Abstract
To identify molecules that play roles in the clearance of apoptotic cells by Drosophila phagocytes, we examined a series of monoclonal antibodies raised against larval hemocytes for effects on phagocytosis in vitro. One antibody that inhibited phagocytosis recognized terribly reduced optic lobes (Trol), a core protein of the perlecan-type proteoglycan, and the level of phagocytosis in embryos of a Trol-lacking fly line was lower than in a control line. The treatment of a hemocyte cell line with a recombinant Trol protein containing the amino acid sequence RGD augmented the phosphorylation of focal adhesion kinase, a hallmark of integrin activation. A loss of integrin βν, one of the two β subunits of Drosophila integrin, brought about a reduction in the level of apoptotic cell clearance in embryos. The presence of integrin βν at the surface of embryonic hemocytes was confirmed, and forced expression of integrin βν in hemocytes of an integrin βν-lacking fly line recovered the defective phenotype of phagocytosis. Finally, the level of phagocytosis in a fly line that lacks both integrin βν and Draper, another receptor required for the phagocytosis of apoptotic cells, was lower than that in a fly line lacking either protein. We suggest that integrin βν serves as a phagocytosis receptor responsible for the clearance of apoptotic cells in Drosophila, independent of Draper.
Keywords: Apoptosis, Cell Surface Receptor, Drosophila, Drosophila Genetics, Integrin, Phagocytosis, Signal Transduction
Introduction
Phagocytic elimination of dead or dying cells plays an important role in animal development and tissue homeostasis (1–3). Phagocytes recognize target cells undergoing apoptosis through the specific binding of phagocytosis receptors to phagocytosis markers or eat-me signals (4, 5). It is therefore essential to identify such molecules to fully understand the mechanism and consequences of this biological event. There are two partly overlapping signaling pathways for the induction of corpse clearance in the nematode Caenorhabditis elegans (6–9), suggesting the presence of two distinct phagocytosis receptors. One receptor is CED-1, a single-path membrane protein with atypical EGF-like repeats in its extracellular region (10). There are counterparts of CED-1 in other species (11), Draper in Drosophila (12, 13), Jedi in mice (14), and MEGF10 in humans (15), the involvement of which in the phagocytosis of apoptotic cells has been reported. However, the other receptor presumably conserved among species remains to be identified. Recently, two membrane proteins, Frizzled (16) and INA-1 (17), were reported to be involved in phagocytosis in C. elegans, and they are strong candidates for the “second” receptor.
We and other investigators previously showed that Draper, a Drosophila homologue of CED-1, is responsible for the phagocytosis of apoptotic cells by hemocytes and glia (12, 13). A loss of Draper expression decreased the level of phagocytosis in embryos by only about one-third (18), suggesting the existence of another mechanism of phagocytosis, presumably one involving the second receptor. A pioneer study of Franc et al. (19, 20) has identified a phagocytosis receptor called Croquemort, but this receptor has no structural similarity to Frizzled or INA-1. To search for the second receptor in Drosophila, we decided to employ a comprehensive strategy. In the present study, we screened a series of monoclonal antibodies that had been raised against larval hemocytes for effects on phagocytosis and found integrin as a likely candidate.
EXPERIMENTAL PROCEDURES
Antibodies
The generation of the antibody recognizing Croquemort, a member of the CD36 superfamily expressed specifically at the surface of Drosophila hemocytes (19, 20), was reported previously (13), and it was used to identify hemocytes in dispersed embryonic cells. The monoclonal antibodies raised against Drosophila larval hemocytes were generated as described previously (21). Briefly, BALB/c mice were immunized with hemocytes of late third instar larvae, and spleen cells were fused with SP2/O myeloma cells. Culture supernatants of the resulting hybridoma were immunochemically screened for the binding to larval hemocytes, and the selected hybridomas were subcloned. The anti-integrin βν antibodies were raised by immunizing rats with an extracellular region (amino acid positions 650–722 with the amino terminus numbered 1) and intracellular region (positions 753–799) of integrin βν that had been expressed in Escherichia coli as proteins fused to GST and purified to homogeneity and used for immunocytochemistry and Western blotting, respectively. The antigen specificity of these two anti-integrin βν antibodies was confirmed (supplemental Fig. 1, A and B). The antibody against FAK56, a Drosophila counterpart of mammalian focal adhesion kinase (FAK),2 was produced by immunizing rats with a portion of FAK56 (positions 881–1200) that had been expressed in E. coli as a GST-fused protein and purified to homogeneity. The anti-phosphorylated (at tyrosine 397) human FAK polyclonal antibody was purchased from Abcam. The antigen specificity of anti-FAK56 and anti-phospho-FAK antibodies was confirmed (supplemental Figs. 1C and 2). The anti-GST monoclonal antibody was purchased from Millipore.
