Abstract
An immunodominant peptide (p185(378–394)) derived from the c-erbB2 gene product, was recognized by an anti-DNA antibody, B3, and importantly by two classical DNA-binding proteins, Tgo polymerase and Pa-UDG. These reactivities were inhibited by DNA, confirming that the peptide mimicked DNA. BALB/c mice immunized with p185(378–394) developed significant titers of IgG anti-dsDNA antibodies. Screening of 39 human lupus sera revealed that 5% of these sera possessed reactivity toward p185(378–394). Representative mouse and human sera with anti-p185(378–394) reactivity bound intact p185, and this binding was inhibited by dsDNA. This is the first demonstration of a naturally occurring autoantigen mimotope. The present study identifies a potential antigenic stimulus that might trigger systemic lupus erythematosus in a subset of patients.
Keywords: Amino Acid, DNA-binding Protein, DNA Enzymes, Peptide Interactions, Protein-Protein Interactions, Anti-DNA Antibodies, Anti-p185 Antibodies, Autoantibodies, dsDNA Mimotope, Systemic Lupus Erythematosus
Introduction
IgG anti-dsDNA antibodies are serological markers of systemic lupus erythematosus (SLE)2 (1), often reflect disease activity (2, 3), and possess the characteristics of antibodies arising in an antigen-driven response, such as high affinity for dsDNA, class switching to IgG, and somatic mutations in the variable region gene segments (4). Given that mammalian dsDNA is a poor immunogen (5) and that some bacterial DNAs although immunogenic in normal mouse strains, do not elicit the production of antibodies cross-reactive with eukaryotic DNA (6, 7), the nature of the original trigger inducing the production of these human disease-specific antibodies, remains unclear. However, a screening of peptide libraries has recently identified some molecular motifs that are recognized by a subset of murine pathogenic antibodies (8) and that generate anti-dsDNA reactivity in nonautoimmune mice following immunization (9). Attempts have also been made to use DNA mimotope peptides for elimination of the B cells producing anti-dsDNA antibodies as a therapy of SLE (10). In our study investigating the role of heavy and light chains of human autoantibodies in determining their anti-DNA/cardiolipin binding activity (11), we employed the Fab construct of an anti-p185 antibody as a negative control. To our surprise, however, the anti-p185 antibody exhibited significant anti-DNA reactivity both against calf thymus DNA in traditional ELISA (12) and against a synthetic oligonucleotide in a novel ELISA (11).
p185 is encoded by a normal cellular gene c-erbB2 present on chromosome 17 (13) and is homologous to, but distinct from, the EGF receptor. c-erbB2 is the human analog of the transforming neu proto-oncogene, found originally in a rat neuroblastoma cell line (14). The product of the c-erbB2 proto-oncogene is a trans-membrane glycoprotein of 185 kDa, which, due to its structure (15) and localization on the membrane, seems to be a receptor for a new growth factor (16). In normal breast cells, c-erbB2 is present as a single gene copy, whereas its amplification and consequent overexpression have been found in 25–30% (17, 18) of primary human breast cancer cases. This evidence stimulated a considerable clinical interest in the role played by the c-erbB2 gene in breast cancer. Different studies (19, 20) have demonstrated that when overexpressed, the c-erbB2 gene product represents a tumor-restricted marker. Anti-p185 antibodies are currently being evaluated for their immunotherapeutic potential against breast cancer (21, 22, 23).
Interestingly, p185-interacting molecules (e.g. EGF) would appear to possess reactivity against molecules similar to DNA. For example, one class of EGF binds heparin, a sulfated polyanion with affinity for DNA-binding proteins in general (24) and interacts with p185 (25). Given the observed anti-DNA activity of anti-p185 antibody, we asked if antibodies reactive to DNA also bind p185. Interestingly, an immunodominant epitope, p185(378–394), derived from the extracellular domain of p185, possessed the conserved part of the consensus motif previously shown to be recognized by a subset of anti-DNA antibodies (26). Using two well characterized autoantibodies, B3 (anti-dsDNA) and UK4 (anti-cardiolipin), in combination with the synthetic p185(378–394) peptide, we now report that such cross-reactivity does indeed exist. These IgG λ mAbs were derived from SLE patients and were selected because of their known distinct and diverse pathogenic properties. The light chains of B3 and UK4 are encoded by the 2A2 gene segment known to be the most commonly used among humans (27) with no known mouse homologue (28). Furthermore, B3 light chain, earlier shown to possess anti-DNA activity (11), possesses the 8.12 idiotope and is encoded by the Vλ2 gene family most commonly expressed in humans (27). The 8.12 idiotope is of particular interest because its presence has been shown in SLE glomerular lesions, and the titers of 8.12+ antibodies are elevated in the serum of up to 50% of patients with SLE (29). We demonstrate that anti-DNA and anti-p185 activities co-exist on the light chain of B3. Importantly, the peptide was recognized by two classical DNA-binding proteins, Tgo polymerase and Pa-UDG, in a DNA-inhibitable manner, confirming that the peptide mimics DNA structurally. To investigate a possible role of p185 in the induction of anti-dsDNA antibodies, we immunized BALB/c mice with the peptide p185(378–394) configured as a multimer on a multiple antigenic peptide (MAP) backbone (30, 31). The immunized BALB/c mice developed significant titers of anti-dsDNA antibodies as well as glomerular immunoglobulin deposition suggestive of lupus nephritis. We now demonstrate that a subset of human lupus sera possess reactivity toward p185(378–394) and that these sera could recognize intact p185 in a dsDNA-inhibitable manner. This is the first demonstration of the presence of an autoantigen (dsDNA) mimotope on a naturally occurring eukaryotic cell molecule in humans. The present study identifies a potential antigenic stimulus that might trigger SLE. This is also the first report of promiscuous binding of a DNA look-alike protein molecule by classical DNA-binding proteins.
EXPERIMENTAL PROCEDURES
The cloning, expression, preparation of the periplasm, and purification of the Fabs of the human autoantibodies B3 and UK4 and the related hybrids have been described previously (11).
