Abstract
Non-structural protein 1 from influenza A virus, NS1A, is a key multifunctional virulence factor composed of two domains: an N-terminal double-stranded RNA (dsRNA)-binding domain and a C-terminal effector domain (ED). Isolated RNA-binding and effector domains of NS1A both exist as homodimers in solution. Despite recent crystal structures of isolated ED and full-length NS1A proteins from different influenza virus strains, controversy remains over the actual biologically relevant ED dimer interface. Here, we report the biophysical properties of the NS1A ED from H3N2 influenza A/Udorn/307/1972 (Ud) virus in solution. Several lines of evidence, including 15N NMR relaxation, NMR chemical shift perturbations, static light scattering, and analytical sedimentation equilibrium, demonstrate that Ud NS1A ED forms a relatively weak dimer in solution (Kd = 90 ± 2 μm), featuring a symmetric helix-helix dimer interface. Mutations within and near this interface completely abolish dimerization, whereas mutations consistent with other proposed ED dimer interfaces have no effect on dimer formation. In addition, the critical Trp-187 residue in this interface serves as a sensitive NMR spectroscopic marker for the concentration-dependent dimerization of NS1A ED in solution. Finally, dynamic light scattering and gel shift binding experiments demonstrate that the ED interface plays a role in both the oligomerization and the dsRNA binding properties of the full-length NS1A protein. In particular, mutation of the critical tryptophan in the ED interface substantially reduces the propensity of full-length NS1A from different strains to oligomerize and results in a reduction in dsRNA binding affinity for full-length NS1A.
Keywords: Mutant, NMR, RNA-binding Protein, Tryptophan, Viral Protein, Dimer Interface, Effector Domain, Influenza A Virus, NS1 Protein
Introduction
Influenza is a contagious respiratory illness in humans caused by influenza A and B viruses that results in an annual average of 36,000 deaths in the United States alone (www.cdc.gov/Flu). Influenza A viruses, infecting birds and mammals, periodically cause widespread pandemics, the most deadly of which was the 1918 “Spanish Flu” pandemic that claimed an estimated 50 million lives (1). In recent years, the appearance of new H5N1 and H1N1 influenza A viruses, known as “avian flu” and “swine flu,” respectively, has fueled fears of an impending deadly influenza pandemic in the 21st century. Moreover, the rapid emergence of influenza virus strains resistant to current antiviral drugs directed against influenza A neuraminidase and M2 ion channel accentuate the need for the development of new classes of influenza antivirals. Toward this end, several recent structural and functional studies of influenza proteins have illuminated new potential drug targets in influenza (2, 3).
One such target is the non-structural protein 1 of influenza A, NS1A, a key multifunctional virulence factor produced in the host infected cell that plays a critical role in evading the host antiviral response (4). This highly conserved protein is composed of two domains, the 73-residue N-terminal double-stranded RNA-binding domain (RBD)4 and the C-terminal (residues 86-end) effector domain (ED), joined by a flexible linker (Fig. 1). By binding non-specifically to dsRNA, the N-terminal RBD functions primarily to inhibit the interferon-induced 2′-5′ oligonucleotide A synthetase/RNase L pathway (5). The C-terminal ED binds a plethora of host cellular proteins, including the 30-kDa subunit of the cleavage and polyadenylation specificity factor (CPSF30) (6, 7), the p85β subunit of phosphatidylinositol 3-kinase (PI3K) (8, 9), protein kinase R (PKR) (10, 11), and human tripartite motif 25, TRIM25, the E3 ubiquitin ligase of the retinoic acid-inducible gene I (RIG-I) (12, 13). All of these activities of NS1A ultimately contribute to the ability of influenza virus to suppress host responses to infection, including interferon production and apoptosis, critical to the life cycle of the virus. The recent developments of novel NS1 antagonists (14) and attenuated influenza viruses containing altered NS1 genes (15) highlight the growing interest in this key viral protein for the design of a new generation of antiviral drugs and vaccines against influenza.
FIGURE 1.
Multiple sequence alignment of NS1A proteins from various influenza virus strains. Multiple sequence alignment of full-length NS1A from influenza A/Udorn/307/1972 (H3N2), influenza A/Puerto Rico/8/1934 (H1N1), influenza A/Duck/Alberta/60/1976 (H12N5), and influenza A/Vietnam/1203/2004 (H5N1) is shown. Conserved residues are shown in red. The RBD and ED domains are outlined in blue and green, respectively. Residue numbering and secondary structural elements from the structures of Ud NS1A RBD (PDB ID 1NS1) and Ud NS1A ED (PDB ID 3EE9) are shown above the sequence. The putative strand-strand and helix-helix ED dimer interfaces are boxed in yellow and blue, respectively. Triangles denote the residues mutated in this study, and the position of Trp-187, important for dimerization of NS1A ED, is marked by an asterisk.
Several structural studies on isolated NS1 RBD and ED domains, as well as the full-length protein, have been reported. The isolated RBD domains from both NS1A (16, 17) and NS1B (the NS1 protein of influenza B virus) (18) form unique six-helical head-to-tail symmetric homodimers featuring a conserved dsRNA-binding epitope (18), which binds the major groove of A-form dsRNA (19). The isolated effector domain of NS1A also forms a homodimer, with each monomer subunit adopting a novel α-helix β-crescent fold (20–22). However, there is some controversy concerning the exact nature of the dimer interface of NS1A ED. The ED of NS1A from H1N1 influenza A/Puerto Rico/8/1934 (PR8 NS1A) was reported to feature an antiparallel strand-strand dimer interface mediated by short β-strands encompassing residues 88–91, located near the N terminus of the domain (20). On the other hand, EDs from H12N5 influenza A/Duck/Alberta/60/1976 (Alb NS1A) and H3N2 influenza A/Udorn/307/1972 (Ud NS1A) exhibit a helix-helix dimer interface involving the C-terminal portion of the long α-helix in each subunit (21, 22). Mutation of a key tryptophan in this helix, Trp-187, was shown to cause monomer formation in solution by analytical gel filtration and static light scattering (21, 23). This same region of the protein forms part of a conserved hydrophobic pocket that binds to the F2F3 fragment of CPSF30 (24) in the tetrameric complex formed between pairs of isolated Ud NS1A EDs and F2F3 domains (25). Yet a third intermolecular ED-ED interface was reported in a recent crystal structure of a R38A/K41A double mutant of the full-length NS1A protein from the highly virulent H5N1 influenza A/Vietnam/1203/2004 (VN NS1A). This structure showed a more twisted variation of the PR8 NS1A ED dimer and featured several electrostatic interactions between the two ED chains (26).