Fly Stocks and Cell Culture
The following fly lines were used in this study: w1118 (Bloomington Drosophila Stock Center, Indiana University, Bloomington, IN), trolnull (22), mys1 (23), mysXG43 (23), betaInt-nu1 (24), betaInt-nu2 (24), srpHemo-GAL4 UAS-srcEGFP (25), drprΔ5 (12), UAS-Crk-IR (Transformant ID 19061, Vienna Drosophila RNAi Center, Vienna, Austria), UAS-Crk-IR (Transformant ID 106498, Vienna Drosophila RNAi Center), UAS-mbc-IR (Transformant ID 16044, Vienna Drosophila RNAi Center), and Act5C-GAL4/CyO (Drosophila Genetic Resource Center (DGRC) number 107727, DGRC, Kyoto, Japan). We established fly lines containing an extra betaInt-nu to be expressed with the GAL4-UAS system using the entire coding region of betaInt-nu cDNA obtained from w1118 and the vector pUAST (26), and one line carrying the transgene on the third chromosome was intercrossed with the fly lines betaInt-nu2 and/or srpHemo-GAL4 UAS-srcEGFP (for hemocyte-specific expression) and used in the experiments. Other fly lines used were generated through mating of the existing lines. Genotypes of the fly lines analyzed are shown in the corresponding figure legends. The cell lines l(2)mbn, established from larval hemocytes, and embryonic-cell derived S2 were maintained at 25 °C with Schneider's Drosophila medium (Invitrogen) as described previously (13). l(2)mbn cells were incubated with 20-hydroxyecdysone (Sigma-Aldrich) (1 μm) for 48 h before being used in an assay for phagocytosis. To induce apoptosis, S2 cells were incubated in the presence of cycloheximide (Sigma-Aldrich) (1.5 μg/ml) for 24 h as described previously (13).
Assays for Phagocytosis
Phagocytosis reactions in vitro with l(2)mbn cells as phagocytes and S2 cells, which had been treated with cycloheximide and surface-labeled with biotin, as targets were carried out at 25 °C for 2 h as described previously (13). To examine the effect of the monoclonal antibodies, 0.2 ml of the culture supernatant of hybridoma cells (RPMI 1640 medium containing 5% (v/v) FBS) was dialyzed against PBS, mixed with 0.1 ml of the medium, and added to the mixture of phagocytes and target cells. An RNAi experiment in vitro was conducted essentially as described previously (13). Double-stranded RNA corresponding to the region between nucleotide positions 5684 and 6203 (with the transcription start site numbered 1) of terribly reduced optic lobes (Trol) mRNA (transcript variant C) or the region between nucleotide positions 1322 and 2463 (with the transcription start site numbered 1) of Draper mRNA was included in the phagocytosis reaction at 25–50 μg/ml. An analysis of phagocytosis with dispersed embryonic cells was conducted according to the procedures described in our previous study with modifications (13); results obtained in this assay for phagocytosis were shown to be consistent with those from the analysis of whole embryos (18). Drosophila embryos were dechorionated, and those at stage 16 were collected. About 50 embryos were homogenized in the presence of collagenase (2.5% (w/v)) and treated with trypsin (2.5% (w/v)), and the homogenates were filtrated through a membrane (BD Falcon cell strainer, BD Biosciences). Cells recovered in the filtrates were mounted on silane-coated glass slides, treated successively with paraformaldehyde, methanol, and Triton X-100, and incubated with swine serum (5% (v/v)) for blocking. The fixed and blocked embryonic cells were cytochemically analyzed with the anti-Croquemort antibody to identify hemocytes and by TUNEL (Millipore) to identify apoptotic cells, and cells positive for both reactions were considered embryonic hemocytes that had phagocytosed apoptotic cells. A microscopic field containing 100 or more embryonic cells that were positive for Croquemort expression was examined for the number of TUNEL-stained cells, and a field containing 100 or more total embryonic cells was examined for the ratios of Croquemort-expressing hemocytes and TUNEL-positive apoptotic cells. A mean of the results with five microscopic fields was obtained in each experiment.
Immunocytochemistry
For immunocytochemical detection of Trol at the surface of ecdysone-treated l(2)mbn cells, cells smeared on silane-coated glass slides were incubated with purified #16 immunoglobulin or normal mouse IgG (50 μg/ml) on ice for 45 min, washed with PBS containing 0.1% (w/v) BSA, and reacted with biotin-labeled anti-mouse IgG antibody (Zymed Laboratories Inc.). The samples were washed, supplemented with FITC-conjugated avidin D (Vector Laboratories), washed again, and examined by fluorescence/phase-contrast microscopy. For the detection of GST-fused Trol proteins bound to the surface of l(2)mbn cells, cells were incubated with GST-fused proteins (5 μm) at 4 °C for 1 h, washed, and incubated with PBS containing 0.1% BSA. The cells were then successively treated with anti-GST antibody and biotin-labeled secondary antibody, washed, treated with Alexa Fluor 488-labeled streptavidin (Molecular Probes), and examined by fluorescence/phase-contrast microscopy. For the detection of integrin βν, l(2)mbn cells or dispersed cells of stage-16 embryos were smeared on silane-coated glass slides, incubated with 1% (w/v) blocking reagent (Roche Applied Science), reacted with the anti-integrin βν antibody (rat antiserum) or preimmune rat serum on ice for 15 min, and washed with PBS. They were then reacted with a biotin-labeled anti-rat IgG antibody (Vector Laboratories), washed with PBS, supplemented with streptavidin-conjugated Alexa Fluor 546 (Molecular Probes), and washed. The final samples were examined by fluorescence/phase-contrast microscopy.
