Abstract
Chronic exposure to high glucose and fatty acid levels caused by dietary sugar and fat intake induces β cell apoptosis, leading to the exacerbation of type 2 diabetes. Oleic acid and linoleic acid are two major dietary fatty acids, but their effects in diabetes are unclear. We challenged β cell-specific glucokinase haploinsufficient (Gck+/−) mice with a diet containing sucrose and oleic acid (SO) or sucrose and linoleic acid (SL) and analyzed β cell apoptosis. In Gck+/− but not wild-type mice, SL significantly decreased the β cell mass and β cell proportion in islet cells arising from increased apoptosis to a greater degree than did SO. The mRNA expression of SREBP-1c was significantly higher, and that of E-cadherin was significantly lower in the islets of Gck+/− mice fed SL compared with mice fed SO. We next evaluated monotherapy with desfluorositagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, in these mouse groups. DPP-4 inhibitor protected against β cell apoptosis, restored the β cell mass, and normalized islet morphology in Gck+/− mice fed SL. DPP-4 inhibition normalized the changes in the islet expression of SREBP-1c and E-cadherin mRNA induced by the SL diet. Furthermore, linoleic acid induced β cell apoptosis to a greater degree in the presence of high glucose levels than in the presence of low glucose levels in vitro in islets and MIN6 cells, whereas a GLP-1 receptor agonist prevented apoptosis. In conclusion, SL exacerbated β cell apoptosis in diabetic Gck+/− mice but not in euglycemic wild-type mice, and DPP-4 inhibition protected against these effects.
Keywords: Apoptosis, Diabetes, Endoplasmic Reticulum (ER), Fatty Acid, Glucokinase, Pancreatic Islets, Peptide Hormones, Dipeptidyl Peptidase-4 (DPP-4), E-cadherin, Sterol Regulatory Element-binding Protein-1c (SREBP-1c)
Introduction
Decreased β cell mass as a result of increased apoptosis is an important characteristic of type 2 diabetes (1). A physiological animal model of β cell apoptosis is crucial for understanding the pathophysiology of diabetes and for developing new strategies for preventing the progression of diabetes. Dietary sugars and fat influence postprandial glucose and β cell function, thereby deteriorating glucose tolerance (2). Palmitic acid, oleic acid, and linoleic acid are the most abundant fatty acids among the total plasma fatty acids, plasma non-esterified fatty acids, plasma triacylglycerol, phospholipids, and plasma cholesteryl esters (3). Palmitic acid is a well known inducer of β cell lipotoxicity. However, the effects of oleic acid and linoleic acid, two major unsaturated fatty acids, on β cells remain obscure. We therefore selected two diet protocols, namely SO2 and SL, to examine the effects of oleic acid and linoleic acid on pancreatic islets (4). The main components of the SO diet are sucrose and oleic acid, whereas those of the SL diet are sucrose and linoleic acid. Both of these diets contain similar amounts of palmitic acids (4).
Desfluorositagliptin (DFS), a dipeptidyl peptidase-4 (DPP-4) inhibitor, acts by inhibiting the breakdown of many regulatory peptides including glucagon-like peptide-1 (GLP-1) (5). The clinically beneficial effects of DPP-4 inhibition on β cells cannot be fully explained by the increase in insulin release alone, and other mechanisms are thought to affect β cell mass and β cell apoptosis (6–9). Treatment with DFS increases the number of insulin-positive β cells in the islets, leading to the normalization of the β cell mass and the β cell-to-α/β cell ratio in mice with high fat diet-streptozotocin-induced diabetes, a mouse model with defects in insulin sensitivity and secretion (10). However, the beneficial effect of DPP-4 inhibition on nutrient-induced β cell apoptosis remains poorly understood.
Haploinsufficiency of β cell-specific glucokinase (Gck+/−) causes impaired insulin secretion in response to glucose in mice fed a standard chow diet despite the mice having a normal β cell mass (11, 12). In this study, we used Gck+/− mice to evaluate the impact of diet on β cell damage in the diabetic state and investigated whether DFS prevented nutrient-induced β cell apoptosis.
EXPERIMENTAL PROCEDURES
Animals and Animal Care
We backcrossed Gck+/− mice, which were generated by distruping the β cell- and brain-specific exon (11), with C57Bl/6J mice more than 10 times. Both the wild-type (WT) and Gck+/− mice were fed a standard chow diet (MF, Oriental Yeast, Tokyo, Japan) until 8 weeks of age and then were given free access to the experimental diets. All the experiments were conducted on male littermates. All the animal procedures were performed in accordance with the guidelines of the Animal Care Committee of Yokohama City University. Animal housing rooms were maintained at a constant room temperature (25 °C) and a 12-h light (7:00 a.m.)/dark (7:00 p.m.) cycle.
Diets
The compositions of the SO and SL diets are described in Table 1. The fat component of the SO and SL diets was derived from safflower oil and high oleic sunflower oil blended with perilla oil, respectively. The two diets were identical except for the type of fat used: oleic acid in the SO diet and linoleic acid in the SL diet. Both diets contained similar amounts of palmitic acids. The experimental diets were freshly prepared weekly. DFS was administrated orally by premixing with SL to a concentration of 1.1% (13).
TABLE 1.
Major components of experimental diets
The two diets were identical except for the type of fat used: linoleic acid in the SL diet and oleic acid in the SO diet.
| Diet |
||
|---|---|---|
| SL | SO | |
| Total milk protein and casein (g/kg diet) | 212.8 | 212.8 |
| Fat (g/kg diet) | 140.4 | 140.4 |
| Carbohydrate (g/kg diet) | 510.7 | 510.7 |
| Fatty acid composition (%) | ||
| Palmitic acid 16:0 | 7.3 | 6.7 |
| Stearic acid 18:0 | 2.6 | 4.2 |
| Oleic acid 18:1 (n-9) | 13.4 | 72.3 |
| Linoleic acid 18:2 (n-6) | 76.4 | 10.7 |
| α-Linolenic acid 18:3 (n-3) | 0.2 | 4.4 |
| Other fatty acids | 0 | 1.7 |
| Carbohydrate composition (%) | ||
| Sucrose | 70 | 70 |
| Dextrin | 30 | 30 |
| Total energy (kJ/g) | 17.8 | 17.8 |
In Vivo Physiological Studies
Plasma glucose levels and blood insulin levels were determined using Glutest Neo Super (Sanwa Chemical Co., Kanagawa, Japan) and an insulin kit (Morinaga Institute of Biological Science, Yokohama, Japan), respectively. Active GLP-1 was assayed using a glucagon-like peptide-1 (active) ELISA kit (Millipore).
