Abstract
Winged-helix transcriptional factors play important roles in the control of gene expression in many organisms. In the plant pathogens Xylella fastidiosa and Agrobacterium tumefaciens, the winged-helix protein BigR, a member of the ArsR/SmtB family of metal sensors, regulates transcription of the bigR operon involved in bacterial biofilm growth. Previous studies showed that BigR represses transcription of its own operon through the occupation of the RNA polymerase-binding site; however, the signals that modulate its activity and the biological function of its operon are still poorly understood. Here we show that although BigR is a homodimer similar to metal sensors, it functions as a novel redox switch that derepresses transcription upon oxidation. Crystal structures of reduced and oxidized BigR reveal that formation of a disulfide bridge involving two critical cysteines induces conformational changes in the dimer that remarkably alter the topography of the winged-helix DNA-binding interface, precluding DNA binding. This structural mechanism of DNA association-dissociation is novel among winged-helix factors. Moreover, we demonstrate that the bigR operon is required for hydrogen sulfide detoxification through the action of a sulfur dioxygenase (Blh) and sulfite exporter. As hydrogen sulfide strongly inhibits cytochrome c oxidase, it must be eliminated to allow aerobic growth under low oxygen tension, an environmental condition found in bacterial biofilms, xylem vessels, and root tissues. Accordingly, we show that the bigR operon is critical to sustain bacterial growth under hypoxia. These results suggest that BigR integrates the transcriptional regulation of a sulfur oxidation pathway to an oxidative signal through a thiol-based redox switch.
Keywords: Crystal Structure, Protein-DNA Interaction, Protein Structure, Sulfur, Transcription Repressor, BigR, Blh, Hydrogen Sulfide, Hypoxia, Winged-helix Repressor
Introduction
Winged-helix proteins belong to a larger ensemble of helix-turn-helix (HTH)4 factors employed by living organisms to sense and respond to diverse environmental cues (1). For example, in bacterial cells, winged-helix transcriptional factors function as metal sensors, which control metal tolerance (2).
In the plant pathogens Xylella fastidiosa and Agrobacterium tumefaciens, the winged-helix repressor protein BigR (biofilm growth-associated repressor) controls the expression of the bigR operon through the recognition of the −10 region in the operator site, thus blocking transcription of the operon genes (3). bigR operons are evolutionarily conserved in some plant-associated bacteria and human opportunistic pathogens and encode BigR itself, membrane transporters, and Blh, a DUF442-β-lactamase domain protein related to the mitochondrial sulfur dioxygenase ETHE1 involved in sulfide detoxification in mammals (3, 4). Previous studies have shown that the bigR operon is actively transcribed in Xylella and Agrobacterium biofilms and that mutants lacking BigR can attach more tightly to glass and root surfaces than normal bacteria. Although these results strongly indicated that the bigR operon plays an important role in bacterial biofilm formation or cell adhesion, the biological function of the operon genes and the signals that modulate the repressor activity of BigR remained elusive (3).
Due to sequence similarities to bacterial metal sensors, BigR was designated a member of the ArsR/SmtB protein family. Nevertheless, the sequence homology to metalloregulatory repressors is restricted to the HTH DNA-binding domain, and BigR does not preserve the metal-binding sites usually found in the metal sensors (5). Moreover, the DNA binding activity of BigR, activation of the operon, and growth of the Agrobacterium bigR− and blh− mutants were not influenced by metal ions (3). These findings led us and others to propose that BigR and a related group of uncharacterized ArsR-like proteins (supplemental Fig. S1) comprise a new subfamily of winged-helix repressors (3, 6, 7).
In this new subfamily of winged-helix repressors, a conserved methionine (Met-18) and two invariant cysteines (Cys-42 and Cys-108) are predicted to be close in the structure, suggesting a structural or functional role in protein activity or regulation (3). Interestingly, the conserved cysteines, which are not present in the metal sensors, are found in the recently characterized SoxR regulator (supplemental Fig. S1) involved in sulfur oxidation (8). Thus, the similarities shared by BigR and Blh with SoxR and ETHE1, respectively, suggested a role of the bigR operon in sulfur metabolism.
Here we show how the conserved cysteines affect the DNA binding activity of BigR through a structural mechanism of DNA association/dissociation that is novel among the winged-helix repressors. In addition, we provide evidence suggesting that Blh is structurally related and functions as a glutathione-dependent sulfur dioxygenase similar to mouse ETHE1 and that its activity is coupled to a sulfite exporter. In summary, our results unveil the biological role of the bigR operon in hydrogen sulfide detoxification under oxygen-limiting conditions. These features may help bacterial cells to form thicker biofilms and to colonize plant tissues with low oxygen tension.
EXPERIMENTAL PROCEDURES
Protein Purification and Crystallization
Xylella BigR was initially purified by ion exchange and hydrophobic interaction chromatographies and crystallized as described previously (9). In an alternative purification protocol, protein extracts were loaded into a Q Sepharose fast flow in 20 mm Tris-HCl, pH 7.5, with 0.5 mm DTT. After elution, BigR was dialyzed in the same buffer, loaded into a Hi-Trap heparin HP column, and eluted with a linear NaCl gradient. The protein was further purified on a Superdex G75-16/60 column, and crystals were obtained by mixing 1 μl of protein solution (6.5 mg/ml in 20 mm Tris-HCl, pH 7.5, 50 mm NaCl, 0.5 mm DTT) with 1 μl of reservoir buffer (100 mm imidazole, pH 8.0, 10% PEG 8000).
X-ray Data Collection and Processing
X-ray diffraction data from native and Se-Met-labeled crystals were collected at the Brazilian Synchrotron Light Laboratory, and although data processing indicated that the crystals belonged to space group P321 (9), structure refinement could only be performed without imposing any crystal symmetry. Thus, diffraction data collected from Se-Met-labeled crystals at the wavelength corresponding to the maximum of f″ was reprocessed in the triclinic space group using the XDS package (10). This data set was chosen due to its higher completeness in space group P1 in comparison with the native data set. Statistics from this data set were labeled as oxidized BigR (see Table 1).
TABLE 1.