Assay for Trol-induced Phosphorylation of FAK56
Recombinant Trol proteins corresponding to a portion of Trol (amino acid positions 1948–2147 or 2846–3035) were expressed in E. coli as a protein fused to GST and purified to homogeneity. PBS containing a Trol protein (0.2 μm) or GST used as a negative control was incubated in a culture container at 4 °C overnight. The dishes were washed with PBS, incubated with 2% BSA at 37 °C for 2 h, and washed with PBS. l(2)mbn cells that had been maintained with an FBS-free medium for 2 h were added to the protein-coated dishes, incubated at 25 °C for 2 h, and lysed with buffer consisting of 10 mm Tris-HCl (pH 7.5), 1% (v/v) Nonidet P-40, 0.1% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 0.15 m NaCl, 1 mm EDTA, 1% (v/v) protease inhibitor mixture (Sigma-Aldrich), and 1% (v/v) phosphatase inhibitor mixture (Sigma-Aldrich). The lysates were analyzed by Western blotting with the anti-FAK56 antibody or anti-phospho-FAK antibody.
Other Methods
Semiquantitative RT-PCR was done according to a standard protocol using total RNA extracted from l(2)mbn cells as a template in RT. We optimized conditions for PCR, i.e. annealing temperature, cycles for DNA synthesis, and the amount of cDNA template, so that amplification occurred at an exponential phase. Western blotting of lysates of cultured cells was done as described previously (13). Flies at various developmental stages were homogenized in a buffer consisting of 50 mm Tris-HCl (pH 7.5), 0.15 m NaCl, 5 mm MgCl2, 0.1% (v/v) Triton X-100, 0.1% Nonidet P-40, and 10% (v/v) glycerol, and the resulting lysates were analyzed by Western blotting as for cultured cells. For the analysis of the growth rate of Drosophila, 100–200 embryos in a vial were developed at 24 °C, and the number of adult flies that emerged was determined each day.
Data Processing and Statistical Analysis
Data from quantitative analyses are expressed as the mean ± S.D. of the results from at least three independent experiments, unless otherwise stated in the text. Other data are representative of at least three independent experiments that yielded similar results. Statistical analyses were performed using Student's t test. Values of p < 0.05 were considered significant and are indicated in the figures.
RESULTS
Identification of Trol Required for Phagocytosis of Apoptotic Cells by Drosophila Phagocytes
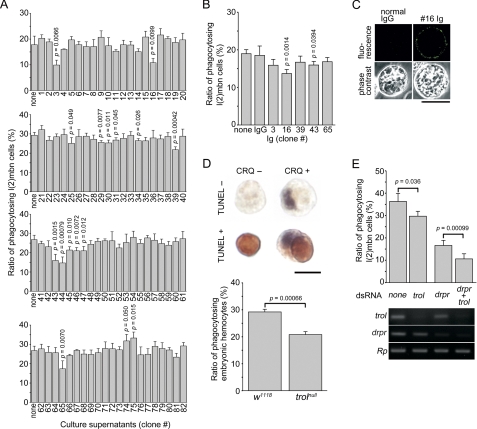
To identify molecules required for Drosophila hemocytes to engulf apoptotic cells, we tested a series of monoclonal antibodies, which had been generated by immunizing mice with larval hemocytes, for effects on the phagocytosis of apoptotic S2 cells by l(2)mbn cells, a cell line derived from larval hemocytes. Of 82 hybridoma clones (operationally renumbered 1–82) analyzed, 16 were found to produce culture supernatants that had inhibitory or stimulatory effect on phagocytosis with statistical significance, whereas the others did not influence phagocytosis (Fig. 1A). We then examined immunoglobulin prepared from the culture supernatants of five hybridoma clones that had a more prominent effect than the others and found that the #16 and #43 antibodies inhibited phagocytosis (Fig. 1B). We further characterized the #16 antibody because the inhibition provided by the #43 antibody was only marginal.
FIGURE 1.
Identification of Trol that plays a role in the phagocytosis of apoptotic cells by Drosophila hemocytes. A, an assay for phagocytosis was conducted with ecdysone-treated l(2)mbn cells as phagocytes and apoptotic S2 cells as targets in the presence and absence of culture supernatants of the indicated hybridoma clones producing monoclonal antibodies raised against larval hemocytes. The ratio of l(2)mbn cells that had accomplished phagocytosis is shown. Significance is versus none. Data from one experiment are presented (S.D. are from data with four microscopic fields). B, the same assay as in A was conducted in the presence of immunoglobulin prepared from culture supernatants of the indicated hybridoma clones or normal mouse IgG (2 μg/ml). The ratio of l(2)mbn cells that had accomplished phagocytosis is shown. Significance is versus control IgG. Data from one of two independent experiments with similar results are presented (S.D. are from data with six microscopic fields). C, ecdysone-treated l(2)mbn cells were subjected to immunocytochemistry with the #16 immunoglobulin or normal rat IgG as a negative control under membrane-nonpermeabilizing conditions. Phase-contrast and fluorescence micrographs of the same fields are shown. Scale bar, 20 μm. D, phagocytosis of apoptotic cells was examined with dispersed embryonic cells of the indicated fly lines. Embryonic cells were cytochemically analyzed with an anti-Croquemort (CRQ) antibody (signals seen in purple) and by TUNEL (signals seen in brown) (top). Scale bar, 5 μm. The ratio of hemocytes that had accomplished phagocytosis is shown (bottom). The genotype of trolnull is trolnull FRT101/Y. E, l(2)mbn cells, which had been treated with double-stranded RNA (dsRNA) containing sequences of mRNA of the indicated genes, were analyzed for the phagocytosis of apoptotic S2 cells as in A (top) as well as for the level of mRNA of the indicated genes by RT-PCR (bottom). In the top panel, data from one of three independent experiments with similar results are presented (S.D. are from data with six microscopic fields). drpr, draper; Rp, gene coding for ribosomal protein S15Ab.
