Abstract
Eukaryotic H ferritins move iron through protein cages to form biologically required, iron mineral concentrates. The biominerals are synthesized during protein-based Fe2+/O2 oxidoreduction and formation of [Fe3+O]n multimers within the protein cage, en route to the cavity, at sites distributed over ∼50 Å. Recent NMR and Co2+-protein x-ray diffraction (XRD) studies identified the entire iron path and new metal-protein interactions: (i) lines of metal ions in 8 Fe2+ ion entry channels with three-way metal distribution points at channel exits and (ii) interior Fe3+O nucleation channels. To obtain functional information on the newly identified metal-protein interactions, we analyzed effects of amino acid substitution on formation of the earliest catalytic intermediate (diferric peroxo-A650 nm) and on mineral growth (Fe3+O-A350 nm), in A26S, V42G, D127A, E130A, and T149C. The results show that all of the residues influenced catalysis significantly (p < 0.01), with effects on four functions: (i) Fe2+ access/selectivity to the active sites (Glu130), (ii) distribution of Fe2+ to each of the three active sites near each ion channel (Asp127), (iii) product (diferric oxo) release into the Fe3+O nucleation channels (Ala26), and (iv) [Fe3+O]n transit through subunits (Val42, Thr149). Synthesis of ferritin biominerals depends on residues along the entire length of H subunits from Fe2+ substrate entry at 3-fold cage axes at one subunit end through active sites and nucleation channels, at the other subunit end, inside the cage at 4-fold cage axes. Ferritin subunit-subunit geometry contributes to mineral order and explains the physiological impact of ferritin H and L subunits.
Keywords: Ion Channels, Iron, Kinetics, Protein Domains, Protein-Metal Ion Interaction, Fe2+/O2 Catalysis, Caged Biominerals, Ferritin
Introduction
Ferritins are an ancient superfamily of protein nanocages that synthesize, reversibly, iron concentrates for cellular use, heme, FeS cluster, and Fe-protein synthesis and provide oxidant protection by consumption of dioxygen or hydrogen peroxide and ferrous iron during stress; the protein cavities containing the minerals are ∼60% of the cage volume (1–4). Ferritins differ in cage size, location, and mechanism of catalytic sites, mineral size, and mineral crystallinity; the Fe2+/O2 oxidoreductase sites are also called ferroxidase sites or FC (ferroxidase centers) sites (1–5). In eukaryotic H ferritins, Fe2+ and dioxygen are substrates for oxidoreductase sites and are catalytically coupled at multiple protein sites to synthesize diferric oxo mineral precursors (Fig. 1). Consumption of both iron and oxygen by ferritins accounts for the antioxidant response and iron-controlled gene regulation of ferritins in eukaryotes (6). Many catalytic proteins use iron and oxygen to produce a variety of organic products. Such products include unsaturated fatty acids such as oleate stearoyl-CoA desaturase-1 (7), deoxyribose (ribonucleotide reductase), and prolyhydroxyl modification of oxygen-sensing DNA transcription factors, e.g. hypoxia-inducible factor-α (8). Only in ferritins are both substrates inorganic. The exclusive use of inorganic substrates may relate to the ancient origins of the ferritins, which are distributed in all kingdoms and in both anaerobes and aerobes; ferritin gene deletion is lethal early in mammalian embryogenesis (9).
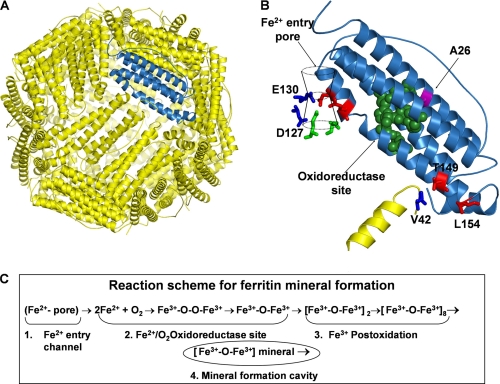
FIGURE 1.
Ferritin nanocage structure. A, frog-M ferritin nanocage structure, viewed from the outside with the 3-fold axis near the center, shown with one subunit highlighted in blue (Protein Data Bank ID code 1MFR). Eight Fe2+ ion channels (10), each formed by residues from three subunits around the 3-fold axes, lead through the protein cage from the outside of the cage to the large (8-nm diameter) central cavity, near several residues of the active sites; B, a single subunit of the frog-M ferritin showing all of the residues studied by site-directed mutagenesis; C, steps in ferritin mineral synthesis.