Data collection and refinement statistics
| Oxidized BigR | Reduced BigR | |
|---|---|---|
| Data statisticsa | ||
| Space group | P1 | P1 |
| Unit cell | a = 34.36 Å; b = 34.37 Å; c = 141.31 Å α = β = 90.01°; γ = 120.01° | a = 40.59 Å; b = 47.84 Å; c = 54.70 Å α = 90.03° β = 89.96°; γ = 105.34° |
| Wavelength (Å) | 0.9795 | 1.433 |
| Resolution range (Å) | 29.01–2.10 (2.22–2.10) | 39.10–2.50 (2.63–2.50) |
| No. of unique reflections | 30801 | 13185 |
| Multiplicity | 2.2 | 3.9 |
| Completeness (%) | 94.0 (92.4) | 93.8 (83.8) |
| <I/σ(I)> | 11.3 (2.8) | 13.2 (4.0) |
| Rmeas (%) | 6.7 (45.8) | 9.7 (43.7) |
| Refinement statistics | ||
| Resolution range (Å) | 18.59–2.10 | 20.86–2.50 |
| No. of reflections | 30782 | 13169 |
| Rwork/Rfree | 0.209/0.242 | 0.194/0.249 |
| r.m.s.d.b bond length (Å) | 0.010 | 0.010 |
| r.m.s.d.b bond angle (°) | 1.03 | 1.17 |
| Mean B-value protein (all atoms) (Å2) | 37.3 | 43.30 |
| Mean B-value solvent (Å2) | 41.3 | 44.86 |
| Protein atoms | 4603 | 2923 |
| Solvent atoms | 133 | 129 |
| Residues in Ramachandran plot regions (%) | ||
| Most favored | 94.25 | 99.18 |
| Allowed | 5.75 | 0.82 |
| Outliers | 0 | 0 |
a Numbers in parentheses are for the highest resolution shell.
b r.m.s.d., root mean square deviation.
The crystals produced by the alternative purification protocol were cryoprotected with 30% (v/v) PEG 400 added to the mother liquor prior to flash-cooling in a 100 K nitrogen stream. The crystals belonged to space group P1 and diffracted up to 2.5-Å resolution. The diffraction data corresponding to reduced BigR were processed with the XDS package, and the statistics are shown in Table 1.
Structure Solution and Refinement
The structure of the oxidized form of BigR was solved by multiwavelength anomalous diffraction method from Se-Met-labeled crystals. Initial phase calculation in the space group P3121 and density modification using autoSHARP (11) resulted in interpretable electron density maps. Nevertheless, although alternating cycles of model building using COOT (12) and refinement with REFMAC5 (13) allowed the modeling of 96 out of the 102 residues expected in the asymmetric unit (a single monomer), Rwork/Rfree values remained at 0.230/0.263, and the addition of water molecules caused the Rfree to rise. Refinement was done against both native and derivative data processed in the space group P3121, and the same problem was encountered. At this point, lower symmetry space groups were applied, and structure refinement could only be accomplished in P1. Diffraction data collected from the Se-Met-labeled crystal at the wavelength corresponding to the maximum of f″ were reprocessed in P1, and the structure refinement was performed with BUSTER 2.9.1 (14). Non-crystallographic symmetry was applied in all steps of the refinement using the local structure similarity restraints method implemented in the program BUSTER. The local structure similarity restraints method involves local distances between pairs of atoms instead of domain separation as in conventional superposition-based non-crystallographic symmetry treatments. The “autoncs” method in BUSTER automatically detects and applies the local structure similarity restraints. Alternating cycles of refinement and model building using COOT (12) allowed the modeling of 603 out of the 612 expected residues for the six monomers in the asymmetric unit. One hundred and thirty three solvent atoms were added during the last refinement cycles, and the R-factor/Rfree values converged to 0.209/0.242. The stereochemistry of the model was analyzed with MolProbity (15), and no outliers were observed in the MolProbity Ramachandran plot, which showed 94.3% of the residues in the most favored region. Additional refinement details are summarized in Table 1.
The structure of reduced BigR was solved by molecular replacement with the program PHASER (16) using the atomic coordinates of an E. coli repressor YgaV (PDB code 3CUO) (17) as the search model. BigR shares 29% identity and 55% similarity at the amino acid level with YgaV. Two copies of the polyalanine dimer used as model were placed in the asymmetric unit. Extensive attempts to use the oxidized form of BigR as a model for molecular replacement were unsuccessful, possibly due to the structure flexibility. Model refinement was performed by alternating cycles of BUSTER with visual inspection of the electron density maps and manual rebuilding with COOT. Non-crystallographic symmetry was applied in all steps of the refinement. A total of 385 residues were modeled out of the 408 expected for the four monomers in the asymmetric unit. The stereochemistry of the model was analyzed with MolProbity, and no outliers were observed in the Ramachandran plot with 99.2% of the residues in the most favored region. Additional refinement details are summarized in Table 1. Electrostatic potentials were calculated using the Adaptive Poisson-Boltzmann Solver (18) through the PDB 2PQR Server (19). The three-dimensional coordinates of oxidized and reduced BigR have been deposited in the Protein Data Bank (accession codes 3PQK and 3PQJ, respectively).
Molecular Modeling and Docking
The crystal structures of the Arabidopsis thaliana ETHE1 protein, 2GCU (20), and Escherichia coli rhodanese YnjE, 3IPP (21), were used as templates for restraint-based modeling of the Blh ETHE1-like and DUF442 domains, respectively, using MODELLER (22). Ten models were generated based on the alignment obtained by the prediction method pGenTHREADER from the PSIPRED server (23–25). Models were evaluated with the discrete optimized protein energy potential, and those with the lower global scores were selected for explicit solvent molecular dynamics simulation using GROMACS (26) to check their stability and consistency. The overall and local quality analyses of the final models were assessed by VERIFY3D (27), PROSA (28), and VADAR (29). Three-dimensional structures were displayed, analyzed, and compared using the program COOT (12).
GSH were modeled into the putative active site of the ETHE1-like domain of Blh using the Molegro Virtual Docker (30). Typical docking runs consisted of a single ligand with the ETHE1-like domain with no solvent molecules. The iron metal in the binding site was considered in the docking runs, taking into account steric (van der Waals) and electrostatic interactions. The docking procedure was randomized with a minimum of 10 runs and 5000 iterations per ligand. The best 10 poses (ligand orientation) generated were recalculated and reranked by analyzing the energy scores, binding affinities, and ligand-residue (H-bond) interactions. Video images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (31).