Comparative Functional Assessment of the Fabs
Anti-DNA Activity
Periplasmic extracts prepared from E. coli with or without IPTG induction were tested in an anti-DNA antibody ELISA involving a biotinylated 35-mer oligonucleotide in its single- or double-stranded form (11) for the evaluation of DNA binding activities of the recombinant Fabs.
Anti-p185(378–394) Activity
p185(378–394) (PESFDGDPASNTAPLQPE) was synthesized (Alta Bioscience, Birmingham, UK) with N terminus biotin conjugation. The polystyrene plates were coated (4 °C, overnight) with streptavidin (5 μg/ml, half of the wells) and Moα-HL (1:1000; one-fourth of the wells). The biotinylated peptide was applied to the streptavidin-coated wells (5 μg/ml, 1 h, 37 °C). The plate was blocked (2% bovine serum albumin (BSA) in PBS, 1 h, 37 °C), and the periplasmic preparations were applied (1 h, 37 °C).
The binding was detected using goat α-HL conjugated to alkaline phosphatase and p-nitrophenyl phosphate as substrate. The DNA/p185(378–394)-specific binding is expressed in relation to that for Moα-HL as percentage of binding using the equation, percentage binding = ((ODDNA/p185 − background)/(ODMoα-Hλ − background)) × 100.
Inhibition Assay
The Fab preparations of BB were mixed with calf thymus dsDNA (the final DNA concentration ranging in doubling dilution from 500 to 1.95 μg/ml), incubated (37 °C, 3 h), and applied (1 h, 37 °C) to the plates precoated with p185(378–394), and the binding was detected as described above.
The first of the two letters in the name of the Fab construct from B3 and UK4 denotes the light chain, and the second of the letters denotes the heavy chain.
Recognition of p185(378–394) Peptide by DNA-binding Proteins
Preparation of Tgo Polymerase and Pa-UDG
The gene encoding for thermostable type B DNA polymerase from Thermococcus gorgonarius (Tgo polymerase) was obtained from Dr. B. A. Connolly (Newcastle, UK) and expressed in E. coli (BL21DE3) (Novagen) using the expression vector pET17b (Novagen) under the control of the T7 promoter. Protein expression and its initial purification were carried out as described previously (32). The protein thus obtained was concentrated in an Amicon Centriprep-50 centrifugal concentrator and loaded onto a Superdex 200 (Amersham Biosciences) column. The fractions containing the pure protein were determined by SDS-PAGE and pooled. The gene encoding for Pyrobaculum aerophilum Pa-UDG was obtained from Dr. J. Jiricny (Institute of Medical Radiobiology, Zürich, Switzerland) on the plasmid pET28-paudg, a derivative of pET28c(+) (Novagen). The protein was expressed in E. coli as an N-terminal His6-tagged Pa-UDG under the control of the Lac operator and the T7 RNA polymerase and was purified to homogeneity by immobilized metal affinity chromatography as described previously (33).
Solid-phase Binding Assay
The binding of Tgo polymerase and Pa-UDG to p185(378–394) was evaluated in a solid-phase binding assay. Polystyrene plates were coated with the purified proteins (20 μg/ml, 250 μl/well, overnight, 4 °C) prepared in a modified Pfu buffer (20 mm Trizma base, pH 8.8, 10 mm potassium chloride, 10 mm ammonium sulfate, 2 mm magnesium sulfate, 2 mm magnesium chloride). The plates were washed (three times) and blocked (1% BSA in the modified Pfu buffer, 37 °C, 2 h).
Titration
The biotinylated p185(378–394) peptide was diluted in the modified Pfu buffer containing 100 μg/ml BSA (six doubling dilutions starting from 80 μg/ml). The different dilutions of the peptide were applied to polystyrene plates precoated with Tgo or Pa-UDG.
Inhibition Assay
Calf thymus dsDNA was diluted (three doubling dilutions starting from 25 μg/ml) in the modified Pfu buffer containing 100 μg/ml BSA and applied to half of the wells (in duplicate) precoated with Tgo or Pa-UDG (37 °C, 1 h). The plates were washed (six times). The peptide was applied either directly to untreated wells in the absence of calf thymus dsDNA or in the presence of the dsDNA dilutions to the wells treated with corresponding dsDNA concentration and incubated (1 h, 37 °C). The binding of the peptide was evaluated using alkaline phosphatase-conjugated streptavidin.
Immunization Experiments
Immunization was as follows. Female BALB/c mice (Harlan, Bicester, UK; age 6 weeks) were acclimatized in the animal facility for 2 weeks before immunization. Mice were immunized with either 100 μg of p185(378–394) MAP peptide emulsified 1:1 (v/v) with complete Freund's adjuvant (n = 5) or with 100 μg of p185(378–394) MAP peptide in saline without adjuvant (n = 5). Control groups of mice received MAP lysine backbone either emulsified with CFA (n = 5) or in saline without adjuvant (n = 5). The mice were boosted on days 7, 14, and 21, with incomplete Freund's adjuvant being substituted for CFA. At each immunization, half the dose was administered subcutaneously, and half was given intraperitoneally. The mice were tail-bled on days 11, 30 and 74 and sacrificed on day 107 with kidneys collected for analysis. All procedures were conducted in accordance with the Animals (Scientific Procedures) Act of 1986.
Anti-DNA Activity
Mouse sera were evaluated for the presence of anti-DNA antibodies by a standard ELISA and a pull-down ELISA as described below.
Anti-DNA Antibody ELISA
Mice sera were tested in an anti-DNA antibody ELISA involving a biotinylated 35-mer oligonucleotide in its single- or double-stranded form (11).