Here, we present extensive biophysical and mutational studies on Ud NS1A ED that conclusively demonstrate that the effector domain from this strain adopts a helix-helix dimer interface in solution. Moreover, we show that the tryptophan at this interface, Trp-187, is critical for dimerization and serves as an excellent spectroscopic marker for the dimerization of this domain. Furthermore, we examine the role of the ED dimerization interface in properties of the full-length NS1A protein in solution. We demonstrate that this ED interface is critical for the concentration-dependent oligomerization of the full-length protein. We also show that this ED interface plays a role in modulating dsRNA binding. The full-length NS1A protein exhibits higher affinity in dsRNA binding than the RBD domain alone, indicating that the ED enhances dsRNA binding. Elimination of the ED interface by mutation of Trp-187 reduces this enhancement.
EXPERIMENTAL PROCEDURES
Sample Preparation
Constructs of wild type influenza A/Udorn/307/1972 NS1A(85–215), hereafter referred to as Ud NS1A ED, for NMR, static light scattering and sedimentation equilibrium studies were cloned with a C-terminal His6 affinity purification tag, expressed, and purified following the standard protocols of the Northeast Structural Genomics (NESG) consortium (27); the largely unstructured 22 C-terminal residues in Ud NS1A were omitted from all work conducted in this study. Briefly, Ud NS1A(85–215) was expressed in codon-enhanced BL21 (DE3) pMGK Escherichia coli cells and cultured in MJ9 minimal medium (28). For the production of U-13C,15N- and U-5%-13C,100%-15N-labeled samples, the medium contained (15NH4)2SO4 and d-[U-13C]glucose as the sole nitrogen and carbon sources. Triple-labeled [U-2H,13C,15N]Ud NS1A(85–215) was expressed in MJ9 medium containing (15NH4)2SO4, d-[U-2H,13C]glucose, and 2H2O. Production of triple-labeled NS1A ED containing protonated Tyr and Phe residues and Ile-δ1, Leu-δ, and Val-γ methyl groups, [U-2H,13C,15N; 1H-Ile-δ1,Leu-δ,Val-γ,Phe,Tyr]Ud NS1A(85–215), was carried out using MJ9 medium containing (15NH4)2SO4, [U-2H,13C]glucose, 2H2O, the precursors [U-13C4, 3,3-2H2]α-ketobutyrate and [U-13C5, 3-2H]α-ketoisovalerate, and U-13C,15N-labeled Tyr and Phe (29). Initial cell growth was carried out at 37 °C, and protein expression was induced at 17 °C by 1 mm isopropyl-β-d-thiogalactopyranoside. Expressed proteins were purified using an ÄKTAxpressTM (GE Healthcare) two-step protocol consisting of HisTrap HP affinity chromatography followed directly by HiLoad 26/60 Superdex 75 gel filtration chromatography. Tagless constructs of full-length influenza A/Udorn/307/1972 NS1A(1–215) and influenza A/Vietnam/1203/2004 NSIA(1–215), hereafter referred to as Ud NS1A and VN NS1A, respectively, for dynamic light scattering experiments were made as SUMO fusion proteins, which were cleaved with yeast SUMO protease and purified by size-exclusion chromatography (30). Single-residue mutations were cloned using the QuikChange site-directed mutagenesis kit (Stratagene) and expressed and purified in the same manner as the wild type proteins. Unless otherwise indicated, all protein samples for NMR spectroscopy, static and dynamic light scattering, and sedimentation equilibrium experiments were exchanged into a buffer containing 20 mm sodium phosphate, 100 mm NaCl, 50 mm arginine, 50 mm glutamic acid, 5 mm DTT, and 1% glycerol at pH 6.9. NMR samples of Ud NS1A ED and its mutants were prepared at protein concentrations ranging from 0.3 to 1.2 mm.
Sedimentation Equilibrium
Three concentrations (6, 12.5, and 25 μm) of wild type Ud NS1A ED were spun at four different speeds (18,000, 25,000, 32,000, and 40,000 rpm). Sedimentation profiles were monitored using the absorbance (280 nm) optical detection system on a Beckman XL-I analytical ultracentrifuge. Samples were spun until the root mean square deviation between consecutive scans, spaced 2 h apart, was less than the inherent noise of the optical system (∼0.02 absorbance units). The analysis was conducted at 20 °C.
The partial specific volume and extinction coefficient of the protein, as well as the density of the buffer, were determined using the program Sednterp (31). The data were processed using Sedfit and analyzed using Sedphat (32). The meniscus position was first optimized for each scan and then fixed for subsequent analysis. Each profile (one concentration at one speed) was initially analyzed using both the monomer model and the monomer-dimer model. In the monomer model, the molecular weight was treated as a floating parameter, and in the monomer-dimer model the Kd was treated as a floating parameter and the molecular weight was fixed at the theoretical value for a monomer. The Kd was determined by globally analyzing all 12 scans simultaneously.
Static Light Scattering
Static light scattering data for wild type and [W187R]Ud5 NS1A ED were collected on a miniDAWN (TREOS) light scattering instrument (Wyatt Technology) coupled with an analytical gel filtration column at λ = 690 nm. All measurements were performed at room temperature and with a flow rate of 0.5 ml/min. Data were analyzed using the ASTRA software package (Wyatt Technology).
NMR Spectroscopy
NMR spectra of Ud NS1A ED and its mutants were acquired on Bruker AVANCE 800- and 600-MHz spectrometers equipped with 5-mm TXI and 1.7-mm TCI cryoprobes, respectively, at 300 K and referenced to internal 2,2-dimethyl-2-silapentane-5-sulfonic acid. All multidimensional NMR spectra were processed with NMRPipe (33) and visualized using Sparky (34).
One-dimensional 15N T1 and T2 Relaxation Measurements
The rotational correlation time, τc, is the time for a protein to rotate one radian. For an approximately spherical globular protein, the rotational correlation time, τc, is related to its effective hydrodynamic radius (a), and thus its oligomerization state, according to the Stokes-Einstein equation (Equation 1), where η is viscosity and T is temperature.
 |
In the limit of slow molecular motion (τc ≫ 0.5 ns), the correlation time of a protein is related to the ratio of the longitudinal (T1) and transverse (T2) 15N relaxation times and nuclear frequency (νN) according to Equation 2 (35, 36).
 |
Here, global 15N T1 and T2 relaxation times were obtained using one-dimensional 15N-edited relaxation experiments (37) by fitting the integrated signal in the backbone amide 1H region of the spectrum as a function of delay time to an exponential decay. The correlation time is then estimated using Equation 2 and compared with a standard curve of τc versus protein molecular weight (Mr) obtained on a series of known monomeric proteins of varying size, taking into account isotopic enrichment and the presence of affinity purification tags, to assess the oligomerization state of the protein (38).
