Abstract
Obesity is frequently associated with an infiltration of macrophages into adipose tissue. Adipocyte dysfunction causes a phenotypic switch of macrophages from an alternatively activated M2-like phenotype towards a proinflammatory M1 phenotype. The cross talk between adipocytes and infiltrating immune cells, in particular macrophages, is thought to contribute to local and eventually systemic inflammation. Here, we tested the phenotypic impact of a lack of adipocytes on the inflammatory status of macrophages. We took advantage of the fat apoptosis through targeted activation of caspase-8 (FAT-ATTAC) mouse model that allows for the inducible system-wide elimination of adipocytes through a proapoptotic mechanism and followed the degree and type of inflammatory response upon ablation of live adipocytes. Analysis of depots 2 wk after elimination of adipocytes resulted in markedly reduced levels of adipose tissue and a robust down-regulation of circulating adipokines. Quantitative PCR and immunohistochemistry on epididymal and inguinal fat depots revealed an increase of the macrophage markers F4/80 and CD11c. Using polychromatic flow cytometry, we observed an up-regulation of alternatively activated M2 macrophage markers (CD206 and CD301) on the majority of F4/80 positive cells. Apoptosis of adipocytes is sufficient to initiate a large influx of macrophages into the remnant fat pads. However, these macrophages are alternatively activated, antiinflammatory M2 macrophages and not M1 cells. We conclude that adipocyte death is sufficient to initiate macrophage infiltration, and live adipocytes are required to initiate and/or sustain a proinflammatory response within the infiltrating macrophages in adipose tissue.
Chronic, low-grade inflammation of adipose tissue plays a key role in the pathogenesis of obesity complications (1). In particular, macrophages accumulate in adipose tissue with increasing body weight in both humans and mice (2, 3). These adipose tissue macrophages (ATM) are the main source of inflammatory cytokine production in adipose tissue and have been identified as key components in the development of insulin resistance (1). As such, genetic ablation of macrophage recruitment and function protects from diet-induced insulin resistance (4–7).
ATM are phenotypically heterogeneous and can be broadly classified into two different phenotypes, M1 or M2 macrophages (8–10). “Classically activated” or M1 macrophages develop in response to stimulation with interferon (IFN)γ and concomitant exposure to microbial products, such as lipopolysaccharide (9). They are the first line of defense against intracellular pathogens and are characterized by enhanced inflammatory cytokine production (TNFα, IL-6, and IL-12) and generation of reactive oxygen species such as nitric oxide via activation of inducible nitric oxide synthase (NOS) (9, 11). “Alternatively activated” or M2 macrophages arise by stimulation with IL-4 and IL-13, TGFβ, glucocorticoids, and diverse stimuli such as apoptotic cells (9). M2 polarized cells have been implicated in tissue remodeling during development and in response to injury, and in the general regulation of tissue homeostasis (12). Macrophages in the M2 configuration typically up-regulate arginase-1 and down-regulate inducible NOS while expressing inflammation-suppressive factors (e.g. IL-10) and matrix metalloproteinases (MMP) (10, 11). The current understanding on ATM polarization is based on the “phenotypic switch” model by Lumeng et al. (11). These authors propose that ATM in the lean, insulin-sensitive state are polarized toward an M2 state (11). Increasing adiposity leads to an accumulation of macrophages with a phenotypic switch toward an M1 phenotype and increased formation of crown-like structures (11). They suggest that the obesity-induced switch to M1 is not due to the conversion of resident M2 macrophages to M1 but rather the localized recruitment of an inflammatory ATM subtype from the circulation (13). Although division of macrophages into two generalized categories is an oversimplification that may neglect the more nuanced subpopulations of cells present in adipose tissue, the broad M1 and M2 categories have proven to be a useful concept for the understanding of the pathological changes associated with chronic macrophage infiltration and its associated consequences.
The molecular mechanisms leading to ATM recruitment are still not completely understood. Increased production of a subset of cytokines and chemoattractants as a consequence of adipocyte hypertrophy and local hypoxia has been implicated (14, 15). More recent data point to an involvement of adipocyte cell death as macrophage formed crown-like structures appear around remnant lipid droplets resulting from adipocyte apoptosis or necrosis (16–18). Knockout of BH3 interacting domain death agonist, a key molecule in apoptosis induction, resulted in an inhibition of adipocyte apoptosis and prevented ATM infiltration (19).