Protein Oxidization and Disulfide Bond Formation
E. coli cells expressing the His6-BigR protein were lysed in the presence of 30 mm oxidized glutathione (GSSG). The lysates were incubated for 30 min on ice, and the protein was purified by affinity chromatography (3). BigR samples were analyzed by mass spectrometry and gel-shift assays.
Molecular Mass Determination by Mass Spectrometry
Purified BigR with reduced and oxidized thiols were treated with 50 mm iodoacetamide and analyzed by a Q-ToF Ultima API spectrometer (Waters Corp.), operated in MS continuum mode. Data acquisition was from m/z 100–3.000 at a scan rate of 1 s and an interscan delay of 0.1 s. The spectra were accumulated over about 300 scans, and the multiple charged data produced on the m/z scale were converted to the mass scale using the maximum entropy-based software (32) supplied with the MassLynx 4.1 software package. The processing parameters were: output mass range was 6.000–20.000 Da at a resolution of 0.1 Da/channel, the simulated isotope pattern model was used with the spectrum blur width parameter set to 0.2 Da, and the minimum intensity ratios between successive peaks were 20% (left and right). The deconvoluted spectrum was smoothed (2 × 3 channels, Savitzky-Golay smooth), and the mass centroid values were obtained using 80% of the peak top and a minimum peak width at half-height of four channels.
Site-directed Mutagenesis and Gel-shift Assays
The Cys-42 and Cys-108 residues were each replaced by serines by site-directed mutagenesis, generating the C42S (M1) and C108S (M2) mutant proteins. The proteins, subcloned into pET28a, were expressed as His6 fusions, purified by affinity chromatography, and used in gel-shift assays as described previously (3), except that those treated with GSSG were also incubated with tris(2-carboxyethyl)phosphine prior to analysis.
Fluorometric Assays
GFP fluorescence as a measurement of the transcriptional activity of the operon was performed as described previously (3). Bacterial cells carrying the reporter plasmid (Blh promoter fused to GFP) were transformed with the wild type and mutated BigR proteins. Cells were grown in LB medium to A600 nm = 1.0, and the expression of the repressors was induced by 0.1 mm isopropyl-1-thio-β-d-galactopyranoside. After growth at 37 °C for 3 h, cells were collected, resuspended in PBS buffer, and lysed with lysozyme (0.2 mg/ml) in the presence of 10 mm GSSG for 2 h on ice. The suspension was disrupted by sonication, and GFP fluorescence was measured in the supernatant.
Growth of Agrobacterium Cells
The wild type A. tumefaciens strain C58 and the insertion mutants defective in BigR (bigR−) and Blh (blh−) production were grown in YEP medium, pH 7.5, as described previously (3), or in YEP supplemented with 50 mm MES buffer, pH 5.8. For growth in gradient plates, 3 μl of a bacterium suspension (A600 nm = 0.05) were spotted on each plate containing ammonium sulfide (0–1 mm), thiosulfate (0–25 mm), or GSSG (0–5 mm), and the cells were grown at 30 °C for 2–4 days.
Sulfite production by the bacterial cells was measured semiquantitatively using the sulfite test strips (Merckoquant, Merck), by visual comparison of the reaction zone of the test strip with the color scale provided by the manufacturer. Bacterial cells were grown at 30 °C for 16 h in buffered YEP, pH 5.8, supplemented with 0.5 mm ammonium sulfide. Cells were removed by centrifugation, and the amount of sulfite was measured in the supernatant.
A. tumefaciens wild type and insertion mutants were grown inside glass vials with screw-capped gas-tight seals. Three μl of the bacterial suspensions were streaked on the surface of the MES-buffered medium. The vials were closed tight and purged with 100% nitrogen at a flow rate of 25 ml/min using inlet and outlet needles. Bacterial cells were grown at 30 °C for different time periods under atmospheric oxygen or after a purge with 0.75 or 1.5 liters of nitrogen.
RESULTS
BigR Shows a Typical Winged-helix Fold but Two Redox States
To gain insights into the structure-function relationship of BigR and to elucidate the structural/regulatory role of the conserved cysteines in this type of winged-helix repressor, the crystal structure of BigR was solved. We observed that BigR purified by two independent procedures produced two types of protein crystals (Table 1). Surprisingly, the three-dimensional structures derived from these crystals revealed that although BigR adopts a typical winged-helix fold of homodimeric repressors of the ArsR family, the protein was found in two redox states. The structure of oxidized BigR revealed an intrachain disulfide bond between the conserved Cys-42 and Cys-108 residues that links helix 2 to helix 5 (Fig. 1, A and B). This observation strongly suggested that the invariant cysteines could indeed play a critical role in the structure and function of the repressor.
FIGURE 1.
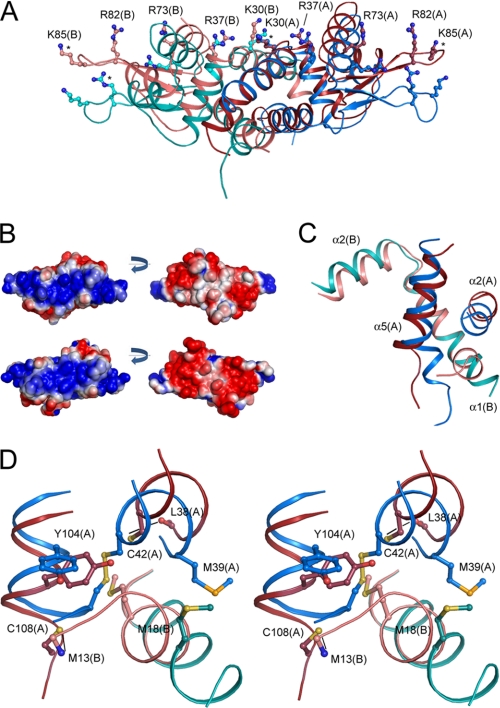
Structure and conformational changes of oxidized and reduced BigR. A, structural comparison of the oxidized (blue) and reduced (red) monomers. The intrachain disulfide bond is represented as sticks, and the corresponding cysteine residues are labeled. Secondary structure elements are indicated. B, 2Fo − Fc electron density map contoured at 1.2σ showing the disulfide between Cys-42 and Cys-108 in the oxidized monomer A. C, superposition of the BigR dimers. Light and dark colors are used to distinguish the homodimer subunits. The figure was produced by superposing the C-α atoms of residues 21–32 (helix 1) from both monomers. The distances between the Gln-67 C-α atoms of helix 4 from opposite subunits are indicated.