Histological Analysis
More than 10 pancreatic tissue sections from each animal were analyzed after fixation and paraffin-embedding. The sections were immunostained with antibodies to insulin, CCAAT/enhancer-binding protein (C/EBP)-homologous protein (CHOP) (Santa Cruz Biotechnology), glucagon (Abcam), single-stranded DNA, Ki67 (Dako, Tokyo, Japan), and E-cadherin (Cell Signaling Technology). Biotinylated secondary antibodies, a VECTASTAIN Elite ABC kit, and a 3,3′-diaminobenzidine (DAB) substrate kit (Vector Laboratories) were used to examine sections using bright field microscopy to determine the β cell mass, and Alexa Fluor 488-, 555-, and 647-conjugated secondary antibodies (Invitrogen) were used for fluorescence microscopy. All images were acquired using a BZ-9000 microscope (Keyence) or Carl Zeiss LSM 510 confocal laser scanning microscope. The proportion of the area of pancreatic tissue occupied by β cells was calculated using BIOREVO software (Keyence) as described previously (12). Briefly, after staining a pancreatic section with insulin antibody, the insulin-positive area relative to the area of the whole pancreatic tissues was calculated for more than five sections per mouse. The immunofluorescence of E-cadherin, relative to insulin, was quantified using the mean gray value with NIH ImageJ software. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining was performed using the ApoAlert DNA Fragmentation Assay kit (Clontech). For CHOP, TUNEL, single-stranded DNA, and Ki67 staining, at least 100 islets per mouse were analyzed using BIOREVO software to assess the proportion of immunostained nuclei among the insulin-positive cells.
Real Time PCR
Islets were isolated as described elsewhere (12). Isolated islets were cultured overnight in RPMI 1640 medium containing 11 mm glucose supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin, and 100 μg/ml streptomycin. Total RNA was isolated from pancreatic islets using an RNase-free DNase and RNeasy kit (Qiagen). cDNA was prepared using the TaqMan reverse transcriptase kit (Applied Biosystems) and subjected to quantitative PCR by performing TaqMan Gene Expression Assays with Universal Master Mix (7500 real time PCR system, Applied Biosystems). All the probes were purchased from Applied Biosystems. Each quantitative reaction was carried out in duplicate. Data were normalized according to the β-actin level.
Immunoblotting
For immunoblotting, isolated islets (100 islets) or MIN6 cells were lysed in ice-cold radioimmune precipitation assay buffer (Cell Signaling Technology) with Complete protease inhibitor mixture (Roche Diagnostics). After centrifugation, the extracts were subjected to immunoblotting with antibodies to CHOP, activating transcription factor 4 (ATF4), C/EBP-β, phosphorylated eIF2α (Santa Cruz Biotechnology), Bcl-2, Bax, cleaved caspase-3 (Cell Signaling Technology), and β-actin (Sigma-Aldrich). Densitometry was performed using NIH ImageJ software.
Glucose-stimulated Insulin Secretion by Islets
Islets were isolated with Liberase RI (Roche Diagnostics). Immediately or after culturing for 12 h in RPMI 1640 medium containing 11 mm glucose supplemented with 10% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma), 10 islets were incubated at 37 °C for 1.5 h in Krebs-Ringer bicarbonate buffer containing 2.8, 8.3, or 22.2 mm glucose with or without 100 nm exendin-4 (Sigma). The insulin concentration of the assay buffer was measured using an insulin ELISA kit (Morinaga Institute of Biological Science).
Treatments of Islets or MIN6 Cells with Fatty Acid and Other Chemicals
Oleic acid, linoleic acid, and palmitic acid were purchased from Sigma. Isolated islets were incubated in RPMI 1640 medium (1000 or 2500 mg/liter glucose) with 0.25 mmol/liter fatty acid plus 0.5% (w/v) BSA or 0.5% (w/v) BSA alone as a control. MIN6 cells were incubated in DMEM (4500 or 1000 mg/liter glucose; Sigma) with 0.25 or 0.5 mmol/liter fatty acid plus 0.5% (w/v) BSA. The preparation of the free fatty acid media has been described elsewhere (14). Drugs were added to the regular growth medium. Exendin-4 was dissolved in PBS, and tunicamycin (Sigma) was dissolved in DMSO.
Analysis of Fatty Acid Composition
Total lipid was extracted from isolated islets (400 islets/sample) or fatty acid-treated MIN6 cells with H2O:chloroform:methanol (0.7:1:1, v/v) containing butylated hydroxytoluene as an antioxidant according to the method of Bligh and Dyer (15). The phospholipids were separated by preparative layer chromatography (silica gel 60F254, 2-mm thickness, Merck) and developed with a solvent of hexane:isopropanol (9:1, v/v). The phospholipid fractions were transmethylated with HCl/methanol at 100 °C for 2 h. The fatty acid methyl esters were separated in a gas-liquid chromatograph (GC-18A, Shimadzu, Kyoto, Japan) with a capillary column (SP2330, Supelco, Bellefonte, PA). Individual fatty acids were identified by comparing the retention time of each peak with those of external standards.
Propidium Iodide/DAPI and Annexin V Cell Death Assays
MIN6 cells were stimulated for 24 h with vehicle alone or with fatty acids at the indicated concentrations. For the last hour of incubation, 10 μg/ml propidium iodide was added directly to the medium. After the incubation, the cells were fixed and mounted on glass slides with VECTASHIELD with DAPI (Vector Laboratories). The cell images were acquired using a BZ-9000 microscope (Keyence). The percentage of cells that had died was calculated as the number of propidium iodide-stained nuclei divided by the total number of DAPI-stained nuclei multiplied by 100 using BIOREVO software (Keyence). At least 1000 cells were counted in each group. Annexin V staining was performed using an Annexin V-FITC Apoptosis Detection kit (Sigma) and analyzed using FACSCanto II (BD Biosciences). The proportions of Annexin V-FITC-positive and propidium iodide-negative apoptotic cell fractions were calculated using FACSDiva software (BD Biosciences).
Statistical Analyses
All the data are reported as the mean ± S.E. and were analyzed using a Student t test or analysis of variance. Differences were considered significant if the p value was <0.05.
RESULTS
Sucrose and Linoleic Acid Diet-induced β Cell Endoplasmic Reticulum (ER) Stress and Apoptosis in Gck+/− Mice
WT mice and Gck+/− mice fed an SO diet or an isocaloric SL diet for 25 weeks were evaluated. No significant differences in body weight gain, fasting blood glucose level, or food intake were observed between the SO group and the SL group of either genotype (16). After 25 weeks, the β cell mass (Fig. 1A) and relative β cell mass as a proportion of the total α cell plus β cell mass (Fig. 1B) were significantly lower in the Gck+/− mice fed the SL diet than in the Gck+/− mice fed the SO diet. The relative loss of β cells was associated with the abnormal distribution of α cells in the islets of the SL-fed Gck+/− mice (supplemental Fig. 1A). Of note, both alterations occurred despite a normal body weight and no exacerbation of insulin sensitivity in the Gck+/− mice (16), and such alterations were not seen in Gck+/− mice fed a high fat diet (HFD32, Clea Japan, Inc.) (12). A similar β cell mass and number of β cells as a proportion of all the islet cells were observed between the SO diet group and SL diet group of WT mice or insulin receptor substrate-1-deficient (IRS-1−/−) mice (Fig. 1, A and B, and data not shown). These results raise the possibility that hyperglycemia as well as fatty acid-induced lipotoxicity is required for β cell abnormalities to develop.
