Abstract
Post-translational modification by covalent attachment of isoprenoid lipids (prenylation) regulates the functions and biological activities of several proteins implicated in the oncogenic transformation and metastatic progression of cancer. The largest group of prenylated proteins contains a CAAX motif at the C-terminal that serves as a substrate for a series of post-translational modifications that convert these otherwise hydrophilic proteins to lipidated proteins, thus facilitating membrane association. C17orf37 (chromosome 17 open reading frame 37), also known as C35/Rdx12/MGC14832, located in the 17q12 amplicon, is overexpressed in human cancer, and its expression correlates with the migratory and invasive phenotype of cancer cells. Here we show that C17orf37 contains a functional CAAX motif and is post-translationally modified by protein geranylgeranyltransferase-I (GGTase-I). Geranylgeranylation of C17orf37 at the CAAX motif facilitates association of the protein to the inner leaflet of plasma membrane, enhances migratory phenotype of cells by inducing increased filopodia formation, and potentiates directional migration. A prenylation-deficient mutant of C17orf37 is functionally inactive and fails to trigger dissemination of tail vein-injected cells in a mouse model of metastasis. These findings demonstrate that prenylation is required for the function of the C17orf37 protein in cancer cells and imply that the post-translational modification may functionally regulate metastatic progression of disease.
Keywords: Cell Migration, Isoprenoid, Membrane Proteins, Plasma Membrane, Tumor Metastases, C17orf37
Introduction
The C17orf37 gene is located in the minus strand of human chromosome 17q12 bounded by the ERBB2 and Grb7 genes. Several studies have reported that a 280-kb minimal region of 17q12 that contains ERBB2 and C17orf37 is frequently amplified in breast and colon cancer (1, 2). C17orf37 expression positively correlates with the grade and stage of breast cancer compared with minimal expression in normal tissues and thus is proposed to be a novel tumor biomarker (3). In patients with metastatic breast cancer, aberrant expression of C17orf37 has been observed in distant metastatic sites such as lungs and liver, suggesting a possible role of C17orf37 protein in metastatic dissemination of cancer cells (3). In prostate cancer, C17orf37 is overexpressed in the higher grades of prostate adenocarcinoma compared with low expression in normal or benign prostatic tissues (4). However, expression of C17orf37 is minimal in 38 different normal tissues examined (3), suggesting C17orf37 as a cancer-specific protein. Although C17orf37 overexpression is linked to genomic amplification of ERBB2 locus (1, 6), abundant expression of C17orf37 protein in ERBB2 nonamplified breast (3) and prostate (4) tumors suggests that C17orf37 has an independent functional promoter. C17orf37 gene encodes a 12-kDa protein that does not have sequence similarity with any known protein. C17orf37 is expressed as a cytosolic protein with predominant membrane localization, and we have previously demonstrated that C17orf37 acts as a signaling molecule channeling signaling through PI3K/Akt pathway, thereby transcriptionally up-regulating NF-κB downstream target genes MMP-9, uPA,3 and VEGF (4).
An interesting feature of C17orf37 is the presence of a consensus sequence for prenylation comprising of the last four amino acids, CVIL, at the C-terminal end. Prenylated proteins belong to the CAAX family of proteins, which are post-translationally modified by the addition of isoprenyl groups. Prenylated proteins are modified at the cysteine residue of the CAAX motif by either farnesylation (addition of 15 carbon chain by protein farnesyltransferase enzyme (or FTase)) (7) or geranylgeranylation with a 20-carbon chain by GGTase-I (8). The C-terminal amino acid (X) of the CAAX motif determines which isoprenoid group is to be added to the candidate protein. If the amino acid X is leucine, the protein is predicted to be geranylgeranylated (7). Hence, C17orf37 is predicted to be geranylgeranylated by GGTase-I at Cys-112. After the isoprenyl group is added, the modified protein undergoes two additional postprenylation processing steps, which include cleavage of the last three C-terminal amino acids by an endoprotease enzyme named Rce1 (Ras-converting enzyme 1) and finally methylation of the prenylated-cysteine by Icmt (isoprenylcysteine-O-carboxyl methyltransferase) enzyme. These final modifications are also thought to be important for correct subcellular localization and biological activities of these proteins (9, 10). The addition of the isoprenyl tail facilitates binding to the cellular membranes and protein-protein interaction and increases the stability of the protein, thereby regulating the overall functional property of these proteins (7). Several oncogenes that undergo prenylation processing are known to promote cancer progression by activating proliferation, migration, and invasion of cancer cells, and inhibiting prenylation of these proteins reduces their functional activity (10). Hence, enzymes involved in the prenylation processing are attractive targets for cancer therapy (10, 11).
We investigated the importance of C-terminal isoprenylation of C17orf37 protein with respect to its function. We show for the first time that C17orf37 is geranylgeranylated by GGTase-I and also undergoes postprenylation processing, and these modifications are important for correct localization of the C17orf37 protein. We also show that membrane-associated C17orf37 increases cellular migration by inducing increased filopodia formation at the leading edge of the migrating cell, whereas prenylation-deficient mutant C17orf37 failed to do so. We further confirm that ectopic expression of C17orf37 modulates the dissemination of cancer cells and facilitates formation of metastatic nodules in a mouse model of metastasis. Our data clearly indicate that C17orf37 prenylation is sufficient to drive cellular migration and colonization of injected nontransformed fibroblast cells to distant organs compared with the inactive mutant C17orf37 protein. These findings directly establish role for C17orf37 in the migration and metastatic dissemination of cancer cells and further elucidates the importance of isoprenyl modification in the function of C17orf37.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of Recombinant C17orf37
Full-length wild type human C17orf37 cDNA of 347 bp was cloned into pGEX-4T-1 vector (GST-C17orf37-WT) and pEGFP-C1 vector (GFP-C17orf37) as described previously (4). The Cys-112 residue in the CVIL motif of C17orf37 (cloned in pGEX-4T1 vector and pEGFP-C1 vector) was mutated to serine, or the entire CVIL motif of C17orf37 (cloned in pEGFP-C1 vector) was deleted by site-directed mutagenesis using a QuikChange site-directed mutagenesis kit (Stratagene). The primer pairs used are as follows: C17orf37C112S, 5′-CAG CCG TCC TCC CAG CGT CAT CCT GTG-3′/5′-GTC GGC AGG AGG GTC GCA GTA GGA CAC-3′; C17orf37Δ112–115, 5′-GCC GTC CTC CCT GAG TCA TCC TGT GAC-3′/5′-CGG CAG GAG GGA CTC AGT AGG ACA CTG-3′. GST-C17orf37-WT (C17WT) and GST-C17orf37-C112S (C17C112S) fused protein was expressed in Escherichia coli Bl-21 strain and purified using glutathione-Sepharose 4B column (GE Healthcare) according to the manufacturer's instructions.
