Abstract
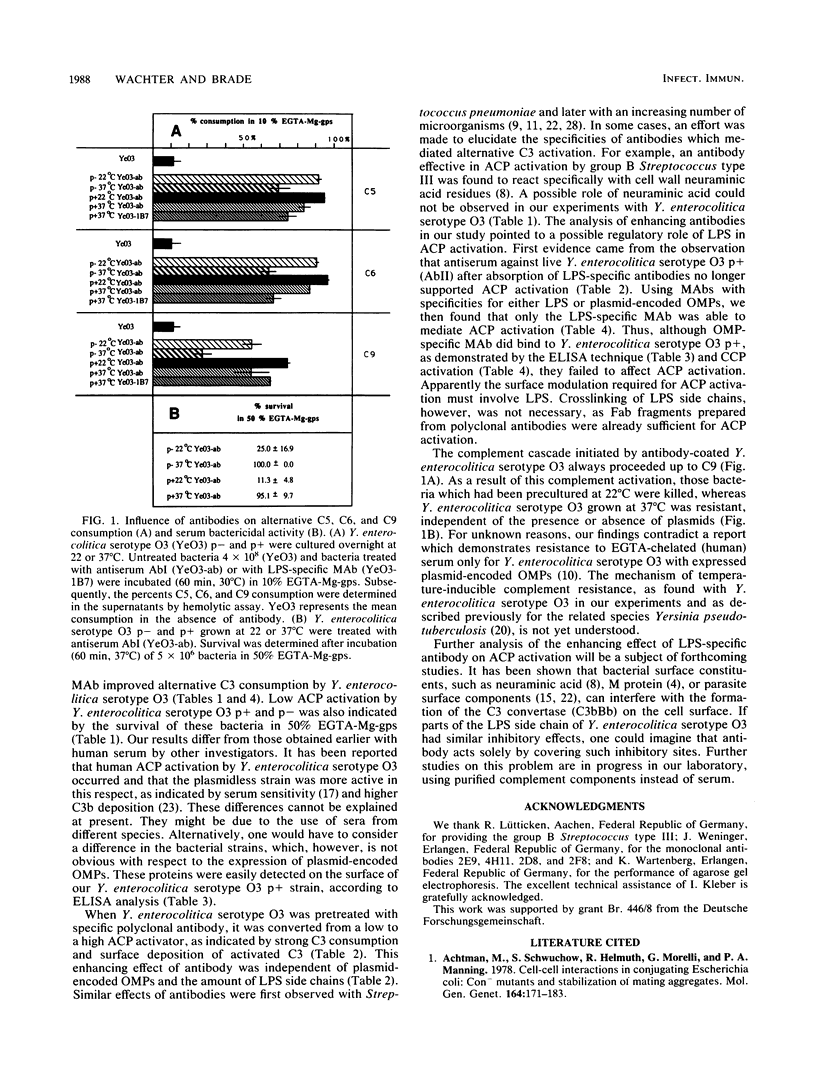
Effector mechanisms resulting from alternative complement pathway (ACP) activation cannot act efficiently against Yersinia enterocolitica serotype O3, as indicated by poor C3 to C9 consumption and by survival in EGTA (ethyleneglycoldiaminetetraacetic acid) Mg-serum. These results were not influenced by the lack or presence of plasmid-encoded outer membrane proteins or lipopolysaccharides (LPS) with different amounts of side chains or by treatment of the bacteria with pronase or neuraminidase. Surface modulation of Y. enterocolitica with polyclonal immunoglobulin G or the immunoglobulin G fragments F(ab')2 and Fab always converted Y. enterocolitica to a high ACP activator, with strong C3 to C9 consumption and surface deposition of activated C3. Killing of Y. enterocolitica as a result of antibody-mediated ACP activation was observed only with bacteria grown at 22 degrees C but not with bacteria from 37 degrees C cultures. The expression of complement resistance in Y. enterocolitica grown at 37 degrees C was not influenced by the presence or absence of plasmids. Using different monoclonal antibodies (MAb), we found that MAb with LPS specificity mediated ACP activation, whereas MAb specific for different plasmid-encoded outer membrane proteins were ineffective, despite surface binding. These results suggest a major inhibitory role of LPS on ACP activation which was neutralized by LPS-specific antibodies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G., Wartenberg K., Knapp W. Zuckerzusammensetzung des Lipopolysaccharids und Feinstruktur der äusseren Membran (Zellwand) bei Yersinia enterocolitica. Zentralbl Bakteriol A. 1980;247(2):229–240. [PubMed] [Google Scholar]
- Beuscher H. U., Brade V. C3 activation by a new factor B-dependent enzyme detected in culture supernatant from guinea-pig peritoneal macrophages. Immunology. 1986 Aug;58(4):545–551. [PMC free article] [PubMed] [Google Scholar]
- Bisno A. L. Alternate complement pathway activation by group A streptococci: role of M-protein. Infect Immun. 1979 Dec;26(3):1172–1176. doi: 10.1128/iai.26.3.1172-1176.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Laroche Y., Balligand G., Sory M. P., Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987 Jan-Feb;9(1):64–87. doi: 10.1093/clinids/9.1.64. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Keller C., Morawa R., Schmidt N., Siemens H. J., Laufs R. Plasmids of human strains of Yersinia enterocolitica: molecular relatedness and possible importance for pathogenesis. J Infect Dis. 1983 Jan;147(1):107–115. doi: 10.1093/infdis/147.1.107. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Goldman R. C., Hammer C. H., Leive L., Frank M. M. Studies of the mechanism of bacterial resistance to complement-mediated killing. V. IgG and F(ab')2 mediate killing of E. coli 0111B4 by the alternative complement pathway without increasing C5b-9 deposition. J Immunol. 1983 Nov;131(5):2563–2569. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marikovsky M., Arnon R., Fishelson Z. Proteases secreted by transforming schistosomula of Schistosoma mansoni promote resistance to killing by complement. J Immunol. 1988 Jul 1;141(1):273–278. [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangburn M. K. Activation of complement via the alternative pathway. Fed Proc. 1983 Jan;42(1):139–143. [PubMed] [Google Scholar]
- Papageorges M., Higgins R., Gosselin Y. Yersinia enterocolitica in two dogs. J Am Vet Med Assoc. 1983 Mar 15;182(6):618–619. [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkman S., Güther M. L., Yoshida N. Mechanism of resistance to lysis by the alternative complement pathway in Trypanosoma cruzi trypomastigotes: effect of specific monoclonal antibody. J Immunol. 1986 Sep 1;137(5):1623–1628. [PubMed] [Google Scholar]
- Tertti R., Eerola E., Lehtonen O. P., Ståhlberg T. H., Viander M., Toivanen A. Virulence-plasmid is associated with the inhibition of opsonization in Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Exp Immunol. 1987 May;68(2):266–274. [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wartenberg K., Weninger J., Röllinghoff M. Plasmids of Yersinia enterocolitica and Yersinia pseudotuberculosis: analysis with restriction endonucleases. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):213–219. doi: 10.1016/s0176-6724(88)80005-1. [DOI] [PubMed] [Google Scholar]
- Weninger J., Schoerner C., Wartenberg K., Röllinghoff M. Detection of cross-reacting epitopes on plasmid-encoded outer membrane proteins of enteropathogenic Yersinia by monoclonal antibodies. Med Microbiol Immunol. 1989;178(1):45–51. doi: 10.1007/BF00202291. [DOI] [PubMed] [Google Scholar]
- Winkelstein J. A., Shin H. S. The role of immunoglobulin in the interaction of pneumococci and the properdin pathway: evidence for its specificity and lack of requirement for the Fc portion of the molecule. J Immunol. 1974 May;112(5):1635–1642. [PubMed] [Google Scholar]
- Zaleska M., Lounatmaa K., Nurminen M., Wahlström E., Mäkelä P. H. A novel virulence-associated cell surface structure composed of 47-kd protein subunits in Yersinia enterocolitica. EMBO J. 1985 Apr;4(4):1013–1018. doi: 10.1002/j.1460-2075.1985.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



