Abstract
Plants have a sensitive system that detects various pathogen-derived molecules to protect against infection. Flagellin, a main component of the bacterial flagellum, from the rice avirulent N1141 strain of the Gram-negative phytopathogenic bacterium Acidovorax avenae induces plant immune responses including H2O2 generation, whereas flagellin from the rice virulent K1 strain of A. avenae does not induce these immune responses. To clarify the molecular mechanism that leads to these differing responses between the K1 and N1141 flagellins, recombinant K1 and N1141 flagellins were generated using an Escherichia coli expression system. When cultured rice cells were treated with recombinant K1 or N1141 flagellin, both flagellins equally induced H2O2 generation, suggesting that post-translational modifications of the flagellins are involved in the specific induction of immune responses. Mass spectrometry analyses using glycosyltransferase-deficient mutants showed that 1,600- and 2,150-Da glycans were present on the flagellins from N1141 and K1, respectively. A deglycosylated K1 flagellin induced immune responses in the same manner as N1141 flagellin. Site-directed mutagenesis revealed that glycans were attached to four amino acid residues (Ser178, Ser183, Ser212, and Thr351) in K1 flagellin. Among mutant K1 flagellins in which each glycan-attached amino acid residue was changed to alanine, S178A and S183A, K1 flagellin induced a strong immune response in cultured rice cells, indicating that the glycans at Ser178 and Ser183 in K1 flagellin prevent epitope recognition in rice.
Keywords: Bacteria, Glycosylation, Immunology, Pathogen-associated Molecular Pattern (PAMP), Receptor Serine/Threonine Kinase, Defense, Flagellin, Rice
Introduction
During development, plants are continuously confronted with diverse pathogens. However, plants are resistant to most microbes and rely entirely on plant immune responses for their defense. Plants have evolved a multilayered defense system that can be activated upon pathogen invasion. The first layer recognizes conserved microbial molecules, referred to as microbe-associated molecular patterns, via pattern recognition receptors (1, 2). Microbe-associated molecular pattern-triggered immunity is key to plant innate immunity (3). Successful pathogens can deliver effectors that suppress these immune responses and contribute to pathogen virulence (4). Another layer recognizes pathogen effector molecules through host resistance genes, triggering a rapid defense response that often includes a localized programmed cell death reaction known as the hypersensitive response (5–7).
Microbe-associated molecular patterns include structures characteristic of pathogens, such as β-glucan, polysaccharide chitin, ergosterol, lipopolysaccharides (LPS), flagellin, and elongation factor Tu (8–13). Among these microbe-associated molecular patterns, flagellin, a main component of the bacterial flagellum, has been the most extensively studied in regard to the recognition mechanism and signal transduction. Arabidopsis recognizes the most conserved N-terminal domain of flagellin that consists of a 22-amino acid peptide (flg22)2 (12). Recognition of this elicitor-active domain depends on flagellin sensing 2 (FLS2) (14). FLS2 encodes a receptor-like kinase composed of an extracellular leucine-rich repeat, a single membrane-spanning domain, and a cytoplasmic serine/threonine kinase domain. FLS2 and flg22 were shown to physically interact by chemical cross-linking and immunoprecipitation studies, suggesting that FLS2 determines the specificity in recognizing flagellin (15).
Acidovorax avenae is a Gram-negative bacterium that causes a seedling disease that is characterized by the formation of brown stripes on the sheaths of infected plants. A. avenae has a wide host range among monocotyledonous plants; however, individual strains of this pathogen infect only one or a few host species (16). For example, strains isolated from rice, such as K1 and H8301, can infect only rice plants (virulent), whereas the N1141 strain isolated from finger millet cannot infect rice even after it is inoculated into rice tissues (avirulent). We reported that a rice avirulent N1141 strain of A. avenae induces several immune responses, such as hypersensitive response cell death, H2O2 generation, and up-regulation of defense genes, whereas the rice virulent K1 and H8301 strains of A. avenae do not induce these immune responses (17–20). To identify the specific recognition molecules that are related to the induction of these immune responses in cultured rice cells, a strain-specific antibody was raised against the avirulent strain and then absorbed with the virulent strain. The specific antibody detected the flagellin protein, which is structurally different between the virulent and avirulent strains of A. avenae (21). Flagellin purified from the avirulent N1141 strain induced rice immune responses, whereas flagellin purified from the virulent K1 strain did not induce these responses. Furthermore, a flagellin-deficient N1141 strain lost the ability to induce immune responses. These data indicate that flagellin from the avirulent N1141 strain of A. avenae specifically induces immune responses in cultured rice cells.
Interestingly, neither flg22 nor flg22-avenae (comprising the flg22 position of N1141 flagellin) induced weak immune responses compared with flagellin from the rice avirulent N1141 strain (22). Full-length sequence analysis of the flagellins from the N1141 and K1 strains showed that both flagellins consisted of 492 amino acids, 14 of which differ between the N1141 and K1 flagellins. All of the substituted amino acid residues are between residues 178 and 382 and are not located in the N-terminal region containing flg22, suggesting that the epitope contributing to specific recognition in rice is present within a region other than flg22. Furthermore, mass spectrometry analysis and sugar chain analysis revealed that the flagellin proteins of the N1141 and H8301 strains were post-translationally glycosylated. The glycosylation pattern should be different between the N1141 and H8301 flagellins because the calculated and measured masses were different between the N1141 and H8301 flagellins (21). However, it is still unknown whether amino acid substitutions and sugar chain modifications in flagellin are involved in specific recognition by rice that induces immune responses.
Because the rice immune response, such as H2O2 generation, was only induced by the avirulent N1141 flagellin, we postulated that the specific induction of immune responses by A. avenae flagellins is due to a structural difference between the N1141 and K1 flagellins. Based on this hypothesis, recombinant N1141 and K1 flagellins were produced in Escherichia coli. Both N1141 recombinant flagellin and K1 recombinant flagellin induced specific immune responses, such as H2O2 generation, indicating that the induction specificity of the immune responses to flagellin is regulated by transcriptional sugar chain modifications. Here, we report that four amino acids residues are glycosylated in the flagellin from the rice virulent K1 strain and that glycosylation of flagellin may prevent epitope recognition in rice.
EXPERIMENTAL PROCEDURES
Plants and Bacteria
Suspension cultures of rice cells (line Oc) were grown at 30 °C under light irradiation (23). The cells were diluted in fresh medium every week, and all experiments were performed 3 or 4 days after transfer. A. avenae strains N1141 (MAFF 301141) and K1 (MAFF 301755) were used as described previously (16, 21).
Purification of Flagellin
Flagellin was purified as described previously with several modifications (21). The A. avenae strains N1141 and K1 were grown for 1.5 days in LB medium at 30 °C on a rotary shaker. The cells were harvested by centrifugation at 6,000 × g for 20 min at 4 °C. The cell pellets were washed with 20 mm Tris-HCl (pH 7.5) containing 137 mm NaCl and 2.68 mm KCl, collected by centrifugation, and then resuspended in 90 ml of the same buffer. Flagella were removed from the cells by shearing for 3 min in a homogenizer (Ultra F Homogenizer HF-93F, Taitec, Saitama, Japan). Intact cells and cellular debris were removed using a two-step centrifugation procedure at 6,000 × g for 30 min and 16,000 × g for 60 min at 4 °C. The flagella were collected by ultracentrifuging at 200,000 × g for 60 min at 4 °C. The pellets were resuspended in distilled water and centrifuged at 20,000 × g for 20 min at 4 °C. After resuspending the pellets in distilled water, the flagellin preparations were stored at −80 °C.
H2O2 Detection and Quantification
H2O2 produced in the medium of cultured cells was monitored based on chemiluminescence due to the ferricyanide-catalyzed oxidation of luminol (5-amino-2,3 dihydro-1,4-phthalazinedione) as described by Schwacke and Hager (24). Ten milligrams of cultured cells were transferred to 1 ml of fresh medium and preincubated for 2 h at 30 °C. Cultured rice cells were incubated with each flagellin (<100 μl) at 30 °C for the indicated periods after treatment. Following this incubation, 10 μl of the medium were harvested; added to 160 μl of 50 mm potassium phosphate buffer (pH 7.9), 10 μl of 1.1 mm luminol, and 20 μl of 14 mm potassium ferricyanide; and immediately analyzed for chemiluminescence using a Genelight55 Lumi-Counter (Microtec Co., Ltd., Chiba, Japan).
Quantitative Real Time RT-PCR
Total RNA was isolated from cultured rice cells using an RNeasy Plant Mini kit (Qiagen, Hilden, Germany) with DNase digestion according to the manufacturer's instructions. Quantitative real time RT-PCR was performed on an Opticon2 instrument (Bio-Rad) using a QuantiTect SYBR Green RT-PCR kit (Qiagen) with PAL gene-specific primers (forward, 5′-ACATCTACGGCGTCACCAC-3′; reverse, 5′-GAAGATTCCGGCGTTGAG-3′) and Cht-1 gene-specific primers (forward, 5′-TTCTACACCTACGACGCCTTC-3′; reverse, 5′-TGGTCTCGTGCGACGTCTG-3′). To eliminate DNA contamination during quantitative RT-PCR, the primer set was designed across an intron. The sizes of the PCR products were examined to confirm that only mRNA was amplified in all quantitative RT-PCR experiments. The fluorescence data produced sigmoidal amplification plots in which the number of cycles was plotted against the fluorescence. The PAL and Cht-1 mRNA levels were calculated using a calibration curve that was prepared using standard PAL and Cht-1 genes of known template amounts (1 ng–0.1 pg) and normalized based on the reference gene Act-1.