The aim of our study is to clarify the interaction of apoptotic adipocytes and surrounding live adipocytes in ATM polarization. We took advantage of our previously described fat apoptosis through targeted activation of caspase-8 (FAT-ATTAC) mouse model, in which selective apoptosis of adipocytes can be induced in a controlled manner: administration of a chemical dimerizer brings two FK506 binding protein-caspase-8 fusion proteins together causing procaspase-8 cleavage and initiation of an apoptotic cascade (20, 21). We demonstrate that inducing adipocyte-specific apoptosis leads to infiltration of M2 macrophages along with an up-regulation of genes that are typically involved in tissue remodeling. This suggests that dead or dying adipocytes are not sufficient to attract and maintain a population of M1 macrophages to adipose tissue. We rather conclude that surrounding active adipocytes are required to trigger an altered population of M1 macrophages under conditions of a rapidly remodeling adipose tissue.
Materials and Methods
Animals
Six- to 8-wk-old male FAT-ATTAC (20) mice and wild-type littermates were used. The Institutional Animal Care and Use Committee of University of Texas Southwestern Medical Center, Dallas approved all animal experiments. Mice were maintained on a 12-h light, 12-h dark cycle and housed in groups of two to four, with unlimited access to water and chow (no. 5058; LabDiet, St. Louis, MO).
Injection protocol for fat ablation
AP21087 (22) (ARIAD Pharmaceuticals, Cambridge, MA) was administered by ip injection at a dose of 0.5 μg/g of body weight. Both wild-type and FAT-ATTAC animals were injected twice daily for 4 d and once per day for additional 10 d.
In vivo scanning and quantification of total, visceral, and sc fat
For in vivo scans, mice were anesthetized by 1% isoflurane inhalation. The whole body (base of the skull, as the spinal canal begins to widen and the distal end of the tibia) of each mouse was scanned at an isotropic voxel size of 93 μm (80 kV, 450 μA, and 100 msec integration time) using the eXplore Locus microcomputer tomography (CT) scanner (GE Healthcare, Princeton, NJ). Selection of the scan energy and voxel size (scanning increment) was based on optimizing the requirements of scanning time and tissue detail and to minimize exposure to radiation. Based on the scan parameters, the estimated radiation exposure was 4 rad (0.04 Gy) for each scan. Three-dimensional images were reconstructed from two-dimensional gray-scale image slices and visualized using Microview Software (GE Healthcare). Density values for soft tissue and bone were calibrated from a phantom (GE Healthcare) containing air bubble, water, and hydroxyl apatite rod. The region of interest (ROI) for each animal was defined based on skeletal landmarks from the gray-scale images. Fat analysis was conducted using microview software, which has an advanced fat analysis tool. For determination of total adipose tissue volume, ROI was drawn around the body of the animal. A histogram was subsequently created of this selected region. The separation of fat regions was obtained from the appropriate gray scale value (upper threshold, −120; and lower threshold, −350) on the histogram. The abdominal muscular wall was used as the differentiation line to separate visceral adipose tissue from sc adipose tissue. The contour lines were drawn around the viscera, and three-dimensional ROI was generated. The visceral fat was determined from the histogram of these segmented viscera using the same thresholds. Subcutaneous fat was obtained by subtracting visceral fat from the total body fat. Color-coded three-dimensional volume of adipose tissues was generated from the histograms using volume rendering tool of microview. A video clip was generated with the Microview Movie Maker Tool.
RNA isolation and analysis
Mice were killed, and tissue was immediately harvested and frozen in liquid nitrogen. Total RNA was isolated after tissue homogenization in Trizol (Invitrogen, Carlsbad, CA) using a TissueLyser (QIAGEN, Valencia, CA) using the RNeasy RNA extraction kit (QIAGEN). The quality and quantity of the RNA were determined by absorbance at 260/280 nm. cDNA was prepared by reverse transcribing 1 μg of RNA with SuperScript III reverse transcriptase (Invitrogen) and oligo(dT)20 (Invitrogen). Primer sequences are given in the Supplemental Appendix, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. For all quantitative RT-PCR experiments, the results were calculated using the threshold cycle method (23) using hypoxanthine phosphoribosyltransferase for normalization. The PCR product was quantified using the Sybr green method (Roche, Indianapolis, IN).