FIGURE 1.
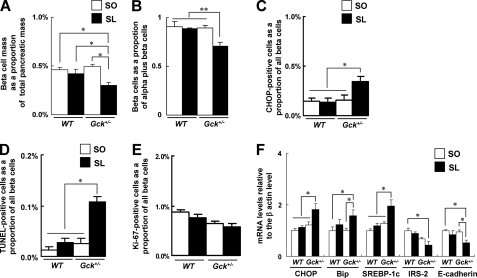
SL diet induced β cell loss, ER stress, and apoptosis in Gck+/− mice. A, β cell mass (n = 6–8). The β cell area is shown as a proportion of the area of the entire pancreas. B, quantification of β cell mass as a proportion of the total α cell plus β cell mass in the islet (n = 6). C, the number of CHOP-positive β cells as a proportion of the total number of β cells in the islet (n = 7). D, the proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the sections (n = 7). E, the proportion of Ki67-positive proliferating cells is shown as a percentage of the total number of insulin-positive cells in the sections (n = 7). D and E, more than 100 islets were counted in each mouse. F, the mRNA expression levels of the indicated molecules in the islets were determined using real time quantitative RT-PCR and were normalized to the level of β-actin mRNA (n = 5). The experiments were performed in WT and Gck+/− mice after 25 weeks on either the SO diet or the SL diet. White bars, SO; black bars, SL. Error bars indicate S.E. *, p < 0.05; **, p < 0.01.
β cell lipotoxicity is reportedly mediated by ER stress, which induces apoptosis (17). The transcription factor CHOP has been implicated as an essential factor in the ER stress response (18, 19). We assessed ER stress and apoptosis by immunostaining pancreatic β cells for CHOP and using TUNEL staining. More CHOP-positive nuclei and TUNEL-positive apoptotic nuclei were detected in Gck+/− mice fed the SL diet than in mice fed the SO diet (Fig. 1, C and D, and supplemental Fig. 1, B and C). To assess β cell proliferation, we counted the number of Ki67-positive cells; no significant differences in the number of Ki67-positive β cells were observed between the SO group and the SL group among either the WT or Gck+/− mice (Fig. 1E and supplemental Fig. 1D). To quantify differences in islet gene expression between the two groups, we measured the mRNA levels using real time PCR (Fig. 1F). Consistent with the results of our immunohistologic analysis, the expression levels of CHOP and immunoglobulin heavy chain-binding protein (Bip) were significantly higher in the Gck+/− mice fed the SL diet than in mice fed the SO diet. Sterol regulatory element-binding protein-1c (SREBP-1c) mRNA was significantly increased in the Gck+/− mice fed the SL diet compared with those fed the SO diet. IRS-2 mRNA expression was unaffected by either diet. We also investigated the level of expression of E-cadherin, an adhesion molecule linking cell-cell interactions in the endocrine pancreas (20), and noted a lower mRNA expression and a weaker protein expression on the surface of the islet cells of Gck+/− mice fed the SL diet compared with those fed the SO diet (supplemental Fig. 1E). By contrast, among the WT mice, no significant differences were observed in the expressions of CHOP, Bip, SREBP-1c, or E-cadherin according to the type of diet. Additionally, we simultaneously evaluated WT and Gck+/− mice fed a standard diet (MF, Oriental Yeast), which contains much lower oleic acid and linoleic acid than SO or SL diet. No significant differences in β cell mass and β cell apoptosis (TUNEL-positive β cells) were observed between the standard diet group and the SO diet group of either genotype (data not shown).
DFS Protected against Sucrose- and Linoleic Acid-induced β Cell ER Stress and Apoptosis in Gck+/− Mice
Not only does GLP-1 receptor signaling preserve the β cell mass in experimental diabetic states, it also directly modulates the ER stress response, promoting β cell adaptation and survival (21–23). To evaluate DFS, a DPP-4 inhibitor, as a treatment for diet-induced β cell damage, we performed a 20-week study of SL or SL plus 1.1% DFS diet-fed WT and Gck+/− mice. At the end of the 20-week DFS treatment period, glucose tolerance and insulin secretion after glucose loading were significantly improved by DFS monotherapy in Gck+/− mice (16).
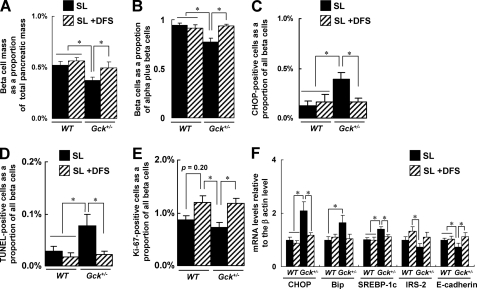
The treatment of SL-fed Gck+/− mice with DFS for 20 weeks produced a significant increase in the β cell mass (Fig. 2A) and the relative β cell mass as a proportion of the total α cell plus β cell mass (Fig. 2B), and the abnormal distribution of pancreatic α cells was also corrected (supplemental Fig. 2A). DFS significantly reduced the proportion of CHOP-positive nuclei and TUNEL-positive apoptotic nuclei among the β cells of Gck+/− mice (Fig. 2, C and D, and supplemental Fig. 2, B and C). The proportion of single-stranded DNA-positive apoptotic nuclei was also reduced by DFS (data not shown). At week 20, the proportion of Ki67-positive β cells in the islets of Gck+/− mice fed the SL diet plus DFS was much higher than in those fed the SL diet alone (Fig. 2E and supplemental Fig. 2D). Next, we examined the levels of CHOP, Bip, SREBP-1c, IRS-2, and E-cadherin mRNA expression in the islets (Fig. 2F). Lower levels of CHOP, Bip, and SREBP-1c were expressed in the islets of DFS-treated Gck+/− mice, but the decrease in Bip did not reach statistical significance. No significant differences in IRS-2 were observed in relation to treatment with DFS. The expression of E-cadherin restored the islet architecture to normal (supplemental Fig. 2E). The expression levels of glucokinase were not affected by DFS administration in mice of both genotypes (data not shown).
FIGURE 2.
DFS improved islet morphology, β cell mass, ER stress, and apoptosis induced in Gck+/− mice by SL diet. A, β cell mass (n = 5–7). The β cell area is shown as a proportion of the area of the entire pancreas. B, quantification of β cell mass as a proportion of the total α cell plus β cell mass in the islet (n = 6). C, the number of CHOP-positive β cells as a proportion of the total number of β cells in the islet (n = 7). D, the proportion of TUNEL-positive cells is shown as a percentage of the total number of insulin-positive cells in the sections (n = 5). E, the proportion of Ki67-positive proliferating cells is shown as a percentage of the total number of insulin-positive cells in the sections (n = 5). D and E, more than 100 islets were counted in each mouse. F, the mRNA expression levels of the indicated molecules in the islets were determined using real time quantitative RT-PCR and were normalized to the level of β-actin mRNA (n = 5). The experiments were performed in WT mice and Gck+/− mice after 20 weeks on the SL diet or the SL plus DFS diet. Black bars, SL; hatched bars, SL + DFS. Error bars indicate S.E. *, p < 0.05.
