Silva JE. 2005. Temperature homeostasis in transgenic mice lacking thyroid hormone receptor α gene products. Endocrinology 146:2872–2884 [DOI] [PubMed] [Google Scholar]
- 10. Gordon CJ. 1993. Temperature regulation in laboratory rodents. New York: Cambridge University Press [Google Scholar]
- 11. Foster DO, Frydman ML. 1978. Brown adipose tissue: the dominant site of nonshivering thermogenesis in the rat. Experientia Suppl 32:147–151 [DOI] [PubMed] [Google Scholar]
- 12. Pelletier P, Gauthier K, Sideleva O, Samarut J, Silva JE. 2008. Mice lacking the thyroid hormone receptor-α gene spend more energy in thermogenesis, burn more fat, and are less sensitive to high-fat diet-induced obesity. Endocrinology 149:6471–6486 [DOI] [PubMed] [Google Scholar]
- 13. Grozovsky R, Ribich S, Rosene ML, Mulcahey MA, Huang SA, Patti ME, Bianco AC, Kim BW. 2009. Type 2 deiodinase expression is induced by peroxisomal proliferator-activated receptor-γ agonists in skeletal myocytes. Endocrinology 150:1976–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hosoi Y, Murakami M, Mizuma H, Ogiwara T, Imamura M, Mori M. 1999. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J Clin Endocrinol Metab 84:3293–3300 [DOI] [PubMed] [Google Scholar]
- 15. Kwakkel J, Van Beeren HC, Ackermans MT, Platvoet-Ter Schiphorst MC, Fliers E, Wiersinga WM, Boelen A. 2009. Skeletal muscle deiodinase type 2 regulation during illness in mice. J Endocrinol 203:263–270 [DOI] [PubMed] [Google Scholar]
- 16. Marsili A, Ramadan W, Harney JW, Mulcahey M, Castroneves LA, Goemann IM, Wajner SM, Huang SA, Zavacki AM, Maia AL, Dentice M, Salvatore D, Silva JE, Larsen PR. 2010. Type 2 iodothyronine deiodinase levels are higher in slow-twitch than fast-twitch mouse skeletal muscle and are increased in hypothyroidism. Endocrinology 151:5952–5960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anunciado-Koza R, Ukropec J, Koza RA, Kozak LP. 2008. Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J Biol Chem 283:27688–27697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Rossmeisl M, McClaine J, Riachi M, Harper ME, Kozak LP. 2003. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest 111:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chin ER, Green HJ, Grange F, Mercer JD, O'Brien PJ. 1994. 6 Technical considerations for assessing alterations in skeletal muscle sarcoplasmic reticulum Ca++-sequestration function in vitro. Mol Cell Biochem 139:41–52 [DOI] [PubMed] [Google Scholar]
- 20. Hemmings SJ. 2001. New methods for the isolation of skeletal muscle sarcolemma and sarcoplasmic reticulum allowing a comparison between the mammalian and amphibian β(2)-adrenergic receptors and calcium pumps. Cell Biochem Funct 19:133–141 [DOI] [PubMed] [Google Scholar]
- 21. Wu FY, Smith SB. 1987. Ionic strength and myofibrillar protein solubilization. J Anim Sci 65:597–608 [DOI] [PubMed] [Google Scholar]
- 22. Netto LE, Stadtman ER. 1996. The iron-catalyzed oxidation of dithiothreitol is a biphasic process: hydrogen peroxide is involved in the initiation of a free radical chain of reactions. Arch Biochem Biophys 333:233–242 [DOI] [PubMed] [Google Scholar]
- 23. Kaplan MM, Visser TJ, Yaskoski KA, Leonard JL. 1983. Characteristics of iodothyronine tyrosyl ring deiodination by rat cerebral cortical microsomes. Endocrinology 112:35–42 [DOI] [PubMed] [Google Scholar]
- 24. Hitomi Y, Kizaki T, Watanabe S, Matsumura G, Fujioka Y, Haga S, Izawa T, Taniguchi N, Ohno H. 2005. Seven skeletal muscles rich in slow muscle fibers may function to sustain neutral position in the rodent hindlimb. Comp Biochem Physiol B Biochem Mol Biol 140:45–50 [DOI] [PubMed] [Google Scholar]
- 25. Bellabarba D, Peterson RE, Sterling K. 1968. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab 28:305–307 [DOI] [PubMed] [Google Scholar]
- 26. Silva JE, Mellen S, Larsen PR. 1987. Comparison of kidney and brown adipose tissue iodothyronine 5′- deiodinases. Endocrinology 121:650–656 [DOI] [PubMed] [Google Scholar]
- 27. Reinwein D, Rall JE. 1966. Nonenzymatic deiodination of thyroxine by hydrogen peroxide. Endocrinology 78:1248–1251 [DOI] [PubMed] [Google Scholar]
- 28. Chiesi M, Inesi G. 1979. The use of quench reagents for resolution of single transport cycles in sarcoplasmic reticulum. J Biol Chem 254:10370–10377 [PubMed] [Google Scholar]
- 29. Rhee SG, Kim KH, Chae HZ, Yim MB, Uchida K, Netto LE, Stadtman ER. 1994. Antioxidant defense mechanisms: a new thiol-specific antioxidant enzyme. Ann NY Acad Sci 738:86–92 [DOI] [PubMed] [Google Scholar]