Histology and immunohistochemistry
Fat pads were fixed in 10% PBS-buffered formalin for 24 h. After paraffin embedding and sectioning (5 μm), tissues were stained with hematoxylin and eosin. Formalin-fixed, paraffin-embedded sections were stained for immunoreactive F4/80, CD11c, CD206, and CD301 using rat antimouse F4/80 (Invitrogen), hamster antimouse CD11c (AbD Serotec, Raleigh, NC), and rat antimouse CD206 and CD301 (AbD Serotec). Binding of primary antibody was visualized using 3,3′ diaminobenzidine chromogen A (Dako, Glostrup, Denmark). Counterstaining was performed with 70% hematoxylin. Images were acquired using the Nikon Coolscope (Nikon, Melville, NY).
Isolation of stromal-vascular cells (SVC) from adipose tissue and flow cytometry
Epididymal fat pads were weighed, rinsed with PBS, and then minced. Liberase LH (Roche) was added for up to 45 min at 37 C shaking. Cell suspensions were centrifuged at 500 × g for 10 min. SVC pellets were then incubated in red blood cell lysis buffer (BD Bioscience, San Jose, CA) for 5 min followed by neutralization by addition of fluorescence-activated cell sorting (FACS) buffer (PBS + 1% BSA). Suspensions were filtered through a 40-μm filter, centrifuged at 500 × g for 5 min, and pellets resuspended in FACS buffer. SVC were incubated with Fc block (eBioscience, San Diego, CA) for 20 min at 4 C before staining with primary antibodies or control IgG for 30 min at 4 C. The following antibodies were used: rat antimouse CD206-Alexa Fluor 488, rat antimouse CD301-biotin and streptavidin-conjugated peridinin chlorophyll protein-Cy5.5, rat antimouse F4/80-APC (AbD Serotec). Cells were washed twice and fixed with 4% paraformaldehyde. FACS analysis was done on FACSCalibur cytometer (BD, Franklin Lakes, NJ).
Immunoblotting
Serum samples collected after 14 d of dimerizer injection were incubated with Laemmli sample buffer followed by boiling for 5 min. Samples were loaded on a Criterion precast gel (Bio-Rad, Hercules, CA), and after SDS-PAGE, the samples were subjected to immunoblot analysis with Immobilon-FL polyvinylidene fluoride transfer membrane (Millipore, Bedford, MA) using 1:1000 polyclonal antiadiponectin antibodies followed by incubation with IRDye 800-coupled goat antirabbit secondary antibodies (Rockland, Gilbertsville, PA). Primary and secondary antibodies were diluted in Tris-buffered saline with 0.1% Tween 20 and 4% nonfat milk. The fluorescent Western blotting protocol from LI-COR Biosciences (document no. 988-07568; LI-COR Biosciences, Lincoln, NE) was followed. The membrane was then scanned by the LI-COR Odyssey infrared imaging system at 700- and 800-nm channels simultaneously.
Statistical analysis
The results are shown as means ± sd. All graphs and statistical analysis were performed by the Student's t test in SigmaPlot 10.0 (Systat Software, Point Richmond, CA). Significance was accepted at P < 0.05.
Results
We used our previously characterized “FAT-ATTAC” mouse model, in which we induce death of adipocytes through a mechanism closely resembling adipocyte turnover under normal physiological conditions. The injection of a FK1012 analog causes dimerization of transgenically expressed adipocyte-specific, membrane-bound caspase-8-FK506 binding protein fusion proteins leading to system-wide coordinated elimination of adipocytes (20). After 2 wk of dimerizer injection, the body weights of FAT-ATTAC animals and wild-type littermates remained comparable (Fig. 1A), but in vivo CT scans revealed a remarkable reduction of adipose tissue depots (Fig. 1B; video clips are provided in the Supplemental Appendix). Consistent with the CT scan, the weights of dissected epididymal and inguinal fat depots were significantly decreased (Fig. 1C). Marked disruption of the adipose tissue architecture was also apparent in both depots (Fig. 1D). The loss of adipocytes by apoptosis resulted in robustly decreased remnant adipose tissue levels of adiponectin mRNA (Fig. 1E) and decreased serum levels of adiponectin (Fig. 1F).