The Cys-42–Cys-108 Disulfide Bridge Induces Conformational Changes in BigR
When the structures of oxidized and reduced BigR were compared with each other, significant changes in the tertiary and quaternary structures of the repressor were observed. The main conformational changes between the two redox-state monomers occur in the N terminus of helix 1, β-hairpin wing, and C terminus of helix 5 (Fig. 1A). The loop preceding helix 4 also adopts a slightly different conformation. Most notably, however, in reduced BigR, the β-hairpin wing approaches helix 4 more closely, whereas in oxidized BigR, the disulfide bond induces a large displacement of helix 5 and introduces a small 310 helix comprising residues Cys-108–Glu-111 (Fig. 1A). In addition, the BigR quaternary structures show a remarkable reorientation of the HTH domain and β-hairpin wing of one monomer relative to the other upon the disulfide bond formation (Fig. 1C). The dimer of reduced BigR is much more compact. For instance, the distance between the C-α atoms of Gln-67 (helix 4) from opposite monomers goes from 34.8 Å in the oxidized structure to 31.2 Å in reduced BigR (Fig. 1C).
Oxidized BigR Does Not Bind to DNA, Allowing Transcription of Its Operon
The fact that BigR was found in two redox states strongly suggested that the Cys-42–Cys-108 disulfide bridge observed in the crystal structure was the basis of a DNA association/dissociation mechanism and transcriptional regulation of the operon. To test this hypothesis, BigR was expressed in the presence and absence of molar excess of GSSG to promote intrachain disulfide bond formation. Mass spectrometry analysis of the purified proteins revealed molecular mass peaks consistent with a mixture of reduced (S–H) and oxidized (S–S) forms of BigR on both GSSG-treated and untreated samples (supplemental Fig. S2), indicating that the GSSG treatment was effective to promote the intrachain disulfide bond formation.
Gel-shift assays showed that BigR from the GSSG-treated sample does not bind to its target DNA, except when in the presence of the reducing agent tris(2-carboxyethyl)phosphine (Fig. 2A). These results indicated that in the oxidized form, BigR loses its affinity to DNA. To confirm this, the Cys-42 and Cys-108 were each replaced by serines, and the DNA binding properties of the mutated proteins were evaluated. As expected, both the C42S and the C108S mutants shifted the target DNA, similar to the wild type BigR in its reduced form (Fig. 2B). In addition, the mutated proteins strongly repressed the transcription of a GFP reporter gene under the control of the BigR target promoter, similar to the wild type BigR (Fig. 2C). Because the mutated proteins cannot form an intrachain disulfide bond, the results confirm that BigR binds to DNA in its reduced form.
FIGURE 2.
Oxidized BigR ceases from binding to DNA and releases transcription. A, gel-shift assay showing that oxidized (S–S) BigR does not bind to the target DNA as reduced (S–H) BigR; however, binding is restored upon tris(2-carboxyethyl)phosphine (TCEP) treatment. Shifted bands are indicated by arrows, and FP is the free probe. B, gel-shift assay showing that both the C42S (M1) and the C108S (M2) mutants bind to the target DNA as the wild type (Wt) protein. Shifted bands are indicated by arrows, and FP is the free probe. C, GFP fluorescence as a measurement of the transcriptional activity of the bigR operon reporter plasmid alone (Rep) or in the presence of the wild type BigR, M1, or M2 proteins. D, GFP reporter gene assay of E. coli cell extracts expressing the wild type or mutated BigR proteins, in the presence (S–S) or absence (S–H) of GSSG. Error bars indicate S.E.
To show, however, that BigR releases transcription upon Cys-42–Cys-108 bond formation, the wild type and mutated proteins were expressed in E. coli carrying the operon reporter plasmid, and the expression of the reporter gene was quantified in response to the GSSG treatment. The results clearly show a significant increase in GFP fluorescence only in the GSSG-treated cell extracts expressing the wild type BigR, thus confirming that in the S–S form, BigR dissociates from DNA, allowing transcription of the operon (Fig. 2D).
The Structural Basis of the Molecular Switch and Redox-regulated DNA Binding
The crystal structures of oxidized and reduced BigR provide the structural basis of the redox-regulated DNA binding. In the oxidized dimer, the side chains of a number of basic residues from both monomers are exposed and arranged in a slightly less compact and continuous basic patch when compared with the reduced dimer (Fig. 3, A and B). These residues are from the HTH domain and β-hairpin wing and are predicted to bind DNA (33). Thus, in the more open conformation of oxidized BigR (Fig. 1C), the side chains of these basic residues are thought to retract from DNA. In addition, a closer look at the Cys-42 and Cys-108 environments reveals the molecular basis of the redox switch. In reduced BigR, the N terminus of helix 1 sits in between helices 2 and 5 of the opposite monomer (Fig. 3, C and D). Accordingly, the C terminus of helix 5 retracts, and the side chains of Cys-42 and Cys-108 are no longer in position to interact. Furthermore, in reduced BigR, the side chain of the conserved Met-18, which is stabilized by a hydrophobic interaction with Tyr-104 from the opposite monomer, occupies almost the exact position of the disulfide bond in the oxidized structure (Fig. 3D). The Cys-42 sulfur acts as a proton donor in a hydrogen bond with the carbonyl oxygen of Leu-38, whereas the Cys-108 sulfur forms a hydrogen bond with the main-chain amide of Met-13 from the opposite monomer. The Cys-108–Met-13 interaction contributes to the stabilization of the twisted conformation of helix 1 in reduced BigR, whereas in oxidized BigR, Met-18 is largely displaced and interacts with Met-39 from the opposite monomer. Met-39 is partially conserved in this subgroup of winged-helix repressors and is replaced in some repressors by a leucine, which conserves the hydrophobic character in this position (supplemental Fig. S1). In summary, a network of interactions involving sulfur-containing residues appears to regulate the redox-induced conformational changes in BigR. This observation, together with the fact that BigR and Blh are both similar to proteins involved in sulfur oxidation, led us to investigate a possible role of BigR and its operon in sulfur metabolism.
FIGURE 3.