For wild type and mutant Ud NS1A ED samples, all one-dimensional 15N T1 and T2 relaxation measurements were acquired at 300 K on a Bruker AVANCE 800-MHz spectrometer with the following typical relaxation delays: T1 spectra, T = 100, 200, 300, 400, 600, 800, 1000, 1500, 2500, 3500, and 5000 ms, and with a relaxation delay of 5 s; T2 spectra with CPMG delays, T = 16, 32, 48, 64, 80, 96, 112, 128, 144, and 160 ms, and with a relaxation delay of 1.5 s. All 15N T1 and T2 relaxation data for the standard set of known NESG monomeric proteins were obtained on Bruker AVANCE 600- and 800-MHz spectrometers at 298 K. In all cases, 15N T1 and T2 relaxation times were extracted from plots of the decay in integrated 1HN intensity between δ ∼8.4 and 9.8 ppm versus delay time by fitting the curves with standard exponential equations using the program t1guide within TopSpin 2.0 (Bruker BioSpin).
NMR Assignments for Ud NS1A(85–215) and [W187R]Ud NS1A(85–215)
Complete 1H, 13C, and 15N resonance assignments for [W187R]Ud NS1A(85–215) were determined using conventional triple resonance NMR methods. Backbone assignments were made by AutoAssign 2.4.0 (39) using peak lists from two-dimensional 1H-15N HSQC and three-dimensional HNCO, HN(CA)CO, HN(CO)CA, HNCA, CBCA(CO)NH, and HNCACB spectra. Side chain assignment was completed manually using three-dimensional HBHA(CACO)NH, HCCH-COSY, HCCH-TOCSY and (H)CCH-TOCSY experiments on U-13C,15N-labeled [W187R]Ud NS1A(85–215). Stereospecific isopropyl methyl assignments for all Val and Leu residues were deduced from characteristic cross-peak fine structures in high resolution two-dimensional 1H-13C HSQC spectra of U-5%-13C,100%-15N-labeled [W187R]Ud NS1A(85–215) (40). Histidine tautomeric states were elucidated by two-dimensional 1H-15N heteronuclear multiple-quantum coherence (HMQC) spectroscopy (41). Resonance assignments were validated using the Assignment Validation Suite (AVS) software package (42) and deposited in the Biological Magnetic Resonance Bank (BMRB accession number 16376).
Near complete backbone and Cβ assignments for wild type Ud NS1A ED were obtained by automated assignment (39) of peak lists from two-dimensional 1H-15N TROSY-HSQC- and TROSY-based versions of three-dimensional HNCO, HN(CA)CO, HN(CO)CA, HNCA, HN(CO)CACB, and HNCACB spectra obtained on [U-2H,13C,15N]Ud NS1A(85–215); experiments featuring Cα/Cβ frequency labeling were performed with 2H decoupling. Isoleucine, leucine, and valine methyl assignments were confirmed by two-dimensional 1H-13C HSQC and three-dimensional 13C- and 15N-edited NOESY spectra of [U-2H,13C,15N; 1H-Ile-δ1,Leu-δ,Val-γ,Phe,Tyr]Ud NS1A(85–215).
1H, 15N Chemical Shift Perturbation Measurements
Composite changes in 1H and 15N backbone amide chemical shifts, Δδcomp, were computed from assigned 1H-15N TROSY-HSQC spectra of wild type and [W187R]Ud NS1A ED using Equation 3 (43).
 |
To minimize isotope and other effects, spectra were recorded on identically labeled protein samples (U-5%-13C,100%-15N) using the same experimental conditions (i.e. spectrometer, buffer, temperature).
Residual Dipolar Coupling Measurements
One-bond N-HN residual dipolar couplings, 1DNH, were obtained on U-5%-13C,100%-15N-labeled [W187R]Ud NS1A(85–215) aligned in 4.2% C12E5 (PEG, Sigma-Aldrich) using standard protocols (44). The residual dipolar couplings were determined from 1J(HN-N) scalar couplings measured from an interleaved pair of two-dimensional 1H-15N TROSY-HSQC acquisitions on isotropic and aligned samples (45).
NMR Structure Determination
The solution NMR structure of [W187R]Ud NS1A(85–215) was calculated using CYANA 3.0 (46, 47) supplied with peak intensities from three-dimensional simultaneous CN NOESY (48) and three-dimensional 13C-edited aromatic NOESY spectra (τm = 100 ms), together with broad (φ ± 30°; ψ ± 30°) dihedral angle constraints computed by TALOS+ (49) and 1DNH residual dipolar couplings for ordered residues. The 20 structures with the lowest target function out of 100 in the final cycle calculated were further refined by restrained molecular dynamics in explicit water with CNS 1.1 (50, 51) and the PARAM19 force field, with a neutral histidine Nδ1H tautomer defined at His-169. Structural statistics and global structure quality factors were computed using the PSVS 1.3 software package (52) and MolProbity 3.15 server (53). The global goodness of fit of the final structure ensembles with the NOESY peak list data and residual dipolar couplings was determined using the RPF analysis (54) and PALES (55) programs, respectively. The final refined ensemble of 20 structures (excluding the C-terminal His6) was deposited in the Protein Data Bank (PDB ID 2KKZ). Three-dimensional protein structure comparison with structures in the Protein Data Bank was conducted using the DaliLite version 3 server (56). All structure figures were made using PyMOL (59).
Dynamic Light Scattering Measurements
All dynamic light scattering experiments were performed on a DynaPro99 series D instrument (Wyatt Technology) at 20 °C at a wavelength of 824.3 nm and a scattering angle of 90°. Each protein sample (15 μl) was centrifuged (10 min at 13,000 × g) and loaded into a 1.5-mm cuvette. Autocorrelations for 20 s were collected, and the data were averaged for three groups of 20 repeats. Points with an intensity fluctuating greater than 15% from the average were eliminated. The scattering data were analyzed with DYNAMICS C5.25.44 software (Wyatt Technology). Final molecular weight values were normalized to the expected molecular weight of the full-length NS1A dimer.
Electrophoretic Mobility Shift Assays (EMSA)
Gel shift assays for dsRNA binding affinity of wild type Ud NS1A(1–215), [W187R]Ud NS1A(1–215), and Ud NS1A(1–73) were performed following previous protocols (57). Briefly, 140-bp dsRNA was prepared by annealing the sense and antisense transcripts of a globin sequence inserted into the pGEM1 vector. Samples of 32P-labeled dsRNA (10,000 cpm, ∼0.36 nm) were incubated with varying concentrations of NS1A protein on ice for 30 min in 20 μl of binding buffer (43 mm Tris, 50 mm KCl, 2.5 mm DTT, 8% glycerol, 0.5 μg/μl tRNA, 0.5 units/μl RNase inhibitor, pH 8.0). All samples were run at 4 °C on a 4% non-denaturing polyacrylamide gel (60:1) for 2–3 h at 150 V using 0.5× Tris-borate-EDTA as the running buffer.