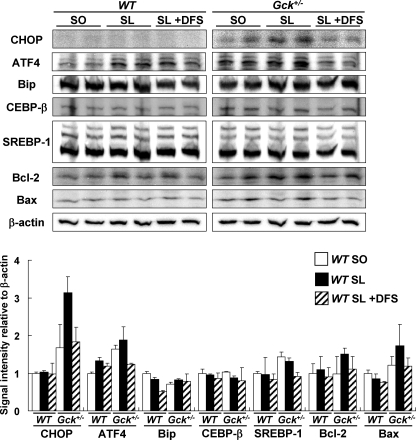
The protein expression levels in islets after a 20-week study of WT and Gck+/− mice fed SO, SL, or SL plus 1.1% DFS diet were also evaluated. The expression of CHOP, ATF4, and Bax tended to be elevated in SL-fed Gck+/− mice compared with SO-fed Gck+/− mice, but such alteration was less evident in WT mice (Fig. 3). The increase in expression of CHOP, ATF4, and Bax was restored by DFS treatment in Gck+/− mice. DFS also tended to suppress the expression of SREBP-1 in SL-fed Gck+/− mice. The expression levels of Bip, Bcl-2, and C/EBP-β were similar in all groups (Fig. 3).
FIGURE 3.
DFS reduced expression of molecules involved in β cell ER stress and apoptosis in SL-fed Gck+/− mice. Upper panel, total cell extracts from isolated islets were subjected to immunoblotting for CHOP, ATF4, Bip, C/EBP-β, SREBP-1, Bcl-2, Bax, and β-actin as indicated. Lower panel, intensity of the signals quantified by densitometry (NIH ImageJ) and corrected by the intensity of the β-actin signal (n = 5). The experiments were performed in WT mice and Gck+/− mice after 20 weeks on the SO diet, the SL diet, or the SL plus DFS diet. White bars, SO; black bars, SL; hatched bars, SL + DFS. Error bars indicate S.E.
Sucrose and Linoleic Acid Diet Increased Arachidonic Acid in Isolated Islets, and DFS Did Not Affect Them
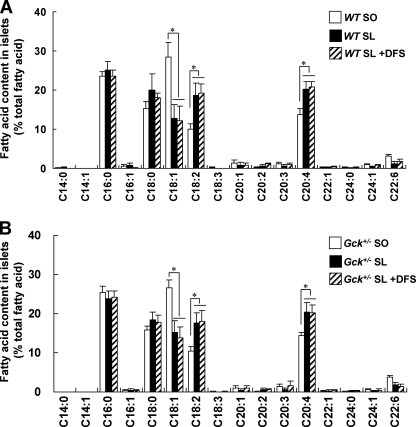
To assess the effects of the diets on fatty acid composition in pancreatic islets, we analyzed fatty acid composition in isolated islets in WT mice and Gck+/− mice fed SO, SL, or SL plus DFS diet for 5 weeks. In WT mice and Gck+/− mice fed SL diet, the composition of linoleic acid (C18:2) and arachidonic acid (C20:4) were significantly increased, and oleic acid (C18:1) was significantly decreased in islets compared with animals fed SO diet (Fig. 4). There were no significant differences in fatty acid compositions of islets between SL plus DFS groups and SL groups in both WT mice and Gck+/− mice (Fig. 4). These results indicated that SL increased arachidonic acid in isolated islets, and DPP-4 inhibition had no effect.
FIGURE 4.
SL diet increased arachidonic acid content in islets in both WT and Gck+/− mice. The fatty acid content in isolated islets from WT mice (A) or Gck+/− mice (B) was analyzed as described under “Experimental Procedures” (n = 7). The experiments were performed in WT mice and Gck+/− mice after 5 weeks on the SO diet, the SL diet, or the SL plus DFS diet. White bars, SO; black bars, SL; hatched bars, SL + DFS. Error bars indicate S.E. *, p < 0.05.
We previously demonstrated that SL diet induced hepatic steatosis in Gck+/− mice (16). Intriguingly, arachidonic acid (C20:4) content in liver as well as in islets was significantly increased in SL-fed mice (supplemental Fig. 3).
Single Oral Dose of DFS Markedly Increased Active GLP-1 Concentration in Gck+/− Mice
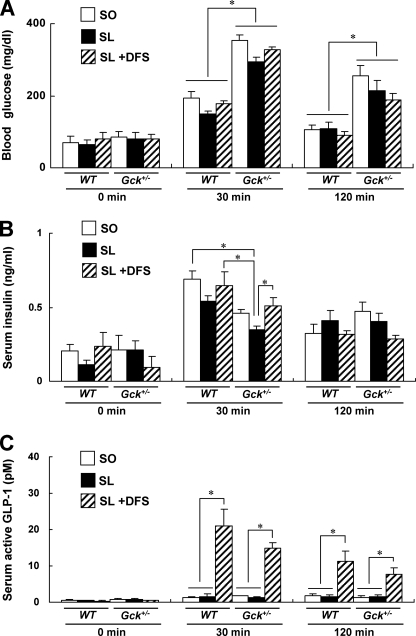
An oleic acid-enriched diet has been reported to increase GLP-1 release in both euglycemic rats and diabetic rats (24, 25). We first measured the serum active GLP-1 concentration after an oral SO or SL meal load (12 mg/g of body weight) in standard chow diet-fed WT and Gck+/− mice. The results showed that the meal-induced rise in the blood glucose level is significantly higher in Gck+/− mice than in WT mice and that the corresponding rise in the serum insulin level is significantly lower (Fig. 5, A and B). In addition, there was a meal-induced rise in serum active GLP-1 of a similar magnitude in both WT and Gck+/− mice that was significantly greater in mice treated with DFS compared with untreated mice (Fig. 5C).
FIGURE 5.
Changes in blood glucose, insulin, and active GLP-1 concentrations in WT mice and Gck+/− mice after oral meal tolerance test. Shown are the blood glucose (A), serum insulin (B), and serum active GLP-1 (C) concentrations at 0 (fasted > 20 h), 30, and 120 min after the oral administration of the SO diet, SL diet, or SL plus DFS diet (12 mg/g of body weight) to 6–8-week-old WT mice and Gck+/− mice (n = 4–5). To obtain a sufficient amount of whole blood to measure the biologically active form of GLP-1, blood was collected from the inferior vena cava under anesthesia with a DPP-4 inhibitor (Millipore) at the time point indicated. The blood glucose and serum insulin levels were also determined using these samples. White bars, SO; black bars, SL; hatched bars, SL + DFS. Error bars indicate S.E. *, p < 0.05.
