Abstract
Earlier investigations have shown that murine natural killer (NK) cells bind to and inhibit the growth of the fungal pathogen Cryptococcus neoformans in vitro and in vivo. To define the stages of NK cell-mediated inhibition of C. neoformans growth and the requirements for the completion of these stages, the events which lead to cryptococcal growth inhibition were compared with those previously elucidated for NK cell-mediated tumor cell lysis. Our data indicate that NK cell-cryptococci binding is a distinct event that precedes inhibition; is temperature independent, although it is slowed at 4 degrees C; and is Mg2+ dependent. In contrast to binding, NK cell-mediated cryptococcal growth inhibition is temperature, Mg2+, and Ca2+ dependent. The removal of Ca2+ by EDTA addition within 3 h after maximal NK cell-cryptococci binding significantly reduced cryptococcal growth inhibition, indicating that Ca2+ is required either late in the NK cell trigger stage or early in the inhibitory stage. These stages and requirements are similar to those previously demonstrated for the model of NK cell-mediated tumor cell lysis; however, the NK cell-cryptococci interactions are somewhat slower than the interactions which culminate in the lysis of the YAC-1 tumor cell targets. These results suggest that C. neoformans cells, although structurally distinct from the standard tumor cell targets, are capable of similar cell-to-cell interactions with NK effector cells as the tumor cell targets.
Full text
PDF


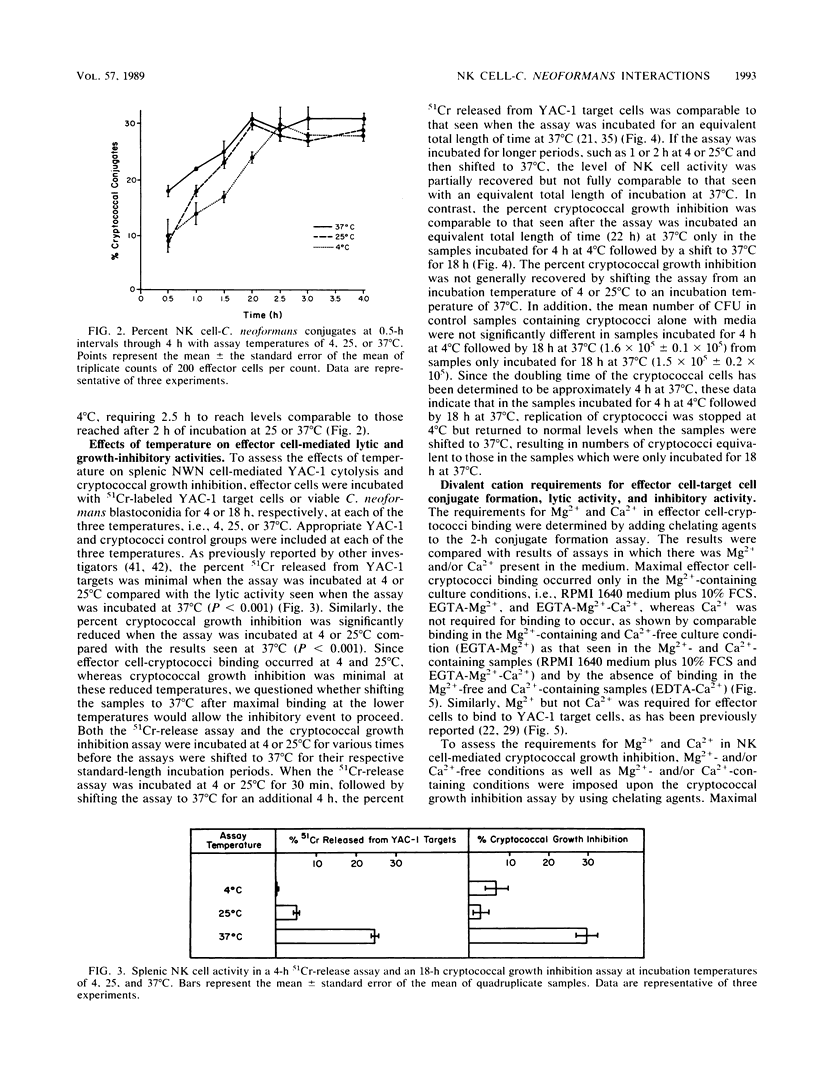
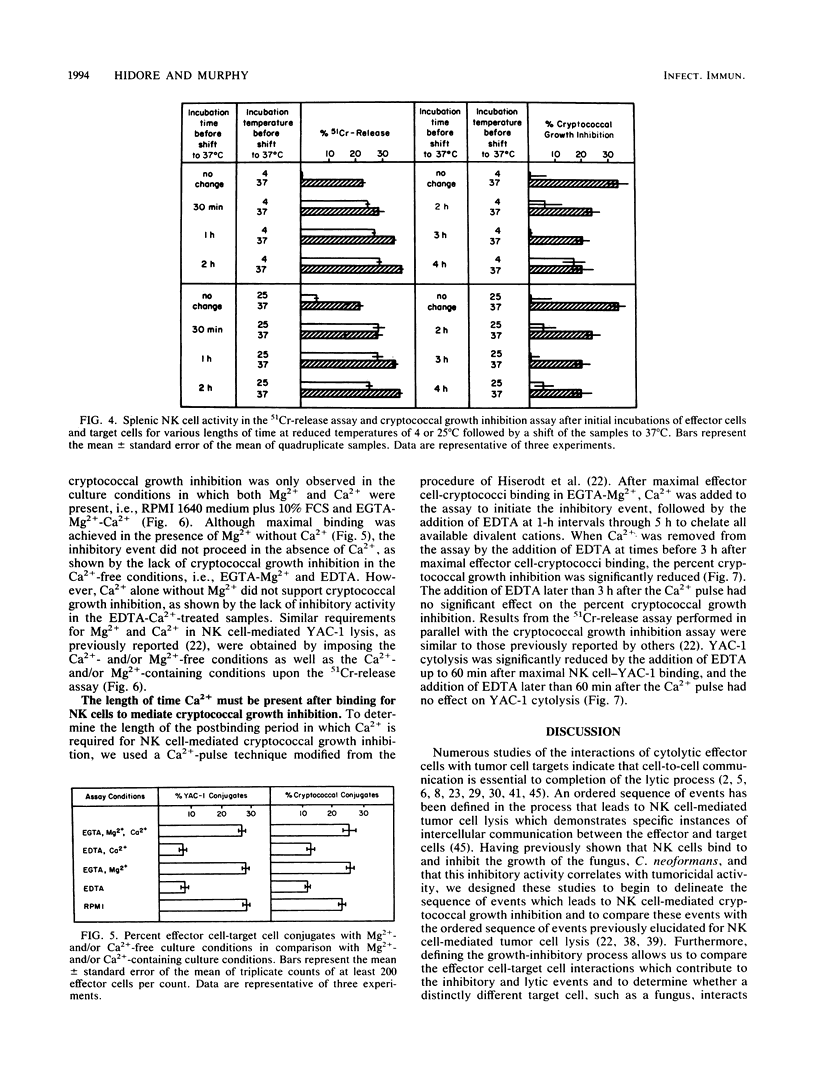
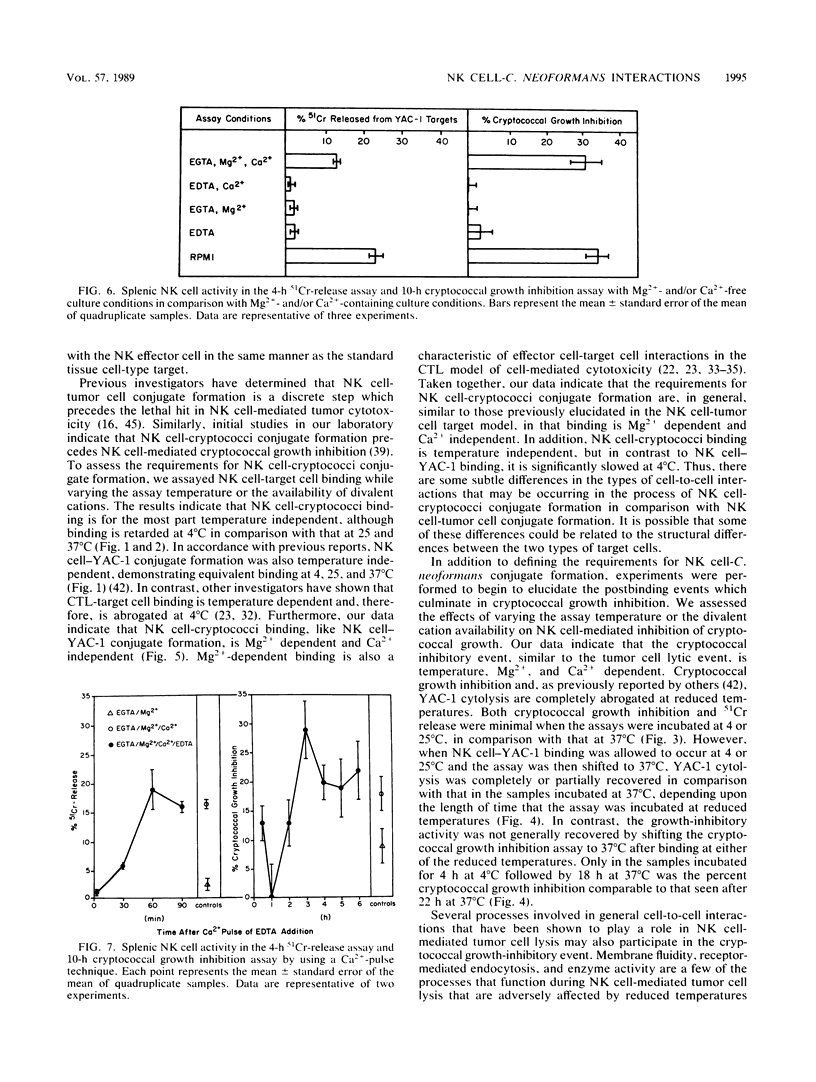


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonavida B., Katz J., Gottlieb M. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. I. Defective trigger on NK cells for NKCF production by target cells, and partial restoration by IL 2. J Immunol. 1986 Aug 15;137(4):1157–1163. [PubMed] [Google Scholar]
- Bonavida B., Wright S. C. Multistage model of natural killer cell-mediated cytotoxicity involving NKCF as soluble cytotoxic mediators. Adv Cancer Res. 1987;49:169–187. doi: 10.1016/s0065-230x(08)60797-6. [DOI] [PubMed] [Google Scholar]
- Brogan M., Targan S. Evidence for involvement of serine proteases in the late stages of the natural killer cell lytic reaction. Cell Immunol. 1986 Dec;103(2):426–433. doi: 10.1016/0008-8749(86)90102-4. [DOI] [PubMed] [Google Scholar]