RESULTS
Ud NS1A ED Forms a Weak Dimer in Solution
The equilibrium constant (Kd) for dimerization of wild type Ud NS1A(85–215) (ED) was determined by sedimentation equilibrium on samples at three protein concentrations spun at four different speeds (supplemental Fig. S1). For the analysis of the individual scans, the weight average Mr ranged from 19,000–21,000 g/mol, significantly greater than the theoretical molecular weight of a monomer (15,943 g/mol). Furthermore, the weight average Mr increased as a function of loading concentration, and there was an improvement in the quality of all of the fits, with the exception of one, when the monomer-dimer model was used instead of the monomer model. Collectively, these observations are in agreement with a system that is in equilibrium between monomer and dimer species. The Kd determined from the global analysis is 89.7 ± 1.8 μm.
Ud NS1A ED Dimerizes through a Helix-Helix Interface
15N T1 and T2 relaxation measurements afford a relatively simple means of assessing the oligomerization state of proteins in solution by providing a measure of the rotational correlation time (τc) of the molecule. A plot of τc determined by this approach versus Mr for Ud NS1A ED and various mutants (blue) as well as known monomeric proteins (red) of varying size is shown in Fig. 2A. Here, a value of τc, which lies on the standard monomer curve, corresponds to a monomer, whereas a point significantly above the curve is indicative of oligomerization. Wild type ED and several mutants, including Y89A, F103A, M106A, G184R, and R193A, all exhibit τc values consistent with dimer formation in solution. However, a handful of mutants, namely K110A, G184D, W187A, and W187R, exist as monomers according to their reduced τc values. When mapped onto the reported structures of NS1A ED, the mutations that abolish dimer formation, highlighted in Fig. 2B in red, all cluster within or near the helix-helix interface, consistent with the helix-helix dimer interface reported in the crystal structures of Alb and Ud NS1A effector domains (21, 22). On the other hand, mutating residues predicted to be important for dimer formation in the crystal structure of PR8 NS1A ED (i.e. Tyr-89 (20)) and full-length VN NS1A (i.e. Arg-193 (26)), shown in Fig. 2B in blue, have no effect on dimer formation. These results indicate that the helix-helix interface is the predominant dimer interface for NS1A ED in solution.
FIGURE 2.
Assessment of wild type and mutant Ud NS1A ED oligomerization state using 15N relaxation. A, plots of rotational correlation time (τc) determined from 15N T1 and T2 relaxation data as a function of protein molecular weight (MW). Blue, wild type Ud NS1A(85–215) and mutants (0.3–0.7 mm in pH 6.9 buffer, 300 K); red, known monomeric proteins solved in the NESG project (38). All NS1A ED samples were U-5%-13C,100%-15N-labeled except W187R-NC, U-13C,15N-labeled [W187R]Ud NS1A(85–215), and WT-ILVFY, [U-2H,13C,15N; 1H-Ile-δ1,Leu-δ,Val-γ,Phe,Tyr]Ud NS1A(85–215); additional isotopic enrichment produces an expected shift to longer τc values. B, effect of mutations on NS1A ED oligomerization state mapped onto literature structures of PR8 NS1A ED (left; PDB ID 2GX9 (20)) and Ud NS1A ED (right; PDB ID 3EE9 (22)). Side chains for mutated residues are colored as follows: red, mutants that are monomeric; yellow, mutants that result in either monomer or dimer formation; blue, mutants that are dimeric.
Using the near complete backbone 1H, 13C, and 15N resonance assignments obtained for wild type NS1A ED and its W187R mutant, we can assess the spectral changes due to dimer formation because the tryptophan mutation causes minimal perturbations to the monomer subunit structure (see below). Perturbations to the 1H-15N TROSY-HSQC spectra of wild type and [W187R]Ud NS1A ED across the sequence of the protein are shown in Fig. 3. Using a composite chemical shift change (Δδcomp) cut-off of 0.1 ppm, 1H/15N chemical shift perturbations predominantly map to the region encompassing the helix-helix interface, providing further evidence in support of the helix-helix interface as the predominant dimer interface for NS1A ED in solution.
FIGURE 3.
NMR chemical shift perturbations in Ud NS1A ED resulting from the mutation of Trp-187. A plot of combined 15N and 1H chemical shift perturbations as a function of residue between wild type Ud NS1A(85–215) and [W187R]Ud NS1A(85–215), obtained from 800 MHz 1H-15N TROSY-HSQC spectra of 0.3–0.4 mm U-5%-13C,100%-15N-labeled protein samples in pH 6.9 buffer at 300 K, is shown. Inset: residues with Δδcomp >0.1 ppm are mapped in red onto the dimer structure of Ud NS1A ED (PDB ID 3EE9 (22)) and cluster within or near the helix-helix dimer interface.
Concentration Dependence of Ud NS1A ED Dimerization
The concentration dependence of dimerization of wild type and [W187R]Ud NS1A ED was further investigated by analytical gel filtration accompanied by static light scattering (Fig. 4). At low protein concentrations (20 μm), both wild type and mutant Ud NS1A EDs behave as monomers in solution with molecular weights in the range expected for the monomer (Mr: wild type, 18,600; W187R, 17,200; expected, 15,900). At concentrations well above the Kd for dimer formation (400 μm), wild type ED shifts to higher molecular weight (Mr 20,000), whereas the W187R mutant remains largely monomeric (Mr 16,600), consistent with the one-dimensional 15N relaxation data presented in Fig. 2A. In addition, the measured molecular weight of wild type NS1A ED increases with increasing protein concentration (data not shown). Hence, the lower than expected molecular weight for the wild type NS1A ED dimer observed by this technique (23) may be attributed to the combination of sample dilution during the experiment and the relatively weak Kd of the dimer (see above).
FIGURE 4.
Analytical gel filtration and static light scattering data for wild type and [W187R]Ud NS1A ED. Data were collected at room temperature on [U-5%-13C,100%-15N]Ud NS1A(85–215) (black, 20 μm; red, 400 μm) and U-5%-13C,100%-15N-labeled [W187R]Ud NS1A(85–215) (blue, 20 μm; green, 400 μm) in pH 6.9 buffer. Note the shift to lower elution volume for the concentrated sample of wild type Ud NS1A ED, indicative of a higher molecular weight species.