Cell Lines, Culture Conditions, Treatment, and Transfection Procedures
DU-145 and SKBR-3 cells were obtained from ATCC and maintained in RPMI1640 supplemented with 10% FBS and 1% penicillin-streptomycin. NIH3T3 mouse fibroblast cells were maintained in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Wild type mouse embryonic fibroblasts (MEFs), Icmt−/−, and Rce1−/− were grown in DMEM supplemented with 15% calf serum, 1% nonessential amino acid, 1% penicillin-streptomycin, and 3.6 μl of β-mercaptoethanol (12). The cells were transfected using Lipofectamine 2000 (Invitrogen) with plasmid DNA for a period of 6 h in OPTI-MEM (Invitrogen). After transfections, the cells were grown in complete medium overnight before mounting on slides using Vectashield (Vector Laboratories, Burlingame, CA) for confocal microscopy.
For generation of stable cells, NIH3T3 cells were transfected using Lipofectamine 2000, with GFP (empty vector), GFP-C17orf37-WT (C17WT), GFP-C17orf37-C112S (C17C112S), or GFP-C17orf37-Δ112–115 (C17 Δ112–115) plasmid DNA for 24 h. Stable transfected cell populations were challenged in complete medium supplemented with 250 μg/ml G418 (Invitrogen) for about 3 weeks. For subsequent experiments, polyclonal pooled clones obtained from the transfected cells were used. DU-145 stable cells expressing C17orf37 or GFP were generated as mentioned previously (4). GGTI-DU-40 and FTI-2148 (Calbiochem) inhibitors were dissolved in Me2SO and mixed with complete medium at the indicated dose prior to treatment for a period of 24 h. The cells were either fixed and stained with C17orf37 antibody for confocal microscopy or subjected to Western immunoblot.
Antibodies and Reagents
Antibodies used for the study are as follows: mouse polyclonal C17orf37 (Abnova, Taiwan; 1:500), rabbit polyclonal anti-PGK, mouse monoclonal anti-Na,K-ATPase (Developmental studies hybridoma bank, Colorado), rabbit polyclonal anti-GFP (Cell Signaling and Roche Applied Science), mouse monoclonal anti-transferrin receptor (Zymed Laboratories Inc.), mouse monoclonal anti-Ki-67 (DAKO), GAPDH (Santa Cruz), Tom20 (13), Lamin A/C (Cell Signaling), and rabbit polyclonal anti-Gαi2 (Santa Cruz). Geranylgeranyl diphosphate (GGPP) was purchased from Biomol, Inc; [3H]GGPP and [3H]FPP from PerkinElmer Life Sciences; [3H]GGOH (American Radiolabeled Chemicals); mevastatin (Sigma); Enhancer (GE Healthcare), and FTI-2148 was purchased from Calbiochem.
Confocal and TIRF Microscopy
GFP-tagged cells were grown on coverslips and mounted on glass slides with Prolong Gold mounting medium (Invitrogen). Confocal images were obtained using Zeiss confocal microscope LSM 510 under 40×, 1.2-numerical aperture water immersion objective at a 0.6-μm Z-section as previously described (12). Lung sections were immunostained with GFP and Ki-67 antibody, and images were obtained under 20× objective. For TIRF microscopy, DU-145 cells were grown on coverslips and then treated with Me2SO or 5, 10, and 20 μm of GGTI for 24 h. The cells were then fixed by 2% paraformaldehyde. Unpermeabilized cells were then washed with PBS and treated with C17orf37 followed by Alexa-568-conjugated secondary antibody. The coverslips were then mounted on specialized cover glass 1 (22 × 50 mm size; Corning, Lowell, MA). For TIRF images, the cells were visualized on an Olympus IX71 microscope with commercial TIRF attachment as described previously (4) by 60× oil immersion objective.
In Vitro Prenylation Analysis
Prenylation of C17orf37 and Rac1 were measured by incorporation of radiolabeled isoprenoid as described previously (14). Briefly, purified mammalian GGTase-I or FTase (10 ng, expressed in Sf9 cells) (15) were used to initiate reactions containing 10 μm GGPP or FPP incubated with either 2.5 μm of purified GST-tagged C17orf37 substrates (wild type or C112S-mutant) or 5 μm GST-tagged Rac1 or Ras. The reactions were incubated for 60 min at 30 °C before precipitation, product termination, and measurement of [3H]GGPP or [3H]FPP incorporation.
Incorporation of [3H]GGOH into DU-145 Cells and Immunoprecipitation of Prenylated Proteins
In vivo labeling of C17orf37 protein with [3H]GGOH in DU-145 cells was performed as described in Ref. 16. Briefly, 2 × 105 DU-145 cells were seeded in 6-well plates and after 12 h in culture, cells were treated with 5 μm mevastatin for 4 h. The cells were then transfected with GFP, C17WT, or C17C112S and after 6 h were incubated in complete medium containing 5 μm mevastatin and 30 μCi/ml of [3H]GGOH. After 40 h, the cells were washed and resuspended in lysis buffer to obtain the whole cell lysate. One half of the lysate was immunoprecipitated using anti-GFP antibody (Roche Applied Science) followed by capture of the protein-IgG complex using protein A/G-agarose beads (Santa Cruz). The beads were washed, and eluted fractions were subjected to SDS-PAGE. The gel was washed, fixed, stained with fluorography enhancer (GE Healthcare), and dried, and labeled proteins were visualized by exposing the gel to imaging film (Denville Scientific) for 3 weeks at −80 °C.
Subcellular Fractionation
Differential centrifugation was performed as described by (17) with minor modifications. Briefly, 10-cm dishes of NIH3T3 cells expressing C17WT and C17C112S were trypsinized and resuspended in ice-cold homogenization buffer (0.25 m sucrose, 10 mm Tris (Cl−), pH 8.0, containing protease and phosphatase inhibitors. The cells were then disrupted using a Dounce homogenizer followed by centrifugation at 1500 × g to obtain nuclei pellet. The supernatant was further subjected to centrifugation for 15 min at 3500 × g to sediment mitochondrial fraction. The supernatant was then centrifuged at 100,000 × g to obtain the membrane pellet.
Isolation of Membrane Microdomains on Sucrose Floatation Gradients
The cells were fractionated, and membrane microdomains were isolated by nondetergent method, according to the previously published protocols with few modifications (18). Briefly the cells were grown to near confluence and lysed in 500 mm sodium carbonate, pH 11, with a mixture of protease and phosphatase inhibitors using a detergent-free procedure. The whole cell lysate was homogenized with 20 strokes in a prechilled Dounce homogenizer followed by homogenization with a polytron homogenizer three times for 10 s with intervals of 10–15 s followed by sonication three times for 20 s with an interval of 60 s. 2 ml of the homogenized whole cell lysate was mixed with 2 ml of 90% sucrose in MBS (25 mm MES, 150 mm NaCl, pH 6.0). A discontinuous sucrose gradient was generated by overlaying with 4 ml of 45% sucrose followed by overlaying with 4 ml of 5% sucrose at the top. The gradient was centrifuged at 40,000 rpm in a SW40Ti rotor (Beckman) for 20 h. In total, twelve fractions of 1 ml each were collected from the top of the tube, and the fractions were subjected to TCA precipitation followed by SDS-PAGE and Western immunoblotting.