Results from a phage-display analysis revealed a cognate antigen for the #16 antibody to be a core protein of the perlecan-type proteoglycan (27) encoded by trol (22). When l(2)mbn cells were immunocytochemically analyzed with the #16 antibody under conditions without membrane permeabilization, they were found to be bound by the antibody but not by the control IgG (Fig. 1C), indicating the presence of Trol at the cell surface. We next examined the phagocytosis of apoptotic cells in embryos of flies lacking Trol. Embryonic cells were dispersed and analyzed simultaneously by immunocytochemistry with an anti-Croquemort antibody and by TUNEL. We considered cells positive for both reactions as embryonic hemocytes that had phagocytosed apoptotic cells (Fig. 1D, top panels), and the results showed that the level of phagocytosis in Trol-lacking flies was lower than in a control fly line (Fig. 1D, bottom panels). We previously identified Draper as a phagocytosis receptor responsible for the elimination of apoptotic cells by hemocytes and glia in Drosophila embryos (13). We thus examined how Trol functionally relates to Draper. For this purpose, l(2)mbn cells were treated with double-stranded RNA with the mRNA sequence of Trol or Draper before they were used in an assay for phagocytosis. Effectiveness of the RNAi-mediated reduction of each mRNA was confirmed by RT-PCR (Fig. 1E, bottom panels). Treatment with either double-stranded RNA brought about a reduction in the phagocytic activity of l(2)mbn cells, and that with a mixture of the two RNA sequences further decreased the level of phagocytosis (Fig. 1E, top panels). These results suggested that Trol and Draper function in l(2)mbn cells independently in the phagocytosis of apoptotic cells. All the above results collectively indicated that Trol, a core protein of Drosophila perlecan, is required for the effective phagocytosis of apoptotic cells by Drosophila embryonic hemocytes.
Involvement of Integrin βν in the Phagocytosis of Apoptotic Cells by Drosophila Hemocytes
The amino acid sequence RGD, a motif conserved among ligands for some groups of integrin (28), repeats three times in Trol (Fig. 2A), suggesting the involvement of integrin in the action of Trol. The RGD motif evokes an increase in the level of the phosphorylated form of FAK in integrin-expressing cells, and this event is considered a hallmark of activation of the RGD-binding integrin (29). We thus examined whether treatment with the RGD-containing Trol protein augments the phosphorylation of FAK in l(2)mbn cells. To do this, an antibody recognizing the phosphorylated form of mammalian FAK was first tested to see whether it detects phosphorylated FAK56, a Drosophila-type FAK (30–32). When whole-cell lysates of l(2)mbn were successively analyzed by immunoprecipitation and Western blotting, the signal corresponding to FAK56 was seen with the anti-phospho-FAK antibody in the sample after immunoprecipitation with the anti-FAK56 antibody but not with normal rat serum (supplemental Fig. 2A). Furthermore, the signals in Western blotting with the anti-phospho-FAK antibody disappeared after treating membranes containing transferred proteins with alkaline phosphatase, whereas the signal detected with the anti-FAK56 antibody was almost unaffected (supplemental Fig. 2B). These results indicated that the phosphorylated form of Drosophila FAK is detectable by Western blotting with the antibody that recognizes the phosphorylated form of mammalian FAK. We first tested whether two Trol proteins containing one of the three RGD motifs (Fig. 2A) bind to l(2)mbn cells. The cells were incubated with GST-fused Trol proteins or GST alone used as a negative control, washed, and immunocytochemically analyzed with anti-GST antibody. The results showed that either Trol protein, but not GST alone, binds to l(2)mbn cells (Fig. 2B, top panels). We then examined the effect of the Trol proteins on the phosphorylation of FAK56 in l(2)mbn cells. To do so, the cells were maintained in culture containers coated with GST-fused proteins for 2 h, and their lysates were analyzed by Western blotting for the levels of phosphorylated and total FAK56. We found that the incubation of cells with either Trol protein resulted in a slight (1.2–1.5-fold) but reproducible increase in the level of phosphorylated FAK56, leaving the total amount of FAK56 unchanged (Fig. 2B, bottom panels). These results suggested that Trol, likely through the RGD motif, activates integrin in l(2)mbn cells. We next extended the analysis of integrin to examine its role in the phagocytosis of apoptotic cells in Drosophila.
FIGURE 2.