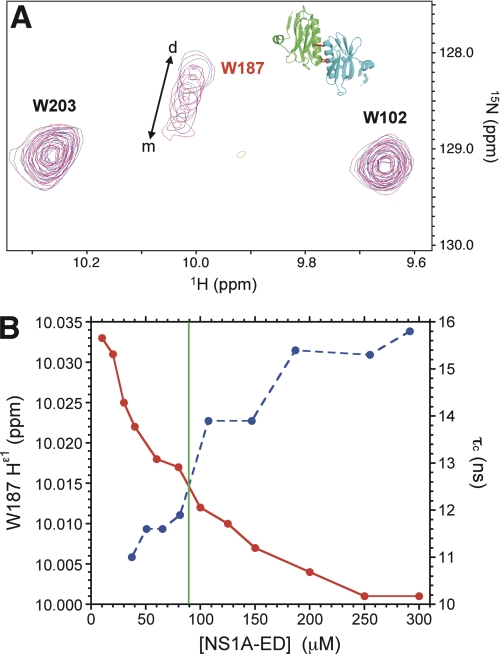
The concentration dependence of the Nϵ1Hϵ1 resonance of Trp-187 serves as an excellent marker of the oligomerization state of wild type NS1A ED. An overlay of the tryptophan Nϵ1Hϵ1 region from the 1H-15N HSQC spectra of Ud NS1A ED at varying protein concentrations is shown in Fig. 5A. The Nϵ1Hϵ1 resonance corresponding to Trp-187 shifts downfield in both 1H and 15N dimensions with decreasing concentration of NS1A ED, consistent with the location of this residue within the helix-helix dimer interface of this protein. The resonance positions of the other two Trp residues in the protein, which are distal to the helix-helix interface, remain unaffected in this experiment and serve as built-in controls. The downfield shift in Hϵ1 chemical shift with decreasing concentration of Ud NS1A ED correlates with a decrease in rotational correlation time, τc, and thus, the transition from dimer to monomer (Fig. 5B).
FIGURE 5.
Trp-187 is a spectroscopic marker for dimerization of Ud NS1A ED. A, overlay of 600 MHz 1H-15N HSQC spectra of Ud NS1A(85–215) at various protein concentrations (pH 6.9 buffer, 300 K) showing the tryptophan Hϵ1/Nϵ1 region. Inset: positions of Trp-187 in the dimer structure of Ud NS1A ED (PDB ID 3EE9 (22)). B, plots of Trp-187 Hϵ1 chemical shift (red) and τc (blue) as a function of Ud NS1A ED concentration. The green line indicates the Kd at ∼90 μm for dimer formation of Ud NS1A(85–215) determined by sedimentation equilibrium.
Solution NMR Structural Studies of [W187R]Ud and Wild Type Ud NS1A(85–215)
To elucidate the structural effects of mutating Trp-187 in NS1A ED, we determined the solution NMR structure of monomeric [W187R]Ud NS1A ED (Fig. 6A and supplemental Table S1). Consistent with reported NS1A ED structures in the literature (20–23), the structure of [W187R]Ud NS1A ED adopts a unique α-helix β-crescent fold (20), featuring a crescent-shaped six-stranded β-sheet cradling the long α-helix that encompasses the helix-helix dimer interface. The solution structure of [W187R]Ud NS1A ED is highly analogous to subunit structures in the crystal structure of the wild type Ud NS1A ED dimer (22) (supplemental Fig. S2), as well as the crystal structures of its W187A and W187Y mutant monomers (23) (Dali Z-scores, 19.7–20.5; Cα root mean square deviations, 1.0–1.7 Å). Hence, mutating Trp-187 causes minimal perturbation to the structure of the monomer subunit of Ud NS1A ED (23), and the spectroscopic effects described above are due to the equilibrium between the monomer and dimer states of this domain.
FIGURE 6.
Solution NMR studies of [W187R]Ud and wild type Ud NS1A(85–215). A, ribbon diagrams of the lowest energy (CNS) conformer from the final solution NMR structure ensemble of [W187R]Ud NS1A(85–215) (PDB ID 2KKZ). The α-helices and β-strands are shown in cyan and magenta, respectively. The side chain of the mutant Arg-187 is shown in red. For clarity, the highly disordered residues at the C terminus of the molecule (after Gly-204) have been omitted. B, strips from the 800-MHz three-dimensional 13C-edited (left) and 15N-edited (right) NOESY spectra of 0.43 mm [U-2H,13C,15N; 1H-Ile-δ1,Leu-δ,Val-γ,Phe,Tyr]Ud NS1A(85–215) showing intermolecular NOESY contacts at the helix-helix dimer interface of Ud NS1A ED. C, close-up view of the helix-helix dimer interface from the x-ray crystal structure of Ud NS1A ED (PDB ID 3EE9 (22)) showing intermolecular Val-180-Trp-187 side chain contacts.
Intermolecular proton-proton NOEs in the spectrum of wild type Ud NS1A ED, obtained using an ED concentration (0.43 mm) favoring dimer formation, are also consistent with a helix-helix dimer interface. In particular, NOE contacts between the Hϵ1 of Trp-187 and Hγ1 methyl of Val-180 are present in NOESY spectra of ILVFY-Ud NS1A ED (Fig. 6B). This is in agreement with the x-ray crystal structure of this dimer (PDB ID 3EE9), where an NOE-generating contact between these side chains is only possible between residues from different subunits (Fig. 6C).
Effects of Mutating Tryptophan in Full-length NS1A on Oligomerization
Next we addressed the question of the role of the helix-helix dimer interface of NS1A ED in properties of the full-length NS1A protein. First, we determined its role in the oligomerization of the full-length protein. It is well established that full-length NS1A is prone to aggregation at increasing protein concentration and is, for example, difficult to work with at concentrations required for structural characterization (26). Consequently, we examined the oligomerization properties of full-length wild type and [W187A]Ud NS1A in solution by dynamic light scattering (Fig. 7A). As expected, the effective molecular weight of wild type NS1A increases dramatically with increasing protein concentration. In fact, the protein begins falling out of solution prior to reaching a concentration of 100 μm. Strikingly, simply mutating Trp-187 to alanine drastically reduces the propensity of NS1A to oligomerize. This mutant form of full-length NS1A can therefore be concentrated to much higher levels than the wild type protein. Moreover, this phenomenon is not strain-specific because the same effect was observed for full-length VN NS1A (Fig. 7B). Wild type VN NS1A is highly aggregated even at the lowest concentration examined (30 μm), yet [W182A]VN NS1A shows little sign of aggregation at concentrations as high as 150 μm. These results dramatically demonstrate that the helix-helix interface of NS1A ED is critical to the oligomerization of the full-length protein in solution.
FIGURE 7.