Pull-down ELISA
Biotinylated or nonbiotinylated 35-mer oligonucleotides (5′-biotin-CCGAATCAGTTCACTTCCAGCCCAGGTATTTAGCC-3′ and its nonbiotinylated complementary strand) (11) were annealed to generate dsDNA. Hybridization of the equimolar concentrations of the two nucleotides was performed in 1 m NaCl, 20 mm monosodium phosphate, 0.1 mm ethylenediaminetetraacetic acid, pH 7 (1 h, 37 °C) as recommended by Pharmacia Biosensor AB (Application Note 306, 1995) to achieve the final dsDNA concentration of 4 μg/μl. Two microliters of each serum were mixed with 50 μl of PBS containing 2 μg of dsDNA and incubated overnight at room temperature. The volume of the mixture was then raised to 500 μl and applied in duplicate (250 μl/well) to the polystyrene plates precoated (4 °C, overnight) with streptavidin (10 μg/ml, 250 μl/well) and blocked with 10% FCS (2 h, 37 °C), and the levels of anti-dsDNA antibodies pulled out by biotinylated dsDNA were detected.
Anti-peptide Antibody ELISA
Binding of the serum IgG (1:150) to p185(378–394) peptide was evaluated as described above for the Fab fragments.
Titration
Mouse sera diluted in PBS (1:150, 1:300, 1:600, 1:1200, and 1:2400) were applied to polystyrene plates precoated with biotinylated dsDNA via streptavidin.
Inhibition Assay
Mouse sera (1:250) mixed with calf thymus dsDNA (final DNA concentration ranging in doubling dilution from 1 to 0.0625 mg/ml) or PBS (controls) were incubated (37 °C, 3 h) and applied to the polystyrene plates precoated with p185(378–394) via streptavidin.
Antibody Specificity ELISA
Purified cardiolipin and lysozyme were obtained commercially. The 96-well microtiter plates were coated with cardiolipin (50 μg/ml in ethanol, dried overnight, 4 °C) or lysozyme (5 μg/ml in bicarbonate buffer, pH 9.6, overnight, 4 °C). The plates were blocked (PBS plus 10% FCS, 1 h, 37 °C), and the mouse sera were applied (1:150, 1 h, 37 °C). The binding was detected using an alkaline phosphatase-conjugated goat antibody to the mouse Fc region.
Antinuclear Antibodies by Immunofluorescence
The binding of serum antibodies to the dsDNA polar body of Crithidia lucilae (Biodiagnostics, Worcestershire, UK) was determined by indirect immunofluorescent assay (11).
Detection of Anti-dsDNA Antibodies in the Kidney
For the anti-DNA ELISA, kidney homogenates prepared in PBS containing 0.1% Tween 20 (0.45 g of tissue per 2 ml of PBS-Tween) were spun in a microcentrifuge (14,000 rpm, 4 °C, 1 h), and the supernatants were filtered (0.22 μm) and applied to polystyrene plates precoated with goat anti-mouse IgG (Fc-specific) (250 μl/well, overnight, 4 °C) and blocked (10% FCS in PBS-Tween, 37 °C, 2 h). Plates were washed (six times, PBS-Tween), treated with DNase I (Type IV, 37 °C, 1 h) washed again (six times, PBS-Tween), and treated with 2 m NaCl (37 °C, three washes of 30 min each) to remove any substances bound nonspecifically by charge interactions to the immunoglobulins. Plates were washed (six times, PBS-Tween), and biotinylated dsDNA was applied (1 μg/ml, 37 °C, 1 h). Binding of the dsDNA to the captured kidney immunoglobulins was detected using alkaline phosphatase-conjugated streptavidin.
Renal Deposition of Immunoglobulins
Four-micrometer-thick renal sections using frozen kidneys from mice immunized with peptide-MAP or MAP backbone alone were cut and stained with goat anti-mouse IgG conjugated to fluorescein isothiocyanate (1:40; Southern Biotechnology; room temperature, 30 min). The slides were washed in PBS, mounted in 10% glycerol, and viewed under UV light (filter set at 530 nm) using an MRC 600 scanning system connected to image analysis software (Bio-Rad).
Detection of Serum Anti-p185(378–394) Antibody Activity
Sera from SLE Patients and Healthy Individuals were obtained as follows. Thirty-nine sera from SLE patients with known clinical history and 20 sera from normal healthy individuals (controls) were analyzed for the presence of the reactivity against p185(378–394). The female/male ratios in the two groups were as follows: 35:4 and 20:0 (controls). The ages (mean ± S.D., years) of the patients in the three groups were as follows: 36.47 ± 12.46 and 40.15 ± 8.8 (controls).
For the anti-peptide antibody ELISA to detect the anti-p185(378–394) activity of human lupus serum IgG, p185(378–394) and a control peptide of similar length (p185(1238–1255), KGTPTAENPEYLGLDVPV; Alta Bioscience, Birmingham, UK) with N terminus biotin conjugation were utilized. The serum anti-p185(378–394) activity was determined using the ELISAs. The polystyrene plates were coated (4 °C, overnight) with streptavidin (5 μg/ml, half of the wells). The two biotinylated peptides (p185(378–394) and p185(1238–1255)) were applied to the streptavidin-coated wells (5 μg/ml, 1 h, 37 °C). The plate was then blocked (2% each casein and BSA in PBS, 1 h, 37 °C), and sera (1:300, diluted in the casein-BSA blocker) were applied in duplicate. The binding of serum IgG was evaluated using a monoclonal anti-human IgG antibody conjugated to alkaline phosphatase and p-nitrophenyl phosphate as substrate. Optical density was measured at 405 nm.
Detection of Serum Activity to Intact p185
Preparation of Intact p185 was as follows. The p185-overexpressing SKBR-3 cell line was obtained from the Cell Line Resource of the Institute of Cancer Research (London, UK). SKBR-3 cells were grown in RPMI 1640 (containing glutamine; Invitrogen) supplemented with 10% FCS and allowed to reach confluence. The cells were then harvested by centrifugation (1500 rpm, 15 min, 4 °C) and lysed using n-octyl β-d-glucopyranoside (30 mm) (34). The cell lysate was dialyzed against PBS and centrifuged (13,000 rpm, 10 min, 4 °C). The supernatant supplemented with glycerol (10% of final concentration) was filtered through a 0.22-μm membrane. The amount of protein was determined using Lowry's method (56). The cell extract was stored at −20 °C.