Mass Spectrometry
All mass spectra were obtained on a VoyagerTM Workstation MALDI-TOF mass spectrometer (Applied Biosystems, Foster City, CA) in a linear, positive mode. The sample solutions were prepared in water containing 0.1% TFA. All samples were prepared by a dried droplet method in which the analyte (intact protein or peptide mixture) was mixed with a 2.3-fold volume of matrix solution (a saturated solution of sinapinic acid in 100% acetonitrile with 0.1% TFA (v/v)), and then the mixture was deposited on a stainless target plate. To prepare for the peptide mass measurements, the monomeric flagellin proteins were incubated at 37 °C overnight with trypsin (Roche Applied Science) (protein:trypsin, 1:5 (w/w) in 50 mm NH4HCO3).
Detection of Glycoproteins
The flagellin concentration was determined with a protein assay kit (Bio-Rad) using BSA as the standard. Five micrograms of each purified flagellin were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (10%, v/v). After SDS-PAGE, the glycoproteins were detected using a GelCode glycoprotein detection kit (Pierce) according to the manufacturer's instructions.
Generation of Fgt and FlaA Deletion Mutants
N1141 and K1 cosmid libraries were screened using an FlaA-specific probe for identification of Fgt genes. Two cosmid clones, N1141-10C6 and K1-12A11, were selected as FlaA-containing clones. Double-stranded sequences were aligned and assembled by using programs in the Genetyx software (Genetyx Co., Ltd., Tokyo, Japan). NFgt and KFgt were located in the 1.5-kbp upstream region of FlaA. Fgt and FlaA deletion mutants for the K1 and N1141 strains were generated using homologous recombination. For the Fgt deletion mutants of the K1 and N1141 strains, ∼6-kbp DNA fragments containing the N or KFgt genes were PCR-amplified using two sets of specific primers (5′-GCCCAGGATGGTGACGATCTCGAA-3′ and 5′-CCAACTGGAACAACTGCATGACCA-3′ and 5′-TCTAGAGAGAAGAAGACCCCGAAGAT-3′ and 5′-ACTTCCCGCTGCTCAGCCTG-3′, respectively). Amplified fragments were cloned into the pCR2.1 TOPO vector (Invitrogen). The resulting plasmids, pN6F (for the N1141 Fgt deletion mutant) and pK6F (for the K1 Fgt deletion mutant), were digested with SmaI and SacII, respectively, and then self-ligated to remove the 2-kbp mutation sites. The resulting plasmid, pN4F (for the N1141 Fgt deletion mutant), was digested with EcoRI and BglII and then cloned into the pK18mobsacB vector (25). pK5F (for the K1 Fgt deletion mutant) was also digested with XbaI and cloned into pK18mobsacB. The resulting plasmids, designated pNΔFgt and pKΔFgt, were electrotransformed into the A. avenae N1141 and K1 strains, respectively. After transformation, the bacterial cells were plated on LB agar plates containing 20 μg/ml kanamycin and incubated for 48 h at 30 °C. The resulting colonies were inoculated into Pseudomonas F liquid medium containing 26% sucrose and incubated for 72 h at 30 °C to excise the plasmid by a second crossover event. The resulting bacteria were designated NΔFgt and KΔFgt. To generate the FlaA deletion mutants, ∼1-kbp DNA fragments located on each side of FlaA were PCR-amplified using two sets of specific primers (5′- TCTAGATAGTGTTCTCGCCCTTGACCG-3′ and 5′-GGATCCTGCCATTGCAAATCTCCTGAA-3′ for the upstream region of N1141 FlaA and 5′-GGATCCCGTTGATTGCGCAGGGCTG-3′ and 5′-TCTAGAAACGCGCTGTTGACGGCGTTG-3′ for the downstream region of N1141 FlaA; 5′-TCTAGAATGCCGTGCTGTTCTCACCCTTG-3′ and 5′-GGATCCTGCCATTGCAAATCTCCTGAA-3′ for the upstream region of K1 FlaA and 5′-GGATCCCGTTGATGTCGCAGGGCTGAA-3′ and 5′-TCTAGACGTCTTCGTGTCCTTGTCGTACTTC-3′ for the downstream region of K1 FlaA). Each amplified DNA fragment was cloned into the pGEM-T vector (Promega, Corp., Madison, WI), and the resulting plasmids, pNU and pND as well as pKU and pKD, were digested with BamHI and NotI. The digested upstream fragment and downstream fragment were ligated and cloned into the pGEM-T vector. The resulting plasmids, pNUD containing DNA fragments lacking NFlaA and pKUD containing DNA fragments lacking KFlaA, were digested with XbaI and then cloned into the pK18mobsacB vector. The resulting plasmids, designated pNΔFlaA and pKΔFlaA, were electrotransformed into the A. avenae N1141 and K1 strains, respectively. After transformation, the bacterial cells were plated on LB agar plates containing 20 μg/ml kanamycin and incubated for 48 h at 30 °C. The resulting colonies were inoculated into Pseudomonas F liquid medium containing 26% sucrose and incubated for 72 h at 30 °C to excise the plasmid through a second crossover event. The resulting bacteria were designated NΔFlaA and KΔFlaA (Table 1). For the swimming assay, each bacterial cell was inoculated onto 0.25% agar LB plates with a needle and incubated for 24 h at 30 °C.
TABLE 1.
Bacterial strains and plasmids used in this study
Kmr, kanamycin resistance.
| Bacterial strains and plasmids | Relevant characteristic | Ref. or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5a | F−λ−φ80dLacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK−mK+) supE44 thi-1 gyrA relA1 | Takara, Kyoto, Japan |
| A. avenae | ||
| N1141 | Wild type | Che et al. (21) |
| NΔFgt | N1141 mutant; ΔFgt | This study |
| NΔFlaA | N1141 mutant; ΔFlaA | This study |
| NΔFlaA-NFlaA | N1141 mutant; NFlaA | This study |
| NΔFlaA-KFlaA | N1141 mutant; KFlaA | This study |
| K1 | Wild type | Che et al. (21) |
| KΔFgt | K1 mutant; ΔFgt | This study |
| KΔFlaA | K1 mutant; ΔFlaA | This study |
| KΔFlaA-KFlaA | K1 mutant; KFlaA | This study |
| KΔFlaA-NFlaA | K1 mutant; NFlaA | This study |
| K1-178Ser/Ala | K1 mutant; S178A | This study |
| K1-183Ser/Ala | K1 mutant; S183A | This study |
| K1-212Ser/Ala | K1 mutant; S212A | This study |
| K1-351Thr/Ala | K1 mutant; T351A | This study |
| K1-178Ser/Ala 183Ser/Ala | K1 mutant; S178A/S183A | This study |
| K1-178,183,212,351Ala | K1 mutant; S178A/S183A/S212A/T351A | This study |
| Plasmids | ||
| pK18mobsacB | Sucrose-sensitive (sacB) Kmr | Schäfer et al. (25) |
| pNΔFgt | 4-kb chimeric PCR product mutating NFgt cloned into pK18mobsacB at EcoRI and BglII sites, Kmr | This study |
| pKΔFgt | 5-kb chimeric PCR product mutating KFgt cloned into pK18mobsacB at XbaI site, Kmr | This study |
| pNΔFlaA | 2.1-kb chimeric PCR product deleting NFlaA cloned into pK18mobsacB at XbaI site, Kmr | This study |
| pKΔFlaA | 2.2-kb chimeric PCR product deleting KFlaA cloned into pK18mobsacB at XbaI site, Kmr | This study |
Generation of Flagellins with Amino Acid Substitutions
To generate the amino acid-substituted flagellins, a KOD-Plus mutagenesis kit (Toyobo, Osaka, Japan) was used. An ∼2-kbp DNA fragment containing the region from 231 bp upstream to 299 bp downstream of KFlaA was PCR-amplified with a specific primer set (5′-CCCGGGGCATTGGGGGGATAA-3′ and 5′-AAGCTTGACGGCATCCACGGCAG-3′) and cloned into the pGEM-T vector. This double-stranded plasmid vector was used as a template to generate mutant flagellins. The DNA for each KFlaA mutant was reverse amplified using the following PCR primer sets containing the noted mutations: K S178A, 5′-GCTGGCGCGGCTACCTCCGGCG-3′ and 5′-GGCCGAGGCCGTCAGTTGGGCGC; K S183A, 5′-GCTGGCGCGTCGGCCGGCTCG-3′ and 5′-GGTAGCCGCGCCGGAGGCCGAG-3′; K S212A, 5′-GCTGGCACGGCCGCCGACATC-3′ and 5′-GGCGGCGACGTTGACGGTCTTG-3′; K T351A, 5′-GCCGGTGCCACCGTGGCCTCG-3′ and 5′-GCCCGCCGCCGTGGCGGT-3′; and K S178A/S183A, 5′-GCTGGCGCGTCGGCCGGCTCG-3′ and 5′-GGTAGCCGCGCCAGCGGCCGAG-3′. The desired mutations were confirmed by DNA sequence analysis, and the plasmids were digested with SmaI and HindIII. For each mutant, a DNA fragment of ∼2 kbp was isolated and ligated into the SmaI and HindIII digestion sites within the pKΔFlaA vector. The resulting plasmids were digested with XbaI and then inserted into the pK18mobsacB vector. The resulting plasmids were transformed into the A. avenae N1141 or K1 strain. After transformation, the bacterial cells were plated on LB agar plates containing 20 μg/ml kanamycin and incubated for 48 h at 30 °C. The colonies were inoculated into Pseudomonas F liquid medium containing 26% sucrose and incubated for 72 h at 30 °C to excise the plasmid by a second crossover event. The resulting bacteria were designated K1-178Ser/Ala, K1-183Ser/Ala, K1-212Ser/Ala, K1-351Thr/Ala, and K1-178Ser/Ala 183Ser/Ala, respectively. Mutants (designated K1-178,183,212,351Ala) with four substitutions (K1-S178A/S183A/S212A/T351A) were also generated using the same method (Table 1).