- 30. Cripps RM, Suggs JA, Bernstein SI. 1999. Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J 18:1793–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saito A, Seiler S, Chu A, Fleischer S. 1984. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol 99:875–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. 1982. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cala SE, Jones LR. 1983. Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl-sepharose. J Biol Chem 258:11932–11936 [PubMed] [Google Scholar]
- 34. Meissner G. 1975. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta 389:51–68 [DOI] [PubMed] [Google Scholar]
- 35. Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. 2009. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol 587:443–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nori A, Valle G, Bortoloso E, Turcato F, Volpe P. 2006. Calsequestrin targeting to sarcoplasmic reticulum of skeletal muscle fibers. Am J Physiol Cell Physiol 291:C245–C253 [DOI] [PubMed] [Google Scholar]
- 37. Howarth FC, Glover L, Culligan K, Qureshi MA, Ohlendieck K. 2002. Calsequestrin expression and calcium binding is increased in streptozotocin-induced diabetic rat skeletal muscle though not in cardiac muscle. Pflugers Arch 444:52–58 [DOI] [PubMed] [Google Scholar]
- 38. Ramadan W, Huang S, Marsili A, Larsen PR, Silva JE. 2009. Mouse skeletal muscle microsomes have type-2 iodothyronine deiodinase (D2) mRNA and activity. Possible role in the hypermetabolism of TRα-deficient mice. Program and Meeting Abstracts, 80th Annual Meeting of the American Thyroid Association, Palm Beach, FL, Sep 23–27 [Google Scholar]
- 39. Pachucki J, Hopkins J, Peeters R, Tu H, Carvalho SD, Kaulbach H, Abel ED, Wondisford FE, Ingwall JS, Larsen PR. 2001. Type 2 iodothyronin deiodinase transgene expression in the mouse heart causes cardiac-specific thyrotoxicosis. Endocrinology 142:13–20 [DOI] [PubMed] [Google Scholar]
- 40. Hillier AP. 1970. The binding of thyroid hormones to phospholipid membranes. J Physiol 211:585–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. 2001. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol 15:2137–2148 [DOI] [PubMed] [Google Scholar]
- 42. Ohba K, Yoshioka T, Muraki T. 2001. Identification of two novel splicing variants of human type II iodothyronine deiodinase mRNA. Mol Cell Endocrinol 172:169–175 [DOI] [PubMed] [Google Scholar]
- 43. Croteau W, Davey JC, Galton VA, St Germain DL. 1996. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salvatore D, Bartha T, Harney JW, Larsen PR. 1996. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology 137:3308–3315 [DOI] [PubMed] [Google Scholar]
- 45. Barker SB, Klitgaard HM. 1952. Metabolism of tissues excised from thyroxine-injected rats. J Physiol 170:81–86 [DOI] [PubMed] [Google Scholar]
- 46. Haman F. 2006. Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol 100:1702–1708 [DOI] [PubMed] [Google Scholar]
- 47. Simonides WS, Thelen MH, van der Linden CG, Muller A, van Hardeveld C. 2001. Mechanism of thyroid-hormone regulated expression of the SERCA genes in skeletal muscle: implications for thermogenesis. Biosci Rep 21:139–154 [DOI] [PubMed] [Google Scholar]
- 48. Silva JE, Ramadan W, Marsili A, Larsen PR. 2010. Type-2 iodoythyronine deiodinase (D2) in skeletal muscle of C57Bl mice: authenticity and increased responses to adrenergic stimulation in thyroid hormone receptor-α null mice (Thra0/0). Proceedings 14th International Thyroid Congress, Paris, Sep 11–16, OC-139, Abstract [Google Scholar]
- 49. Wilkie GS, Schirmer EC. 2008. Purification of nuclei and preparation of nuclear envelopes from skeletal muscle. Methods Mol Biol 463:23–41 [DOI] [PubMed] [Google Scholar]
- 50. Wu X, Bers DM. 2006. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res 99:283–291 [DOI] [PubMed] [Google Scholar]
- 51. Escobar-Morreale HF, Obregón MJ, Escobar del Rey F, Morreale de Escobar G. 1995. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomize rats. J Clin Invest 96:2828–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang LC, Kernell D. 2001. Fibre type regionalisation in lower hindlimb muscles of rabbit, rat and mouse: a comparative study. J Anat 199:631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Finke C, Juge C, Goumaz M, Kaiser O, Davies R, Burger AG. 1987. Effects of rifampicin on the peripheral turnover kinetics of thyroid hormones in mice and in men. J Endocrinol Invest 10:157–162 [DOI] [PubMed] [Google Scholar]
- 54. Ramadan W, Marsili A, Larsen PR, Zavacki AM, Silva JE. 2011. Type-2 iodothyronine 5′ deiodinase (D2) in skeletal muscle of C57Bl/6 mice. II. Evidence for a role of D2 in the hypermetabolism of thyroid hormone receptor α-deficient mice. Endocrinology 152:3093–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]