Fig. 1.
Targeted deletion of adipocytes in FAT-ATTAC mice. Six-week-old wild-type (wt) (n = 5) or FAT-ATTAC (n = 6) mice were subjected to AP20187 dimerizer treatment for 14 d. A, Body weights. B, Magnetic resonance imaging analysis from representative wild-type and FAT-ATTAC mice showing loss of fat signal. C, Weights of epididymal (eWAT) and inguinal (iWAT) white adipose tissue depots were taken. Data are expressed as mean ± sd; *, P < 0.01 wild type vs. FAT-ATTAC. D, Representative hematoxylin and eosin stains of epididymal and inguinal adipose depots. Scale bars, 50 μm. E, mRNA expression of adiponectin in epididymal and inguinal adipose depots. Data are expressed as mean ± sd; *, P < 0.01 wild type vs. FAT-ATTAC. F, Adiponectin serum samples from three representative wild-type and FAT-ATTAC mice were analyzed by Western blotting using an antiadiponectin antibody.
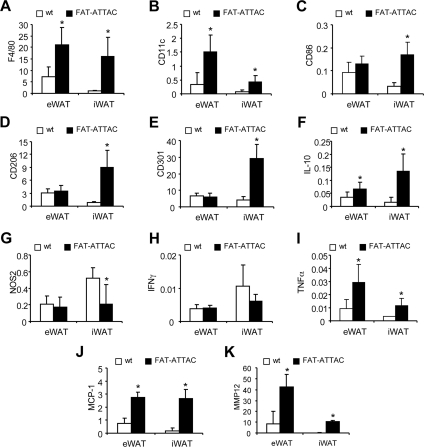
Loss of adipocytes caused an infiltration of macrophages into epididymal and inguinal adipose tissue depots as shown by increased mRNA expression of macrophages markers F4/80 (Fig. 2A), CD11c (Fig. 2B), and CD86 (Fig. 2C), consistent with our previous findings (20). The expression of the M2 markers CD206, CD301, and IL-10 was significantly up-regulated in remnant inguinal depots of FAT-ATTAC animals (Fig. 2, D–F), paralleled by a down-regulation of markers more characteristic of M1 macrophages, such as NOS2 (Fig. 2G) and IFNγ (Fig. 2H). The expression of TNFα was increased upon induction of apoptosis in both fat depots (Fig. 2I). Gene expression of genes involved in tissue repair and remodeling, such as monocyte chemotactic protein (MCP)-1 and MMP12 (Fig. 2, J and K) were significantly up-regulated in epididymal and inguinal adipose tissue depots under these conditions. All these changes were readily detected after 2 d of dimerizer injection (Supplemental Fig. 1).
Fig. 2.
mRNA expression of macrophage markers is up-regulated upon apoptosis induction in FAT-ATTAC mice. Six-week-old wild-type (wt) (n = 5) or FAT-ATTAC (n = 6) mice were subjected to AP20187 dimerizer treatment for 14 d. Total RNA was isolated from epididymal (eWAT) and inguinal (iWAT) white adipose tissue. Quantitative PCR analysis was performed using primer pairs specific for macrophage markers (A) F4/80, (B) CD11c, (C) CD86, M2 macrophage markers (D) CD206, (E) CD301, (F) IL-10, M1 macrophage markers (G) NOS2, (H) IFNγ, (I) TNFα, and remodeling marker genes (J) MCP-1 and (K) MMP12. Data are represented as relative amount of mRNA expression normalized to hypoxanthine phosphoribosyltransferase. Each bar represents mean ± sd; *, P < 0.05 wild type vs. FAT-ATTAC.