Electron Microscopy
For negative staining, 50-μl droplets of the sample solution were used. Samples were allowed to absorb for 1 min onto collodion-coated grids supported with carbon, which rendered the carbon surface hydrophilic. The grids were stained for 1 min with 1% (w/v) phosphotungstic acid (pH 6.9) (Merck) and washed with two drops of distilled water. Images were taken as digitized pictures with a Hitachi H-7100 transmission electron microscope operated at 80 kV using a 2K charge-coupled device camera (XR-41, Advanced Microscopy Technique Corp.)
RESULTS
Induction of Immune Responses by Recombinant Flagellins Produced in E. coli
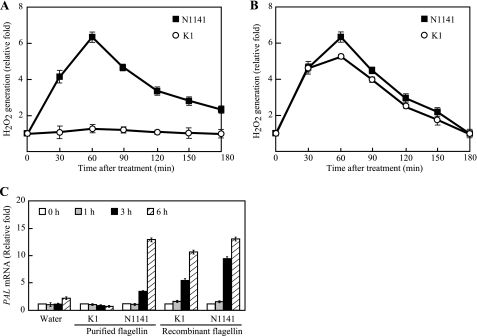
We previously reported that rice immune responses are induced by flagellin from the rice avirulent N1141 strain but not by flagellin from the rice virulent K1 and H8301 strains. Furthermore, the flagellins of the N1141 and K1 strains are composed of 492 amino acids with only 14 residues differing between these flagellins (21). To determine whether these differences in the amino acid sequences between the N1141 and K1 flagellins primarily cause the specific immune responses in rice cells, His-tagged N1141 and K1 flagellins were produced in E. coli and purified using HisTrap affinity chromatography. The MALDI-TOF mass spectrum of the purified recombinant N1141 and K1 flagellins showed that the recombinant N1141 flagellin had a molecular mass of 50,450 Da, whereas recombinant K1 flagellin had a mass of 50,310 Da (data not shown). The measured masses of the recombinant N1141 and K1 flagellins are highly similar to the calculated molecular masses (50,454 and 50,309 Da, respectively), suggesting that the N1141 and K1 flagellins are not modified. To clarify the role of the amino acids that differ between the N1141 and K1 flagellins in the specific induction of immune responses, H2O2 generation was examined as a readout for the plant immune responses using a luminol chemiluminescence assay (24). When N1141 flagellin was added to cultured rice cells, H2O2 was rapidly generated within 60 min after treatment, and the ratio of H2O2 generation gradually decreased until 3 h after treatment. In contrast, K1 flagellin did not cause a detectable increase in H2O2 generation until 3 h after treatment (Fig. 1A). When cultured rice cells were treated with the recombinant His-tagged N1141 flagellins, H2O2 was rapidly generated as observed with the purified N1141 flagellin. Interestingly, the recombinant His-tagged K1 flagellin also caused rapid H2O2 generation in cultured rice cells to the same degree as the recombinant N1141 flagellin (Fig. 1B).
FIGURE 1.
Induction of immune responses in cultured rice cells by flagellin from A. avenae. A, time course of H2O2 generation in cultured rice cells that were treated with flagellin purified from the avirulent N1141 strain (solid squares) or virulent K1 strain (open circles). The y axis represents the -fold change in H2O2 in cultured rice cells relative to the levels before flagellin treatment. B, time course of H2O2 generation in cultured rice cells treated with recombinant flagellin from the avirulent N1141 strain (solid squares) or virulent K1 strain (open circles) produced in E. coli. H2O2 was detected using a luminol chemiluminescence assay. The y axis represents the -fold change in H2O2 in cultured rice cells relative to the levels before flagellin treatment. C, PAL mRNA levels in cultured rice cells that were treated with flagellin purified from the N1141 or K1 strain and recombinant flagellin of the N1141 or K1 strain. The mRNA levels were calculated from the threshold point located in the log-linear range of RT-PCR. Standard samples with known template amounts were used to quantitate the PAL mRNA levels. The y axis represents the -fold change in PAL mRNA levels relative to the levels in water-treated cultured rice cells. White columns, 0 h after treatment; gray columns, 1 h after treatment; black columns, 3 h after treatment; hatched columns, 6 h after treatment. The error bars in all figures indicate the S.D. for five experiments.
We have reported that expression of PAL, which encodes phenylalanine ammonia-lyase and catalyzes the first step in the biosynthesis of lignin monomers and certain classes of phytoalexins, was increased upon the addition of purified N1141 flagellin but not purified K1 flagellin (20). To clarify whether the N1141 flagellin-specific induction of the PAL gene depends on the amino acid residues that are different between the N1141 and K1 flagellins, quantitative real time RT-PCR was performed using the purified and recombinant flagellins. Purified N1141 and K1 flagellins were added to cultured rice cells, and total RNA was extracted after 0, 1, 3, and 6 h. PAL transcripts were induced 1 h after inoculation with the flagellin purified from the N1141 strain, and the expression levels gradually increased up to 6 h, whereas PAL mRNA did not increase upon treatment with the flagellin purified from the K1 strain (Fig. 1C). In contrast, when the recombinant flagellins were used for this experiment, the PAL mRNA levels increased not only with the N1141 recombinant flagellin but also with the K1 recombinant flagellin. Thus, the increased PAL mRNA levels together with the comparable H2O2 generation showed that the specific induction of immune responses between N1141 and K1 flagellins did not depend on the amino acids that differ between these flagellins.
Identification of Flagellin Glycosylation Genes
Because the amino acid substitutions between N1141 and K1 flagellins did not regulate the induction specificity of the rice immune response, we assumed that post-translational modification(s) of these flagellins is involved in the specificity of the immune responses. We reported that flagellins of the A. avenae N1141 and K1 strains were glycosylated and that the glycans differed between N1141 flagellin and K1 flagellin (21). Therefore, we next clarified whether the glycosylation of flagellins in A. avenae affects the induction specificity of the immune response in rice cells.
The N1141 and K1 flagellin sequences showed no evidence of a classic eukaryotic N-linked sequence or the more recently defined prokaryotic N-linked consensus sequences, suggesting that the glycan was O-linked (26). The glycan was removed from the flagellins by chemical degradation or enzymatic digestion. However, these trials were not successful because the flagellin proteins were degraded or aggregated by these treatments (data not shown). It was reported that the glycosyltransferase gene within the flagellar gene operon of Pseudomonas aeruginosa or Pseudomonas syringae is necessary for flagellin glycosylation and that deleting this gene results in the production of non-glycosylated flagellin (27, 28). Therefore, we next identified the glycosyltransferase gene within the flagellar operon in the A. avenae N1141 and K1 strains. Genomic clones from DNA cosmid libraries of the N1141 and K1 strains were screened using the PCR product for each FlaA gene that encodes flagellin (21) as a DNA probe, and then the nucleotide sequences upstream of FlaA were determined. The sequence analysis of the region upstream of the N1141 and K1 strains showed that putative glycosyltransferase genes, designated NFgt and KFgt, were located near the flagellar motor gene MotA, which is located in the 1.5-kbp upstream region of FlaA (supplemental Fig. 2). NFgt contained an open reading frame of 4,077 bp that encoded a 1,359-amino acid protein with a calculated molecular weight of 150,768. KFgt is composed of 3,795 bp and is predicted to encode a protein of 1,265 amino acids with a calculated molecular weight of 139,780. The deduced amino acid sequences showed that the sequence identity between these N1141 and K1 glycosyltransferases is relatively low (61%).