Scratch Wound Healing Migrating Assay
NIH3T3 and DU-145 cells were grown on coverslips coated with fibronectin (5 μg/ml) in a monolayer, and scratches were created using a 100-μl pipette tip. The cells were then incubated in serum-free medium after removing the floating cells and then imaged at indicated time points after the creation of wound. For filopodia assay, the cells were fixed, stained with rhodamine-phalloidin (Molecular Probes), and subjected to confocal microscopy.
In Vitro Migration Assay
Transwell® migration was assay performed using HTS FuoroBlok multiwell insert system (Falcon; 8-μm pore size). The cells were prelabeled with 4 μg/ml of calcein AM (Molecular Probes), and 2.5 × 104 cells/well suspended in DMEM were added to the upper chamber, and 10% FBS was added to the bottom chambers as chemoattractant. After incubation for 24 h at 37 °C, the nonmigratory cells were removed from the top chamber, and the cells that successfully migrated to the lower side of the filter were imaged and counted.
Experimental Lung Metastasis
All of the animal experiments were performed in accordance with a protocol approved by the University of North Texas Health Science Center committee on animal health. 6–8-week-old athymic nude mice were purchased from Harlan Laboratories. To perform lung metastasis, 5 × 105 cells (resuspended in PBS) were injected into the tail vein of mice, and after 56 days (for DU-145 cells) or 11 weeks (for NIH3T3 cells), the animals were sacrificed. Isolated lungs were perfused and metastasis was quantified using a Kodak fluorescence imager within 3 h of specimen isolation according to the published report (19).
Immunohistochemsitry
The animals were sacrificed according to the protocol approved by the University of North Texas Health Science Center committee on animal health, and tissues were either embedded in optical cutting temperature (OCT) compound and snap frozen or preserved at −80 °C or paraffin-embedded. 5-μm sections were treated with indicated antibodies and imaged using confocal microscopy.
RESULTS
The C17orf37 Protein Is Prenylated by GGTase-I Enzyme
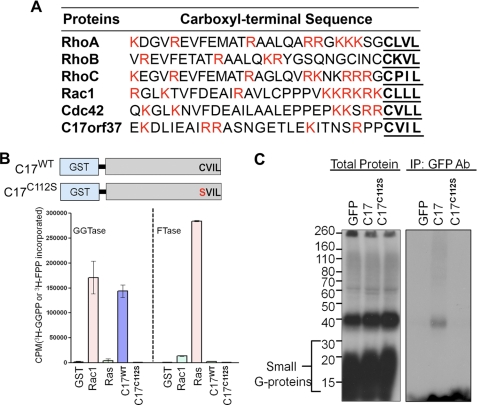
C17orf37 localization in cells has been reported to be both cytosolic and associated with membranes (4). In a survey of several computational algorithms, including SOSUI, TMAP, TMHMM, TMpred, and TopPRED, we could not identify a hydrophobic or putative transmembrane domain responsible for the membrane binding of C17orf37. We did, however, note a prenylation motif, CVIL, at the C terminus of the protein, a motif predicting the protein to be prenylated. In addition to the CAAX motif, certain upstream clusters of polybasic amino acids are also known to enhance efficient membrane association of the prenylated proteins (12, 20). Comparison of the 30 amino acids of C17orf37 preceding the CAAX motif with the known geranylgeranylated proteins RhoA, RhoB, RhoC, Rac1, and Cdc42 revealed the presence of multiple basic amino acids in C17orf37 protein as well (Fig. 1A). Because the last amino acid of CAAX box is leucine, C17orf37 is predicted to be prenylated by GGTase-I at Cys-112 compared with FTase, whereas GGTase-II, which requires two cysteine residues in the CAAX box for isoprenyl group addition, did not show any match. To directly determine whether GGTase-I could catalyze the addition of a geranylgeranyl isoprenoid to Cys-112 of the C17orf37 CAAX motif, we performed an in vitro prenylation assay using GST-fused C17orf37 (C17WT) and mutant form of C17orf37 in which Cys-112 was substituted with Ser (C17C112S) (supplemental Fig. S1A). C17WT protein with functional CVIL motif efficiently incorporated geranylgeranyl from [3H]GGPP in presence of GGTase-I, comparable with a known geranylgeranylated protein, Rac1 (Fig. 1B). However, mutation of Cys-112 to Ser (C17C112S) abolished the prenylation by GGTase-I (Fig. 1B and supplemental Fig. S1B). Both C17WT and C17C112S failed to incorporate [3H]FPP in presence of FTase, suggesting that C17orf37 protein is specifically prenylated by GGTase-I. We confirmed our in vitro findings by labeling DU-145 cells with [3H]GGOH that were transfected with either GFP, C17WT, or C17C112S (supplemental Fig. S1c). Electrophoretic analysis of immunoprecipitated eluates showed significant incorporation of [3H]GGOH by C17WT, whereas C17C112S failed to do so (Fig. 1C). Collectively, these findings indicate that C17orf37 is geranylgeranylated by GGTase-I.
FIGURE 1.
C17orf37 is geranylgeranylated by GGTase-I at Cys 112. A, sequence alignment of last 30 amino acids of representative geranylgeranylated proteins. Basic amino acids are shown in red, and the CAAX motif is in bold and underlined. B, schematic diagram showing that the CAAX box located at the C-terminal end of C17orf37 was mutated to serine (C112S), and GST-fused recombinant protein C17orf37 wild type (C17WT) and C17orf37-C112S (C17C112S) was used for in vitro prenylation assay. Direct geranylgeranylation or farnesylation of C17WT (2.5 μm) was achieved in the presence of recombinant GGTase-I enzyme containing [3H]geranylgeranyl pyrophosphate or FTase enzyme containing [3H]FPP. Rac1 (5 μm) and Ras were used as positive controls, and GST protein was used as negative control. C, incorporation of [3H]GGOH into geranylgeranylated proteins was achieved by labeling DU-145 cells transfected with GFP, C17WT, and C17C112S constructs. Left panel, SDS-PAGE followed by autoradiography showing total protein that incorporated [3H]GGOH. Right panel, immunoprecipitation (IP) of the whole cell lysate using GFP antibody showing the incorporation of [3H]GGOH only in C17WT.