Identification of integrin βν required for the phagocytosis of apoptotic cells in Drosophila embryos. A, the structure of Trol (a product from transcript variant C) is schematically exhibited with the positions of the motif RGD. Shown below are portions of Trol prepared as recombinant proteins fused to GST (GST-Trol 1 and GST-Trol 2). The numbers are amino acid positions with the amino terminus numbered 1. B, top, l(2)mbn cells that had been incubated with the indicated proteins were immunocytochemically analyzed for the bound proteins using anti-GST antibody. Phase-contrast and fluorescence micrographs of the same fields are shown. The arrowheads point to the positive signals. Scale bar, 10 μm. Bottom, lysates (20 μg of protein) of l(2)mbn cells that had been incubated in culture containers coated with the indicated proteins were analyzed by Western blotting with the anti-phospho-FAK antibody and anti-FAk56 antibody. The arrowheads indicate the positions of phosphorylated FAK56 and total FAK56. The intensity of the signals was determined, and averages from three independent experiments are shown relative to the result with a negative control (GST). C, lysates (0.13 mg of protein) of larvae of the indicated fly lines were analyzed by Western blotting with the anti-integrin βν antibody. The arrowhead indicates the position of integrin βν. D, dispersed embryonic cells of the indicated fly lines were analyzed for the phagocytosis of apoptotic cells by hemocytes (top), the population of hemocytes (middle), and the number of apoptotic cells (bottom). The ratios in percentage terms of hemocytes that had accomplished phagocytosis (top), of hemocytes to all embryonic cells (middle), and of apoptotic cells to all embryonic cells (bottom) are shown. Genotypes of the fly lines analyzed are w; betaInt-nu1 (betaInt-nu1), w; betaInt-nu2 (betaInt-nu2), mys1/Y (mys1), and sn mysXG43/Y (mysXG43). Significance is versus w1118.
Integrin is a heterodimer of α and β subunits, both of which are single-path membrane proteins (33). There exist five α and two β subunits for the Drosophila integrin (34), and we began by searching for the β subunit, βν or βPS, involved in the phagocytosis of apoptotic cells. We analyzed two mutant alleles of betaInt-nu coding for βν as well as of myospheroid (mys) coding for βPS. It was already known that either mys allele is null for the expression of integrin βPS (23), but the level of integrin βν expression needed to be determined for the two betaInt-nu alleles. To do so, we generated an anti-integrin βν antibody and analyzed lysates of Drosophila larvae by Western blotting. The βν subunit appeared as a signal with a molecular mass of about 110 kDa in the lysate of w1118 flies but was undetectable in the two mutants (Fig. 2C), indicating that each betaInt-nu allele is null for integrin βν expression. We then determined the level of phagocytosis in embryos of mutant flies lacking βν or βPS. The level in the βν mutants was decreased, whereas that in the βPS mutants was almost the same as in embryos of control w1118 flies (Fig. 2D, top panel). The reduction in phagocytosis in embryos of the βν mutants seemed not to be due to a change in the level of hemocytes or apoptosis because the numbers of hemocytes and apoptotic cells relative to all dispersed cells remained unaltered in the mutant animals (Fig. 2D, middle and bottom panels).
Independent Actions of Integrin βν and Draper in Hemocytes
Integrin βν appeared to be continuously expressed in w1118 flies throughout the developmental process, as is Draper (18), and its level was higher in embryos and lower in pupae (Fig. 3A). We next immunocytochemically examined the presence of integrin βν at the surface of hemocytes. When l(2)mbn cells were analyzed under conditions without membrane permeabilization, punctate signals were seen with the anti-integrin βν antibody but not with the preimmune rat serum (Fig. 3B, left panels). These signals almost disappeared when the analysis was done in the presence of the corresponding antigen protein but not in the presence of a protein used as a negative control (supplemental Fig. 1C), indicating the specificity of the antibody used. We then similarly analyzed dispersed embryonic cells of a fly line where GFP was expressed under the promoter of serpent that codes for a hemocyte-specific protein called Serpent and found the presence of integrin βν as patches at the surface of GFP-positive cells (Fig. 3B, right panels). These results suggested that integrin βν plays a role at the surface of hemocytes, most likely as a receptor for phagocytosis. To further examine this, we overexpressed integrin βν in hemocytes of a null mutant and found that the phagocytosis almost completely recovered (Fig. 3C). These results collectively suggested that integrin βν functions in embryonic hemocytes, most probably as a receptor, to achieve the effective phagocytosis of apoptotic cells.
FIGURE 3.
Presence and function of integrin βν in hemocytes. A, lysates (0.1 mg of protein) of w1118 flies at the indicated developmental stages were analyzed by Western blotting with the anti-integrin βν antibody. The arrowhead points to the position of integrin βν. B, l(2)mbn cells (left) and dispersed embryonic cells of y w; srpHemo-GAL4 UAS-srcEGFP flies (right) were subjected to immunocytochemistry with the anti-integrin βν antibody or preimmune rat serum as a negative control under membrane-nonpermeabilizing conditions. Embryonic hemocytes were identified based on the expression of GFP driven by the promoter of serpent. Note that most GFP-positive cells were also positive for the expression of Croquemort (data not shown). Phase-contrast and fluorescence micrographs of the same fields are shown. The arrowheads point to the signals derived from the anti-integrin βν antibody. Scale bars, 10 μm. C, dispersed embryonic cells of the indicated flies were analyzed for the phagocytosis of apoptotic cells by hemocytes. The ratio of hemocytes that had accomplished phagocytosis to total hemocytes is shown. Genotypes of the fly lines analyzed are w; betaInt-nu2 (betaInt-nu2 GAL4− UAS−), w; betaInt-nu2/betaInt-nu2 srpHemoGAL4 UAS-srcEGFP (betaInt-nu2 GAL4+ UAS−), w; betaInt-nu2; UAS-betaInt-nu/+ (betaInt-nu2 GAL4− UAS+), and w; betaInt-nu2/betaInt-nu2 srpHemoGAL4 UAS-srcEGFP; UAS-betaInt-nu/+ (betaInt-nu2 GAL4+ UAS+).