Generation Fgt and FlaA Deletion Mutants
To examine whether the NFgt and KFgt genes are responsible for flagellin glycosylation in A. avenae, we generated Fgt deletion mutants and FlaA deletion mutants using homologous recombination. The regions up- and downstream of each gene were PCR-amplified from N1141 and K1 genomic DNA. The purified products were joined and then ligated into the pK18mobsacB plasmid. Isogenic A. avenae N1141 and K1 mutants were made in each corresponding pK18mobsacB plasmid. Two Fgt deletion mutants, designated NΔFgt (from N1141) and KΔFgt (from K1), and two FlaA deletion mutants, designated NΔFlaA and KΔFlaA, were generated.
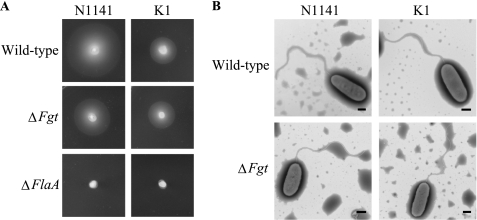
All mutant strains had normal viability and growth. The motility of the four mutants was examined based on a swimming assay on soft agar plates. For the NΔFlaA and KΔFlaA strains in which the FlaA gene was deleted, the cells were completely non-motile as demonstrated by the small, sharply delineated colonies that are typical of non-motile cells on soft agar (Fig. 2A). In contrast, the NΔFgt and KΔFgt strains had a diffuse spreading growth pattern that is characteristic of motile bacteria and that was similar to the parental strains (Fig. 2A). The morphological features of the NΔFgt and KΔFgt strains were observed by transmission electron microscopy using negative staining and compared with the parental strains. These were characteristic polar flagella on the NΔFgt and KΔFgt strains, and the length and diameter of the flagella on the NΔFgt and KΔFgt strains were very similar to those of the parental cells (Fig. 2B).
FIGURE 2.
Functions of FlaA and Fgt genes in A. avenae N1141 and K1 strains. A, swimming motility of the wild-type, ΔFgt, and ΔFlaA strains of A. avenae. Bacterial cell densities were adjusted to an A610 of 1.0, and 2-μl aliquots were inoculated on LB soft agar plates. Photographs were taken after 24 h at 30 °C. B, transmission electron micrograph of wild-type and ΔFgt strains of A. avenae N1141 and K1. The bars represent 500 nm.
Characterization of Deglycosylated Flagellins in NΔFgt and KΔFgt Strains
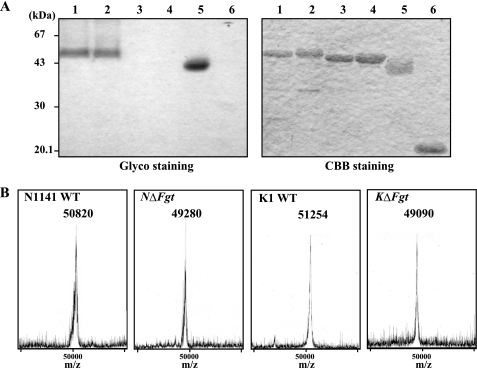
To analyze the effects of deleting the glycosyltransferase genes on the sugar chain of each flagellin, the flagellins were separated by SDS-PAGE and stained using a glycoside detection kit (Pierce). SDS-PAGE and subsequent Coomassie Brilliant Blue R-250 staining showed that the flagellin protein from the NΔFgt mutant migrated at a position that was ∼1.5 kDa smaller than the protein from the wild-type N1141 strain. Furthermore, the flagellin protein from KΔFgt was ∼2 kDa smaller than that of the flagellin protein from the wild-type K1 strain. When the flagellin proteins were separated by SDS-PAGE and stained with a glycoside detection kit, the N1141 and K1 wild-type flagellins and horseradish peroxidase (as a positive glycosylated protein control) were stained (Fig. 3A). In contrast, the NΔFgt and KΔFgt flagellins and the soybean trypsin inhibitor (as a non-glycosylated protein control) could not be detected using this staining method (Fig. 3A), suggesting that the flagellin proteins of the NΔFgt and KΔFgt strains were deglycosylated.
FIGURE 3.
Structural analysis of flagellin purified from N1141 wild-type, N1141 ΔFgt, K1 wild-type, and K1 ΔFgt strains of A. avenae. A, detection of sugar moieties in the N1141 wild-type, N1141 ΔFgt, K1 wild-type, and K1 ΔFgt flagellins of A. avenae. SDS-PAGE and Coomassie Brilliant Blue R-250 (CBB) (right) or glycoprotein (Glyco) staining (left) are shown. Lane 1, flagellin of the N1141 wild-type strain; lane 2, flagellin of K1 wild-type strain; lane 3, flagellin of the N1141 ΔFgt strain; lane 4, flagellin of the K1 ΔFgt strain; lane 5, horseradish peroxidase (positive control); lane 6, soybean trypsin inhibitor (negative control). Five micrograms of protein were loaded into each lane, and a prestained protein marker was used to identify the molecular weights. B, MALDI-TOF MS analysis of flagellins purified from the N1141 wild-type (N1141 WT), N1141 ΔFgt (NΔFgt), K1 wild-type (K1 WT), and K1 ΔFgt (KΔFgt) strains of A. avenae. The molecular ion peaks obtained by MALDI-TOF MS analyses showed that molecular weights of flagellins purified from the N1141 wild-type, N1141 ΔFgt, K1 wild-type, and K1 ΔFgt strains of A. avenae are ∼50,820, 49,280, 51,254, and 49,090, respectively.
To determine the molecular mass of the sugar chains in the flagellin proteins, MALDI-TOF MS analysis was performed. The mass spectrum of the N1141 wild-type flagellin showed that the molecular mass of the mature-type N1141 flagellin is 50,820 Da, which is greater than the calculated molecular mass by ∼1,600 Da. The molecular mass of K1 flagellin is 51,254 Da, which is also greater than the calculated molecular mass by ∼2,150 Da (Fig. 3B). Mass spectrometry analysis also showed that the molecular masses of the NΔFgt and KΔFgt flagellins are 49,280 and 49,090 Da, respectively, which are highly similar to the respective calculated masses (Fig. 3B). The mass spectrum data together with the mobility shift in the SDS-PAGE analysis revealed that no glycans are present on the flagellins from the NΔFgt and KΔFgt strains.
Function of Flagellin Glycosylation in Induction of Immune Responses in Rice
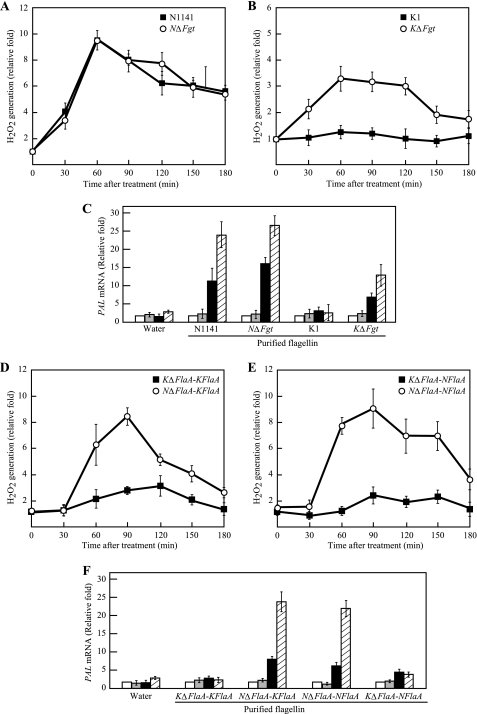
To clarify the specific induction mechanism by A. avenae flagellin in rice cells, the ability of the deglycosylated flagellins to induce H2O2 was measured. When the flagellin purified from the NΔFgt strain was added to cultured rice cells, H2O2 was rapidly generated within 60 min after treatment, and the ratio of H2O2 generation gradually decreased until 3 h after treatment. The same pattern of H2O2 generation was observed with the N1141 mature-type flagellin purified from the N1141 wild-type strain (Fig. 4A). Interestingly, when the cultured rice cells were treated with the deglycosylated flagellin from KΔFgt, H2O2 was rapidly generated, whereas the K1 mature-type flagellin purified from the K1 wild-type strain did not cause a detectable increase in H2O2 until 3 h after treatment (Fig. 4B). Additionally, the H2O2 patterns generated by the deglycosylated K1 flagellin and N1141 flagellin were consistent.
FIGURE 4.