- Deem R. L., Niederlehner A., Targan S. R. Active target cell processes, possibly involving receptor-mediated endocytosis, are critical for expression of cytotoxicity by natural killer cell-derived cytolytic factor. Cell Immunol. 1986 Oct 1;102(1):187–197. doi: 10.1016/0008-8749(86)90337-0. [DOI] [PubMed] [Google Scholar]
- Deem R. L., Targan S. R. Analysis of sequential substages of the natural killer cell lethal hit. Adv Exp Med Biol. 1985;184:239–256. doi: 10.1007/978-1-4684-8326-0_16. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Sellins K. S., Cohen J. J. Cytotoxic lymphocyte-derived lytic granules do not induce DNA fragmentation in target cells. J Immunol. 1988 Oct 1;141(7):2191–2194. [PubMed] [Google Scholar]
- Grimm E., Price Z., Bonavida B. Studies on the induction and expression of T cell-mediated immunity. VIII. Effector-target junctions and target cell membrane disruption during cytolysis. Cell Immunol. 1979 Aug;46(1):77–99. doi: 10.1016/0008-8749(79)90247-8. [DOI] [PubMed] [Google Scholar]
- Hadden J. W. Transmembrane signals in the activation of T lymphocytes. Adv Exp Med Biol. 1987;213:69–83. doi: 10.1007/978-1-4684-5323-2_8. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Reynolds C. W., Ortaldo J. R. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–680. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Correlation of natural killer cell activity and clearance of Cryptococcus neoformans from mice after adoptive transfer of splenic nylon wool-nonadherent cells. Infect Immun. 1986 Feb;51(2):547–555. doi: 10.1128/iai.51.2.547-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Natural cellular resistance of beige mice against Cryptococcus neoformans. J Immunol. 1986 Dec 1;137(11):3624–3631. [PubMed] [Google Scholar]
- Hiserodt J. C., Britvan L. J., Targan S. R. Characterization of the cytolytic reaction mechanism of the human natural killer (NK) lymphocyte: resolution into binding, programming, and killer cell-independent steps. J Immunol. 1982 Oct;129(4):1782–1787. [PubMed] [Google Scholar]