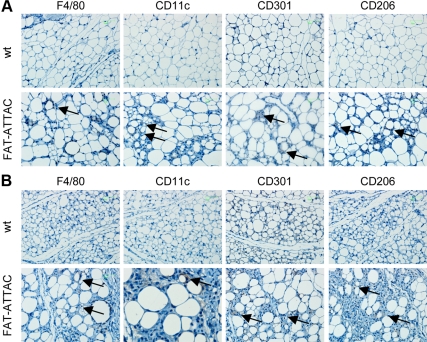
Immunohistochemistry results demonstrated accumulation of F4/80 and CD11c positive macrophages in FAT-ATTAC mice upon apoptosis induction (Fig. 3, A and B). As expected, macrophages formed crown-like structures around dying fat cells. Interestingly, apoptosis induction resulted in high expression of the M2 markers CD206 and CD301 in both epididymal and inguinal adipose tissue depots (Fig. 3, A and B). Combined, these findings suggest that macrophages infiltrating adipose tissue due to widespread apoptosis of the vast majority of local adipocytes are predominantly in the alternatively activated M2 state. This indicates that the previously reported enrichment of M1-type macrophages in the context of obese fat pads critically depends on the presence of live adipocytes to attract and maintain a population of macrophages that have undergone this phenotypic switch to the M1 phenotype.
Fig. 3.
M2 macrophage markers are up-regulated upon apoptosis induction in FAT-ATTAC mice. Six-week-old wild-type (wt) or FAT-ATTAC mice were subjected to AP20187 dimerizer treatment for 14 d. Immunohistochemical analysis was performed on (A) epididymal or (B) inguinal adipose tissue samples using anti-F4/80, anti-CD11c, anti-CD206, and anti-CD301. Representative stainings are shown. Brown, Antibody labeling (arrowheads); blue, hematoxylin counterstain.
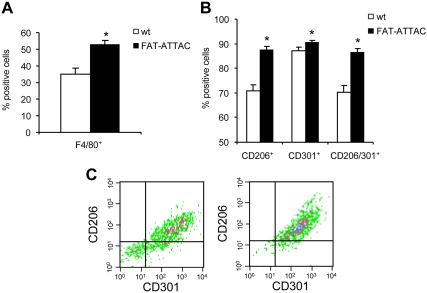
To further establish this phenomenon, we studied the expression of CD206 and CD301 at the single cell level using polychromatic flow cytometry. SVC were isolated from epididymal adipose tissue from either wild-type mice or FAT-ATTAC animals after 2 wk of dimerizer exposure. The number of F4/80+ cells was significantly increased upon induction of apoptosis in FAT-ATTAC animals (Fig. 4A), consistent with our mRNA and immunohistochemistry data. We also examined the expression of the M2 surface markers CD206 and CD301 on F4/80+ macrophages. The number of cells that express either CD206 or CD301, as well as the population of cells on which both markers are expressed simultaneously, was significantly increased upon dimerizer injection into FAT-ATTAC mice relative to wild-type littermates (Fig. 4, B and C). These observations demonstrate that widespread apoptosis of adipocytes leads to an infiltration of macrophages into adipose tissue with a distinct subpopulation of macrophages with a predominant M2 phenotype.
Fig. 4.
F4/80 macrophages recruited to adipose tissue upon fat cell apoptosis express the M2 markers, CD206 and CD301. Six-week-old wild-type (wt) or FAT-ATTAC mice were subjected to AP20187 dimerizer treatment for 14 d. SVC from epididymal adipose tissue were obtained by collagenase digestion. A, Quantification of F4/80 positive macrophages by flow cytometry. B and C, M2 macrophage marker expression. F4/80 macrophages were gated and analyzed for CD206, CD301, or CD206/CD301 expression. n = 4 mice per group. *, P < 0.05. C, Representative plot demonstrating CD206 and CD301 expression on F4/80 macrophages. Left, Wild-type; right, FAT-ATTAC.