To assess the acute effects of DFS on GLP-1 release, we performed an oral SL plus 1.1% DFS meal tolerance test (12 mg/g of body weight) in these mice. The serum insulin concentration of the Gck+/− mice was significantly higher 30 min after DFS administration, but no significant differences in the blood glucose levels were observed at any time (Fig. 5, A and B). DFS markedly increased the serum active GLP-1 concentration in WT and Gck+/− mice (Fig. 5C).
To investigate the long term effects of an SO or SL diet on GLP-1 release, we performed an oral SO or SL meal tolerance test in WT and Gck+/− mice after the mice had been fed either diet for 20 weeks. The results showed no significant differences in the serum active GLP-1 concentration at 30 min between the SO-fed and the SL-fed groups in both WT and Gck+/− mice (supplemental Fig. 4). Interestingly, after 20 weeks of the SO or SL diet, GLP-1 release in the Gck+/− mice was higher than that in the WT mice.
We also evaluated the insulinotropic effect of GLP-1 receptor signaling on islets by analyzing glucose-stimulated insulin secretion in the presence or absence of a potent GLP-1 receptor agonist, exendin-4, at 2.8, 8.3, or 22.2 mm glucose (supplemental Fig. 5). Experiments were performed using two protocols: (a) immediately after islet isolation and (b) after a 12-h culture in medium containing 11 mm glucose after islet isolation. Exendin-4 did not affect glucose-stimulated insulin secretion at 2.8 mm glucose and tended to increase glucose-stimulated insulin secretion at 22.2 mm glucose in both genotypes of islets. The increase in insulin secretion by exendin-4 in response to 8.3 mm glucose was much lower in the Gck+/− islets than in the WT islets.
Requirement of High Glucose Concentration for Linoleic Acid-induced β Cell ER Stress and Apoptosis in Vitro and Mitigation by GLP-1 Receptor-mediated Signals
Palmitic acid and, to a lesser extent, oleic acid induce ER stress and apoptosis in vitro (17), and GLP-1 receptor signaling activated by exendin-4 prevents ER stress and apoptotic cell death in β cells both in vivo and in vitro (21). We investigated whether linoleic acid induced ER stress and apoptosis and whether GLP-1 receptor signaling regulated fatty acid-induced ER stress and apoptosis in pancreatic islets and β cell lines.
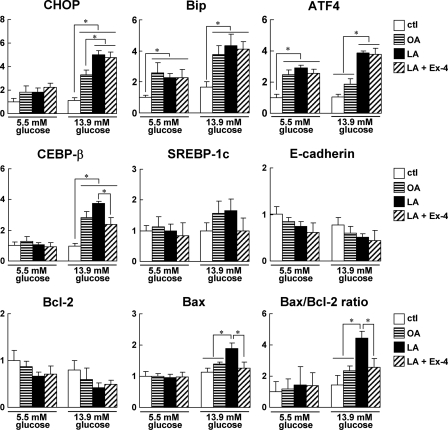
Pancreatic islets from WT mice were exposed to fatty acids for 16 h in the presence of 5.5 or 13.9 mm glucose, and mRNA expression levels were analyzed (Fig. 6). The expressions of CHOP, Bip, and C/EBP-β were increased to a greater degree by fatty acids at 13.9 mm glucose than at 5.5 mm glucose. Linoleic acid significantly increased the expressions of CHOP and ATF4 compared with oleic acid at 13.9 mm glucose. However, the expressions of SREBP-1c and E-cadherin did not change significantly in vitro. The Bax/Bcl-2 ratio was also significantly increased in linoleic acid-treated islets at 13.9 mm glucose. In Gck+/− islets, the inductions of ER stress-related genes and apoptosis-related genes by fatty acids were similar to those in WT islets (data not shown). These results suggested that linoleic acid and high glucose act synergistically to induce β cell ER stress and apoptosis. Furthermore, exendin-4 significantly decreased the Bax/Bcl-2 ratio and C/EBP-β but did not affect the expressions of CHOP, Bip, and ATF4 (Fig. 6).
FIGURE 6.
High glucose and linoleic acid levels induced β cell ER stress and apoptosis in islets in vitro and impacted by exendin-4. Islets were isolated from WT mice treated with vehicle (ctl), 0.25 mm oleic acid (OA), or 0.25 mm linoleic acid (LA) in the absence or presence of 50 nm exendin-4 (Ex-4) for 16 h in the presence of 13.9 or 5.5 mm glucose. The mRNA expression levels of the indicated molecules in the islets were determined using real time quantitative RT-PCR and were normalized to the level of β-actin mRNA (n = 5). White bars, vehicle; horizontal striped bars, 0.25 mm oleic acid; black bars, 0.25 mm linoleic acid; hatched bars, 0.25 mm linoleic acid + 50 nm exendin-4. Error bars indicate S.E. *, p < 0.05.
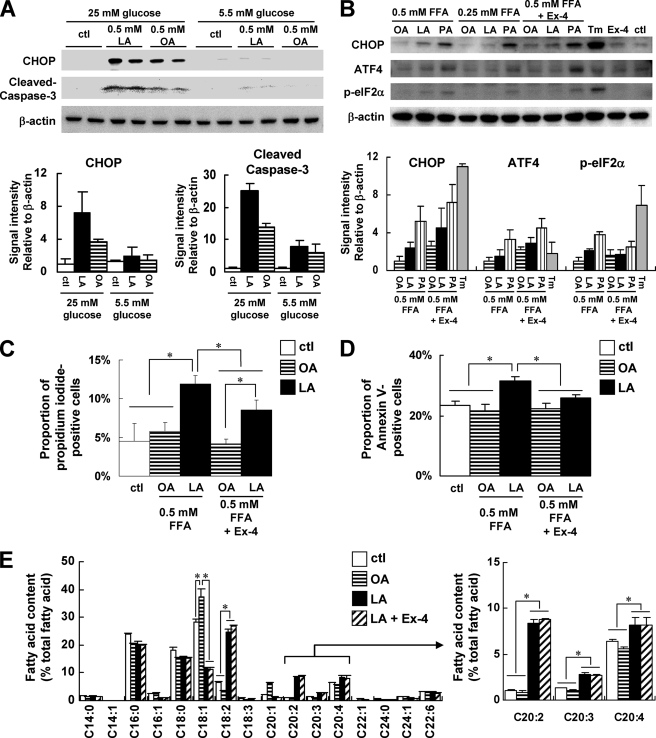
MIN6 cells were also exposed to fatty acids for 24 h. In the presence of 25 mm glucose, linoleic acid up-regulated the expressions of CHOP and cleaved caspase-3 more efficiently than oleic acid. However, such differences were not observed in the presence of 5.5 mm glucose (Fig. 7A). These results supported the hypothesis that a high glucose concentration was required for the sufficient induction of ER stress and apoptosis by linoleic acid. The expressions of ATF4 and phosphorylated eukaryotic initiation factor 2α increased to a greater degree in the presence of 0.25 and 0.5 mm linoleic acid than in the presence of the same concentration of oleic acid but to a lesser degree than in the presence of the same concentration of palmitic acid in the presence of 25 mm glucose (Fig. 7B). Tunicamycin markedly induced the expression of CHOP and the phosphorylation of eIF2α. Exendin-4 increased the magnitude of CHOP and ATF4 induction in the presence of each fatty acid; however, the level of phosphorylated eIF2α induced by linoleic acid or palmitic acid was reduced by exendin-4. These findings were consistent with a previous report that exendin-4 potentiated the ATF4-CHOP-GADD34-mediated ER stress pathway and promoted translational recovery from the ER stress-mediated repression of protein synthesis by reducing the levels of phospho-eIF2α (21).