Effects of mutating tryptophan on the oligomerization of full-length NS1A. Plots of effective molecular weight obtained from dynamic light scattering versus NS1A concentration are shown. A, Ud NS1A(1–215). Blue, wild type protein; red, [W187A] mutant. B, VN NS1A(1–215). Blue, wild type protein; red, [W182A] mutant. For both influenza strains, mutation of the conserved tryptophan dramatically reduces the aggregation property of full-length NS1A.
Effects of Mutating and Removing the Effector Domain on the dsRNA Affinity of Full-length NS1A
Finally, we investigated the role of the effector domain in the affinity of full-length NS1A for dsRNA. Gel shift experiments on 32P-labeled 140-bp dsRNA in the presence of varying amounts of wild type Ud NS1A(1–215), [W187R]Ud NS1A(1–215), and the RBD alone, Ud NS1A(1–73), which completely lacks the ED, are shown in Fig. 8. In these experiments, the lower band represents uncomplexed dsRNA, whereas the upper band constitutes fully complexed dsRNA. Completely removing the effector domain from the protein, to produce the intact 73-residue N-terminal NS1A RBD, results in an ∼5-fold reduction in dsRNA binding affinity (compare Fig. 8A and 8C), demonstrating that the ED of NS1A is required for highest affinity dsRNA binding. When the ED dimer interface is perturbed by mutating Trp-187 to Arg in the full-length protein, the enhancement of dsRNA binding by the ED is consistently reduced, by ∼2-fold (compare Fig. 8A and 8B), indicating that the helix-helix ED interface plays a role in dsRNA binding by the full-length protein. Moreover, the sharpness of the transitions observed in Fig. 8A and 8B as compared with Fig. 8C suggest higher cooperativity of dsRNA binding in the full-length NS1A protein due to ED-ED interactions.
FIGURE 8.
EMSA gel shift dsRNA binding experiments on full-length and truncated Ud NS1A. A, wild type Ud NS1A(1–215). B, [W187R]Ud NS1A(1–215). C, Ud NS1A(1–73). The protein monomer concentrations (in nm) are indicated in each lane. The experiment was repeated twice, yielding identical results each time. Approximate monomer protein concentrations required for 50% complex formation with dsRNA are as follows: wild type, 14 nm; [W187R]Ud mutant, 28 nm; NS1A RBD, 65 nm.
DISCUSSION
A number of important conclusions can be drawn from these biophysical studies carried out in solution on the NS1A ED and full-length NS1A proteins. First, the Kd for dimerization of the isolated Ud NS1A ED, accurately determined for the first time in this study by sedimentation equilibrium, is relatively weak (Kd ∼90 μm). This has important ramifications for drug development against influenza NS1A. Screening of effective potential antiviral drug candidates would be performed in the micromolar or submicromolar range, a range where our data clearly show that the isolated ED is largely monomeric in solution. Hence, this property of the NS1A ED obviates the need to design monomeric mutant EDs for small molecule ligand screening experiments (23).
Second, we have provided clear evidence from NMR (15N relaxation, chemical shift perturbation and NOE data) and static light scattering techniques that the helix-helix dimer interface is the relevant interface for ED dimer formation in solution. This conclusion is consistent with the x-ray crystal structures of Alb NS1A ED (21) and Ud NS1A ED (22). In addition, our results reveal that the critical Trp-187 residue at this interface, one from each monomer that juts into a deep hydrophobic pocket in the adjacent subunit (20–22), serves as a unique marker for monitoring ED dimer formation by NMR spectroscopy and can potentially function as a sensitive internal reporter in ligand screening experiments. This illustrates the power of NMR for obtaining accurate insights at an atomic level into the nature of protein-protein interactions in solution, especially for weak dimers. This is a particular problem in x-ray crystallography, where it is often difficult to discriminate between a biologically relevant interface and crystal-packing interfaces in crystal lattices (21). In fact, close inspection of the crystal structures of PR8 NS1A ED and full-length VN NS1A (20, 26) reveals that the helix-helix interface is present as an alternative interdomain interaction surface, which was interpreted as a crystal lattice interaction rather than a biologically relevant interaction. Our results demonstrate that this interpretation is not correct and that the helix-helix interface is in fact the correct ED dimerization surface in solution. Furthermore, the extremely high amino acid sequence conservation in the helix-helix dimer region (Fig. 1) supports our conclusion that this interface is the biologically relevant interface in solution across all influenza A virus strains. The high conservation of these interactions argues strongly against the notion that different viral strains utilize different ED dimer/oligomerization interfaces.
In addition, our results show that the helix-helix ED dimer interface plays a role in the properties of the full-length NS1A protein. The dynamic light scattering results on full-length NS1A proteins from two influenza A virus strains, Ud and VN, demonstrate that the same interface that functions in dimer formation in the ED alone is also critical for oligomerization of the full-length NS1A protein. Moreover, electrophoretic mobility shift assay data demonstrate that the ED dimer interface plays a role in dsRNA binding by the full-length NS1A protein. The ED enhances dsRNA binding affinity by ∼5-fold, and mutation of Trp-187 alone reduces this enhancement by ∼2-fold. The fact that the Trp-187 mutation does not eliminate the ED-mediated enhancement of dsRNA binding affinity indicates that residue Trp-187 in the ED dimer interface is not solely responsible for this enhancement and that other regions of the ED probably play a role in cooperative binding to dsRNA.
Finally, our results demonstrate that the same interface responsible for dimerization and oligomerization of the ED and full-length NS1A, respectively, includes the region of the NS1A protein that participates in binding to the F2F3 region of CPSF30 (25). This has significant biological consequences and leads us to propose a new model for the function of full-length NS1A in the host cell. Specifically, we propose that during dsRNA binding by the full-length NS1A protein, which is cooperative (Fig. 8A), the NS1A protein oligomerizes via intermolecular ED-ED complex formation mediated by the helix-helix interface (as illustrated in the schematic diagram of Fig. 9). The protein would form a tube, via alternating RBD and ED dimers tethered by a flexible linker, a concept previously proposed from analysis of the x-ray crystal structure of a mutated version of full-length VN NS1A that is incapable of binding dsRNA (26). Our model, however, is distinct from this previous model in a number of ways. In particular, based on the nature of the conserved dsRNA-binding surface of the RBD, we propose that the RBD is on the inside of the oligomer with its conserved tracks of basic residues determining the orientation of its interaction with dsRNA (Fig. 9, left inset), as established in recent structural studies of NS1A RBD-dsRNA complexes (18, 19). In fact, in the previous model, the orientation of the RBD with respect to the bound dsRNA is not consistent with the orientation of dsRNA relative to the conserved basic surface of the RBD defined by structures of NS1A RBD-dsRNA complexes (18, 19). Moreover, in our model, the CPSF30-binding pocket is buried because NS1A oligomerization is mediated by this same interface. At low NS1A concentrations, the intermolecular ED-ED interactions responsible for its oligomerization can dissociate, yielding a dimer that is linked only through the RBD and exposing the hydrophobic pocket in each ED for binding to CPSF30. Hence, we postulate that dsRNA binding with concomitant oligomerization of full-length NS1A and CPSF30 binding are mutually exclusive events regulated by the concentration of the NS1A protein, leading to the prediction that NS1A protein concentration plays an important role in the multiple spatial and temporal functions of the NS1A protein in infected cells.