Induction of immune responses in cultured rice cells by flagellins purified from N1141 wild-type, N1141 ΔFgt, K1 wild-type, and K1 ΔFgt strains of A. avenae. A, time course of H2O2 generation in cultured rice cells that were treated with flagellin purified from the N1141 wild-type (solid squares) or N1141 ΔFgt (open circles) strain. B, time course of H2O2 generation in cultured rice cells that were treated with flagellin purified from the K1 wild-type strain (solid squares) or K1 ΔFgt (open circles) strain. H2O2 was detected using a luminol chemiluminescence assay. C, PAL mRNA levels in cultured rice cells that were treated with flagellins purified from the N1141 wild-type, N1141 ΔFgt, K1 wild-type, and K1 ΔFgt strains of A. avenae. The mRNA levels were calculated from the threshold point located in the log-linear range of RT-PCR. Standard samples with known template amounts were used to quantitate the PAL mRNA levels. The y axis represents the -fold change relative to the PAL mRNA levels of water-treated cultured rice cells. White columns, 0 h after treatment; gray columns, 1 h after treatment; black columns, 3 h after treatment; hatched columns, 6 h after treatment. D, time course of H2O2 generation in cultured rice cells that were treated with K1-type flagellin purified from the KΔFlaA-KFlaA (KΔFlaA strain carrying the K1 FlaA expression vector) (solid squares) or NΔFlaA-KFlaA (NΔFlaA strain carrying the K1 FlaA expression vector) (open circles) strain. E, time course of H2O2 generation in cultured rice cells that were treated with N1141-type flagellins purified from the KΔFlaA-NFlaA (KΔFlaA strain carrying the N1141 FlaA expression vector) (solid squares) or NΔFlaA-NFlaA (NΔFlaA strain carrying the N1141 FlaA expression vector) (open circles) strain. F, PAL mRNA levels in cultured rice cells that were treated with flagellins purified from the KΔFlaA-KFlaA, NΔFlaA-KFlaA, NΔFlaA-NFlaA, and KΔFlaA-NFlaA strains of A. avenae. The mRNA levels were calculated from the threshold point located in the log-linear range of RT-PCR. Standard samples with known template amounts were used to quantitate the PAL mRNA levels. The y axis represents the -fold change relative to the PAL mRNA levels of water-treated cultured rice cells. White columns, 0 h after treatment; gray columns, 1 h after treatment; black columns, 3 h after treatment; hatched columns, 6 h after treatment. The error bars in all figures indicate the S.D. for five experiments.
Induction of PAL gene expression was also examined by quantitative real time RT-PCR (Fig. 4C). PAL transcripts were induced 1 h after treatment with N1141 flagellin and the deglycosylated N1141 flagellin, and these expression levels gradually increased up to 6 h after treatment. In contrast, the PAL mRNA levels did not increase upon treatment with K1 flagellin, whereas the K1 deglycosylated flagellin purified from KΔFgt induced PAL mRNA but to a lesser extent compared with the levels measured in response to N1141 flagellin (Fig. 4C). These data suggest that deglycosylation determines the ability of K1 flagellin to induce immune responses.
To clarify whether the characteristic glycan form and the attachment site in each bacterial strain of A. avenae are involved in the specific induction of rice immune responses, each N1141 FlaA and K1 FlaA expression vector was introduced into the NΔFlaA and KΔFlaA strains, and the flagellin was purified from each bacterial strain. The K1-type flagellin purified from the KΔFlaA-KFlaA strain did not induce H2O2 in the same manner as the K1 wild-type flagellin, whereas the K1-type flagellin purified from the NΔFlaA-KFlaA strain rapidly induced H2O2 that continued for 90 min after treatment (Fig. 4D). In contrast, when the N1141-type flagellin that was purified from the KΔFlaA-NFlaA strain was added to cultured rice cells, there was no observable H2O2, whereas the flagellin from the NΔFlaA-NFlaA strain caused rapid H2O2 generation in the same manner as the N1141 wild-type flagellin (Fig. 4E). Furthermore, PAL gene expression was also induced by flagellins purified from the NΔFlaA-NFlaA and NΔFlaA-KFlaA strains, whereas no induction of PAL mRNA was observed when cultured rice cells were treated with the flagellin purified from the KΔFlaA-KFlaA and KΔFlaA-NFlaA strains (Fig. 4F). These data together with the data of H2O2 induction clearly indicate that the characteristic glycan form or glycan attachment site in the K1 strain contributes to flagellin recognition by rice.
Determination of Glycan Attachment Site in K1 Flagellin
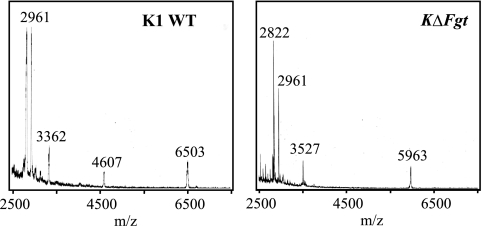
The induction specificity of the rice immune responses observed between the N1141 and K1 flagellins depended on the glycan form and attachment sites in K1 flagellin. Therefore, we next examined the exact glycan attachment sites and characterized the glycans in K1 flagellin. To determine the exact sites of glycan attachment, the flagellins purified from the K1 and KΔFgt strains were digested with trypsin, and the molecular mass of each digested peptide was measured by mass spectrometry. Three peaks were detected as specific fragments that differed between the K1 and KΔFgt strains. Three sharp peaks were observed at m/z 2,822, 3,527, and 5,963 in the KΔFgt flagellin digestion mixtures, whereas ion peaks of m/z 3,362, 4,607, and 6,503 were detected in K1 flagellin digestion mixtures (Fig. 5). A prediction analysis of tryptic peptides from KΔFgt flagellin revealed that the obtained ion peaks at m/z 2,822, 3,527, and 5,963 could be readily assigned as Thr206–Arg236 (expected molecular mass, 2,821 Da), Thr166–Lys205 (expected molecular mass, 3,526 Da), and Leu296–Arg358 (expected molecular mass, 5,962 Da), respectively. In contrast, three specific peaks in the tryptic K1 flagellin did not match any expected tryptic peptides, suggesting that a glycan was attached to the three fragments of K1 flagellin, Thr206–Arg236, Thr166–Lys205, and Leu296–Arg358. In each case, the molecular mass difference in each peak of the tryptic KΔFgt flagellin and corresponding peak for the tryptic K1 flagellin was ∼540 Da (Thr206–Arg236), 1,080 (Thr166–Lys205), and 540 (Leu296–Arg358). Because the molecular mass difference in the Thr166–Lys205 fragments between K1 and KΔFgt flagellin is a multiple of that of Thr206–Arg236 or Leu296–Arg358, the same two glycans may be present in the Thr166–Lys205 fragment of K1 flagellin. In addition, the 51,254-Da molecular mass of intact K1 flagellin determined by mass spectrometry was 2,164 Da larger than that predicted from the K1 flagellin sequence or that of KΔFgt (Fig. 3B), suggesting that glycans were attached to all three fragments of K1 flagellin.
FIGURE 5.
MALDI-TOF MS analysis of trypsin-digested K1 wild-type flagellin (left) and K1 deglycosylated flagellin (right). K1 WT, K1 wild-type flagellin; KΔFgt, K1 ΔFgt flagellin. Each flagellin was digested with trypsin at 37 °C, and the mass spectra of the mixed flagellin digested peptides were measured using a Voyager Workstation MALDI-TOF mass spectrometer (Applied Biosystems) in a linear, positive mode with sinapinic acid as the matrix.
Identification of Glycosylated Residues in K1 Flagellin of A. avenae
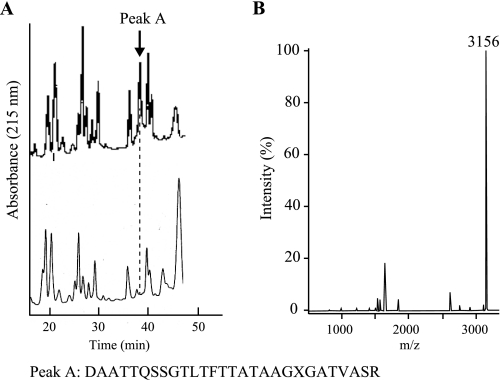
To determine the location of the attached glycan, purified flagellin proteins from the KΔFgt and K1 strains were digested with trypsin and aspartic N-peptidase and then analyzed by reverse-phase HPLC. As shown in Fig. 6A, a comparison of the retention times of the peptide fragments produced by the digestion revealed one specific peak (retention time of 38 min) only in the chromatogram for K1 flagellin. N-terminal sequencing analysis revealed that this fragment corresponded to Asp331–Arg358. In this sequencing analysis, the Thr351 residue was not identified due to incorrect retention times, suggesting that this threonine residue is likely modified (Fig. 6A). MALDI-TOF MS analysis showed that the molecular mass of the fragment is 3,156 Da, which is 540 Da larger than the mass predicted based on the fragment sequence (Fig. 6B). These data indicated that a glycan with a molecular weight of ∼540 may be attached to Thr351 in K1 flagellin.
FIGURE 6.
Detection of amino acids harboring glycan attachment site in flagellin from A. avenae K1 strain. A, HPLC chromatograms of a flagellin fragment derived from trypsin and aspartic N-peptidase digestion of each flagellin. After digesting K1 wild-type flagellin (upper chromatogram) and K1 ΔFgt flagellin (lower chromatogram) with trypsin and aspartic N-peptidase, the peptide fragments were detected by reverse-phase HPLC as described under “Experimental Procedures.” Peak A was detected only in the chromatogram for the K1 wild-type flagellin. The bottom amino acid sequence represents the amino acid sequence of peak A obtained with N-terminal sequencing analysis using a peptide sequencer (Applied Biosystems 492cLC). The X residue in the amino acid sequence for peak A (bottom) indicates an amino acid residue that was not identified in the N-terminal sequencing analysis. B, mass spectrum of peak A. The molecular mass ion 3,156 is 540 larger than that predicted from the peak A fragment.