Discussion
Adipose tissue inflammation is an important step in the pathogenesis of obesity complications (1–3, 24). Many studies in rodents as well as in humans have shown that macrophages infiltrate adipose tissue upon adipose tissue expansion and play an important role in tissue housekeeping and remodeling (2, 25). The population of adipose tissue immune cells is phenotypically heterogeneous (8–10). Only a small number of macrophages is present in the lean state, representing mostly the alternatively activated M2 phenotype (11, 13). Increasing adiposity is associated with an accumulation of macrophages in adipose tissue, formation of crown-like structures, and a phenotypic switch toward an M1 phenotype (11, 13). The ATM characteristics have been well described under different conditions, such as high-fat diet and genetic models (10, 11, 13, 26). However, the molecular mechanisms leading to ATM recruitment and the switch in macrophage phenotype are still not completely understood. Recent data suggest an involvement of adipocyte cell death, because macrophages surround dead adipocytes (16–18).
Employing an in vivo model of induced adipocyte cell death (20, 21), we demonstrate that adipocyte apoptosis causes infiltration of F4/80+ macrophages into adipose tissue. These macrophages represented the alternative activated M2 phenotype. Moreover, adipocyte apoptosis was paralleled by an up-regulation of genes that are typically involved in tissue remodeling and repair.
Our study reveals that the concerted induction of apoptosis in adipocytes is sufficient as an initial event to trigger macrophage infiltration in adipose tissue. Earlier studies were not able to distinguish whether adipocyte cell death is a late event and thus a consequence of inflammation or a process that actually initiates and drives ATM recruitment (16–18). In line with our results, Alkhouri et al. (19) demonstrated that inhibition of apoptosis achieved by knockout of the proapoptotic molecule BH3 interacting domain death agonist prevents not only ATM accumulation but also protects against the development of systemic insulin resistance and hepatic steatosis.
The macrophages that accumulate in adipose tissue upon apoptosis induction resemble the M2 phenotype as judged by the expression of the well-established markers characteristic of alternatively activated macrophages, CD206 (8) and CD301 (27), at the single cell level. The mRNA expression profile of epididymal and inguinal adipose depots supports this finding at the tissue level, because we detected an up-regulation of the classical M2 cytokine IL-10 and down-regulation of the M1 marker genes NOS2 and IFNγ. Our data fits well into the classical concept of a rapid clearing of apoptotic cells without a concomitant inflammatory response. Upon embarking on a path of apoptotic cell death, phagocytosis of apoptotic debris is carried out by professional phagocytes, such as macrophages (28, 29). These polarized cells resemble the M2 phenotype, with the production of IL-10 and other markers (30, 31). The interaction of apoptotic cells with macrophages actively suppresses an inflammatory response by suppressing the release of proinflammatory mediators and provoking production of antiinflammatory mediators (30, 31).
Strissel et al. (16) linked adipocyte death induced by high-fat feeding to recruitment of macrophages and a proinflammatory milieu in adipose tissue. In this study, dead adipocytes were identified as cells with perilipin-negative lipid droplets (16). This method provides an unspecific assessment for cell death, because it does not distinguish between apoptotic and necrotic cell death (17). Cinti et al. (17) suggest that obesity is associated with a necrotic type of cell death. Dead adipocytes display several features of necrosis, including ruptured membranes, dilated endoplasmic reticulum, and cell debris in the extracellular space, whereas typical signs of apoptosis, such as membrane blebbing, chromatin condensation, and apoptotic bodies, were not present (17). Our FAT-ATTAC mouse model enables us to specifically induce caspase-8-driven apoptosis (20). Apoptosis of adipocytes was clearly associated with the M2 phenotype of macrophages. This leads us to conclude that apoptosis of fat cells might be the initial event in macrophage recruitment, but it is not responsible for the obesity-induced phenotypic switch toward the M1 phenotype. This suggests that obesity-associated necrosis is a causative for the shift in macrophage polarization. Alternatively, because we eliminate the vast majority of live fat cells in our mice, it could also suggest that living adipocytes are required to reprogram macrophages toward the M1 phenotype. Furthermore, adipocyte hypertrophy and subsequent hypoxia might contribute to these processes (15, 17). Interestingly, hypertrophy alone, independently of obesity, is sufficient to induce cell death of adipocytes as shown in adipose sections of HSL knockout mice, which are characterized by hypertrophy of adipocytes without obesity (17). However, many other biochemical processes induced by obesity, such as mitochondrial dysfunction (32), endoplasmatic reticulum stress (33), or lipotoxicity (34), might be involved in macrophage polarization in adipose tissue as well.