We previously identified Draper, a Drosophila homologue of the C. elegans phagocytosis receptor CED-1, as a receptor required for the phagocytosis of apoptotic cells by hemocytes and glia in embryos (13). The relationship between integrin βν and Draper was examined next. Flies lacking either integrin βν or Draper showed almost the same level of phagocytosis, which was about two-thirds of that in control w1118 (Fig. 4A). We then analyzed a double null mutant and found that the simultaneous loss of these two receptors further decreased phagocytosis, down to about one-third of the control level (Fig. 4A). These results suggested that integrin βν and Draper act independently, rather than activating the same signaling pathway, in the phagocytosis of apoptotic cells in Drosophila embryos. Draper was shown to lie upstream of CED-6 and CED-10 (18), one of the two partly overlapping pathways for phagocytosis originally found in C. elegans. It is therefore likely that integrin βν resides the furthest upstream of the other engulfment pathway CED-2-CED-5-CED-12 in C. elegans. We previously showed that hemocytes lacking Elmo, a Drosophila counterpart of CED-12, normally phagocytose apoptotic cells (18). We thus examined the involvement of Crk and Mbc, Drosophila homologues of C. elegans CED-2 and CED-5, respectively, in the integrin βν-mediated phagocytosis. When embryos of fly lines in which RNAi for Crk and mbc was induced in hemocytes were analyzed, the level of phagocytosis of apoptotic cells was almost equal to that in embryos of a control fly line (supplemental Fig. 3). Inhibition of the expression of Crk and Mbc in the RNAi-induced hemocytes seemed likely because the same fly lines barely developed into adults after induction of RNAi in all cell types (supplemental Table 1), as reported previously for null mutants of Crk (35) and mbc (36). These results indicate that Crk and Mbc do not seem to participate in the phagocytosis of apoptotic cells at least by embryonic hemocytes. Consequently, the existence of the pathway Crk-Mbc-Elmo downstream of integrin βν remained uncertain. Finally, flies lacking these two major receptors for the phagocytosis of apoptotic cells did not show a crucial defect in development but took longer to develop into adults than w1118 or each single mutant (Fig. 4B).
FIGURE 4.
Independent actions of integrin βν and Draper. A, dispersed embryonic cells of the indicated flies were analyzed for the phagocytosis of apoptotic cells by hemocytes. The ratio of hemocytes that had accomplished phagocytosis to total hemocytes is shown. B, periods in the development of Drosophila from embryos to adults were determined with the indicated fly lines. Genotypes of the fly lines analyzed are w; betaInt-nu2 (betaInt-nu2), w; +; drprΔ5 (drprΔ5), and w; betaInt-nu2; drprΔ5 (betaInt-nu2 drprΔ5).
DISCUSSION
In the present study, we identified Trol, a core protein of proteoglycan, and integrin βν, a β subunit of integrin, as components required for Drosophila phagocytes to achieve effective phagocytosis of apoptotic cells. The involvement of integrin in the phagocytosis of apoptotic cells has been known for mammals; integrins αvβ3 and αvβ5 serve as a receptor for milk fat globule EGF factor 8, which binds on one side to integrin via the RGD motif and on the other side to phosphatidylserine exposed at the surface of apoptotic cells (37). Trol or Trol-containing proteoglycan might serve to connect apoptotic cells and Drosophila hemocytes, although the binding of Trol to apoptotic cells remains to be shown. Recently, INA-1, an α subunit of C. elegans integrin, was reported to be responsible for the phagocytosis of cell corpses (17). It is thus most probable that integrin is an evolutionally conserved receptor for the phagocytosis of apoptotic cells, and our finding has filled in a blank for insects.
The two partly overlapping pathways for the induction of phagocytosis in C. elegans are presumably conserved among species (9, 38). Mammalian integrin αvβ5 (39) and nematode INA-1 (17) were shown to connect with a pathway consisting of CrkII-DOCK180-ELMO-Rac1 for mammals and CED-2-CED-5-CED-12-CED-10 for C. elegans. Our data indicated an independent action of integrin βν from Draper that activates the pathway Ced-6-Rac1/Rac2 corresponding to CED-6-CED-10 of C. elegans (18), suggesting that integrin βν is located upstream of the other pathway. However, it is unclear whether a presumed pathway in Drosophila, Crk-Mbc-Elmo-Rac1/Rac2, lies downstream of integrin βν because the involvement of this pathway itself in the phagocytosis by Drosophila phagocytes still remains ambiguous. The situation becomes more complicated when we consider how phagocytes recognize apoptotic cells through the actions of the two presumed receptors. In mammals, milk fat globule EGF factor 8 is simultaneously bound by integrin and phosphatidylserine to connect phagocytes and apoptotic cells. A similar bridging protein was recently found in C. elegans (40). This protein, called TTR-52, binds to phosphatidylserine but seemingly serves as a ligand for CED-1, not INA-1. In addition, phosphatidylserine-binding phagocytosis receptors in mammals seem to activate either of the two conserved pathways (41, 42). It is thus likely that utilization of the two pathways is defined not by markers but by receptors for phagocytosis. On the other hand, the binding of Trol seemed to activate integrin and increase the level of FAK phosphorylation. The requirement of FAK has been shown for mammals in the integrin αvβ5-mediated phagocytosis of apoptotic cells (43) or spent photoreceptor outer segment fragments (44) and for insects in the integrin-mediated phagocytosis of bacteria (45). Therefore, it is possible that phosphorylated FAK is included in a signaling pathway activated by integrin βν, although INA-1 does not seem to need FAK to induce phagocytosis (17). The intracellular region of β subunits contains the amino acid sequence NPXY that is bound by the cytoskeleton protein talin (46), and this binding is believed essential for the activation of integrin. It is therefore worth knowing whether talin interacts with signal mediators of the engulfment pathways, although there is a report that the activation of integrin β5 in the phagocytosis of apoptotic cells occurs independent of the NPXY motif (47).