- Hiserodt J., Britvan L., Targan S. Studies on the mechanism of the human natural killer cell lethal hit: a new method for rapid physical isolation of lethally hit K562 targets and inhibition of cytolysis by reduced temperature or trypsin. Cell Immunol. 1984 Jan;83(1):43–51. doi: 10.1016/0008-8749(84)90223-5. [DOI] [PubMed] [Google Scholar]
- Howell D. M., Martz E. Low calcium concentrations support killing by some but not all cytolytic T lymphocytes, and reveal inhibition of a postconjugation step by calcium antagonists. J Immunol. 1988 Mar 15;140(6):1982–1988. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kalina M., Berke G. Contact regions of cytotoxic T lymphocyte-target cell conjugates. Cell Immunol. 1976 Jul;25(1):41–51. doi: 10.1016/0008-8749(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Klein E., Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kiyohara T., Dennis J. W., Boegman R. J., Roder J. C. An exoglycosidase-sensitive triggering site on NK cells which is coupled to transmethylation of membrane phospholipids. J Immunol. 1985 Jul;135(1):659–664. [PubMed] [Google Scholar]
- Kiyohara T., Dennis J. W., Roder J. C. Double restriction in NK cell recognition is linked to transmethylation and can be triggered by asparagine-linked oligosaccharides on tumor cells. Cell Immunol. 1987 May;106(2):223–233. doi: 10.1016/0008-8749(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Luini W., Boraschi D., Alberti S., Aleotti A., Tagliabue A. Morphological characterization of a cell population responsible for natural killer activity. Immunology. 1981 Aug;43(4):663–668. [PMC free article] [PubMed] [Google Scholar]
- Martz E., Benacerraf B. T-lymphocyte mediated cytolysis: temperature dependence of killer cell dependent and independent phases and lack of recovery from the lethal hit at low temperatures. Cell Immunol. 1975 Nov;20(1):81–91. doi: 10.1016/0008-8749(75)90086-6. [DOI] [PubMed] [Google Scholar]
- Martz E. Early steps in specific tumor cell lysis by sensitized mouse T lymphocytes. I. Resolution and characterization. J Immunol. 1975 Jul;115(1):261–267. [PubMed] [Google Scholar]
- Martz E., Heagy W., Gromkowski S. H. The mechanism of CTL-mediated killing: monoclonal antibody analysis of the roles of killer and target-cell membrane proteins. Immunol Rev. 1983;72:73–96. doi: 10.1111/j.1600-065x.1983.