The structural basis for the redox-regulated DNA binding in BigR. A, basic residues potentially involved in DNA interaction in oxidized (blue) and reduced BigR (red) are represented in ball-and-sticks format. Dark and light colors are used to distinguish monomers A and B, respectively. Residues marked with an asterisk are replaced by alanines in the refined structure, and their side chains were modeled to produce this image. B, electrostatic surface of reduced (top) and oxidized (bottom) BigR showing differences in the basic DNA-binding region as well as in the negatively charged surface of the opposite face of the dimers. The bonds for potential contour map visualization are ± 2 kT/e. C, comparison of the secondary structure elements in oxidized (blue) and reduced (red) BigR showing the N terminus of helix 1 in between helices 2 and 5 in the reduced structure. Dark and light colors correspond to monomers A and B, respectively. D, stereo view of the Cys-42 and Cys-108 neighborhood depicting a network of sulfur-containing residues.
The bigR Operon Is Required for Hydrogen Sulfide Detoxification
Despite the low sequence identity observed between the β-lactamase domain of Blh and the sulfur dioxygenase ETHE1, protein sequence alignments show that the amino acid residues that are involved in metal binding or that have been shown to affect the function of the human ETHE1 protein (20) are conserved in Blh (supplemental Fig. S3). Moreover, molecular modeling not only indicates that Blh is structurally related to ETHE1 but supports previous data showing that these are glutathione-dependent enzymes (34) (supplemental Fig. S3). Thus, to test whether Blh would play a role similar to mammalian ETHE1 in hydrogen sulfide oxidation, we employed the Agrobacterium blh− and bigR− mutants. Although the blh− mutant does not produce Blh, the bigR− mutant expresses high levels of the operon proteins (3). The Agrobacterium wild type and mutant cells were grown in the presence of bismuth (BiGGY agar), an indicator of hydrogen sulfide production. As sulfide combines with bismuth, brown to black pigmented colonies develop. In agreement with the operon regulation, the bigR− mutant produces fewer pigmented colonies when compared with the wild type bacteria (Fig. 4A), suggesting that when the operon is activated, hydrogen sulfide does not accumulate. By contrast, the blh− mutant grows much darker colonies in BiGGY agar than the wild type and bigR− cells, indicating that it accumulates higher levels of hydrogen sulfide.
FIGURE 4.
The bigR operon is required for hydrogen sulfide detoxification. A, growth of A. tumefaciens wild type and bigR− and blh− insertion mutants in BiGGY agar medium, showing that the blh− cells accumulate higher levels of hydrogen sulfide relative to wild type (wt) and bigR− mutant. B and C, growth of the wild type, bigR−, and blh− cells in thiosulfate (0–25 mm) or ammonium sulfide (0–1 mm) gradient plates, respectively. D, effect of pH on the toxicity of 1 mm ammonium sulfite relative to control (no ammonium sulfide). The growth of the blh− cells is affected by the acidic pH only. Bacterial cells were plated as indicated in A. E, sulfite levels in the culture supernatants of wild type, bigR−, and blh− cells estimated by the sulfite test strip, according to the scale. Culture medium without bacterial growth (control) is shown for comparison.
To further verify whether hydrogen sulfide affects bacterial growth, the A. tumefaciens wild type and mutant cells were grown under increased amounts of thiosulfate or ammonium sulfide, which at acidic pH generate hydrogen sulfide. It was found that although blh− cells are more sensitive to thiosulfate and ammonium sulfide, bigR− cells are able to tolerate higher amounts of both compounds relative to the wild type bacteria (Fig. 4, B and C), indicating that the bigR operon is important for hydrogen sulfide detoxification. The toxic effects of ammonium sulfide and thiosulfate are pH-dependent and were observed at pH ∼5.8, (Fig. 4D), which is close to the pH where molecular hydrogen sulfide predominates in solution. Because blh− cells accumulate higher levels of hydrogen sulfide, their growth is inhibited by the low pH (Fig. 4D).
These results indicated that Blh acts as a sulfur dioxygenase similar to mouse ETHE1, which oxidizes hydrogen sulfide to sulfur dioxide (4). Because sulfur dioxide readily interconverts into sulfite and one of the proteins of the bigR operon carries a TauE domain found in sulfite exporters (35), we tested whether the bacterial cells would export sulfite. Surprisingly, higher amounts of sulfite were detected in the culture supernatants of the bigR− relative to wild type and blh− cells in medium supplemented with ammonium sulfide (Fig. 4E), strongly indicating that when the operon is active, sulfite is exported. Taken together, the results show that the bigR operon is important for hydrogen sulfide detoxification through the action of a sulfur dioxygenase that operates in conjunction with a sulfite exporter.
The bigR Operon Is Critical to Sustain Growth under Hypoxia
Hydrogen sulfide is a potent inhibitor of cytochrome c oxidase (36). Because X. fastidiosa and A. tumefaciens are obligate aerobic organisms (37, 38), we thought that hydrogen sulfite accumulation due to metabolic processes might become a limiting factor for bacterial growth in environments of low oxygen tension, particularly in places where these organisms live. For instance, oxygen levels in vascular tissues and roots can vary considerably, depending on the organism, plant age, tissue type, and cortex architecture, and can be as low as 0.5% (39–43). To test whether the bigR operon plays an adaptive role under oxygen-limiting conditions, the A. tumefaciens wild type and mutant cells were grown under nitrogen-purged atmospheres. As observed in Fig. 5A, the growth of the blh− mutant was impaired in nitrogen-purged air, relative to the wild type and bigR− mutant. On the other hand, the bigR− mutant grew faster under nitrogen-purged air and recovered faster from lack of oxygen than the wild type and blh− mutant after subsequent cultivation in atmospheric oxygen (Fig. 5B). Thus, the results show that low oxygen tension is a limiting factor for the growth of hydrogen sulfide-producing bacteria and that the bigR operon, which detoxifies hydrogen sulfide, allows bacteria to survive in oxygen-limited environments.
FIGURE 5.
The bigR operon is critical for growth under oxygen-limiting conditions. A, growth of A. tumefaciens wild type and bigR− and blh− mutants under nitrogen-purged atmospheres (N2). Bacterial cells were grown for the time periods indicated either under atmospheric oxygen or after a purge of nitrogen. B, after incubation under nitrogen-purged air (0.75 or 1.5 liters of N2) for the time periods indicated, the flasks were opened, and the cells were further grown for 2–4 days to show that oxygen restored their growth. Atmos. O2, under atmospheric oxygen.