There are two types of protein channels that move iron into and through the 24 subunit cages during synthesis of [Fe3+O]n in eukaryotic ferritins. The two functional types of ferritin channels are: (i) ion entry channels around the 3-fold axes, for Fe2+ substrate access to oxidoreductase sites (Asp127, Glu130) and (ii) Fe3+O nucleation channels, distal to the active sites on the long axes of each subunit bundle (Ala26, Val42, Thr149), where diferric oxo mineral precursors produced by oxidoreduction fuse to tetramers and larger multimers before exiting from the protein cage for mineral growth.
Recent high resolution structural studies show that ferritin is a soluble ion channel protein with lines of multiple metal ions (10), much like K+ and other membrane ion channel proteins. The eight Fe2+ entry channels (Fig. 1) suggest roles beyond just electrostatics for the conserved carboxylates, such as ion selectivity at the channel constriction or directing Fe2+ substrate ions to active sites in three subunits that also form the entry channels (10). The Fe3+O nucleation channels were recently identified using 13C-13C solution NMR spectroscopy in the presence and absence of Fe3+, by the disappearance of resonances within 5 Å of Fe3+O moving away from the active sites (11). Nucleation channel exits into the cavity are clustered around the 4-fold axes of the cage, which facilitates ordered mineral growth. Functional studies of carboxylate residues in the Fe2+ ion entry channels have been mostly limited to later stages after diferric peroxo (DFP)4 decay (12–14) except (15), and none has examined the function of residues in the Fe3+O nucleation channel. Several recent reviews addressing the general topic of ferroxidation and catalysis in ferritin proteins include Refs. 1–4.
We now report the functional effects of amino acid substitutions on the initial Fe2+/O2 reaction intermediate, diferric peroxo (Fe3+O-O-Fe3+), and mineral precursors (Fe3+O)n (Fig. 1C), during multiple catalytic turnovers, for residues in the Fe2+ entry channels, identified by Co2+,Mg2+ protein co-crystallography (10) and earlier studies (16, 17), and on residues in the Fe3+ postoxidation/nucleation channel, identified by NMR spectroscopy (11). Our results show that conserved carboxylates in the Fe2+ ion entry channels, connecting the external cage surface to the central, mineralization cavity (Fig. 1, A and B), have two different functions, i.e. Fe2+ entry and Fe2+ distribution among multiple active sites. In addition, we show that amino acid substitutions in the Fe3+ postoxidation/nucleation channels affect oxidation and decay of the diferric peroxo intermediate. Concepts of ferritin function have usually considered that movement of iron through the protein cage depended on residues in localized regions within each 4-helix bundle subunit. The results of this study, by contrast, reveal functional residues spanning the length of each 4-helix bundle subunit, from Ala26 to Thr149, in the assembled ferritin protein cage.
EXPERIMENTAL PROCEDURES
Mutagenesis
Frog-M ferritin was used as the template because Fe-protein interactions have been studied extensively by Mössbauer, resonance Raman, EXAFS, UV-visible spectroscopies and x-ray crystallography as reviewed in Ref. 5, and more recently by active site amino acid substitutions (18), MCD/CD (19) and very high resolution x-ray crystallography (10). Site-directed amino acid substitutions in frog-M ferritin protein cages were generated by PCR on the expression plasmid pET-3a frog-M, using the QuikChange® II site-directed mutagenesis kit (Stratagene). Coding regions of all protein expression vectors were analyzed for DNA sequence confirmation (UC Berkeley DNA sequencing facility). The forward primers that were used are listed in the supplemental text, with bold nucleotides representing the mutated codon; reverse primers were complementary to the forward primers.