FIGURE 7.
Effects of exendin-4 on ER stress and apoptosis induced by combination of high glucose and linoleic acid in MIN6 cells. A, upper panel, mouse MIN6 insulinoma cells were treated with vehicle (ctl), linoleic acid (LA), or oleic acid (OA) in the presence of 25 or 5.5 mm glucose for 24 h. Total cell extracts (10 μg) were subjected to immunoblotting for CHOP, cleaved caspase-3, and β-actin as indicated. Lower panel, intensities of the CHOP signal and cleaved caspase-3 signal quantified by densitometry (NIH ImageJ) and corrected by the intensity of the β-actin signal. The results shown are the means of three independent experiments. B, MIN6 cells were treated with the vehicle (ctl), oleic acid, linoleic acid, palmitic acid (PA), or tunicamycin (Tm) in the absence or presence of 50 nm exendin-4 (Ex-4) in the presence of 25 mm glucose for 24 h. The final concentration of tunicamycin was 0.5 μg/ml. Total cell extracts (10 μg) were subjected to immunoblotting and quantified as in A. Results shown are the means of three independent experiments. C, quantitation of the percentage of propidium iodide-stained nuclei relative to the proportion of DAPI-stained nuclei of MIN6 cells treated with the vehicle alone (ctl) and with 0.5 mm oleic acid or linoleic acid in 25 mm glucose for 24 h in the absence or presence of 50 nm exendin-4. D, flow cytometric analysis of the proportion of Annexin V-FITC-stained and propidium iodide-negative apoptotic MIN6 cells treated with vehicle alone (ctl) or the 0.5 mm oleic acid or linoleic acid in 25 mm glucose for 24 h in the absence or presence of 50 nm exendin-4. E, fatty acid content in MIN6 cells treated with vehicle alone (ctl) or the 0.5 mm oleic acid or linoleic acid in 25 mm glucose for 24 h in the absence or presence of 50 nm exendin-4. Error bars indicate S.E. *, p < 0.05.
We next investigated fatty acid-induced cell death using propidium iodide staining in the presence and absence of exendin-4 for 24 h. More cell death was observed after exposure to 0.5 mm linoleic acid than after exposure to 0.5 mm oleic acid, and exendin-4 significantly reduced the linoleic acid-induced cell death (Fig. 7C and supplemental Fig. 6A). Annexin V staining revealed that the rescue from linoleic acid-induced cell death by extendin-4 was at least in part attributable to the inhibition of apoptosis (Fig. 7D and supplemental Fig. 6B). We also analyzed fatty acid composition in MIN6 cells after exposure to fatty acids for 24 h. Arachidonic acid (C20:4) and its precursors 11,14-eicosadienoic acid (C20:2) and 11,14,17-eicosatrienoic acid (C20:3) were significantly increased after exposure to 0.5 mm linoleic acid (C18:2) compared with exposure to 0.5 mm oleic acid (C18:1) or vehicle control in MIN6 cells (Fig. 7E). Treatment with exendin-4 did not affect the compositions of arachidonic acid and its precursors in linoleic acid-exposed MIN6 cells (Fig. 7E).
DISCUSSION
In this study, we established a model in which a diet rich in sucrose and linoleic acid induced the apoptosis of β cells in Gck+/− mice. Because considerable and progressive deficits in the β cell mass supposedly occur during the development of human type 2 diabetes before the manifestation of patent hyperglycemia (26, 27), our model should be a good model for the diet-induced exacerbation of diabetes. SL was found to exacerbate β cell ER stress and apoptosis in Gck+/− mice but not in euglycemic WT or IRS-1−/− mice, a finding that was consistent with previous reports that fatty acid-induced β cell ER stress is amplified by high glucose concentrations (28, 29).
We also demonstrated that high glucose concentrations were required for linoleic acid-induced β cell apoptosis in vitro. Because the fatty acid-induced ER stress response is reportedly not modified by high glucose concentrations (30), a factor(s) other than glucose may be involved in linoleic acid-induced β cell ER stress in diabetic Gck+/− mice. Linoleic acid is reportedly converted to arachidonic acid in vivo, and arachidonic acid is converted to prostaglandins, leukotrienes, and other lipid mediators by lipoxygenase or cyclooxygenase (31). In fact, we noted that the content of arachidonic acid in islets was significantly increased in mice fed the SL diet compared with those fed the SO diet. SL diet-induced β cell apoptosis may be mediated in part by the activation of the arachidonic acid cascade. This hypothesis is supported by reports that SREBP-1c-induced calcium-independent phospholipase A2 (iPLA2β) plays a key role in spontaneous ER stress and apoptosis in β cells (32, 33). To clarify the precise mechanisms for SL diet-induced β cell apoptosis, further study using inhibitors of cyclooxygenase or lipoxygenases and mice deficient in phospholipases A2 will be required. A palmitic acid-rich diet may also contribute to further elucidation of the mechanism of nutrient-induced β cell lipotoxicity.
The results of this study also showed that DFS protected against SL diet-induced β cell ER stress and apoptosis in Gck+/− mice. GLP-1 and an analog have been reported to prevent β cell ER stress and apoptosis (21, 22, 28). Despite the massive increase in active GLP-1 induced by a single administration of DFS in both WT and Gck+/− mice, their serum insulin and blood glucose levels remained largely unchanged. The glucose-dependent insulinotropic effects of GLP-1 may be attenuated by normoglycemia in WT mice. Because glucokinase is the major glucose sensor of β cells, we hypothesized that glucokinase is also a key molecule in the glucose-dependent insulinotropic action of GLP-1 and that the elevated GLP-1 level was still insufficient to stimulate insulin secretion in Gck+/− mice. Consistent with this hypothesis, a GLP-1 receptor agonist attenuated the effects of incretin on insulin secretion in response to 8.3 mm glucose from Gck+/− islets compared with WT islets. Because DFS or exendin-4 in mice or MIN6 cells did not affect increased arachidonic acid content induced by linoleic acid, the protective effects of DFS or exendin-4 might be independent of the modulation of the arachidonic acid cascade. Whether an increase in glucose-dependent insulinotropic polypeptide in response to DPP-4 inhibitor contributes to the protection of β cells remains unexplored.