DISCUSSION
To successfully colonize the plant vascular tissue and to induce crown galls, which are made of numerous vascular bundles, X. fastidiosa and A. tumefaciens, respectively, have to adapt to these particular niches (44, 45). Here we show that the bigR operon in these pathogens, previously shown to influence bacterial biofilm formation (3), is required for hydrogen sulfide detoxification to allow bacterial growth under oxygen-limited conditions. Because hydrogen sulfide inhibits respiration, aerobic obligate bacteria such as Xylella and Agrobacterium must eliminate it to be able to grow under hypoxia, an environmental condition encountered by these pathogens in the interior of plant tissues (39, 41, 43). The data presented here indicate that hydrogen sulfide is oxidized to sulfite by the sulfur dioxygenase Blh and that sulfite, which is also toxic to the cells, is exported. This mechanism of hydrogen sulfide detoxification has not been reported before, and it highlights the adaptive role of the BigR operon in the colonization of the plant vascular tissues. In addition, production of hydrogen sulfide in bacterial biofilms may inhibit cell growth if oxygen levels in the biofilm layers are limited. This explains why the bigR operon is expressed at higher levels in Xylella and Agrobacterium biofilms (3). At least in the Xylella-citrus interaction, biofilm formation inside xylem vessels is the main cause of disease. Curiously, substantial quantities of sulfur were detected in Xylella biofilms inside the xylem vessels, and sulfur-linked structures on the surface of bacterial cells were suggested to promote bacterium adhesion and aggregation (44). Because the bigR− mutant appears to be more “sticky,” it is possible that the bigR operon may also favor bacterial aggregation by increasing the external sulfur contents through the export of sulfite.
Related BigR operons occur in other plant and human opportunistic pathogens, and in a number of cases, the DUF442 domain of Blh is found separate from the ETHE1-like domain, an indication that these domains have independent but coupled enzymatic activities (3). Although Blh seems to function as a sulfur dioxygenase, the precise role of DUF442 is still unknown. DUF442 is structurally related to protein-tyrosine phosphatases (46); however, the C-(X)5-R motif of the catalytic loop of classical protein-tyrosine phosphatases is replaced by C-(X)4-R in Blh. Thus, we searched for proteins having the C-(X)4-R consensus and found that rhodaneses (sulfurtransferases) have highly conserved active-site loops with a CRXGX(R/T) motif (47). Surprisingly, the superposition of the Blh DUF442 with the catalytic domain of E. coli rhodanese YnjE (21) shows that the two domains have a similar fold and a remarkable conservation of the active-site residues, including the catalytic cysteine (supplemental Fig. S4), suggesting that DUF442 could display a rhodanese-like activity. Considering that rhodaneses catalyze the transfer of sulfane sulfurs and have been implicated in cyanide detoxification (48), it is possible that by acting as a rhodanese, DUF442 could provide an extra protection against cytochrome c oxidase inhibition under low oxygen tension.
In addition to the role played by the bigR operon, the three-dimensional structures of the BigR repressor presented here show that although BigR has a typical winged-helix fold of homodimeric repressors, its DNA binding activity is modulated by the redox status of a cysteine pair. To our knowledge, this is the first report on a winged-helix repressor whose mechanism of DNA association/dissociation is controlled by a redox switch involving a disulfide bond. Thus, we propose that BigR and related proteins be considered as a new subfamily of HTH repressors, named redox switches. In these repressors, a disulfide bridge between helices 2 and 5 induces large conformational changes in the dimer that lead to a retraction of the DNA-binding structures, precluding DNA interaction.
Conformational changes in the quaternary structure of homodimeric wing-helix repressors were reported for the metal sensors SmtB and CzrA (2, 49). A comparison of the quaternary structures of BigR, SmtB, and CzrA shows that BigR in its open conformation (oxidized form) is more compact than both apo and zinc SmtB structures and becomes even more compact when reduced (closed conformation) (Fig. 6A). On the other hand, although the conformations of apo and zinc CzrA are virtually the same (49), the quaternary structures of zinc CzrA and CzrA bound to DNA are similar to the oxidized and reduced BigR structures, respectively (Fig. 6B). Thus, despite the fold similarities found between BigR and metal sensors, the dimer conformation associated with high DNA binding affinity does not appear to be conserved among these repressors because the closed conformation of SmtB bound to metal has the lower DNA binding affinity (2). Considering that charge distribution in the winged-HTH domain of BigR is not substantially altered between the low and high DNA binding affinity conformations (Fig. 3B), the binding-site topography and relative orientation of the winged-HTH domains become relevant for DNA regulation. In this respect, it is notable that BigR footprints a 22-bp palindrome extending ∼75 Å (3), a distance that matches more closely the length of the DNA-binding site of reduced BigR than the oxidized BigR, estimated to be ∼84 Å long. Furthermore, the consensus BigR box has two conserved TATA elements separated by ∼28 Å (3). Because the heads of helices 4, predicted to contact the major groove of the DNA (33, 49), are ∼31 Å apart in reduced BigR (Fig. 1C), it is reasonable to suggest that they would recognize the two TATA elements of the BigR box. Interestingly, the distance between the two winged-helix domains of the CzrA dimer in complex with its target DNA is slightly wider than that of reduced BigR (Fig. 6B), which is consistent with the fact that CzrA recognizes a longer 28-bp palindrome (49). Therefore, it appears that BigR and related proteins change the aperture of their winged-HTH domains not only to control their DNA binding affinity but also to match their target DNA sequences.
FIGURE 6.
Conformational changes of BigR and related metal sensors. A, superposition of the BigR and SmtB dimers showing the conformational changes in the quaternary structures of reduced (red) and oxidized (blue) BigR relative to the structures of SmtB in its apo (purple) and zinc-bound forms (pink). Oxidized BigR adopts a more compact conformation than the apo and zinc SmtBs, as judged by the distances between the C-α atoms of Gln-67 (34.8 Å) and equivalent His-78 in the apo (40.9 Å) and zinc SmtB (37.4 Å). B, superposition of BigR and CzrA dimers showing that oxidized and reduced BigR have quaternary conformations similar to zinc CzrA (orange) and CzrA bound to DNA (yellow), respectively, indicating that the more compact (closed) conformation associated with high DNA binding affinity is conserved between BigR and CzrA. The figures were produced by superposing the C-α atoms of helix 1 of both monomers.