Lipid modification of CAAX proteins facilitates association with the plasma membrane and to the biomembranes of intracellular organelles. Confocal microscopy of DU-145 cells showed strong association of C17orf37 protein with the plasma membrane, particularly to the inner leaflet of plasma membrane facing the cytosol (Fig. 2A). Some C17orf37 staining was also observed in intracellular vesicles, because postprenylation processes are known to occur in endoplastic reticulum. We next sought to identify whether prenylation of C17orf37 by GGTase-I enzyme is required for its proper localization in the cell. To this end, we employed breast cancer cells SKBR-3 and prostate cancer cells DU-145 endogenously expressing increased amounts of C17orf37 protein and treated them with the selective GGTI DU-40 (21) or the FTI-2148 (22, 23). In cells treated with vehicle control Me2SO, C17WT protein was always excluded from the nucleus and predominantly localized surrounding the plasma membrane (Fig. 2B). However, inhibition of GGTase-I, but not FTase, increased C17orf37 nuclear accumulation both in SKBR-3 and DU-145 cells (Fig. 2B). Further, combinatorial treatment by GGTI and FTI did not show any synergism and was similar to the effect observed with GGTI treatment alone. Next we performed TIRF microscopy to detect the distribution of C17orf37 protein in the plasma membrane. In the TIRF microscopy, the evanescent wave produced by the visible light at the cell/substratum interface penetrates only 100–200 nm with decaying intensity with the distance, allowing detection of protein molecules associated with the plasma membrane (24). TIRF microscopy was performed in DU-145 cells treated with either vehicle control Me2SO or an increasing dose of GGTI. DU145 cells treated with vehicle (Me2SO) showed numerous dense spots of C17orf37 protein, whereas increasing doses of GGTI treatment particularly at 10 and 20 μm concentration reduced membrane association of C17orf37 (supplemental Fig. S2). These results demonstrate that C17orf37 is indeed a substrate of GGTase-I and that modification by GGTase-I is required for localization of C17orf37 to the plasma membrane.
FIGURE 2.
Geranylgeranylation of C17orf37 is required for correct subcellular localization. A, DU-145 cells grown on coverslips were labeled with C17of37 antibody (green) and plasma membrane marker (red) followed by confocal microscopy. B, immunofluorescence staining of C17orf37 in SKBR-3 and DU-145 cells, and overlay with nuclear DAPI (blue) staining of the same field (Merge). SKBR-3 and DU-145 cells were treated with dimethyl sulfoxide (DMSO), GGTI DU-40 at a dose of 10 μm, FTI-2148 at a dose of 100 nm, or a combination of both GGTI (10 μm) and FTI (100 nm) for 24 h followed by confocal microscopy to image subcellular localization of C17orf37. Scale bars, 20 μm.
Postprenylation Processing Is Required for Proper Subcellular Localization of C17orf37
For CAAX proteins, prenylation is followed by two additional important biochemical processes (25). Proteolytic cleavage of the last three amino acids (AAX) by Rce1 and carboxyl methylation of the prenylated cysteine by Icmt are important postprenylation events required for the correct localization and biological functions of many CAAX proteins (12, 26). Gene disruption studies in mice have confirmed that Icmt and Rce1 enzymes are essential for mouse development (27–29). To determine whether geranylgeranyl modification of C17orf37 is followed by Rce1-mediated cleavage and carboxymethylation, we evaluated the subcellular localization of C17orf37 in MEF deficient in Rce1 and Icmt enzymes. Wild type MEF cells transiently transfected with GFP-tagged C17orf37 showed evenly distributed C17orf37 in the membrane and cytosol. However, in Rce1−/− cells, C17orf37 localization was abrogated and displayed increased nuclear and cytoplasmic accumulation (Fig. 3A). Surprisingly, in Icmt−/− cells, C17orf37 was found to be localized as dense punctuated spots, presumably some form of vesicles, and also in the cytosol and nucleus (Fig. 3B). It seems likely that the absence of carboxylmethylation results in a tendency for C17orf37 protein to associate with vesicles. We also generated a GFP-tagged C17orf37 mutant in which Cys-112 was mutated to Ser (C17C112S). Expression of this form of the protein in wild type, Rce1−/−, and Icmt−/− MEFs showed significant nuclear accumulation (Fig. 3, A and B, bottom panels), demonstrating mistargeting of the mutant protein. Taken together, these findings provide evidence that efficient membrane association of geranylgeranylated C17orf37 is also dependent on postprenylation processing mediated by Rce1 and Icmt enzymes.
FIGURE 3.
Post-translational modifications are necessary for membrane association of C17orf37 protein. Rce1+/+ and Rce1−/− (A) and Icmt+/+ and Icmt−/− (B) MEFs were transiently transfected with GFP-fused expression constructs of blank vector alone (GFP), wild type C17orf37 (C17WT), and C17orf37-C112S mutant (C17C112S), and confocal microscopy was performed to visualize the localization of the tagged protein. The images shown are representative of three independent experiments. Scale bars, 10 μm.
Prenylated C17orf37 Is Associated with Plasma Membrane
To demonstrate that the Cys-112 residue is required for C17orf37 prenylation and subsequent association with the plasma membrane, we generated stable polyclonal pooled populations of C17orf37-null NIH3T3 cells, expressing GFP vector, GFP-C17orf37, or the C17orf37 mutants C17C112S and C17orf37Δ112–115, in which the entire CAAX box was deleted (supplemental Fig. S3). Although C17orf37Δ112–115 mutant behaved as expected, the abundance of the protein recovered from NIH3T3 cells was low, suggesting that deletion of entire CAAX box affected protein stability (supplemental Fig. S3). Subcellular fractionation of cells by differential centrifugation revealed distribution of C17WT protein exclusively in the cytosolic and plasma membrane fractions, whereas C17C112S is mostly cytosolic (Fig. 4A) and very less in the membrane fractions. Surprisingly, C17C112S mutant was not found to be in the nuclear fraction probably because of increased molecular weight caused by fusion with GFP. GAPDH, TOM20, Na-K/ATPase, and Lamin A/C were used to check the purity of the cytosolic, mitochondrial, plasma membrane, and nuclear fractions.
FIGURE 4.