FIGURE 9.
Working model for the oligomerization of NS1A upon binding to dsRNA. Views into (top) and along (bottom) the dsRNA helical axis of a model of the complex between full-length NS1A and dsRNA (black) are shown. The RBD and ED domains are rendered as cylinders and surfaces, respectively. Domains corresponding to full-length NS1A dimers (mediated by RBD-RBD interactions) are shown in similar colors; ED subunits are labeled. The model was made using the coordinates from the x-ray crystal structures of PR8 NS1A RBD-dsRNA complex (PDB ID 2ZKO (19)), preserving the known dsRNA binding-topology observed in this complex, and Ud NS1A ED (PDB ID 3EE9 (22)), featuring a helix-helix dimer interface, by overlaying multiple copies of the RBD-dsRNA complex coordinates onto canonical dsRNA and manually docking the ED coordinates onto separate RBDs. In this schematic model, the protein wraps around the dsRNA via its RBD, forming a tube with the dsRNA in the center. Each RBD dimer binds the major groove of dsRNA (19) via highly conserved tracks of amino acid residues from helices 2 and 2′ (18). The oligomer is held together by intermolecular contacts mediated by the helix-helix interface between adjacent ED domains. Left inset: view of the dsRNA-binding epitope of NS1A RBD. The relative orientation of the dsRNA parallel to the axes of helices 2 and 2′ is determined by highly conserved tracks of basic residues (18) and other polar dsRNA-binding residues (19) lining the dsRNA-binding face, shown in dark and light blue, respectively. Right inset: view of the intermolecular ED-ED interface (22). The side chains of the critical Trp-187 residues from each subunit are shown in red and indicated by an arrow.
Supplementary Material
Acknowledgments
We thank Paolo Rossi, Christopher Barbieri, Arthur Clark, Brian Radvansky, Alexander Lemak, and Rongjin Guan for valuable scientific discussions. Yeast SUMO protease was kindly provided by Prof. Steven Brill. Structures were determined through the Northeast Structural Genomics Consortium (NESG), which is supported by the National Institutes of Health through the PSI-Biology program of the Protein Structure Initiative.
Addendum
While this manuscript was under review, Kerry et al. (58) published an article (PLoS One 6, e17946) that strongly supports our hypothesis that the multiple functions of NS1A may be autoregulated by its oligomerization state.
This work was supported, in whole or in part, by National Institutes of Health Grant U54-GM094597 through the NIGMS Protein Structure Initiative (to G. T. M.) and National Institutes of Health Grants U01 AI074497 (to G. T. M. and R. M. K.) and R01 AI11772 (to R. M. K.).
The atomic coordinates and structure factors (code 2KKZ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. S1–S2.
Throughout this study, the designation [W187R]Ud indicates substitution where the mutation W187R is inserted in the Ud NS1A protein, and [W182A]VN indicates substitution where the mutation W182A is inserted in the VN NS1A protein.
- RBD
- RNA-binding domain
- ED
- effector domain
- HSQC
- heteronuclear single quantum coherence
- TROSY
- transverse relaxation optimized spectroscopy
- SUMO
- small ubiquitin-like modifier.
REFERENCES
- 1. Taubenberger J. K., Morens D. M. (2006) Emerg. Infect. Dis. 12, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krug R. M., Aramini J. M. (2009) Trends Pharmacol. Sci. 30, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das K., Aramini J. M., Ma L. C., Krug R. M., Arnold E. (2010) Nat. Struct. Mol. Biol. 17, 530–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hale B. G., Randall R. E., Ortín J., Jackson D. (2008) J. Gen. Virol. 89, 2359–2376 [DOI] [PubMed] [Google Scholar]
- 5. Min J. Y., Krug R. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nemeroff M. E., Barabino S. M., Li Y., Keller W., Krug R. M. (1998) Mol. Cell 1, 991–1000 [DOI] [PubMed] [Google Scholar]
- 7. Noah D. L., Twu K. Y., Krug R. M. (2003) Virology 307, 386–395 [DOI] [PubMed] [Google Scholar]
- 8. Hale B. G., Jackson D., Chen Y. H., Lamb R. A., Randall R. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14194–14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shin Y. K., Li Y., Liu Q., Anderson D. H., Babiuk L. A., Zhou Y. (2007) J. Virol. 81, 12730–12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Min J. Y., Li S., Sen G. C., Krug R. M. (2007) Virology 363, 236–243 [DOI] [PubMed] [Google Scholar]
- 11. Li S., Min J. Y., Krug R. M., Sen G. C. (2006) Virology 349, 13–21 [DOI] [PubMed] [Google Scholar]
- 12. Gack M. U., Albrecht R. A., Urano T., Inn K. S., Huang I. C., Carnero E., Farzan M., Inoue S., Jung J. U., García-Sastre A. (2009) Cell Host Microbe 5, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo R. L., Zhao C., Malur M., Krug R. M. (2010) Virology 408, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basu D., Walkiewicz M. P., Frieman M., Baric R. S., Auble D. T., Engel D. A. (2009) J. Virol. 83, 1881–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richt J. A., García-Sastre A. (2009) Curr. Top. Microbiol. Immunol. 333, 177–195 [DOI] [PubMed] [Google Scholar]
- 16. Chien C. Y., Tejero R., Huang Y., Zimmerman D. E., Ríos C. B., Krug R. M., Montelione G. T. (1997) Nature Struct. Biol. 4, 891–895 [DOI] [PubMed] [Google Scholar]
- 17. Liu J., Lynch P. A., Chien C. Y., Montelione G. T., Krug R. M., Berman H. M. (1997) Nature Struct. Biol. 4, 896–899 [DOI] [PubMed] [Google Scholar]
- 18. Yin C., Khan J. A., Swapna G. V., Ertekin A., Krug R. M., Tong L., Montelione G. T. (2007) J. Biol. Chem. 282, 20584–20592 [DOI] [PubMed] [Google Scholar]