The concept of a continuous turnover of adipocytes is a relatively recent one (35). Measuring the integration of 14C derived from nuclear tests in genomic DNA by accelerator mass spectrometry, Spalding et al. (35) showed that approximately 10% of fat cells are renewed annually. Tissue homeostasis includes elimination of old cells and supply of new cells from progenitors followed by differentiation into mature adipocytes. Indeed, there is a large pool of preadipocytes that are interspersed among adipocytes in human adipose tissue (14). The potential existence of a pool of early committed adipocyte precursors in the mural cell compartment of the adipose tissue vasculature represents an additional potential source for new adipocytes (15). In our experiments presented here, apoptosis of adipocytes was accompanied by an up-regulation of genes in adipose tissue that are typically involved in tissue repair and remodeling, such as MCP-1 and MMP12 (10, 36, 37), rather than by an outright proinflammatory response. We conclude that live adipocytes surrounding the dead or dying adipocytes as instigators of the proinflammatory response seen under conventional conditions associated with an obese fat pad. In our particular case with widespread apoptosis, the loss of adipocytes was counterbalanced by a remodeling process that involves the recruitment of precursor cells and reorganization of the extracellular matrix (35).
In an elegant paper, Shoelson and co-workers (38) have used a slightly different lipodystrophy model to study the nature of macrophages infiltrating adipose tissue. In this case, they used a congenital model of lipodystrophy, using the aP2-nSREBP-1c mouse that Shimomura et al. (39) developed. Although this model shares the widespread feature of a high rate of adipocyte death with the FAT-ATTAC model, it is different in that it is a congenital model in which the adipocyte turnover occurs throughout the lifespan of the mouse. The fat pads retain a significant proportion of functional adipocytes. However, just like in our FAT-ATTAC mice, there is widespread infiltration of macrophages into adipose tissue. These authors also report relatively unique characteristics of these macrophages that are clearly distinct from those observed in ob/ob and lean mice. The higher level of inflammation seen in the aP2-nSREBP-1c fat pads is a reflection of the larger number of live adipocytes present in these mice compared with the FAT-ATTAC mice where the concerted action of caspase-8 activation leads to a more complete reduction of live adipocytes. Results from both the aP2-nSREBP-1c and the FAT-ATTAC model lends further support to an active involvement of live adipocytes toward shaping the characteristics of the macrophage population involved in the clearance of cellular debris left behind from necrotic adipocytes.
It is unclear at this point which specific adipocyte-derived factors play a predominant role in shaping the local macrophage milieu and how the overall local hormonal milieu is uniquely affected by the loss of all adipocytes. Adiponectin is a candidate that is abundantly expressed locally, and many antiinflammatory properties have been ascribed to it. These properties may relate to its ability to activate a ceramidase activity within its cognate receptors adiponectin receptor 1 and 2, thereby lowering the pool of proinflammatory ceramides and increasing the level of sphingosines-1-P (40). However, future experiments will have to address whether we affect the balance of these lipid moieties upon ablation of adipocytes and how that affects the local macrophage population.
In summary, apoptosis of adipocytes causes an accumulation of alternatively activated, antiinflammatory M2 macrophages. Apoptosis of adipocytes per se is a healthy process contributing to normal tissue homeostasis without causing a proinflammatory milieu. Other and/or additional factors must therefore be responsible for switching toward a M1 proinflammatory macrophage phenotype observed in obesity.
Acknowledgments
We thank Martin Wabitsch (Division of Pediatric Endocrinology, Ulm University, Ulm, Germany) and our colleagues in the Touchstone Diabetes Center for critical comments on the manuscript.