Anti-p185 Antibody Assay
The serum reactivity to the intact p185 molecule was determined using an ELISA. The polystyrene plates were coated (1 μg/ml, 4 °C, overnight) with a mouse monoclonal antibody (Sigma/Genosys, Cambridge, UK) to the cytoplasmic domain (residues 1238–1255) of p185. The SKBR-3 cell extract was applied to half of the wells (10 μg/ml, 1 h, 37 °C). The plate was then blocked (2% each casein and BSA in PBS, 1 h, 37 °C). Two sera earlier determined to possess anti-p185(378–394) activity, one representative of immunized mice and one human (serum number 23) (1:300, diluted in the casein-BSA blocker) were applied in duplicate to p185-coated or non-coated wells.
Inhibition Assay
The above described mouse and human sera (1:250) mixed with calf thymus dsDNA (final DNA concentration ranging in doubling dilution from 0.5 to 0.125 mg/ml) or PBS (controls), were incubated (37 °C, 3 h) and applied to the polystyrene plates precoated as described above, with intact p185. The binding of serum IgG was evaluated using alkaline phosphatase-conjugated goat anti-mouse or monoclonal anti-human IgG antibody.
All chemicals were purchased from Sigma unless otherwise mentioned. Polystyrene plates (Maxisorp) were purchased from Nunc (Roskilde, Denmark). Moα-HL was from Immunostics (London, UK).
RESULTS
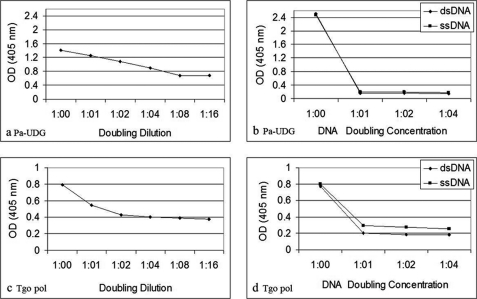
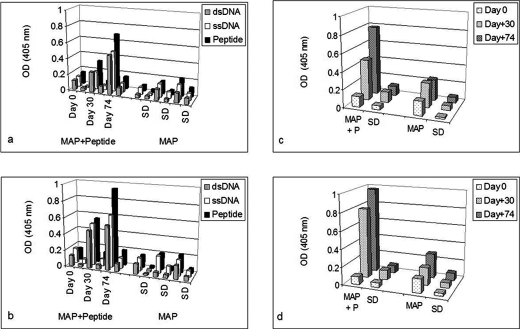
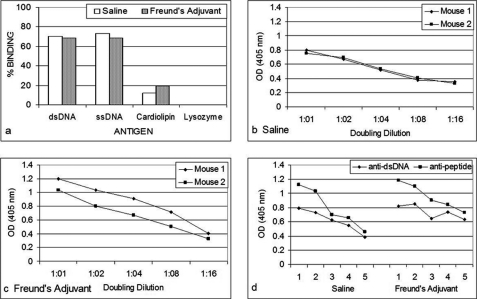
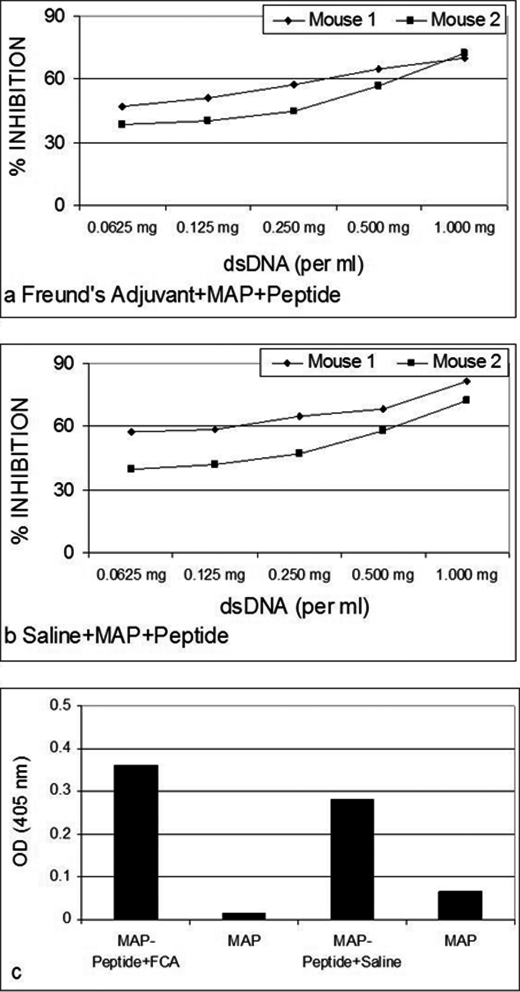
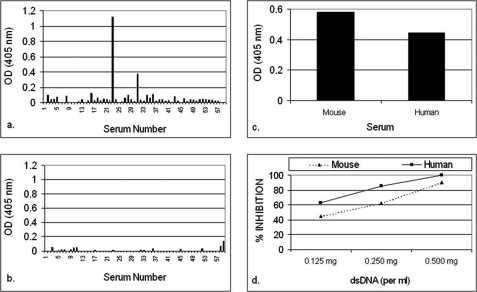
Only B3 exhibited significant anti-p185(378–394) activity (Fig. 1a). The anti-p185(378–394) activity of hybrid Fab BU containing B3 light chain and UK4 heavy chain was comparable with that of BB. The anti-p185(378–394) activity of BB was inhibited in the presence of dsDNA in an inhibitor concentration-dependent manner (Fig. 1b). Both of the DNA-binding proteins (Pa-UDG, Fig. 2a; Tgo, Fig. 2c) tested, especially Pa-UDG, exhibited significant binding to the peptide. These reactivities were inhibited by dsDNA in a concentration-dependent manner (Fig. 2, b and d). BALB/c mice immunized with p185(378–394) peptide mixed with saline (Fig. 3, a and c) or FCA (Fig. 3, b and d), developed significant titers of IgG anti-dsDNA antibodies (Fig. 3, a and d) as determined by traditional (Fig. 3, a and b) or a novel pull-down (Fig. 3, c and d) anti-dsDNA antibody ELISA. Although both of the immunoassays yielded consistent results, the pull-down ELISA was observed to be more sensitive, yielding significantly higher OD values. The mice also developed significant titers of IgG anti-ssDNA and anti-peptide antibodies (Fig. 3, a and b). Although the antibody titers were relatively higher in mice that received peptide with CFA compared with those receiving the peptide in saline on day 30, the titers of the two groups were generally comparable on day 74. The mice also developed low titers of anti-cardiolipin antibodies; however, none of the sera exhibited convincing reactivity to lysozyme that was used as a negative antigen control (Fig. 4a). Four