Cys-112 of the CVIL domain is required for C17orf37 prenylation and membrane association. A, NIH3T3 cells were stably transfected with GFP-fused expression constructs of wild type C17orf37 (C17WT) and C17orf37-C112S mutant (C17C112S), and polyclonal pools were selected using G-418 antibiotic. Pooled clones were lysed and subjected to subcellular fractionation by differential centrifugation. Collected fractions of plasma membrane (PM), mitochondria (MT), cytosolic (CY), and nuclear (NU) were subjected to SDS-PAGE followed by Western immunoblot using anti-C17orf37, GAPDH (cytosolic), Na-K/ATPase (PM), Tom20 (MT), and Lamin A/C (nucleus). L denotes molecular weight standard (ladder). B and C, NIH3T3 cells stably expressing GFP-tagged wild type C17orf37 (C17WT) (B) and C17orf37-C112S mutant (C17C112S) (C) were lysed, and equal amounts of total protein were fractionated on a 5–45% discontinuous sucrose gradient. A total of 12 fractions were collected based on their density in sucrose gradient and blotted with antibodies C17orf37, Gαi2 (Gαi2), transferrin receptor (Tfr), and phosphoglycerate kinase (PGK). Localization of the marker proteins were used to classify the membrane microdomains: Gαi2 protein (lipid rafts) enriched in fractions 2–4; transferrin receptor protein in fractions 5–8; and PGK (cytosolic) protein predominantly localized in fractions 9–12.
The plasma membrane is a dynamic structure composed of extremely complex set of 500 different lipid species with proteins embedded within (30). Most prenylated proteins have the ability to interact with cellular membranes, although many maintain a substantial cytoplasmic soluble pool (31). Studies have shown that farnesylated Ras protein has the ability to aggregate in cholesterol-enriched membrane microdomains known as “lipid rafts,” and there is a dynamic relation between raft and nonraft associated proteins (32). We determined the spatial and temporal distribution of prenylated C17orf37 in the membrane microdomains. Total protein recovered from NIH3T3 cells stably expressing either C17WT or C17C112S were subjected to differential centrifugation on a discontinuous sucrose gradient, and fractions collected based on their density on sucrose gradients were analyzed. The cholesterol-rich low density membrane fractions (fractions 2–4) were distributed at the interface between 5 and 45% sucrose and contain an enrichment of the raft marker Gαi2; the heavier membrane fractions (fractions 5–8) show a predominant expression of transferrin receptor, whereas most cellular protein were localized to the bottom of the gradient in fractions (fractions 9–12) (Fig. 4, B and C). C17WT was distributed predominantly in two different pools: as soluble fraction in the cytosol (fractions 9–12) and in the membrane fractions enriched in transferrin receptor (fractions 5–8) and less in the raft fractions (Fig. 4B). As expected, C17C112S was mostly localized in the soluble fractions comprised of cytosolic proteins (fractions 9–12) and less in the membrane (Fig. 4C). Our studies provide evidence that prenylated C17orf37 localizes outside the cholesterol-enriched raft region at the plasma membrane, consistent with previous reports that geranylgeranylated proteins cluster in microdomains outside the lipid rafts (33, 34).
C17orf37 Prenylation Regulates Functional Response of the Protein
We next investigated whether the function of C17orf37 shows a dependence on the post-translational modifications at the CAAX motif. Ectopic expression of C17orf37 increases the migratory ability of cancer cells (4). Hence, in the first set of studies to examine the role of C17orf37 prenylation, we assessed migration of NIH3T3 cells stably expressing GFP vector, C17WT, or C17C112S (supplemental Fig. S4) in scratch wound assays. Scratch assays are commonly used to study the ability of cells to polarize and migrate into the wound with time (35, 36). Cells expressing the C17orf37WT were able to migrate efficiently and cover nearly all of the wounded area within 18 h (∼2-fold increase in cell migration), whereas cells expressing the GFP vector or the C17C112S were much less efficient in this process (Fig. 5, A and B). Further, in a Transwell® migration assay, expression of C17orf37WT led to a ∼2.5-fold increase in migration compared with GFP vector or C17C112S in NIH3T3 cells (Fig. 5, C and D), indicating that the ability of C17orf37 to enhance cell migration is dependent on its prenylation.
FIGURE 5.
Expression of prenylation-deficient C17orf37 mutant inhibits cell migration. A, scratch wound assays were performed in NIH3T3 cells stably expressing vector (GFP), GFP-fused wild type C17orf37 (C17WT), or GFP-fused C17orf37-C112S mutant (C17C112S). Confluent monolayers of cells were wounded, and healing of the wound by cell migration was monitored for 18 h. Images were taken at different time points: 0 h (left column), 9 h (middle column), and 18 h (right column). B, migrated cells in the wound area at 9 h (open bar) and 18 h (gray bar) were counted from five different fields and expressed as the means ± S.E. of five independent experiments. C and D, NIH3T3 cells expressing different constructs as mentioned for A were prelabeled with calcein AM fluorescent dye and Transwell® migration assay performed with 10% serum as a chemoattractant in the lower chamber. C, representative images of migrated cells from two different fields were obtained after 24 h, and the fold change of migration was calculated normalized to parental NIH3T3 cells and expressed as the means ± S.E. of three independent experiments as shown in D.
Membrane-bound Prenylated C17orf37 Induces Cell Migration by Increased Filopodia Formation
Cell migration depends on coordinated polymerization of actin filaments resulting in protrusive structures at the leading edge of motile cell called lamellipodia (37). From the lamellipodia arise thin finger-like projections filled with parallel bundles of F-actin, known as filopodia (38). Surprisingly, the ectopic expression of C17WT in NIH3T3 cells dramatically increased filopodia formation in more than 70% of the cells counted (Fig. 6, A and C, open bars). However, in cells expressing GFP vector or C17C112S, only 15–20% counted cells showed filopodia formation (Fig. 6, A and C, open bars), and there was a ∼4-fold decrease in the number of filopodia/cell when compared with C17WT (Fig. 5D, open bars). Filopodia are often referred to as “tentacles” used by the migrating cells to probe their microenvironment and known to facilitate directional movement (34). Inducing migration by wounding scratch in NIH3T3 cells, we observed increased actin polymerization and stress fiber formation in both wild type and mutant C17orf37 (Fig. 6B). Filopodia originating from the migratory cells were found to protrude toward to the wound, supporting a directional migration (Fig. 6B); however, the number of filopodia radiating from C17WT cells was significantly higher than that of C17C112S cells (Fig. 6, B and D, gray bars), suggesting the involvement of prenylated C17orf37 in filopodium formation. We also observed a global increase in the formation of filopodia, particularly in cells surrounding the wound (Fig. 6D, gray bars), and the number of cells with filopodium increased upon induction of migration (Fig. 6B, gray bars), supporting the notion that filopodia formation is a well regulated process by the dynamic balance of actin polymerization (39). Nevertheless, our findings strongly suggest a mechanism by which prenylated C17orf37 induces increased migratory behavior in cells.
FIGURE 6.