- 19. Cheng A., Wong S. M., Yuan Y. A. (2009) Cell Res. 19, 187–195 [DOI] [PubMed] [Google Scholar]
- 20. Bornholdt Z. A., Prasad B. V. (2006) Nat. Struct. Mol. Biol. 13, 559–560 [DOI] [PubMed] [Google Scholar]
- 21. Hale B. G., Barclay W. S., Randall R. E., Russell R. J. (2008) Virology 378, 1–5 [DOI] [PubMed] [Google Scholar]
- 22. Xia S., Monzingo A. F., Robertus J. D. (2009) Acta Crystallogr. D Biol. Crystallogr. 65, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia S., Robertus J. D. (2010) Arch. Biochem. Biophys. 494, 198–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Twu K. Y., Noah D. L., Rao P., Kuo R. L., Krug R. M. (2006) J. Virol. 80, 3957–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Das K., Ma L. C., Xiao R., Radvansky B., Aramini J., Zhao L., Marklund J., Kuo R. L., Twu K. Y., Arnold E., Krug R. M., Montelione G. T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bornholdt Z. A., Prasad B. V. (2008) Nature 456, 985–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Acton T. B., Xiao R., Anderson S., Aramini J., Buchwald W. A., Ciccosanti C., Conover K., Everett J., Hamilton K., Huang Y. J., Janjua H., Kornhaber G., Lau J., Lee D. Y., Liu G., Maglaqui M., Ma L., Mao L., Patel D., Rossi P., Sahdev S., Shastry R., Swapna G. V., Tang Y., Tong S., Wang D., Wang H., Zhao L., Montelione G. T. (2011) Methods Enzymol. 493, 21–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jansson M., Li Y. C., Jendeberg L., Anderson S., Montelione G. T., Nilsson B. (1996) J. Biomol. NMR 7, 131–141 [DOI] [PubMed] [Google Scholar]
- 29. Goto N. K., Gardner K. H., Mueller G. A., Willis R. C., Kay L. E. (1999) J. Biomol. NMR 13, 369–374 [DOI] [PubMed] [Google Scholar]
- 30. Panavas T., Sanders C., Butt T. R. (2009) Methods Mol. Biol. 497, 303–317 [DOI] [PubMed] [Google Scholar]
- 31. Laue T. M., Shah B. D., Ridgeway T. M., Pelletier S. L. (1992) in Analytical Ultracentrifugation in Biochemistry and Polymer Science (Harding S., Rowe A., Horton J. eds) pp. 90–125, Royal Society of Chemistry, Cambridge, UK [Google Scholar]
- 32. Vistica J., Dam J., Balbo A., Yikilmaz E., Mariuzza R. A., Rouault T. A., Schuck P. (2004) Anal. Biochem. 326, 234–256 [DOI] [PubMed] [Google Scholar]
- 33. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 34. Goddard T. D., Kneller D. G. (2006) Sparky 3, University of California, San Francisco, CA [Google Scholar]
- 35. Kay L. E., Torchia D. A., Bax A. (1989) Biochemistry 28, 8972–8979 [DOI] [PubMed] [Google Scholar]
- 36. Fushman D., Weisemann R., Thüring H., Rüterjans H. (1994) J. Biomol. NMR 4, 61–78 [DOI] [PubMed] [Google Scholar]
- 37. Farrow N. A., Muhandiram R., Singer A. U., Pascal S. M., Kay C. M., Gish G., Shoelson S. E., Pawson T., Forman-Kay J. D., Kay L. E. (1994) Biochemistry 33, 5984–6003 [DOI] [PubMed] [Google Scholar]
- 38. Rossi P., Swapna G. V., Huang Y. J., Aramini J. M., Anklin C., Conover K., Hamilton K., Xiao R., Acton T. B., Ertekin A., Everett J. K., Montelione G. T. (2010) J. Biomol. NMR 46, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moseley H. N., Monleon D., Montelione G. T. (2001) Methods Enzymol. 339, 91–108 [DOI] [PubMed] [Google Scholar]
- 40. Neri D., Szyperski T., Otting G., Senn H., Wüthrich K. (1989) Biochemistry 28, 7510–7516 [DOI] [PubMed] [Google Scholar]
- 41. Pelton J. G., Torchia D. A., Meadow N. D., Roseman S. (1993) Protein Sci. 2, 543–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moseley H. N., Sahota G., Montelione G. T. (2004) J. Biomol. NMR 28, 341–355 [DOI] [PubMed] [Google Scholar]
- 43. Farmer B. T., 2nd, Constantine K. L., Goldfarb V., Friedrichs M. S., Wittekind M., Yanchunas J., Jr., Robertson J. G., Mueller L. (1996) Nat. Struct. Biol. 3, 995–997 [DOI] [PubMed] [Google Scholar]
- 44. Rückert M., Otting G. (2000) J. Am. Chem. Soc. 122, 7793–7797 [Google Scholar]
- 45. Kontaxis G., Clore G. M., Bax A. (2000) J. Magn. Reson. 143, 184–196 [DOI] [PubMed] [Google Scholar]
- 46. Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 47. Herrmann T., Güntert P., Wüthrich K. (2002) J. Mol. Biol. 319, 209–227 [DOI] [PubMed] [Google Scholar]
- 48. Pascal S. M., Muhandiram D. R., Yamazaki T., Forman-Kay J. D., Kay L. E. (1994) J. Magn. Reson. Ser. B 103, 197–201 [Google Scholar]
- 49. Shen Y., Delaglio F., Cornilescu G., Bax A. (2009) J. Biomol. NMR 44, 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 51. Linge J. P., Williams M. A., Spronk C. A., Bonvin A. M., Nilges M. (2003) Proteins 50, 496–506 [DOI] [PubMed] [Google Scholar]
- 52. Bhattacharya A., Tejero R., Montelione G. T. (2007) Proteins 66, 778–795 [DOI] [PubMed] [Google Scholar]
- 53. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang Y. J., Powers R., Montelione G. T. (2005) J. Am. Chem. Soc. 127, 1665–1674 [DOI] [PubMed] [Google Scholar]
- 55. Zweckstetter M. (2008) Nat. Protoc. 3, 679–690 [DOI] [PubMed] [Google Scholar]
- 56. Holm L., Kääriäinen S., Rosenström P., Schenkel A. (2008) Bioinformatics 24, 2780–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang W., Riedel K., Lynch P., Chien C. Y., Montelione G. T., Krug R. M. (1999) RNA 5, 195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kerry P. S., Ayllon J., Taylor M. A., Hass C., Lewis A., García-Sastre A., Randall R. E., Hale B. G., Russell R. J. (2011) PLoS One 6, e17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DeLano W. L. (2010) The PyMOL Molecular Graphics System, version 1.3r1, Schrödinger, LLC, New York [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.