representative sera, two from each group of mice (receiving peptide in Freund's adjuvant or in saline), were further analyzed in the inhibition assays; the sera exhibited a decrease in their binding to dsDNA in a concentration-dependent manner when applied in doubling dilutions (Fig. 4, b and c). Interestingly, the anti-peptide and anti-dsDNA activities exhibited a similar pattern of antigen reactivity when plotted in a decreasing order of anti-peptide activities of the sera (Fig. 4d), indicating that the two activities were associated. The anti-peptide activities in the four sera were inhibited by dsDNA in a concentration-dependent manner (Fig. 5, a–c). The presence of anti-dsDNA antibodies could be detected in the extracts of the kidneys only of the mice immunized with the peptide (Fig. 4d). The sera from the mice immunized with the peptide and not those from the control mice receiving MAP backbone alone also reacted with dsDNA in Crithidia assays, and immunoglobulin deposition was present in renal glomeruli only from these mice (data not shown). Two (serum numbers 23 and 31) of the 39 human sera from the patients with lupus but none of the 20 sera from healthy individuals possessed significant reactivity to p185(378–394) (Fig. 6a). None of the 59 sera possessed convincing reactivity to the control peptide (Fig. 6b). One of the representative sera from mice exhibiting anti-p185(378–394) activity and the human lupus serum number 23 were further analyzed for their ability to recognize the intact p185 molecule derived from SKBR3 cells. Both of these sera bound the intact p185 (Fig. 6c), and this reactivity was inhibited by dsDNA (Fig. 6d).
FIGURE 1.
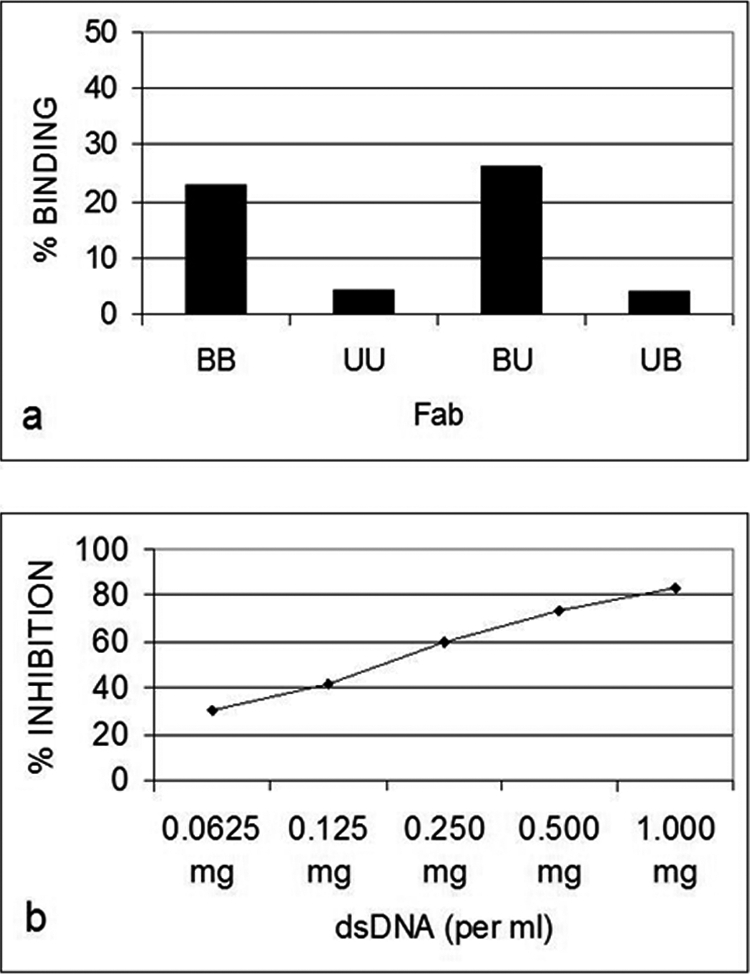
a, quantitative comparative assessment as percentage of binding in relation to total binding to anti-human λ monoclonal antibody, of the recombinant Fab proteins in their native and hybrid forms, in ELISA, to an immunodominant peptide (p185(378–394)) derived from the extracellular domain of human c-erbB2. b, inhibition of the binding of the antibody BB to p185(378–394) by calf thymus dsDNA in ELISA. B and U, B3 and UK4, respectively; the first of the two letters that name the Fab construct denotes the light chain, and the second of the letters denotes the heavy chain.
FIGURE 2.
Binding of p185(378–394) peptide to the DNA repair enzyme Pa-UDG (a) or DNA polymerase Tgo (c) in its doubling dilutions starting from 80 μg/ml. Shown is inhibition of the binding of the peptide p185(378–394) used at 20 μg/ml to the enzymes Pa-UDG (b) or DNA polymerase Tgo (d) by three doubling concentrations of calf thymus dsDNA starting from 6.25 μg/ml (shown as 1:04).
FIGURE 3.
Binding of the sera from mice immunized with MAP + p185(378–394) peptide or MAP alone constituted in saline (a and c) or Freund's adjuvant (b and d), to dsDNA, ssDNA, or p185(378–394) peptide in traditional (a and b) or pull-down (dsDNA) (c and d) ELISAs. MAP + P, MAP + p185(378–394) peptide. Vertical bars represent the mean of (and S.D. is based on) five data points obtained for five mice.