The islet architecture of Gck+/− mice was restored, and the reduction of E-cadherin expression was recovered in mice fed the SL diet plus DFS. E-cadherin expression on β cells plays an important role in glucose-stimulated insulin secretion (34–36). Because E-cadherin-mediated cell adhesion is controlled by β-catenin and the Wnt signaling pathway (37) and an interaction between GLP-1 signaling and the TCF7L2-dependent Wnt signaling pathway has been reported in pancreatic β cells (38–40), the Wnt/β-catenin network may be involved in the DFS-mediated normalization of islet architecture and E-cadherin expression. SREBP-1c activation caused by ER stress has been implicated in β cell lipotoxicity (41, 42). Although the SREBP-1c mRNA level was significantly increased in the Gck+/− mice fed the SL diet, the expressions of SREBP-1c and E-cadherin in vitro were not changed by the fatty acids. This discrepancy raised the possibility that the ER stress signaling mechanisms may differ notably between in vivo (chronic reaction) and in vitro (acute reaction) situations. Indeed, a previous study demonstrated that the expression of CHOP was decreased in vivo by treatment with exendin-4 but was increased in vitro under ER stress (21).
We previously reported a reduction in amyloid deposition in Gck+/− islets (43), suggesting that Gck+/− islets may be partially protected against glucose toxicity via the glycolytic pathway. Consequently, the high glucose-induced amplification of ER stress and apoptosis in our model might be independent of glucokinase signaling. On the other hand, β cell glucokinase plays a dominant role in the induction of IRS-2 in response to high fat diet-induced insulin resistance, and the antiapoptotic effects of exendin-4 have been implicated in the enhancement of the IRS-2/Akt signaling pathway (12, 44). We also observed an increase in the expression of IRS-2 in response to tunicamycin- or fatty acid-induced ER stress in MIN6 cells and isolated islets.3 Although the SL diet or DFS administration did not affect the mRNA expression of IRS-2 in the Gck+/− islets in this study, insulin signaling might be involved in the regulation of ER stress and apoptosis via fatty acids or GLP-1 receptor signaling. Thus, the modifications of ER stress by GLP-1 receptor activation in vivo have been controversial. Therefore, further research is needed to clarify the link between DPP-4 inhibition and ER stress in our model.
In summary, we created a model of nutrient-induced β cell apoptosis in a diabetic state and showed that DPP-4 inhibition with DFS ameliorated apoptosis. The results of the current study demonstrate the novel therapeutic potential of DPP-4 inhibitors for the treatment of diabetes.
Supplementary Material
Acknowledgments
We are grateful to Dr. Junichi Miyazaki (University of Osaka) for providing us with the MIN6 cells and Dr. Naoto Kubota and Prof. Takashi Kadowaki (University of Tokyo) for contributing to the discussion and providing us with Gck+/− mice. We thank Mitsuyo Kaji and Eri Sakamoto for technical assistance and Misa Katayama for secretarial assistance. We also thank Merck & Co., Inc. (Rahway, NJ) for providing DFS and for encouraging our research.
This work was supported in part by Grants-in-aid for Scientific Research (B) 19390251 and (B) 21390282 from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a medical award from the Japan Medical Association, a grant-in-aid from the Japan Diabetes Foundation, a grant-in-aid from the Suzuken Memorial Foundation, a grant-in-aid from the Naito Foundation, a grant-in-aid from the Uehara Memorial Foundation (to Y. T.), and a grant-in-aid for Japan Society for the Promotion of Science fellows (to J. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–6.
J. Shirakawa, K. Orime, and Y. Terauchi, unpublished data.
- SO
- sucrose and oleic acid
- SL
- sucrose and linoleic acid
- ATF4
- activating transcription factor 4
- Bip
- immunoglobulin heavy chain-binding protein
- C/EBP
- CCAAT/enhancer-binding protein
- CHOP
- C/EBP-homologous protein
- DFS
- desfluorositagliptin
- DPP-4
- dipeptidyl peptidase-4
- ER
- endoplasmic reticulum
- Gck
- glucokinase
- GLP-1
- glucagon-like peptide-1
- IRS
- insulin receptor substrate
- eIF2α
- eukaryotic initiation factor 2α
- SREBP-1c
- sterol regulatory element-binding protein-1c.
REFERENCES
- 1. Donath M. Y., Halban P. A. (2004) Diabetologia 47, 581–589 [DOI] [PubMed] [Google Scholar]
- 2. Grundy S. M., Abate N., Chandalia M. (2002) Am. J. Med. 113, Suppl. 9B, 25S–29S [DOI] [PubMed] [Google Scholar]
- 3. Hodson L., Skeaff C. M., Fielding B. A. (2008) Prog. Lipid Res. 47, 348–380 [DOI] [PubMed] [Google Scholar]
- 4. Sato K., Arai H., Mizuno A., Fukaya M., Sato T., Koganei M., Sasaki H., Yamamoto H., Taketani Y., Doi T., Takeda E. (2007) J. Nutr. 137, 1908–1915 [DOI] [PubMed] [Google Scholar]
- 5. Kim D., Wang L., Beconi M., Eiermann G. J., Fisher M. H., He H., Hickey G. J., Kowalchick J. E., Leiting B., Lyons K., Marsilio F., McCann M. E., Patel R. A., Petrov A., Scapin G., Patel S. B., Roy R. S., Wu J. K., Wyvratt M. J., Zhang B. B., Zhu L., Thornberry N. A., Weber A. E. (2005) J. Med. Chem. 48, 141–151 [DOI] [PubMed] [Google Scholar]
- 6. Xu G., Stoffers D. A., Habener J. F., Bonner-Weir S. (1999) Diabetes 48, 2270–2276 [DOI] [PubMed] [Google Scholar]
- 7. Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U., Perfetti R. (2002) Endocrinology 143, 4397–4408 [DOI] [PubMed] [Google Scholar]
- 8. Ahrén B. (2004) Horm. Metab. Res. 36, 842–845 [DOI] [PubMed] [Google Scholar]
- 9. Stoffers D. A., Kieffer T. J., Hussain M. A., Drucker D. J., Bonner-Weir S., Habener J. F., Egan J. M. (2000) Diabetes 49, 741–748 [DOI] [PubMed] [Google Scholar]