More issues remain to be clarified regarding how integrin βν acts to induce the phagocytosis of apoptotic cells by Drosophila hemocytes. A Trol protein containing the RGD motif bound to the surface of l(2)mbn cells (Fig. 2B), but direct interaction between this protein and a protein corresponding to the extracellular region of integrin βν was not observed (data not shown). This suggests that integrin βν needs to form a heterodimer with an α subunit to bind Trol. It is necessary to find which of five Drosophila α subunits forms a complex and cooperates with integrin βν. In addition, the fact that a significant level of phagocytosis exists in embryos lacking both Draper and integrin βν indicates the presence of other mechanisms for the phagocytosis of apoptotic cells in Drosophila. Finally, our data showed that a loss of both Draper and integrin βν caused a delay in eclosion. This suggests the involvement of the phagocytic removal of apoptotic cells in the development of Drosophila.
Supplementary Material
Acknowledgments
We thank Danny Brower, Nicholas Brown, Marc Freeman, Herbert Jäckle, Nobert Perrimon, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, and the DGRC for fly stocks and the National Institute of Genetics for EST clones. We are grateful to Takayuki Kuraishi for suggestions and Kunizo Arai for contributing to our study at the initial stage. We also acknowledge the use of FlyBase.
This study was supported by Grants-in-Aid for Scientific Research 19370051, 22370049, and 22657033 from the Japan Society for the Promotion of Science (to Y. N.), the Hungarian National Research Fund Országos Tudomónyos Kutatási Alap (National Scientific Research Fund) Grant NK78024 (to I. A.), and a grant from the Fuji Foundation for Protein Research (to K. N.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3 and Table 1.
- FAK
- focal adhesion kinase
- Trol
- terribly reduced optic lobes.
REFERENCES
- 1. Savill J., Fadok V. (2000) Nature 407, 784–788 [DOI] [PubMed] [Google Scholar]
- 2. Liao D. J. (2005) Med. Hypotheses 65, 23–28 [DOI] [PubMed] [Google Scholar]
- 3. Nakanishi Y., Nagaosa K., Shiratsuchi A. (2011) Dev. Growth Differ. 53, 149–160 [DOI] [PubMed] [Google Scholar]
- 4. Lauber K., Blumenthal S. G., Waibel M., Wesselborg S. (2004) Mol. Cell 14, 277–287 [DOI] [PubMed] [Google Scholar]
- 5. Ravichandran K. S., Lorenz U. (2007) Nat. Rev. Immunol. 7, 964–974 [DOI] [PubMed] [Google Scholar]
- 6. Reddien P. W., Horvitz H. R. (2004) Annu. Rev. Cell Dev. Biol. 20, 193–221 [DOI] [PubMed] [Google Scholar]
- 7. Kinchen J. M., Hengartner M. O. (2005) Curr. Top. Dev. Biol. 65, 1–45 [DOI] [PubMed] [Google Scholar]
- 8. Mangahas P. M., Zhou Z. (2005) Semin. Cell Dev. Biol. 16, 295–306 [DOI] [PubMed] [Google Scholar]
- 9. Lettre G., Hengartner M. O. (2006) Nat. Rev. Mol. Cell Biol. 7, 97–108 [DOI] [PubMed] [Google Scholar]
- 10. Zhou Z., Hartwieg E., Horvitz H. R. (2001) Cell 104, 43–56 [DOI] [PubMed] [Google Scholar]
- 11. Callebaut I., Mignotte V., Souchet M., Mornon J. P. (2003) Biochem. Biophys. Res. Commun. 300, 619–623 [DOI] [PubMed] [Google Scholar]
- 12. Freeman M. R., Delrow J., Kim J., Johnson E., Doe C. Q. (2003) Neuron 38, 567–580 [DOI] [PubMed] [Google Scholar]
- 13. Manaka J., Kuraishi T., Shiratsuchi A., Nakai Y., Higashida H., Henson P., Nakanishi Y. (2004) J. Biol. Chem. 279, 48466–48476 [DOI] [PubMed] [Google Scholar]
- 14. Wu H. H., Bellmunt E., Scheib J. L., Venegas V., Burkert C., Reichardt L. F., Zhou Z., Fariñas I., Carter B. D. (2009) Nat. Neurosci. 12, 1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamon Y., Trompier D., Ma Z., Venegas V., Pophillat M., Mignotte V., Zhou Z., Chimini G. (2006) PLoS ONE 1, e120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cabello J., Neukomm L. J., Günesdogan U., Burkart K., Charette S. J., Lochnit G., Hengartner M. O., Schnabel R. (2010) PLoS Biol. 8, e1000297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu T. Y., Wu Y. C. (2010) Curr. Biol. 20, 477–486 [DOI] [PubMed] [Google Scholar]