FIGURE 4.
a, quantitative comparative assessment as a percentage of binding in relation to binding to p185(378–394) peptide of the sera from mice immunized with MAP + p185(378–394) peptide or MAP alone constituted in saline or Freund's adjuvant, to dsDNA, ssDNA, cardiolipin, or lysozyme in ELISAs. Shown is binding of four representative sera (two from each group of mice receiving MAP + p185(378–394) peptide in saline (b) or in Freund's adjuvant (c) in their doubling dilutions to dsDNA. Anti-peptide and anti-dsDNA activities of the four sera are plotted in decreasing order of their anti-peptide activities (d), indicating that the two activities were associated.
FIGURE 5.
Inhibition of the binding of the four representative sera, two from each group of mice receiving MAP + p185(378–394) peptide in Freund's adjuvant (a) or in saline (b), to peptide p185(378–394) by doubling concentrations of calf thymus dsDNA. Shown is binding of the IgG eluted in kidney extracts of the mice immunized with MAP + p185(378–394) peptide in Freund's adjuvant or in saline (c) to dsDNA.
FIGURE 6.
Binding of 39 human lupus sera and 20 control sera from healthy individuals in ELISA to p185(378–394) (a) or control peptide (p185(1238–1255)) (b). Serums number 23 and 31 react with p185(378–394). c, binding of a representative p185(378–394)-immunized mouse serum and the human serum number 23 with intact p185 molecule derived from SKBR-3 cells. The inhibition of the reactivity of the mouse and human sera to intact p185 molecule by dsDNA is shown in d.
DISCUSSION
Although anti-dsDNA antibodies are the hallmark of SLE, it has been difficult to identify an antigen capable of eliciting this specificity upon immunization. A number of studies have been focused on investigating the importance of DNA itself (6, 35, 36, 37) as the crucial antigen. However, immunization with DNA has routinely failed to elicit anti-dsDNA antibodies in non-autoimmune hosts (5). Immunization of nonautoimmune mice with unmodified mammalian or bacterial dsDNA does not elicit high affinity anti-DNA IgG antibodies that bind eukaryotic DNA and are thus dissimilar to the anti-DNA antibodies found in lupus (6, 7). Further, eukaryotic DNA alone is relatively nonimmunogenic, although DNA (hapten) complexed to a DNA-binding protein (carrier) has been shown to possess immunogenic potential in some nonautoimmune models (35–37). The DNA-binding protein was thus the requirement for the recognition by T cells and to help elicit the B cell response to dsDNA.
The recognition of the peptide p185(378–394) by the anti-DNA antibody BB, by its hybrid BU with constituent BB light chain previously shown to possess anti-DNA activity (11), and, most importantly, by the two classical DNA-binding proteins that use DNA as substrate for its replication (polymerase; Tgo polymerase) or repair (Pa-UDG) in a DNA-inhibitable manner, confirm the peptide to be a DNA “look-alike” (mimotope). Using a random decapeptide phage display library, two consensus motifs, (D/E)W(D/E)Y(G/S) and (D/E)G(D/E)WP, recognized by two murine anti-DNA antibodies, have recently been identified (26). (D/E)X(D/E) (highlighted in the peptides) is the only common motif present in the two consensus peptides. Interestingly, the peptide p185(378–394) (biotin-PESFDGDPASNTAPLQPE) possesses this common motif, in addition to a G residue between the two D residues present in the second of the two consensus peptides. Further, the residues APL and QPE (present in the p185(378–394) peptide; shown in italic type) have recently been found to be the constituents of the peptides recognized by serum from a human lupus patient, using a heptamer phage display library.3 The peptide thus possesses an epitope/motif recognizable by a subset of anti-DNA antibodies and also by non-sequence-specific DNA-binding enzymes. Given that 5% of the lupus sera indeed possessed significant reactivity to p185(378–394), by analogy, this epitope could be a natural elicitor and/or the target antigen for a subset of anti-DNA antibodies. p185 is known to be released from carcinoma cells (38) and may thus be accessible to the host immune system to mount an immune response.
Peptide ligands recognized by mAbs (39) are rarely mimotopes for the original antigen eliciting the antibody response. For example, mice immunized with such peptides isolated using a neutralizing antibody against pertussis toxin (40), a neutralizing mAb against HIV gp120 (41), or a protective anti-Cryptococcus neoformans capsular glucoronoxylomannan antibody 2H1 (42) led only to a peptide-specific response without reactivity against the original antigen. The present study demonstrates that p185(378–394) is a peptide surrogate for dsDNA and that not only was this peptide a mimetic of the nucleic acid, but it could also elicit anti-dsDNA antibody production. Immunization with the peptide resulted in a lupus-like syndrome in nonautoimmune mouse; these mice developed serological autospecificities of SLE and Ig deposition in the kidney. Inhibition studies demonstrate that antibodies induced by p185(378–394) peptide immunization are broadly cross-reactive because the anti-peptide activity of serum IgG could be significantly inhibited by dsDNA. This observation suggests the existence of common epitopes on these antigens and that the p185(378–394) antigen could trigger the generation of a subset of anti-dsDNA antibodies.
Increased levels of p185HER2 in breast, ovarian, stomach, and colorectal carcinomas (43, 44) and elevated serum levels of c-ErbB2 in 13–25% of the patients with these as well as lung and prostate cancers (45) have been reported. Data from in vitro experiments indicate that overexpression of either EGF receptor or p185c-neu (or the human homolog c-erbB2) transforms cell lines (43). The physical and functional interaction of p185c-neu and EGF receptor leads to the formation of a highly active, heterodimeric tyrosine kinase complex that synergistically activates cellular transformation. Anti-receptor antibodies have shown potential utility in the down-modulation of these cell surface proteins (46) and suppression of the malignant phenotype (47). p185 has been proposed to provide a useful target for serotherapy (48). The cross-reactivity of anti-DNA and anti-p185 we describe here has an important implication. The production of such dual specific antibodies, following the onset of SLE, might influence the incidence of the appearance of neoplastic phenotype among the patients overexpressing c-erbB2.