C17orf37 prenylation stimulates filopodia formation. A, NIH3T3 cells stably expressing vector (GFP), GFP-fused C17orf37 (C17WT), or GFP-fused C17orf37-C112S mutant (C17C112S) were plated on coverglass coated with fibronectin (5 μg/ml) and then fixed and stained with rhodamine-phalloidin (left column, images shown in monochrome). Middle column, localization of GFP-fused proteins. Right column, overlay with phalloidin staining (red) of the same field. Scale bars, 10 μm. B, NIH3T3 cells expressing different constructs as mentioned for A were grown in a monolayer to confluency and then wounded. Six h later, the cells were fixed and stained with rhodamine-phalloidin. Two left columns, actin stained (monochrome) cells surrounding the wound. The far-left column contains magnified images of the boxed areas. The arrows indicate direction of cell migration. Middle column, localization of GFP-fused protein in the migrating cells (green). Right column, overlay of actin (red) and GFP (green). Scale bars, 10 μm. C, NIH3T3 cells stably expressing constructs as mentioned for A stained with rhodamine-phalloidin were counted to determine percentage of cells with filopodia. In a total of five fields, with more than 80 cells/field were counted, and the values represent [(cells with at least five filopodia/cell)/(total number of cells selected)] × 100 and are expressed as the means ± S.E. of three independent experiments. The open bars indicate nonmigrating cells as mentioned for A, and the gray bars represent migrating cells as mentioned for B. D, NIH3T3 cells stably expressing constructs as mentioned for A stained with rhodamine-phalloidin were counted to determine number of filopodia/cell. In total, five fields with more than 80 cells/field were counted and expressed as the means ± S.E. The open bars indicate nonmigrating cells as mentioned for A, and the gray bars represent migrating cells as mentioned for B.
C17orf37 Prenylation Enhances Metastatic Dissemination and Colonization of Cells
Migration and invasion of cancer cells are the hallmark of metastasis, and ectopic expression of C17orf37 increases the migratory and invasive behavior of cancer cells. In clinical specimens, increased expression of C17orf37 protein has been detected in patients with metastatic cancer (3). To determine whether C17orf37 prenylation could drive oncogenesis and metastatic progression of the disease in vivo, we used the mouse model of experimental lung metastasis. NIH3T3 cells stably expressing GFP vector, C17WT, or C17C112S were injected into the tail veins of athymic nude mice. NIH3T3 is a nontransformed cell line and does not form tumors or lung metastasis when injected into nude mice (40). However, stable overexpression of different oncogenes has been known to induce oncogenic transformation of NIH3T3 cells (40). Eleven weeks after the injection, we did not observe any change in animal behavior, and the animals looked healthy. The isolated lungs were free of any visible external metastatic nodules (Fig. 7B, top panels). However, quite unexpectedly, we observed increased colonization of C17WT-expressing cells in the lung parenchyma when compared with mice injected with cells expressing the GFP vector alone or C17C112S (Fig. 7A). Most of the C17WT cells densely populated the lung parenchyma, predominantly infiltrating from the blood vessels (Fig. 7, A, bottom panels, and B, middle panels), whereas minimal GFP stain was observed in GFP vector or C17C112S lung sections (Fig. 7B, middle panels). Ki-67 staining of the lung sections was basal (Fig. 7B, bottom panels), and no significant differences were found between the groups (supplemental Fig. S5B). This clearly suggests that C17orf37 prenylation markedly increases the migratory ability of cells, an aggressive trait required for dissemination and metastatic colonization. On the other hand, preventing prenylation of C17orf37 by genetically altering the CAAX box completely suppressed this effect.
FIGURE 7.
The C112S mutation impairs C17orf37 function in vivo. A and B, NIH3T3 cells stably expressing GFP vector (Vector, left column), GFP-fused C17orf37 (C17WT, middle column), or GFP-fused C17orf37-C112S (C17C112S, right column) were injected into the tail vein of nude mice (n = 6). A, upper two panels, representative images of murine lungs (maximum and minimum, fluorescence observed in the animal group) to visualize GFP labeled NIH3T3 cells, 11 weeks after tail vein injection. Bottom panels, hematoxylin- and eosin-stained sections of lungs shown in top panel. The arrows indicate the colonized cells observed. Scale bars, 1 mm (white); 100 μm (black). B, murine lung images as mentioned for A, showing the absence of visible tumor nodules (upper panels), and corresponding GFP- and Ki-67-immunostained sections are shown in the bottom panels. C–F, DU-145 cells stably expressing GFP vector only (Vector) (n = 5) or GFP-tagged C17orf37 (C17orf37) (n = 7) were injected into the tail vein of nude mice. Two mice from C17orf37 group died after 50 days, and the experiment was terminated after 56 days. C, upper two panels, representative images of murine lungs (maximum and minimum, fluorescence observed) to visualize GFP-labeled DU-145 cells 56 days after tail vein injection. Bottom panels, hematoxylin- and eosin-stained sections of lungs shown in top panel. The arrows indicate metastatic foci. Scale bars, 1 mm (white); 100 μm (black). D, representative images of murine lungs injected with vector-expressing (left) and C17orf37-expressing cells (right), and the corresponding GFP- and Ki-67-immunostained sections shown in the top panels. Scale bars, 100 μm. E, quantification of metastatic nodules in the lungs and expressed as fold lung metastasis, mean ± S.E. F, Ki-67-positive cells were counted, total five fields/section in vector (n = 5) and C17orf37 (n = 7), and expressed as the means ± S.E. Ab, antibody.
When DU-145 prostate cancer cells stably expressing GFP-fused C17orf37 (C17WT) (supplemental Fig. S3) were injected in athymic nude mice via tail vein, increased lung metastasis was observed 56 days postinjection compared with the DU-145 cells expressing GFP vector alone (Fig. 7C). Animals injected with C17WT showed enhanced lung colonization of tumor cells (Fig. 7C), increased number of metastatic lesions, and a ∼4-fold increase in lung metastasis (Fig. 7, D and E). Two mice injected with DU-145 expressing C17WT cells died 50 days postinjection because of increased lung metastasis (animals 3 and 7) (supplemental Fig. S5A). Microscopic examination of the lung sections from animals injected with C17WT-expressing cells confirmed abundant expression of GFP-positive cells in the metastatic lesions, compared with few GFP-expressing cells in lung tissues of animal injected with vector alone (Fig. 7D, middle panels). Although C17orf37 expression does not significantly alter cancer cell proliferation and growth in DU-145 cells in vitro,4 we observed a ∼4-fold increase in Ki-67 staining (Fig. 7F) in metastatic lesions of the lungs expressing C17WT, compared with minimal stain in the vector alone (Fig. 7, D, bottom panel, and F). This increased proliferation may be attributed to the tumorigenic ability of parental DU-145 cells, suggesting that ectopic expression of C17orf37 enhanced the migratory and metastatic ability of the cells to colonize to the lungs, whereas proliferation and increased Ki-67 staining are due to the inherent malignant phenotype of DU-145 cells. Taken together, our findings strongly indicate that overexpression of C17orf37 functionally predisposes cells to increased migratory and metastatic phenotype and that the prenylation at CVIL motif is critical for the C17orf37-mediated effect.