Redox-sensitive transcriptional factors belonging to other protein families have been described. The prokaryotic OxyR and yeast Yap1, which play roles as hydrogen peroxide sensors, are also modulated by intramolecular disulfide bonds (50, 51). In the case of OxyR, the disulfide bond causes a structural change in the DNA-binding domain of the repressor, affecting DNA regulation (52). Similarly, reversal of disulfide bond formation between distant cysteines is an effective way to induce large conformational changes in the DNA-binding domain of BigR. The transition between the two BigR redox states can be viewed in the animation depicting the molecular movement displayed by the BigR dimer, which resembles a butterfly movement (supplemental Movie S1).
A question that remains unanswered is what oxidizes BigR. Structural comparisons between the reduced and oxidized dimers reveal that although there is a channel leading to the disulfide bridge in oxidized BigR, the unbound cysteines are inaccessibly buried in the structure of the reduced protein (supplemental Fig. S5). This helps to explain why BigR is easily reduced but difficult to oxidize. Although we have some clues of what factors might have contributed to the oxidation of BigR during its purification, how BigR is oxidized in vivo is presently unknown. Hydrogen sulfide is unlikely to be the oxidizing agent itself due to its redox potential. Nevertheless, its toxicity also involves the formation of reactive oxygen species as a consequence of the electron transport chain inhibition (53). We believe that hydrogen sulfide-induced reactive oxygen species could play a role in BigR oxidation. Thiol groups of redox-sensitive cysteines have characteristic pKa values as low as ∼3.5. Thus, at pH ∼5.8, where toxicity of hydrogen sulfide was observed, the thiolate anions are highly susceptible to oxidation by reactive oxygen species and can undergo various oxidative modifications including disulfide bonds (54). This idea is consistent with the fact that GSSG oxidized BigR in cell extracts and that blh− mutants are more sensitive to GSSG than the wild type and bigR− cells (supplemental Fig. S6). Alternatively, BigR could be a target of a thiol peroxidase similar to Gpx3, a hydroperoxide sensor that promotes the intramolecular disulfide bond that activates Yap1 (51). Proteins similar to Gpx3 exist in most Xylella and Agrobacterium strains.
In summary, we have described a novel winged-helix repressor that integrates the transcriptional regulation of a sulfur oxidation operon to an oxidative signal through a thiol-based redox switch. Furthermore, because the BigR operon is important for bacterial growth under hypoxia and influences biofilm formation, BigR could become a target to block the operon expression. Small ligands could be identified to either hold helix 1 in between helices 2 and 5 or directly prevent the disulfide bond formation, keeping the repressor bound to its target DNA. This approach might be useful to control biofilm formation in hydrogen sulfide-producing bacteria.
Supplementary Material
Acknowledgments
We gratefully acknowledge Thais Caroline Dallabona Dombroski and Simone Bau Betim for technical assistance in mass spectrometry and nitrogen gas experiments, respectively, and Jörg Kobarg and José Xavier Neto for critical reading of the manuscript.
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
The atomic coordinates and structure factors (codes 3PQK and 3PQJ) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and a supplemental movie.
- HTH
- helix-turn-helix
- BigR
- biofilm growth-associated repressor
- Blh
- β-lactamase-like hydrolase
- ArsR
- arsenic resistance repressor.
REFERENCES
- 1. Gajiwala K. S., Burley S. K. (2000) Curr. Opin. Struct. Biol. 10, 110–116 [DOI] [PubMed] [Google Scholar]
- 2. Eicken C., Pennella M. A., Chen X., Koshlap K. M., VanZile M. L., Sacchettini J. C., Giedroc D. P. (2003) J. Mol. Biol. 333, 683–695 [DOI] [PubMed] [Google Scholar]
- 3. Barbosa R. L., Benedetti C. E. (2007) J. Bacteriol. 189, 6185–6194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tiranti V., Viscomi C., Hildebrandt T., Di Meo I., Mineri R., Tiveron C., Levitt M. D., Prelle A., Fagiolari G., Rimoldi M., Zeviani M. (2009) Nat. Med. 15, 200–205 [DOI] [PubMed] [Google Scholar]
- 5. Busenlehner L. S., Pennella M. A., Giedroc D. P. (2003) FEMS Microbiol. Rev. 27, 131–143 [DOI] [PubMed] [Google Scholar]
- 6. Campbell D. R., Chapman K. E., Waldron K. J., Tottey S., Kendall S., Cavallaro G., Andreini C., Hinds J., Stoker N. G., Robinson N. J., Cavet J. S. (2007) J. Biol. Chem. 282, 32298–32310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu T., Chen X., Ma Z., Shokes J., Hemmingsen L., Scott R. A., Giedroc D. P. (2008) Biochemistry 47, 10564–10575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandal S., Chatterjee S., Dam B., Roy P., Das Gupta S. K. (2007) Microbiology 153, 80–91 [DOI] [PubMed] [Google Scholar]
- 9. Barbosa R. L., Rinaldi F. C., Guimarães B. G., Benedetti C. E. (2007) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 63, 596–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kabsch W. (1993) J. Appl. Crystallogr. 26, 795–800 [Google Scholar]
- 11. Vonrhein C., Blanc E., Roversi P., Bricogne G. (2006) in Macromolecular Crystallography Protocols (Doublié S. ed) Vol. 2, pp. 215–230, Humana Press, Totowa, NJ [Google Scholar]
- 12. Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 13. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 14. Bricogne G., Blanc E., Brandl M., Flensburg C., Keller P., Paciorek W., Roversi P., Smart O. S., Vonrhein C., Womack T. O. (2009) BUSTER, version 2.9.1 Global Phasing Ltd., Cambridge, UK [Google Scholar]