Given that p185(378–394)-induced antibodies could recognize intact p185 (Fig. 6c), the onset of SLE with the associated anti-DNA antibodies possessing anti-p185 cross-reactivity could cause down-modulation of p185 with the consequence of suppression of the malignant phenotype. Such an occurrence might explain the documented infrequent association of SLE and solid tumors (49) and the significantly reduced cancer mortality among 2513 relatives of 56 Japanese patients with SLE (50). The reduced cancer mortality in this study was suggested to be associated with an increased immune surveillance in SLE patients (50). Further, the patients with lupus (serum numbers 23 and 31) that possessed significant anti-p185 activity also did not exhibit detectable malignancy. In contrast, the onset of cancer with associated overexpression of p185, however, may lead to the development of cross-reactive anti-p185(378–394) antibodies, which, by inducing anti-dsDNA reactivity, may lead to SLE. There indeed have been reports where the carcinoma of the breast led to lupus-like syndrome (51) or Sjogren's syndrome-like symptoms (52) in the course of the neoplastic disease. There is a dichotomy in the literature as to whether SLE is associated with an increased risk of malignancy (53–55). It would be of interest to determine whether the link, if it does exist, is dependent upon the overexpression of p185.
In conclusion, lupus-like anti-dsDNA reactivity can be generated in nonautoimmune mice by immunization with a peptide antigen derived from a naturally occurring extracellular eukaryotic cell molecule. The present study identifies p185(378–394) as a potential antigenic stimulus that might trigger SLE and demonstrates that a subset of lupus sera possess cross-reactivity to this epitope. The recognition of a DNA mimetic protein molecule by key cell DNA polymerase/repair enzymes has been demonstrated for the first time.
This work was supported by the Arthritis Research Campaign, UK.
A. Sharma, D. A. Isenberg, and B. Diamond, unpublished observations.
- SLE
- systemic lupus erythematosus
- MAP
- multiple antigenic peptide.
REFERENCES
- 1. Isenberg D. A., Horsfall A. (1998) in Oxford Textbook of Rheumatology (Maddison P. J., Isenberg D. A., Woo P., Glass D. eds) pp. 1145–1180, Oxford University Press, Oxford, UK [Google Scholar]
- 2. Swaak A. J., Aarden L. A., Statius van Eps L. W., Feltkamp T. E. (1979) Arthritis Rheum. 22, 226–235 [DOI] [PubMed] [Google Scholar]
- 3. Isenberg D. A., Garton M., Reichlin M. W., Reichlin M. (1997) Br. J. Rheumatol. 36, 229–233 [DOI] [PubMed] [Google Scholar]
- 4. Diamond B., Katz J. B., Paul E., Aranow C., Lustgarten D., Scharff M. D. (1992) Annu. Rev. Immunol. 10, 731–757 [DOI] [PubMed] [Google Scholar]
- 5. Madaio M. P., Hodder S., Schwartz R. S., Stollar B. D. (1984) J. Immunol. 132, 872–876 [PubMed] [Google Scholar]
- 6. Pisetsky D. S., Grudier J. P., Gilkeson G. S. (1990) Arthritis Rheum. 33, 153–159 [DOI] [PubMed] [Google Scholar]
- 7. Gilkeson G. S., Pippen A. M., Pisetsky D. S. (1995) J. Clin. Invest. 95, 1398–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaynor B., Putterman C., Valadon P., Spatz L., Scharff M. D., Diamond B. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 1955–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Putterman C., Diamond B. (1998) J. Exp. Med. 188, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dimitrova I., Gesheva V., Nikolova K., Mihaylova N., Todorov T., Nikolova M., Tchorbanov A. (2010) Lupus 19, 1261–1271 [DOI] [PubMed] [Google Scholar]
- 11. Kumar S., Kalsi J., Ravirajan C. T., Rahman A., Athwal D., Latchman D. S., Isenberg D. A., Pearl L. H. (2000) J. Biol. Chem. 275, 35129–35136 [DOI] [PubMed] [Google Scholar]
- 12. Le Page S. H., Williams W., Parkhouse D., Cambridge G., MacKenzie L., Lydyard P. M., Isenberg D. A. (1989) Clin. Exp. Immunol. 77, 314–318 [PMC free article] [PubMed] [Google Scholar]
- 13. Fukushige S., Matsubara K., Yoshida M., Sasaki M., Suzuki T., Semba K., Toyoshima K., Yamamoto T. (1986) Mol. Cell. Biol. 6, 955–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Padhy L. C., Shih C., Cowing D., Finkelstein R., Weinberg R. A. (1982) Cell 28, 865–871 [DOI] [PubMed] [Google Scholar]
- 15. Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. (1986) Science 232, 1644–1646 [DOI] [PubMed] [Google Scholar]
- 16. Gullick W. J., Berger M. S., Bennett P. L., Rothbard J. B., Waterfield M. D. (1987) Int. J. Cancer 40, 246–254 [DOI] [PubMed] [Google Scholar]
- 17. van de Vijver M. J., Mooi W. J., Peterse J. L., Nusse R. (1988) Eur. J. Surg. Oncol. 14, 111–114 [PubMed] [Google Scholar]
- 18. Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. (1987) Science 235, 177–182 [DOI] [PubMed] [Google Scholar]
- 19. De Potter C. R., Van Daele S., Van de Vijver M. J., Pauwels C., Maertens G., De Boever J., Vandekerckhove D., Roels H. (1989) Histopathology 15, 351–362 [DOI] [PubMed] [Google Scholar]