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Martz E. Immune T lymphocyte to tumor cell adhesion. Magnesium sufficient, calcium insufficient. J Cell Biol. 1980 Mar;84(3):584–598. doi: 10.1083/jcb.84.3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz E., Parker W. L., Gately M. K., Tsoukas C. D. The role of calcium in the lethal hit of T lymphocyte-mediated cytolysis. Adv Exp Med Biol. 1982;146:121–147. doi: 10.1007/978-1-4684-8959-0_9. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., McDaniel D. O. In vitro reactivity of natural killer (NK) cells against Cryptococcus neoformans. J Immunol. 1982 Apr;128(4):1577–1583. [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadimitriou J. M., Robertson T. A., Kletter Y., Aronson M., Walters M. N. An ultrastructural examination of the interaction between macrophages and Cryptococcus neoformans. J Pathol. 1978 Feb;124(2):103–109. doi: 10.1002/path.1711240206. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Argov S., Klein M., Petersson C., Kiessling R., Andersson K., Hansson M. Target-effector cell interaction in the natural killer cell system. V. Energy requirements, membrane integrity, and the possible involvement of lysosomal enzymes. Immunology. 1980 May;40(1):107–116. [PMC free article] [PubMed] [Google Scholar]
- Roder J. C., Kiessling R., Biberfeld P., Andersson B. Target-effector interaction in the natural killer (NK) cell system. II. The isolation of NK cells and studies on the mechanism of killing. J Immunol. 1978 Dec;121(6):2509–2517. [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Sanders V. M., Snyder J. M., Uhr J. W., Vitetta E. S. Characterization of the physical interaction between antigen-specific B and T cells. J Immunol. 1986 Oct 15;137(8):2395–2404. [PubMed] [Google Scholar]
- Tirosh R., Berke G. Immune cytolysis viewed as a stimulatory process of the target. Adv Exp Med Biol. 1985;184:473–492. doi: 10.1007/978-1-4684-8326-0_31. [DOI] [PubMed] [Google Scholar]
- Zagury D. Direct analysis of individual killer T cells: susceptibility of target cells to lysis and secretion of hydrolytic enzymes by CTL. Adv Exp Med Biol. 1982;146:149–169. doi: 10.1007/978-1-4684-8959-0_10. [DOI] [PubMed] [Google Scholar]
- de Fries R. U., Golub S. H. Characteristics and mechanism of IFN-gamma-induced protection of human tumor cells from lysis by lymphokine-activated killer cells. J Immunol. 1988 May 15;140(10):3686–3693. [PubMed] [Google Scholar]