This work was supported by National Institutes of Health (NIH) Grants R01-DK55758, R01-CA112023, RC1-DK086629, and P01-DK088761 (to P.E.S.). P.F.-P. is supported by the Ministry of Science, Research, and Art Baden-Württemberg and the European Social Fund and by the German Research Association (Fi 1700/1-1). I.W.A. was also supported by a fellowship from the Throne-Holst Foundation and the Swedish Research Council (2006-3931). J.M.R. is supported by the NIH Postdoctoral Fellowship F32DK085935-01A1.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATM
- Adipose tissue macrophage
- CT
- computer tomography
- FACS
- fluorescence-activated cell sorting
- FAT-ATTAC
- fat apoptosis through targeted activation of caspase-8
- IFN
- interferon
- MCP
- monocyte chemotactic protein
- MMP
- matrix metalloproteinase
- NOS
- nitric oxide synthase
- ROI
- region of interest
- SVC
- stromal-vascular cell.
References
- 1. Shoelson SE, Herrero L, Naaz A. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180 [DOI] [PubMed] [Google Scholar]
- 2. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr 2006. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. 2005. IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198 [DOI] [PubMed] [Google Scholar]
- 6. Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. 2007. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6:386–397 [DOI] [PubMed] [Google Scholar]
- 7. Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610–614 [DOI] [PubMed] [Google Scholar]
- 8. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. 2009. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 58:2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- 10. Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. 2010. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet–induced obesity in mice. Diabetes 59:1171–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. 2008. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skurk T, Alberti-Huber C, Herder C, Hauner H. 2007. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab 92:1023–1033 [DOI] [PubMed] [Google Scholar]
- 15. Wood IS, de Heredia FP, Wang B, Trayhurn P. 2009. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc 68:370–377 [DOI] [PubMed] [Google Scholar]
- 16. Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. 2007. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918 [DOI] [PubMed] [Google Scholar]
- 17. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. 2005. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 18. Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. 2008. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res 49:1562–1568 [DOI] [PubMed] [Google Scholar]
- 19. Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. 2010. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem 285:3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. 2005. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med 11:797–803 [DOI] [PubMed] [Google Scholar]
- 21. Trujillo ME, Pajvani UB, Scherer PE. 2005. Apoptosis through targeted activation of caspase 8 (“ATTAC-mice”): novel mouse models of inducible and reversible tissue ablation. Cell Cycle 4:1141–1145 [DOI] [PubMed] [Google Scholar]
- 22. Clackson T, Yang W, Rozamus LW, Hatada M, Amara JF, Rollins CT, Stevenson LF, Magari SR, Wood SA, Courage NL, Lu X, Cerasoli F, Jr, Gilman M, Holt DA. 1998. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc Natl Acad Sci USA 95:10437–10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 24. Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- 25. Neels JG, Olefsky JM. 2006. Inflamed fat: what starts the fire? J Clin Invest 116:33–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. 2007. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56:16–23 [DOI] [PubMed] [Google Scholar]
- 27. Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. 2009. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206:3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rathmell JC, Thompson CB. 1999. The central effectors of cell death in the immune system. Annu Rev Immunol 17:781–828 [DOI] [PubMed] [Google Scholar]
- 29. Savill J, Dransfield I, Gregory C, Haslett C. 2002. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2:965–975 [DOI] [PubMed] [Google Scholar]
- 30. Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. 1997. Immunosuppressive effects of apoptotic cells. Nature 390:350–351 [DOI] [PubMed] [Google Scholar]
- 31. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. 1998. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest 101:890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bournat JC, Brown CW. 2010. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 17:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hotamisligil GS. 2010. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kewalramani G, Bilan PJ, Klip A. 2010. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care 13:382–390 [DOI] [PubMed] [Google Scholar]
- 35. Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. 2008. Nature 453:783–787 [DOI] [PubMed] [Google Scholar]
- 36. Moali C, Hulmes DJ. 2009. Extracellular and cell surface proteases in wound healing: new players are still emerging. Eur J Dermatol 19:552–564 [DOI] [PubMed] [Google Scholar]
- 37. Schober A. 2008. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol 28:1950–1959 [DOI] [PubMed] [Google Scholar]
- 38. Herrero L, Shapiro H, Nayer A, Lee J, Shoelson SE. 2010. Inflammation and adipose tissue macrophages in lipodystrophic mice. Proc Natl Acad Sci USA 107:240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. 1998. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Gene Dev 12:3182–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SS, Scherer PE. 2011. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]