- 20. Natali P. G., Nicotra M. R., Bigotti A., Venturo I., Slamon D. J., Fendly B. M., Ullrich A. (1990) Int. J. Cancer 45, 457–461 [DOI] [PubMed] [Google Scholar]
- 21. Zhou X. X., Ji F., Zhao J. L., Cheng L. F., Xu C. F. (2010) J. Gastroenterol. Hepatol. 257, 1266–1275 [DOI] [PubMed] [Google Scholar]
- 22. Goldenberg M. M. (1999) Clin. Ther. 21, 309–318 [DOI] [PubMed] [Google Scholar]
- 23. Park B. W., Zhang H. T., Wu C., Berezov A., Zhang X., Dua R., Wang Q., Kao G., O'Rourke D. M., Greene M. I., Murali R. (2000) Nat. Biotechnol. 18, 194–198 [DOI] [PubMed] [Google Scholar]
- 24. Wells A. (1999) Int. J. Biochem. Cell Biol. 31, 637–643 [DOI] [PubMed] [Google Scholar]
- 25. Ethier S. P., Langton B. C., Dilts C. A. (1996) Mol. Carcinog. 15, 134–143 [DOI] [PubMed] [Google Scholar]
- 26. Spatz L., Iliev A., Saenko V., Jones L., Irigoyen M., Manheimer-Lory A., Gaynor B., Putterman C., Bynoe M., Kowal C., Kuo P., Newman J., Diamond B. (1997) Methods 11, 70–78 [DOI] [PubMed] [Google Scholar]
- 27. Ignatovich O., Tomlinson I. M., Jones P. T., Winter G. (1997) J. Mol. Biol. 268, 69–77 [DOI] [PubMed] [Google Scholar]
- 28. Williams S. C., Frippiat J. P., Tomlinson I. M., Ignatovich O., Lefranc M. P., Winter G. (1996) J. Mol. Biol. 264, 220–232 [DOI] [PubMed] [Google Scholar]
- 29. Isenberg D., Williams W., Axford J., Bakimer R., Bell D., Casaseca-Grayson T., Diamond B., Ebling F., Hahn B., Harkiss G. (1990) J. Autoimmun. 4, 393–414 [DOI] [PubMed] [Google Scholar]
- 30. Tam J.P. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 5409–5413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tam J. P. (1996) J. Immunol. Methods 196, 17–32 [DOI] [PubMed] [Google Scholar]
- 32. Greagg M. A., Fogg M. J., Panayotou G., Evans S. J., Connolly B. A., Pearl L. H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 9045–9050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sartori A. A., Schär P., Fitz-Gibbon S., Miller J. H., Jiricny J. (2001) J. Biol. Chem. 276, 29979–29986 [DOI] [PubMed] [Google Scholar]
- 34. Gould R. J., Ginsberg B. H., Spector A. A. (1981) Biochemistry 20, 6776–6781 [DOI] [PubMed] [Google Scholar]
- 35. Desai D. D., Krishnan M. R., Swindle J. T., Marion T. N. (1993) J. Immunol. 151, 1614–1626 [PubMed] [Google Scholar]
- 36. Marchini B., Puccetti A., Dolcher M. P., Madaio M. P., Migliorini P. (1995) Clin. Exp. Rheumatol. 13, 7–10 [PubMed] [Google Scholar]
- 37. Rekvig O. P., Moens U., Fredriksen K., Traavik T. (1997) Methods 11, 44–54 [DOI] [PubMed] [Google Scholar]
- 38. Zabrecky J. R., Lam T., McKenzie S. J., Carney W. (1991) J. Biol. Chem. 266, 1716–1720 [PubMed] [Google Scholar]
- 39. Scott J. K. (1992) Trends Biochem. Sci. 17, 241–245 [DOI] [PubMed] [Google Scholar]
- 40. Felici F., Luzzago A., Folgori A., Cortese R. (1993) Gene 128, 21–27 [DOI] [PubMed] [Google Scholar]
- 41. Lundin K., Samuelsson A., Jansson M., Hinkula J., Wahren B., Wigzell H., Persson M. A. (1996) Immunology 89, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valadon P., Nussbaum G., Boyd L. F., Margulies D. H., Scharff M. D. (1996) J. Mol. Biol. 261, 11–22 [DOI] [PubMed] [Google Scholar]
- 43. Dougall W. C., Qian X., Greene M. I. (1993) J. Cell Biochem. 53, 61–73 [DOI] [PubMed] [Google Scholar]
- 44. Lewis G. D., Figari I., Fendly B., Wong W. L., Carter P., Gorman C., Shepard H. M. (1993) Cancer Immunol. Immunother. 37, 255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Molina R., Jo J., Filella X., Bruix J., Castells A., Hague M., Ballesta A. M. (1997) Tumour Biol. 18, 188–196 [DOI] [PubMed] [Google Scholar]
- 46. Ohnishi Y., Nakamura H., Yoshimura M., Tokuda Y., Iwasawa M., Ueyama Y., Tamaoki N., Shimamura K. (1995) Br. J. Cancer 71, 969–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mountain A., Adair J. R. (1992) Biotechnol. Genet. Eng. Rev. 10, 1–142 [DOI] [PubMed] [Google Scholar]
- 48. Bast R. C., Jr., Boyer C. M., Jacobs I., Xu F. J., Wu S., Wiener J., Kohler M., Berchuck A. (1993) Cancer 71, 1597–1601 [DOI] [PubMed] [Google Scholar]
- 49. Sulkes A., Naparstek Y. (1991) Cancer 68, 1389–1393 [DOI] [PubMed] [Google Scholar]
- 50. Tanaka K., Nishigouri S., Kameda S., Kajiyama K., Kunihiro K., Jimi S., Tokudome S., Yamaguchi M. (1983) Am. J. Epidemiol. 118, 728–731 [DOI] [PubMed] [Google Scholar]
- 51. Wallach H. W. (1977) Arch. Intern. Med. 137, 532–535 [PubMed] [Google Scholar]
- 52. Calderoni A., Altermatt H. J., Pirovino M. (1994) Dtsch. Med. Wochenschr. 119, 1194–1198 [DOI] [PubMed] [Google Scholar]
- 53. Sultan S. M., Ioannou Y., Isenberg D. A. (2000) Rheumatology 39, 1147–1152 [DOI] [PubMed] [Google Scholar]
- 54. Ramsey-Goldman R., Mattai S. A., Schilling E., Chiu Y. L., Alo C. J., Howe H. L., Manzi S. (1998) J. Investig. Med. 46, 217–222 [PubMed] [Google Scholar]
- 55. Mellemkjaer L., Andersen V., Linet M. S., Gridley G., Hoover R., Olsen J. H. (1997) Arthritis Rheum. 40, 761–768 [DOI] [PubMed] [Google Scholar]
- 56. Bollag D. M., Edelstein S. J. (1991) Protein Methods, Wiley-Liss, Inc., New York [Google Scholar]