In the HPLC analysis of the digested K1 and KΔFgt flagellins, we did not detect specific peaks with the exception of a peak consistent with Asp331–Arg358. Therefore, the other glycosylated residues were identified by performing site-directed mutagenesis of candidate glycosylated residues within the Thr166–Lys205 and Thr206–Arg236 fragments of K1 flagellin. A comparison of the amino acid sequences between K1 and N1141 flagellins showed that three amino acids (Ser178, Ser183, and Ser186) and one amino acid (Ser212) were substituted within the Thr166–Lys205 and Thr206–Arg236 fragments of K1 flagellin, respectively. Therefore, we assumed that these were primary candidates for glycosylated amino acid residues in K1 flagellin. Expression vectors encoding mutant flagellins in which the candidate serines were replaced with alanines were generated using the KOD-Plus mutagenesis kit and introduced into the K1 flagellin deletion mutant KΔFlaA. Each flagellin protein was purified from the corresponding mutant, and immunoblot analysis was performed using an anti-flagellin antibody. Monomeric flagellin proteins from single S178A, S183A, and S212A substitutions had a lower molecular mass than the K1 strain, whereas the molecular mass of the S186A mutant flagellin was not reduced (supplemental Fig. 1).
To confirm that the three serine residues in K1 flagellin were glycosylated, MALDI-TOF MS analyses were performed. The molecular masses of flagellins from the S178A, S183A, and S212A substituted mutants were ∼1,616 Da larger than that predicted based on K1 flagellin, whereas the molecular mass of the S186A mutant flagellin was the same as that of K1 flagellin (Table 2). The Thr351 residue that is predicted to be attached to a glycan with a molecular weight of 540 was also replaced with an alanine residue. Consistent with our expectations, the molecular mass of the T351A mutant flagellin was ∼540 Da lower than that of intact K1 flagellin (Table 2). These data suggest that the three serine residues at positions 178, 183, and 212 and the one threonine residue at position 351 in flagellin from the A. avenae K1 strain are glycosylated by glycans with molecular weights of approximately 540.
TABLE 2.
Molecular masses of intact flagellin (Ala2–Arg492) and peptide fragments (Thr166–Lys205, Thr206–Arg236, and Leu296–Arg358)
M, major molecular masses (in Da) measured by MALDI-TOF MS; P, molecular masses (in Da) predicted by deduced amino acid sequence.
| A. avenae | Intact (Ala2–Arg492) |
Δ1–2 | Fragment (Thr166–Lys205) |
Fragment (Thr206–Arg236) |
Fragment (Leu296–Arg358) |
Δ3–4, Δ5–6, Δ7–8 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| M1 [M + H]+ | P2 [M + H]+ | M3 [M + H]+ | P4 [M + H]+ | M5 [M + H]+ | P6 [M + H]+ | M7 [M + H]+ | P8 [M + H]+ | |||
| N1141 | ||||||||||
| Wild type | 50,820 | 49,258 | 1,562 | 4,668 | 3,568 | —a | — | 6,584 | 6,034 | 1,100, —, 550 |
| NΔFgt | 49,280 | 49,258 | 22 | 3,567 | 3,568 | — | — | 6,034 | 6,034 | −1, —, 0 |
| NΔFlaA-KFlaA | 51,370 | 49,113 | 2,257 | 4,626 | 3,526 | 3,370 | 2,820 | 6,512 | 5,962 | 1,100, 550, 550 |
| K1 | ||||||||||
| Wild type | 51,254 | 49,113 | 2,141 | 4,606 | 3,526 | 3,360 | 2,820 | 6,502 | 5,962 | 1,080, 540, 540 |
| KΔFgt | 49,090 | 49,113 | −23 | 3,526 | 3,526 | 2,820 | 2,820 | 5,962 | 5,962 | 0, 0, 0 |
| S178A | 50,711 | 49,097 | 1,614 | 4,050 | 3,510 | — | — | — | — | 540, —, — |
| S183A | 50,702 | 49,097 | 1,605 | 4,049 | 3,510 | — | — | — | — | 539, —, — |
| S212A | 50,670 | 49,097 | 1,573 | — | — | 2,804 | 2,804 | — | — | —, 0, — |
| T351A | 50,649 | 49,083 | 1,566 | — | — | — | — | 5,930 | 5,932 | —, —, −2 |
| S178A/S183A/S212A/T351A | 49,055 | 49,035 | 20 | 3,494 | 3,494 | 2,804 | 2,804 | 5,931 | 5,932 | 0, 0, −1 |
| KΔFlaA-NFlaA | 50,898 | 49,258 | 1,640 | 4,648 | 3,568 | — | — | 6,573 | 6,034 | 1080, —, 539 |
a —, measured molecular mass and predicted molecular mass are identical.
Induction of Immune Responses in Cultured Rice Cells by Flagellins with Mutated Glycan Attachment Site
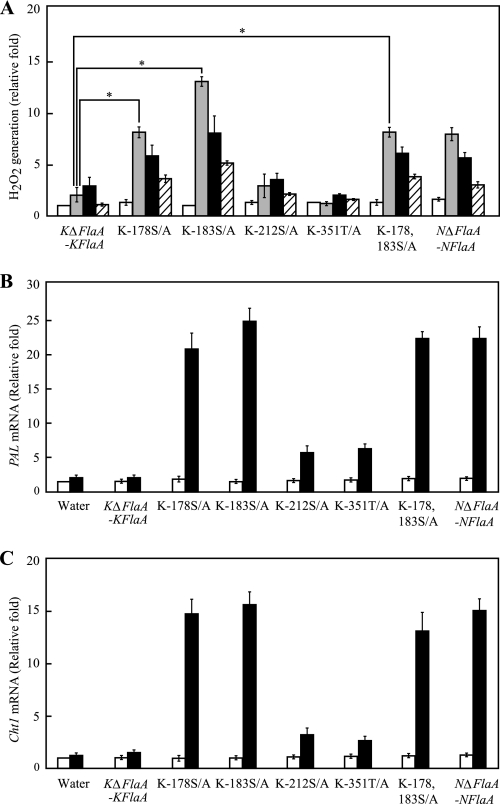
Cultured rice cells that were treated with the S178A and S183A K1 flagellins produced higher levels of H2O2 than cells treated with K1 flagellin from the KΔFlaA-KFlaA strain 1 h after treatment, and the ratio of H2O2 generation gradually decreased until 3 h after treatment. The H2O2 generated by the S178A and S183A K1 substituted flagellins was similar to that induced by N1141 flagellin, and the ability of the S178A and S183A K1 substituted flagellins to stimulate H2O2 production was higher than that of N1141 flagellin from the NΔFlaA-NFlaA strain. Interestingly, the S178A/S183A K1 double substituted flagellin also induced H2O2 generation, and the ratio of H2O2 generation was similar to that induced by S178A K1 substituted flagellin. In contrast, the S212A and T351A K1 flagellins did not induce remarkable H2O2 generation until 3 h after treatment, which was the same as K1 flagellin from the KΔFlaA-KFlaA strain (Fig. 7A).
FIGURE 7.
Induction of immune responses in cultured rice cells by several mutant flagellins. A, time course of H2O2 generation in cultured rice cells that were treated with several mutant flagellins. White columns, 0 h after treatment; gray columns, 1 h after treatment; black columns, 3 h after treatment; hatched columns, 6 h after treatment. The y axis represents the -fold change in H2O2 in cultured rice cells relative to the levels before flagellin treatment. Asterisks indicate a significant increase (t test; p < 0.05) in H2O2 generation with the amino acid-substituted flagellins. B, PAL mRNA levels in cultured rice cells that were treated with several mutant flagellins. The mRNA levels were calculated from the threshold point located in the log-linear range of RT-PCR. Standard samples with known template amounts were used to quantitate the PAL mRNA levels. The y axis represents the -fold change relative to the PAL mRNA levels of water-treated cultured rice cells. White columns, 0 h after treatment; black columns, 6 h after treatment. C, Cht-1 mRNA levels in cultured rice cells that were treated with several mutant flagellins. The mRNA levels were calculated from the threshold point located in the log-linear range of RT-PCR. Standard samples with known template amounts were used to quantitate the Cht-1 mRNA levels. The y axis represents the -fold change relative to the Cht-1 mRNA levels of water-treated cultured rice cells. White columns, 0 h after treatment; black columns, 6 h after treatment. The error bars indicate the S.D. for five experiments. KΔFlaA-KFlaA, K1-type flagellin purified from the KΔFlaA strain possessing the K1 FlaA expression vector; K-178S/A, S178A substituted flagellin; K-183S/A, S183A substituted K1 flagellin; K-212S/A, S212A substituted K1 flagellin; K-351T/A, T351A substituted K1 flagellin; K-178,183S/A, S178A/S183A double substituted flagellin and N1141 flagellin purified from the NΔFlaA strain carrying the N1141 FlaA expression vector.
Induction of PAL gene expression by the substituted flagellins was also examined by quantitative real time RT-PCR (Fig. 7B). Induction of PAL mRNA expression was observed 6 h after treatment with the S178A and S183A K1 flagellins and S178A/S183A K1 double substituted K1 flagellin, whereas significant induction of PAL mRNA was not detected at 6 h after treatment with the S212A and T351A K1 flagellins, which was the same as K1 flagellin from the KΔFlaA-KFlaA strain.