- 10. Mu J., Woods J., Zhou Y. P., Roy R. S., Li Z., Zycband E., Feng Y., Zhu L., Li C., Howard A. D., Moller D. E., Thornberry N. A., Zhang B. B. (2006) Diabetes 55, 1695–1704 [DOI] [PubMed] [Google Scholar]
- 11. Terauchi Y., Sakura H., Yasuda K., Iwamoto K., Takahashi N., Ito K., Kasai H., Suzuki H., Ueda O., Kamada N., Jishage K., Komeda K., Noda M., Kanazawa Y., Taniguchi S., Miwa I., Akanuma Y., Kodama T., Yazaki Y., Kadowaki T. (1995) J. Biol. Chem. 270, 30253–30256 [DOI] [PubMed] [Google Scholar]
- 12. Terauchi Y., Takamoto I., Kubota N., Matsui J., Suzuki R., Komeda K., Hara A., Toyoda Y., Miwa I., Aizawa S., Tsutsumi S., Tsubamoto Y., Hashimoto S., Eto K., Nakamura A., Noda M., Tobe K., Aburatani H., Nagai R., Kadowaki T. (2007) J. Clin. Investig. 117, 246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamont B. J., Drucker D. J. (2008) Diabetes 57, 190–198 [DOI] [PubMed] [Google Scholar]
- 14. Wrede C. E., Dickson L. M., Lingohr M. K., Briaud I., Rhodes C. J. (2002) J. Biol. Chem. 277, 49676–49684 [DOI] [PubMed] [Google Scholar]
- 15. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 16. Shirakawa J., Fujii H., Ohnuma K., Sato K., Ito Y., Kaji M., Sakamoto E., Koganei M., Sasaki H., Nagashima Y., Amo K., Aoki K., Morimoto C., Takeda E., Terauchi Y. (2011) Diabetes 60, 1246–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karaskov E., Scott C., Zhang L., Teodoro T., Ravazzola M., Volchuk A. (2006) Endocrinology 147, 3398–3407 [DOI] [PubMed] [Google Scholar]
- 18. Oyadomari S., Mori M. (2004) Cell Death Differ. 11, 381–389 [DOI] [PubMed] [Google Scholar]
- 19. Laybutt D. R., Preston A. M., Akerfeldt M. C., Kench J. G., Busch A. K., Biankin A. V., Biden T. J. (2007) Diabetologia 50, 752–763 [DOI] [PubMed] [Google Scholar]
- 20. Perl A. K., Wilgenbus P., Dahl U., Semb H., Christofori G. (1998) Nature 392, 190–193 [DOI] [PubMed] [Google Scholar]
- 21. Yusta B., Baggio L. L., Estall J. L., Koehler J. A., Holland D. P., Li H., Pipeleers D., Ling Z., Drucker D. J. (2006) Cell Metab. 4, 391–406 [DOI] [PubMed] [Google Scholar]
- 22. Tsunekawa S., Yamamoto N., Tsukamoto K., Itoh Y., Kaneko Y., Kimura T., Ariyoshi Y., Miura Y., Oiso Y., Niki I. (2007) J. Endocrinol. 193, 65–74 [DOI] [PubMed] [Google Scholar]
- 23. Cunha D. A., Ladrière L., Ortis F., Igoillo-Esteve M., Gurzov E. N., Lupi R., Marchetti P., Eizirik D. L., Cnop M. (2009) Diabetes 58, 2851–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cancelas J., Prieto P. G., Villanueva-Peñacarrillo M. L., Valverde I., Malaisse W. J. (2006) Horm. Metab. Res. 38, 98–105 [DOI] [PubMed] [Google Scholar]
- 25. Prieto P. G., Cancelas J., Villanueva-Peñacarrillo M. L., Valverde I., Malaisse W. J. (2005) Endocrine 26, 107–115 [DOI] [PubMed] [Google Scholar]
- 26. Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 27. Ritzel R. A., Butler A. E., Rizza R. A., Veldhuis J. D., Butler P. C. (2006) Diabetes Care 29, 717–718 [DOI] [PubMed] [Google Scholar]
- 28. Buteau J., El-Assaad W., Rhodes C. J., Rosenberg L., Joly E., Prentki M. (2004) Diabetologia 47, 806–815 [DOI] [PubMed] [Google Scholar]
- 29. Bachar E., Ariav Y., Ketzinel-Gilad M., Cerasi E., Kaiser N., Leibowitz G. (2009) PLoS One 4, e4954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunha D. A., Hekerman P., Ladrière L., Bazarra-Castro A., Ortis F., Wakeham M. C., Moore F., Rasschaert J., Cardozo A. K., Bellomo E., Overbergh L., Mathieu C., Lupi R., Hai T., Herchuelz A., Marchetti P., Rutter G. A., Eizirik D. L., Cnop M. (2008) J. Cell Sci. 121, 2308–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitz G., Ecker J. (2008) Prog. Lipid Res. 47, 147–155 [DOI] [PubMed] [Google Scholar]
- 32. Lei X., Zhang S., Bohrer A., Ramanadham S. (2008) J. Biol. Chem. 283, 34819–34832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lei X., Zhang S., Barbour S. E., Bohrer A., Ford E. L., Koizumi A., Papa F. R., Ramanadham S. (2010) J. Biol. Chem. 285, 6693–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bosco D., Rouiller D. G., Halban P. A. (2007) J. Endocrinol. 194, 21–29 [DOI] [PubMed] [Google Scholar]
- 35. Jain R., Lammert E. (2009) Diabetes Obes. Metab. 11, Suppl. 4, 159–167 [DOI] [PubMed] [Google Scholar]
- 36. Rogers G. J., Hodgkin M. N., Squires P. E. (2007) Cell. Physiol. Biochem. 20, 987–994 [DOI] [PubMed] [Google Scholar]
- 37. Brembeck F. H., Rosário M., Birchmeier W. (2006) Curr. Opin. Genet. Dev. 16, 51–59 [DOI] [PubMed] [Google Scholar]
- 38. Liu Z., Habener J. F. (2008) J. Biol. Chem. 283, 8723–8735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villareal D. T., Robertson H., Bell G. I., Patterson B. W., Tran H., Wice B., Polonsky K. S. (2010) Diabetes 59, 479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pilgaard K., Jensen C. B., Schou J. H., Lyssenko V., Wegner L., Brøns C., Vilsbøll T., Hansen T., Madsbad S., Holst J. J., Vølund A., Poulsen P., Groop L., Pedersen O., Vaag A. A. (2009) Diabetologia 52, 1298–1307 [DOI] [PubMed] [Google Scholar]
- 41. Wang H., Kouri G., Wollheim C. B. (2005) J. Cell Sci. 118, 3905–3915 [DOI] [PubMed] [Google Scholar]
- 42. Kato T., Shimano H., Yamamoto T., Ishikawa M., Kumadaki S., Matsuzaka T., Nakagawa Y., Yahagi N., Nakakuki M., Hasty A. H., Takeuchi Y., Kobayashi K., Takahashi A., Yatoh S., Suzuki H., Sone H., Yamada N. (2008) Diabetes 57, 2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Andrikopoulos S., Verchere C. B., Terauchi Y., Kadowaki T., Kahn S. E. (2000) Diabetes 49, 2056–2062 [DOI] [PubMed] [Google Scholar]
- 44. Natalicchio A., De Stefano F., Orlando M. R., Melchiorre M., Leonardini A., Cignarelli A., Labarbuta R., Marchetti P., Perrini S., Laviola L., Giorgino F. (2010) Endocrinology 151, 2019–2029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.