- 18. Kuraishi T., Nakagawa Y., Nagaosa K., Hashimoto Y., Ishimoto T., Moki T., Fujita Y., Nakayama H., Dohmae N., Shiratsuchi A., Yamamoto N., Ueda K., Yamaguchi M., Awasaki T., Nakanishi Y. (2009) EMBO J. 28, 3868–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franc N. C., Dimarcq J. L., Lagueux M., Hoffmann J., Ezekowitz R. A. (1996) Immunity 4, 431–443 [DOI] [PubMed] [Google Scholar]
- 20. Franc N. C., Heitzler P., Ezekowitz R. A., White K. (1999) Science 284, 1991–1994 [DOI] [PubMed] [Google Scholar]
- 21. Kurucz E., Zettervall C. J., Sinka R., Vilmos P., Pivarcsi A., Ekengren S., Hegedüs Z., Ando I., Hultmark D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2622–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voigt A., Pflanz R., Schäfer U., Jäckle H. (2002) Dev. Dyn. 224, 403–412 [DOI] [PubMed] [Google Scholar]
- 23. Bunch T. A., Salatino R., Engelsgjerd M. C., Mukai L., West R. F., Brower D. L. (1992) Genetics 132, 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devenport D., Brown N. H. (2004) Development 131, 5405–5415 [DOI] [PubMed] [Google Scholar]
- 25. Brückner K., Kockel L., Duchek P., Luque C. M., Rørth P., Perrimon N. (2004) Dev. Cell 7, 73–84 [DOI] [PubMed] [Google Scholar]
- 26. Brand A. H., Perrimon N. (1993) Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- 27. Iozzo R. V., Cohen I. R., Grässel S., Murdoch A. D. (1994) Biochem. J. 302, 625–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown N. H. (2000) Matrix Biol. 19, 191–201 [DOI] [PubMed] [Google Scholar]
- 29. Legate K. R., Wickström S. A., Fässler R. (2009) Genes Dev. 23, 397–418 [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto J., Sawamoto K., Okabe M., Takagi Y., Tezuka T., Yoshikawa S., Ryo H., Okano H., Yamamoto T. (1999) J. Biol. Chem. 274, 29196–29201 [DOI] [PubMed] [Google Scholar]
- 31. Fox G. L., Rebay I., Hynes R. O. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14978–14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer R. H., Fessler L. I., Edeen P. T., Madigan S. J., McKeown M., Hunter T. (1999) J. Biol. Chem. 274, 35621–35629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shattil S. J., Kim C., Ginsberg M. H. (2010) Nat. Rev. Mol. Cell Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown N. H., Gregory S. L., Martin-Bermudo M. D. (2000) Dev. Biol. 223, 1–16 [DOI] [PubMed] [Google Scholar]
- 35. Ishimaru S., Ueda R., Hinohara Y., Ohtani M., Hanafusa H. (2004) EMBO J. 23, 3984–3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nolan K. M., Barrett K., Lu Y., Hu K. Q., Vincent S., Settleman J. (1998) Genes Dev. 12, 3337–3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. (2002) Nature 417, 182–187 [DOI] [PubMed] [Google Scholar]
- 38. Kinchen J. M., Ravichandran K. S. (2007) J. Cell Sci. 120, 2143–2149 [DOI] [PubMed] [Google Scholar]
- 39. Akakura S., Singh S., Spataro M., Akakura R., Kim J. I., Albert M. L., Birge R. B. (2004) Exp. Cell Res. 292, 403–416 [DOI] [PubMed] [Google Scholar]
- 40. Wang X., Li W., Zhao D., Liu B., Shi Y., Chen B., Yang H., Guo P., Geng X., Shang Z., Peden E., Kage-Nakadai E., Mitani S., Xue D. (2010) Nat. Cell Biol. 12, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park S. Y., Kang K. B., Thapa N., Kim S. Y., Lee S. J., Kim I. S. (2008) J. Biol. Chem. 283, 10593–10600 [DOI] [PubMed] [Google Scholar]
- 42. Park D., Tosello-Trampont A. C., Elliott M. R., Lu M., Haney L. B., Ma Z., Klibanov A. L., Mandell J. W., Ravichandran K. S. (2007) Nature 450, 430–434 [DOI] [PubMed] [Google Scholar]
- 43. Wu Y., Singh S., Georgescu M. M., Birge R. B. (2005) J. Cell Sci. 118, 539–553 [DOI] [PubMed] [Google Scholar]
- 44. Finnemann S. C. (2003) EMBO J. 22, 4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Metheniti A., Paraskevopoulou N., Lambropoulou M., Marmaras V. J. (2001) FEBS Lett. 496, 55–59 [DOI] [PubMed] [Google Scholar]
- 46. Dupuy A. G., Caron E. (2008) J. Cell Sci. 121, 1773–1783 [DOI] [PubMed] [Google Scholar]
- 47. Singh S., D'mello V., van Bergen en Henegouwen P., Birge R. B. (2007) Biochem. Biophys. Res. Commun. 364, 540–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.