- 15. Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., Read R. J. (2007) J. Appl. Crystallogr. 40, 658–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gueuné H., Durand M. J., Thouand G., DuBow M. S. (2008) Appl. Environ. Microbiol. 74, 1954–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baker N. A., Sept D., Joseph S., Holst M. J., McCammon J. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dolinsky T. J., Nielsen J. E., McCammon J. A., Baker N. A. (2004) Nucleic Acids Res. 32, W665–W667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCoy J. G., Bingman C. A., Bitto E., Holdorf M. M., Makaroff C. A., Phillips G. N., Jr. (2006) Acta Crystallogr. D. Biol. Crystallogr. 62, 964–970 [DOI] [PubMed] [Google Scholar]
- 21. Hänzelmann P., Dahl J. U., Kuper J., Urban A., Müller-Theissen U., Leimkühler S., Schindelin H. (2009) Protein Sci. 18, 2480–2491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiser A., Sali A. (2003) Methods Enzymol. 374, 461–491 [DOI] [PubMed] [Google Scholar]
- 23. Jones D. T. (1999) J. Mol. Biol. 292, 195–202 [DOI] [PubMed] [Google Scholar]
- 24. Bryson K., McGuffin L. J., Marsden R. L., Ward J. J., Sodhi J. S., Jones D. T. (2005) Nucleic Acids Res. 33, W36–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lobley A., Sadowski M. I., Jones D. T. (2009) Bioinformatics 25, 1761–1767 [DOI] [PubMed] [Google Scholar]
- 26. Lindahl E., Hess B., van der Spoel D. (2001) J. Mol. Model. 7, 306–317 [Google Scholar]
- 27. Eisenberg D., Lüthy R., Bowie J. U. (1997) Methods Enzymol. 277, 396–404 [DOI] [PubMed] [Google Scholar]
- 28. Wiederstein M., Sippl M. J. (2007) Nucleic Acids Res. 35, W407–W410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willard L., Ranjan A., Zhang H., Monzavi H., Boyko R. F., Sykes B. D., Wishart D. S. (2003) Nucleic Acids Res. 31, 3316–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomsen R., Christensen M. H. (2006) J. Med. Chem. 49, 3315–3321 [DOI] [PubMed] [Google Scholar]
- 31. Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. (2004) J. Comput. Chem. 25, 1605–1612 [DOI] [PubMed] [Google Scholar]
- 32. Ferrige A. G., Seddon M. J., Green B. N., Jarvis S. A., Skilling J., Staunton J. (1992) Rapid Commun. Mass. Spectrom. 6, 707–711 [Google Scholar]
- 33. Cook W. J., Kar S. R., Taylor K. B., Hall L. M. (1998) J. Mol. Biol. 275, 337–346 [DOI] [PubMed] [Google Scholar]
- 34. Rohwerder T., Sand W. (2003) Microbiology 149, 1699–1710 [DOI] [PubMed] [Google Scholar]
- 35. Weinitschke S., Denger K., Cook A. M., Smits T. H. (2007) Microbiology 153, 3055–3060 [DOI] [PubMed] [Google Scholar]
- 36. Lloyd D. (2006) Trends Microbiol. 14, 456–462 [DOI] [PubMed] [Google Scholar]
- 37. Wells J. M., Raju B. C., Hung H. Y., Weisburg W. G., Mandelco-Paul L., Brenner D. J. (1987) Int. J. Syst. Bacteriol. 37, 136–143 [Google Scholar]
- 38. Kanvinde L., Sastry G. R. (1990) Appl. Environ. Microbiol. 56, 2087–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thomson C. J., Greenway H. (1991) Plant Physiol. 96, 1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eklund L. (2000) Trees Structure and Function 14, 177–180 [Google Scholar]
- 41. van Dongen J. T., Schurr U., Pfister M., Geigenberger P. (2003) Plant Physiol. 131, 1529–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spicer R., Holbrook N. M. (2007) J. Exp. Bot. 58, 1313–1320 [DOI] [PubMed] [Google Scholar]
- 43. Armstrong W., Webb T., Darwent M., Beckett P. M. (2009) Ann. Bot. 103, 281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leite B., Ishida M. L., Alves E., Carrer H., Pascholati S. F., Kitajima E. W. (2002) Braz. J. Med. Biol. Res. 35, 645–650 [DOI] [PubMed] [Google Scholar]
- 45. Pavlovkin J., Okamoto H., Wächter R., Läuchli A., Ullrich C. I. (2002) J. Exp. Bot. 53, 1143–1154 [DOI] [PubMed] [Google Scholar]
- 46. Krishna S. S., Tautz L., Xu Q., McMullan D., Miller M. D., Abdubek P., Ambing E., Astakhova T., Axelrod H. L., Carlton D., Chiu H. J., Clayton T., DiDonato M., Duan L., Elsliger M. A., Grzechnik S. K., Hale J., Hampton E., Han G. W., Haugen J., Jaroszewski L., Jin K. K., Klock H. E., Knuth M. W., Koesema E., Morse A. T., Mustelin T., Nigoghossian E., Oommachen S., Reyes R., Rife C. L., van den Bedem H., Weekes D., White A., Hodgson K. O., Wooley J., Deacon A. M., Godzik A., Lesley S. A., Wilson I. A. (2007) Proteins 69, 415–421 [DOI] [PubMed] [Google Scholar]
- 47. Cipollone R., Ascenzi P., Visca P. (2007) IUBMB Life 59, 51–59 [DOI] [PubMed] [Google Scholar]
- 48. Wilson K., Mudra M., Furne J., Levitt M. (2008) Dig. Dis. Sci. 53, 277–283 [DOI] [PubMed] [Google Scholar]
- 49. Arunkumar A. I., Campanello G. C., Giedroc D. P. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18177–18182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Choi H., Kim S., Mukhopadhyay P., Cho S., Woo J., Storz G., Ryu S. E. (2001) Cell 105, 103–113 [DOI] [PubMed] [Google Scholar]
- 51. Delaunay A., Pflieger D., Barrault M. B., Vinh J., Toledano M. B. (2002) Cell 111, 471–481 [DOI] [PubMed] [Google Scholar]
- 52. Lee C., Lee S. M., Mukhopadhyay P., Kim S. J., Lee S. C., Ahn W. S., Yu M. H., Storz G., Ryu S. E. (2004) Nat. Struct. Mol. Biol. 11, 1179–1185 [DOI] [PubMed] [Google Scholar]
- 53. Eghbal M. A., Pennefather P. S., O'Brien P. J. (2004) Toxicology 203, 69–76 [DOI] [PubMed] [Google Scholar]
- 54. Brandes N., Schmitt S., Jakob U. (2009) Antioxid. Redox Signal. 11, 997–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.