Cht-1 encodes the chitinase enzyme, which hydrolyzes the β-1,4-linkage between N-acetyl-d-glucosamine residues of chitin, and is expressed following N1141 flagellin infection (20). When cultured rice cells were treated with the S178A and S183A K1 flagellins and S178A/S183A K1 double substituted K1 flagellin, Cht-1 mRNA expression was induced 6 h after treatment. In contrast, the S212A and T351A K1 flagellins did not induce remarkable Cht-1 mRNA expression (Fig. 7C). These results showed that glycans at either Ser178 or Ser183 in K1 flagellin disrupt flagellin recognition in rice.
DISCUSSION
Protein glycosylation imparts novel physical properties and biological roles to both eukaryotic and prokaryotic proteins. Recently, glycoproteins from bacteria have received considerable attention, particularly glycoproteins in pathogenic species and those localized on the bacterial cell surface where they may interact with the host. In Gram-negative bacteria, examples of surface-associated glycoproteins include the pilins of P. aeruginosa and Neisseria spp. (29, 30), the adhesins TibA and Aida-1 of E. coli (31, 32) and HMW1 of Haemophilus influenza (33), and the flagellins of A. avenae and many other pathogenic bacteria (7, 34, 35). Although the full significance of the glycosylation of these proteins has not been determined, a number of reports have shown that these modifications are involved in virulence and motility. A glycosylation-defective mutant of P. syringae pv. tabaci retained its swimming ability but displayed defective swarming (36). The glycosylation of flagellin in several pathogens including Campylobacter jejuni, Helicobacter pylori, and Aeromonas caviae, which all colonize the gastrointestinal tract, is also involved in motility (37, 38). In addition, the flagellin glycans of P. aeruginosa and P. syringae pv. tabaci 6605 have been implicated in virulence (39, 40). We have shown that although flagellin from a K1 Fgt deletion mutant, KΔFgt, was deglycosylated the motility of the KΔFgt mutant was not affected. Furthermore, when rice cells were infected with the KΔFgt strain, this mutant produced a seedling disease characterized by the formation of brown stripes on the sheathes that was similar to the K1 wild-type strain, and both the KΔFgt and K1 wild-type strains had the same growth rate (supplemental Fig. 3). These data indicate that the flagellin glycan in A. avenae K1 strain does not influence virulence and motility. Thus, the role of the glycan in flagellin may be different in each bacterial strain, and the glycan in flagellin may play a pleiotropic role. Although the flagellin from the KΔFgt strain could induce the rapid immune response, such as H2O2 generation, the KΔFgt strain still caused disease in rice plant that was similar to the K1 wild-type strain. A similar observation was reported in the case of Arabidopsis FLS2 mutant. The pathogenic bacterium P. syringae pv. tomato DC3000 is a virulent strain to Arabidopsis and causes the symptom of disease. When P. syringae pv. tomato DC3000 was used to inoculate the surface of Arabidopsis mutated in FLS2, which is the receptor for flg22, susceptibility to this strain was increased. However, this increment of susceptibility to P. syringae pv. tomato DC3000 was only partial (41). Although the flagellin is an important pathogen-associated molecular pattern that induces several immune responses, the role of flagellin in determination of the host specificity between the plant and the pathogenic bacterial strain may be restrictive.
The bacterial flagellum is composed of a filament that is attached to a molecular motor. Flagellar filaments are composed of 11 protofilaments that wrap together to form the filament. Each protofilament is composed almost entirely of flagellin monomers that consist of four linearly connected domains, D0, D1, D2, and D3. The core D0 and D1 domains are the most highly conserved and are responsible for filament assembly. The flagellar filaments must be properly assembled for bacterial motility and polymorphisms. The middle D2 domain may affect the stability of the filament shape, and the central domains (D3) in adjacent filament subunits are not connected to each other. The D2 and D3 domains correspond with the hypervariable region located on the surface of the flagellin filament. Several lines of evidence indicate that the exposed D3 domain contains the major epitopes of the H antigen. More recent studies also suggest that Toll-like receptor 5 (TLR5), which is involved in inducing the innate immune system upon recognition of flagellin as a pathogen-associated molecular pattern, specifically recognizes the flagellin D1 domain. When the glycosylated Ser178 and Ser183 residues of the K1 flagellin of A. avenae were changed to alanine, both substituted flagellins induced immune responses in rice. The remaining S212A and T351A K1 mutant flagellins did not induce plant immune responses. The Ser178 and Ser183 residues are located within the N-terminal D2 domain in K1 flagellin. These data indicate that the glycan moieties attached to the Ser178 or Ser183 residue in the D2 domain may be involved in flagellin recognition in rice. We demonstrated that the C-terminal D2 and D1 regions of flagellin in the A. avenae N1141 strain induced immune responses in cultured rice cells, whereas flagellin fragments containing the middle or N-terminal region did not induce immune responses (21). Because the D2 domain contributes to the stability of the filament shape and flagellar structure, deglycosylation of the two serine residues within the N-terminal D2 domain may cause structural changes and denudation of the epitope within the C-terminal D2 and D1 domains. Therefore, it is important to identify the detailed flagellin region recognized by rice plants and to clarify the effects of glycans on the tertiary structure of flagellin.
A structural characterization of flagellin glycosylation for a number of bacterial species has demonstrated that the glycan is linked to the protein backbone through an O-linkage to serine/threonine in all cases. However, a more recent study showed that the flagellin structural proteins FlaA, -B1, -B2, and -B3 and the S-layer protein of Methanococcus voltae are uniquely modified with a novel N-linked trisaccharide (34). The prokaryotic primary consensus amino acid sequence for N-glycosylation is (D/E)YNX(S/T) where X and Y can be any amino acid except proline (26). In contrast, the distinct consensus sequence for O-glycosylation is still unknown. The amino acid sequence of flagellin in the A. avenae K1 strain contains 35 asparagine residues, but there was no apparent consensus sequence for N-glycosylation, indicating that K1 flagellin is modified by an O-linked glycan. The studies have revealed significant diversity in the composition of the attached glycans. The flagellins of C. jejuni and H. pylori are glycosylated with pseudaminic acid (5,7-diacetamideo-3,5,7,9,-tetradeoxy-l-glycero-l-mannononulosonic acid) and related derivatives, whereas Listeria monocytogenes flagellin is glycosylated with GlcNAc, and P. aeruginosa type-a flagellin is glycosylated with a rhamnose-linked complex glycan (37, 42–44). More recently, flagellin of the plant pathogenic bacteria P. syringae pv. tabaci was also shown to be a glycoprotein that contains an O-linked trisaccharide composed of rhamnosyl and 4,6-dedeoxy-4-(3-hydroxybutanamido)-2-O-methylglucosyl residues (45). An analysis of the molecular mass of flagellins from K1 wild-type and KΔFgt mutant strains showed that Ser178, Ser183, Ser212, and Thr351 are glycosylated by an ∼540-Da glycan (Fig. 7). Because the molecular masses of the glycans in flagellins from P. syringae pv. tabaci and the K1 strain of A. avenae were accurately predicted (538 and 540 Da, respectively), the glycan moiety of K1 flagellin may be a derivative of the glycan of P. syringae pv. tabaci.
In this study, we demonstrated that the N1141-type flagellin produced by the KΔFlaA-NFlaA strain (N1141 FlaA expression vector-possessing KΔFlaA strain) contained a glycan of ∼1,600 Da, which is the nearly same as that of the N1141 flagellin produced by the N1141 wild-type strain. Similarly, the molecular weight of the glycan attached to the K-type flagellin from the NΔFlaA-KFlaA strain (K1 FlaA expression vector-possessing NΔFlaA strain) is nearly the same as that of flagellin from the K1 wild-type strain (Table 2). An amino acid sequence analysis of glycosyltransferases that catalyze the transfer of the glycan unit to serine or threonine residues in flagellin showed that the glycosyltransferases from the K1 and N1141 strains have comparatively low identity. Furthermore, NFgt contained an open reading frame of 4,077 bp that encoded a 1,359-amino acid protein, whereas KFgt is composed of 3,795 bp with 1,265 predicted amino acids. These data indicated that the glycosyltransferases of the N1141 and K1 strains catalyze the formation of the glycan linkage that is dependent on the flagellin amino acid sequence even though there are structural differences between the N1141 and K1 glycosyltransferases. Interestingly, mass spectrometry analysis using flagellins from the NΔFlaA-KFlaA, KΔFlaA-NFlaA, and wild-type strains revealed that the glycan structures in the N1141 and K1 flagellins are different because the molecular weight of the glycan in K1 flagellin was predicted to be 540, whereas the molecular weight of the glycan in N1141 flagellin was estimated to be 550. The K-type flagellin purified from the NΔFlaA-KFlaA strain induced immune responses, such as H2O2 generation, whereas the flagellin from the KΔFlaA-KFlaA strain did not induce H2O2 to the same degree as the K1 wild-type flagellin in Fig. 4E. These data clearly indicate that the glycan moiety linked by the K1 glycosyltransferase disrupts flagellin recognition by rice that causes the induction of immune responses. In this respect, we conclude that O-glycosylation of flagellin by the glycosyltransferase in the K1 strain contributes to recognition. Identifying the glycan structure associated with flagellin in the A. avenae K1 strain will be important to further understand how flagellin is recognized by rice.