DISCUSSION
Information concerning the importance of the novel gene C17orf37 in cancer biology is still emerging. Prompted by the finding that the gene is located in an important amplicon of chromosome 17q12 known as the “hot spot locus of cancer,” previous studies identified C17orf37 to be overexpressed in breast, colon, and prostate cancer (1, 2, 4) and postulated that it could play an important role in cancer. In an attempt to understand the post-translational modifications of C17orf37 that may regulate its function, we identified a consensus prenylation motif CVIL at the C-terminal end. Both in vitro and in vivo prenylation assays confirmed C17orf37 is a substrate for the GGTase-I enzyme that catalyzes addition of 20-carbon isoprenyl group to the cysteine residue of CVIL motif (Fig. 1). Pharmacologic inhibition of GGTase-I and genetic inhibition of Icmt and Rce1 enzymes revealed that post-translational modifications are required for membrane association of C17orf37 protein (Figs. 2 and 3). When this paper was under revision, Katz et al. (41) identified another motif at the N terminus of C17orf37 protein called immunoreceptor tyrosine-based activation motif, which is cross-phosphorylated at two tyrosine residues, and they show that phosphorylation is important for C17orf37 function. Therefore, it is quite evident that post-translational modification codes regulate the activity and stability of C17orf37 protein.
Functionally, C17orf37 protein enhances the migratory and invasive ability of prostate cancer cells. Mutation of the Cys residue that is the site of prenylation (C112S) results in a protein that fails to be modified by GGTase-I and is functionally inactive (Fig. 5). Through both in vitro and in vivo studies, we find that prenylated C17orf37 enhances the migratory and metastatic ability of tumor cells to increase their colonization to distant organs, whereas a prenylation-defective mutant of C17orf37 fails to do so (Fig. 7). Because prenylated C17orf37 promotes migratory ability not proliferation of cells, its role may be restricted to the intravasation and extravasation steps of tumor metastasis.
In a migrating cancer cell, C17orf37 preferentially localizes to the leading edge of the cell (4), which is comprised of protrusive structures formed by polymerization of actin cytoskeleton. Mechanistic studies reveal that prenylated C17orf37 modulates filopodia formation and facilitates directional migration of cancer cells (Fig. 6). Filopodia formation is a tightly regulated process mediated by cortical actin polymerization in response to stimuli. Several proteins have been identified to mediate the assembly and disassembly of actin, which includes small GTPases like Rho family of proteins, Rac1, and Arp2/3 (actin-related protein) (38). Interestingly, most of these proteins are post-translationally modified by isoprenyl group addition important for protein-protein interaction and GTP loading. Although C17orf37 protein does not have a canonical GTP-binding site, it will be important to identify how C17orf37 protein induces filopodia formation. Because it is known that C17orf37 can act as a signaling molecule through the PI3K/Akt axis and activate NF-κB downstream genes MMP-9, uPA, and VEGF, it will be interesting to find whether C17orf37-induced filopodium formation is a direct effect mediated by the Akt pathway or by other regulatory processes engaged by C17orf37.
Membrane is a vast dynamic structure composed of different classes of lipids. Our studies indicate that a majority of the prenylated C17orf37 protein localize to the membrane low in cholesterol composition (30), whereas the mutant C17orf37 accumulates in the cytosol. Recently, there has been a great debate in the field to better understand the raft and nonraft lipid compositions and re-evaluate our understanding of the membrane microdomains (42). Although it is clear that rafts are enriched in cholesterol and glycophosphatidylinositol (34), the lipid composition of non-cholesterol-rich membrane fraction is poorly understood (42). Studies in prostate cancer cells have that revealed protein kinase B/Akt located in different compartments of plasma membrane (5) regulates distinctive downstream signaling pathways. Our studies indicate that prenylated C17orf37 localizes outside the raft region (Fig. 4), and further studies are required to identify interactors of C17orf37 and lipid composition that favor its binding to the plasma membrane.
Collectively, the findings of the present study broaden our understanding regarding the importance of C17orf37 in metastatic progression of cancer and the implications of post-translational isoprenylation on the functional activity of the protein. The present data also indicate that membrane association of C17orf37 is dependent on its post-translational modification at the C-terminal prenylation domain, which facilitates cellular migration by inducing filopodia formation and subsequent dissemination of cancer cells to distant organs. Because metastasis of cancer cells increases the mortality rate of human carcinomas, it is plausible that therapeutic strategies targeting C17orf37 may prove to be clinically useful to impede metastasis.
Supplementary Material
Acknowledgments
We greatly appreciate the generous gifts of the Icmt+/+, Icmt−/−, Rce1+/+, and Rce1−/− MEFs from Dr. Stephen Young (UCLA) and Dr. Channing Der and Dr. Adrienne D. Cox (University of North Carolina at Chapel Hill). We thank the confocal microscopy, animal facility and histology facility of University of North Texas Health Science Center for technical assistance. We thank Dr. Bert O'Malley (Baylor College of Medicine) for supporting S. Dasgupta to perform some of the experiments in his laboratory.
This work was supported, in whole or in part, by National Institutes of Health Grant MD 001633 (to J. K. V.). This work was also supported by Department of Defense Grant PC081282 (to S. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
S. Dasgupta and J. K. Vishwanatha, unpublished observations.
- uPA
- urokinase-type plasminogen activator
- GGTase-I
- geranylgeranyltransferase-I
- FTase
- farnesyltransferase
- MEF
- mouse embryonic fibroblast
- GGPP
- geranylgeranyl diphosphate
- TIRF
- total internal reflection fluorescent
- MES
- 2-(N-morpholino)ethanesulfonic acid
- GGTI
- GGTase-I inhibitor
- FTI
- FTase inhibitor
- FPP
- farnesyl pyrophosphate.