Supplementary Material
Acknowledgments
We thank Dr. Mitsuru Yoshida for helpful discussions and Hiromi Morii for excellent technical support.
This work was supported in part by Grant-in-aid for Scientific Research (B) 21380073 from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Grant 500107 from the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- flg22
- conserved N-terminal domain of flagellin that consists of a 22-amino acid peptide
- FLS2
- flagellin sensing 2
- PAL
- phenylalanine ammonia-lyase.
REFERENCES
- 1. Schwessinger B., Zipfel C. (2008) Curr. Opin. Plant Biol. 11, 389–395 [DOI] [PubMed] [Google Scholar]
- 2. Boller T., Felix G. (2009) Annu. Rev. Plant Biol. 60, 379–406 [DOI] [PubMed] [Google Scholar]
- 3. Lacombe S., Rougon-Cardoso A., Sherwood E., Peeters N., Dahlbeck D., van Esse H. P., Smoker M., Rallapalli G., Thomma B. P., Staskawicz B., Jones J. D., Zipfel C. (2010) Nat. Biotechnol. 28, 365–369 [DOI] [PubMed] [Google Scholar]
- 4. Jones J. D., Dangl J. L. (2006) Nature 444, 323–329 [DOI] [PubMed] [Google Scholar]
- 5. Dodds P. N., Lawrence G. J., Catanzariti A. M., Teh T., Wang C. I., Ayliffe M. A., Kobe B., Ellis J. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 8888–8893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ade J., DeYoung B. J., Golstein C., Innes R. W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2531–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Logan S. M., Hui J. P., Vinogradov E., Aubry A. J., Melanson J. E., Kelly J. F., Nothaft H., Soo E. C. (2009) FEBS J. 276, 1014–1023 [DOI] [PubMed] [Google Scholar]
- 8. Klarzynski O., Plesse B., Joubert J. M., Yvin J. C., Kopp M., Kloareg B., Fritig B. (2000) Plant Physiol. 124, 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaku H., Nishizawa Y., Ishii-Minami N., Akimoto-Tomiyama C., Dohmae N., Takio K., Minami E., Shibuya N. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 11086–11091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laquitaine L., Gomès E., François J., Marchive C., Pascal S., Hamdi S., Atanassova R., Delrot S., Coutos-Thévenot P. (2006) Mol. Plant Microbe Interact. 19, 1103–1112 [DOI] [PubMed] [Google Scholar]
- 11. Silipo A., Molinaro A., Sturiale L., Dow J. M., Erbs G., Lanzetta R., Newman M. A., Parrilli M. (2005) J. Biol. Chem. 280, 33660–33668 [DOI] [PubMed] [Google Scholar]
- 12. Felix G., Duran J. D., Volko S., Boller T. (1999) Plant J. 18, 265–276 [DOI] [PubMed] [Google Scholar]
- 13. Kunze G., Zipfel C., Robatzek S., Niehaus K., Boller T., Felix G. (2004) Plant Cell 16, 3496–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gómez-Gómez L., Boller T. (2000) Mol. Cell 5, 1003–1011 [DOI] [PubMed] [Google Scholar]
- 15. Chinchilla D., Bauer Z., Regenass M., Boller T., Felix G. (2006) Plant Cell 18, 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kadota I., Mizuno A., Nishiyama K. (1996) Ann. Phytopathol. Soc. Jpn. 62, 425–428 [Google Scholar]
- 17. Che F. S., Iwano M., Tanaka N., Takayama S., Minami E., Shibuya N., Kadota I., Isogai A. (1999) Plant Cell Physiol. 40, 1036–1045 [Google Scholar]
- 18. Tanaka N., Nakajima Y., Kaneda T., Takayama S., Che F. S., Isogai A. (2001) Plant Biotech. 18, 295–299 [Google Scholar]
- 19. Iwano M., Che F. S., Goto K., Tanaka N., Takayama S., Isogai A. (2002) Mol. Plant Pathol. 3, 1–8 [DOI] [PubMed] [Google Scholar]
- 20. Tanaka N., Che F. S., Watanabe N., Fujiwara S., Takayama S., Isogai A. (2003) Mol. Plant Microbe Interact. 16, 422–428 [DOI] [PubMed] [Google Scholar]
- 21. Che F. S., Nakajima Y., Tanaka N., Iwano M., Yoshida T., Takayama S., Kadota I., Isogai A. (2000) J. Biol. Chem. 275, 32347–32356 [DOI] [PubMed] [Google Scholar]
- 22. Takai R., Isogai A., Takayama S., Che F. S. (2008) Mol. Plant Microbe Interact. 21, 1635–1642 [DOI] [PubMed] [Google Scholar]
- 23. Baba A., Hasezawa S., Syono K. (1986) Plant Cell Physiol. 27, 463–472 [Google Scholar]
- 24. Schwacke R., Hager A. (1992) Planta 187, 136–141 [DOI] [PubMed] [Google Scholar]
- 25. Schäfer A., Tauch A., Jäger W., Kalinowski J., Thierbach G., Pühler A. (1994) Gene 145, 69–73 [DOI] [PubMed] [Google Scholar]
- 26. Kowarik M., Young N. M., Numao S., Schulz B. L., Hug I., Callewaert N., Mills D. C., Watson D. C., Hernandez M., Kelly J. F., Wacker M., Aebi M. (2006) EMBO J. 25, 1957–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arora S. K., Bangera M., Lory S., Ramphal R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 9342–9347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeuchi K., Taguchi F., Inagaki Y., Toyoda K., Shiraishi T., Ichinose Y. (2003) J. Bacteriol. 185, 6658–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qutyan M., Henkel M., Horzempa J., Quinn M., Castric P. (2010) J. Bacteriol. 192, 5972–5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chamot-Rooke J., Rousseau B., Lanternier F., Mikaty G., Mairey E., Malosse C., Bouchoux G., Pelicic V., Camoin L., Nassif X., Duménil G. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherlock O., Vejborg R. M., Klemm P. (2005) Infect. Immun. 73, 1954–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berthiaume F., Leblond M. F., Harel J., Mourez M. (2010) FEMS Microbiol. Lett. 311, 176–184 [DOI] [PubMed] [Google Scholar]
- 33. Grass S., Lichti C. F., Townsend R. R., Gross J., St Geme J. W., 3rd (2010) PLoS Pathog. 6, e1000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voisin S., Houliston R. S., Kelly J., Brisson J. R., Watson D., Bardy S. L., Jarrell K. F., Logan S. M. (2005) J. Biol. Chem. 280, 16586–16593 [DOI] [PubMed] [Google Scholar]
- 35. Verma A., Schirm M., Arora S. K., Thibault P., Logan S. M., Ramphal R. (2006) J. Bacteriol. 188, 4395–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Taguchi F., Takeuchi K., Katoh E., Murata K., Suzuki T., Marutani M., Kawasaki T., Eguchi M., Katoh S., Kaku H., Yasuda C., Inagaki Y., Toyoda K., Shiraishi T., Ichinose Y. (2006) Cell Microbiol. 8, 923–938 [DOI] [PubMed] [Google Scholar]
- 37. Schirm M., Soo E. C., Aubry A. J., Austin J., Thibault P., Logan S. M. (2003) Mol. Microbiol. 48, 1579–1592 [DOI] [PubMed] [Google Scholar]
- 38. Ewing C. P., Andreishcheva E., Guerry P. (2009) J. Bacteriol. 191, 7086–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verma A., Arora S. K., Kuravi S. K., Ramphal R. (2005) Infect. Immun. 73, 8237–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taguchi F., Yamamoto M., Ohnishi-Kameyama M., Iwaki M., Yoshida M., Ishii T., Konishi T., Ichinose Y. (2010) Microbiology 156, 72–80 [DOI] [PubMed] [Google Scholar]
- 41. Forsyth A., Mansfield J. W., Grabov N., de Torres M., Sinapidou E., Grant M. R. (2010) Mol. Plant Microbe Interact. 23, 1545–1552 [DOI] [PubMed] [Google Scholar]
- 42. Thibault P., Logan S. M., Kelly J. F., Brisson J. R., Ewing C. P., Trust T. J., Guerry P. (2001) J. Biol. Chem. 276, 34862–34870 [DOI] [PubMed] [Google Scholar]
- 43. Schirm M., Kalmokoff M., Aubry A., Thibault P., Sandoz M., Logan S. M. (2004) J. Bacteriol. 186, 6721–6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schirm M., Arora S. K., Verma A., Vinogradov E., Thibault P., Ramphal R., Logan S. M. (2004) J. Bacteriol. 186, 2523–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeuchi K., Ono H., Yoshida M., Ishii T., Katoh E., Taguchi F., Miki R., Murata K., Kaku H., Ichinose Y. (2007) J. Bacteriol. 189, 6945–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.