REFERENCES
- 1. Kauraniemi P., Kallioniemi A. (2006) Endocr. Relat. Cancer 13, 39–49 [DOI] [PubMed] [Google Scholar]
- 2. Maqani N., Belkhiri A., Moskaluk C., Knuutila S., Dar A. A., El-Rifai W. (2006) Mol. Cancer Res. 4, 449–455 [DOI] [PubMed] [Google Scholar]
- 3. Evans E. E., Henn A. D., Jonason A., Paris M. J., Schiffhauer L. M., Borrello M. A., Smith E. S., Sahasrabudhe D. M., Zauderer M. (2006) Mol. Cancer Ther. 5, 2919–2930 [DOI] [PubMed] [Google Scholar]
- 4. Dasgupta S., Wasson L. M., Rauniyar N., Prokai L., Borejdo J., Vishwanatha J. K. (2009) Oncogene. 28, 2860–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cinar B., Mukhopadhyay N. K., Meng G., Freeman M. R. (2007) J. Biol. Chem. 282, 29584–29593 [DOI] [PubMed] [Google Scholar]
- 6. Kauraniemi P., Kuukasjärvi T., Sauter G., Kallioniemi A. (2003) Am. J. Pathol. 163, 1979–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang F. L., Casey P. J. (1996) Annu. Rev. Biochem. 65, 241–269 [DOI] [PubMed] [Google Scholar]
- 8. Casey P. J., Seabra M. C. (1996) J. Biol. Chem. 271, 5289–5292 [DOI] [PubMed] [Google Scholar]
- 9. Cushman I., Casey P. J. (2009) J. Biol. Chem. 284, 27964–27973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winter-Vann A. M., Casey P. J. (2005) Nat. Rev. Cancer 5, 405–412 [DOI] [PubMed] [Google Scholar]
- 11. Konstantinopoulos P. A., Karamouzis M. V., Papavassiliou A. G. (2007) Nat. Rev. Drug Discov. 6, 541–555 [DOI] [PubMed] [Google Scholar]
- 12. Roberts P. J., Mitin N., Keller P. J., Chenette E. J., Madigan J. P., Currin R. O., Cox A. D., Wilson O., Kirschmeier P., Der C. J. (2008) J. Biol. Chem. 283, 25150–25163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothermund C. A., Gopalakrishnan V. K., Eudy J. D., Vishwanatha J. K. (2005) BMC Urol. 5, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thissen J. A., Casey P. J. (1996) Anal. Biochem. 243, 80–85 [DOI] [PubMed] [Google Scholar]
- 15. Zhang F. L., Moomaw J. F., Casey P. J. (1994) J. Biol. Chem. 269, 23465–23470 [PubMed] [Google Scholar]
- 16. Woo J. T., Nakagawa H., Krecic A. M., Nagai K., Hamilton A. D., Sebti S. M., Stern P. H. (2005) Biochem. Pharmacol. 69, 87–95 [DOI] [PubMed] [Google Scholar]
- 17. Pei Z., Oey N. A., Zuidervaart M. M., Jia Z., Li Y., Steinberg S. J., Smith K. D., Watkins P. A. (2003) J. Biol. Chem. 278, 47070–47078 [DOI] [PubMed] [Google Scholar]
- 18. Ostrom R. S., Liu X. (2007) Methods Mol. Biol. 400, 459–468 [DOI] [PubMed] [Google Scholar]
- 19. Valastyan S., Reinhardt F., Benaich N., Calogrias D., Szász A. M., Wang Z. C., Brock J. E., Richardson A. L., Weinberg R. A. (2009) Cell 137, 1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Der C. J., Cox A. D. (1991) Cancer Cells 3, 331–340 [PubMed] [Google Scholar]
- 21. Peterson Y. K., Kelly P., Weinbaum C. A., Casey P. J. (2006) J. Biol. Chem. 281, 12445–12450 [DOI] [PubMed] [Google Scholar]
- 22. Sun J., Blaskovich M. A., Knowles D., Qian Y., Ohkanda J., Bailey R. D., Hamilton A. D., Sebti S. M. (1999) Cancer Res. 59, 4919–4926 [PubMed] [Google Scholar]
- 23. Carrico D., Ohkanda J., Kendrick H., Yokoyama K., Blaskovich M. A., Bucher C. J., Buckner F. S., Van Voorhis W. C., Chakrabarti D., Croft S. L., Gelb M. H., Sebti S. M., Hamilton A. D. (2004) Bioorg. Med. Chem. 12, 6517–6526 [DOI] [PubMed] [Google Scholar]
- 24. Axelrod D. (2001) J. Biomed. Opt. 6, 6–13 [DOI] [PubMed] [Google Scholar]
- 25. Ashby M. N. (1998) Curr. Opin. Lipidol. 9, 99–102 [DOI] [PubMed] [Google Scholar]
- 26. Kim E., Ambroziak P., Otto J. C., Taylor B., Ashby M., Shannon K., Casey P. J., Young S. G. (1999) J. Biol. Chem. 274, 8383–8390 [DOI] [PubMed] [Google Scholar]
- 27. Bergo M. O., Leung G. K., Ambroziak P., Otto J. C., Casey P. J., Gomes A. Q., Seabra M. C., Young S. G. (2001) J. Biol. Chem. 276, 5841–5845 [DOI] [PubMed] [Google Scholar]
- 28. Bergo M. O., Lieu H. D., Gavino B. J., Ambroziak P., Otto J. C., Casey P. J., Walker Q. M., Young S. G. (2004) J. Biol. Chem. 279, 4729–4736 [DOI] [PubMed] [Google Scholar]
- 29. Lin X., Jung J., Kang D., Xu B., Zaret K. S., Zoghbi H. (2002) Gastroenterology 123, 345–351 [DOI] [PubMed] [Google Scholar]
- 30. Mayor S., Rao M. (2004) Traffic 5, 231–240 [DOI] [PubMed] [Google Scholar]
- 31. Magee A. I., Seabra M. C. (2003) Biochem. J. 376, e3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prior I. A., Hancock J. F. (2001) J. Cell Sci. 114, 1603–1608 [DOI] [PubMed] [Google Scholar]
- 33. Zacharias D. A., Violin J. D., Newton A. C., Tsien R. Y. (2002) Science 296, 913–916 [DOI] [PubMed] [Google Scholar]
- 34. van Meer G. (2002) Science 296, 855–857 [DOI] [PubMed] [Google Scholar]
- 35. Magdalena J., Millard T. H., Machesky L. M. (2003) J. Cell Sci. 116, 743–756 [DOI] [PubMed] [Google Scholar]
- 36. Magdalena J., Millard T. H., Etienne-Manneville S., Launay S., Warwick H. K., Machesky L. M. (2003) Mol. Biol. Cell 14, 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chhabra E. S., Higgs H. N. (2007) Nat. Cell Biol. 9, 1110–1121 [DOI] [PubMed] [Google Scholar]
- 38. Mattila P. K., Lappalainen P. (2008) Nat. Rev. Mol. Cell Biol. 9, 446–454 [DOI] [PubMed] [Google Scholar]
- 39. Mallavarapu A., Mitchison T. (1999) J. Cell Biol. 146, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bradley M. O., Kraynak A. R., Storer R. D., Gibbs J. B. (1986) Proc. Natl. Acad. Sci. U. S.A. 83, 5277–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Katz E., Dubois-Marshall S., Sims A. H., Faratian D., Li J., Smith E. S., Quinn J. A., Edward M., Meehan R. R., Evans E. E., Langdon S. P., Harrison D. J. (2010) Br. J. Cancer 103, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shaikh S. R., Edidin M. A. (2006) Chem. Phys. Lipids 144, 1–3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.