Protein Expression
pET-3a constructs encoding wild-type frog-M ferritin and mutants were transformed into Escherichia coli BL21(DE3)pLysS cells and subsequently cultured in LB medium containing ampicillin (0.1 mg/ml). Cells were grown at 37 °C, until A600 nm reached 0.6–0.8. Induction, at 30 °C with isopropyl 1-thio-β-d-galactopyranoside (0.5 mm final concentration), was 4 h, when cells were harvested (14, 20). Recombinant ferritins were purified as described previously (14, 20–22). In short, cells were broken by sonication, and the cell free extract obtained after centrifugation (2 h, 18,000 rpm, 4 °C) was incubated for 15 min at 65 °C as first purification step. After removal of the aggregated proteins (30 min, 18,000 rpm, 4 °C), ferritin was precipitated with 65% ammonium sulfate, resolubilized in 25 mm bis-Tris-propane, pH 7.5, and dialyzed against the same buffer. Next, the sample was loaded onto a Q-Sepharose column (Vc = 200 ml) and eluted with a linear NaCl gradient of 0–1 m in bis-tris-propane, pH 7.5. Fractions containing ferritin (monitored by SDS-PAGE) were combined, precipitated with ammonium sulfate (65%), resolubilized in volumes of 100 mm MOPS, pH 7.0, containing 100 mm NaCl, and dialyzed against the same buffer. Protein concentration was determined with a Bradford assay, and iron content was analyzed after boiling in 1 n HCl, as the Fe2+-1,10-phenanthroline complex (23, 24).
Fe2+/O2 Catalysis and Fe3+O Mineralization
Single turnover iron oxidation (uptake of 48 Fe2+/ferritin cage, 2 Fe2+/subunit), in frog-M ferritin, wild type or with amino acid substitutions, was monitored as the change in A650 nm (diferric peroxo or DFP) (12, 25) or A350 nm (Fe3+O) after rapidly mixing (<5 ms) equal volumes of 100 μm protein subunits (4.16 μm protein cages) in 200 mm MOPS, pH 7.0, containing 200 mm NaCl with a freshly prepared 200 μm ferrous sulfate in 1 mm HCl in a UV/visible, stopped-flow spectrophotometer (18). Generally, 2000 data points were collected during 10 s. The incubation time between subsequent additions of Fe2+ aliquots (48 Fe2+/nanocage) was 4–16 h (12, 25). The progress of oxidation (DFP + diferric oxo + Fe3+O tetramers + mineral nuclei + mineral) was monitored using the nonspecific absorbance between 300 and 450 nm, at ΔA350 nm. Absorbance curves for Fe3+O between 300 and 450 nm were also collected for solutions of 240 μm protein subunits (10 μm ferritin cages) with an iron concentration of 480 μm (2 Fe2+/subunit; 48 Fe2+/protein cage).
Initial rates of DFP and [Fe3+O]n species formation were determined from the linear fitting of the initial phases of 650 nm and 350 nm trace (0.01–0.03 s). The rate of decay of DFP complex was determined by exponential fitting of the decreasing part of the progress curve at 650 nm (0.1–1.5 s). The data presented are averages from a minimum of 4–6 experiments each using a minimum of two or more independent protein preparations, and the error is presented as the S.D.
Fe3+O Mineral Dissolution/Chelation
Empty ferritin protein cages were mineralized with ferrous sulfate (480 Fe2+/cage), in 100 mm MOPS, pH 7.0, containing 100 mm NaCl, as described previously (20). Mineral dissolution was monitored as formation of Fe2+-bipyridyl, at A522 nm, outside of the ferritin nanocavity after reduction of Fe3+ as described previously (20, 21, 26). A mixture of reductant (NADH and FMN) was added to a solution of 0.5 μm mineralized ferritin (0.25 mm Fe) containing 2.5 mm bipyridyl at 25 °C; final concentrations of NADH and FMN were 2.5 mm. Initial rates of iron release were calculated from the linear part of the Fe2+-bipyridyl formation (522 nm trace). The data were averaged from a minimum of four experiments using a minimum of two independent protein preparations.
RESULTS
Fe2+ Enters Ferritin Ion Channels Controlled by Glu130 and Is Directed to Active Sites by Asp127
Negatively charged ferritin residues Glu130 and Asp127 were originally identified by sequence conservation and location in 3-fold pores (16, 17). The three Glu130 residues are in the middle of the Fe2+ ion channels, one from each of the three subunits that form the channels, at a constriction (Fig. 1B); the 8 channels are formed by the juxtaposition of the helix3-loop helix4 regions of three subunits around the 3-fold axes of the protein cage. Three Asp127 residues are at the end of the channels at the entries to the large central cavity (8-nm diameter) where mineral forms. The influence of Glu130 and Asp127 on catalysis at the Fe2+/O2 oxidoreductase sites, i.e. on diferric peroxo formation, has never been studied, although substitutions at Glu130 and Asp127 are known to inhibit formation of the multiple Fe3+O species measured in the range A305–450 nm (27, 28), where Asp127 and Glu130 are equivalent to Asp131 and Glu134 in the human ferritin studied. The three Glu130 residues and the three Asp127 residues from each of the three subunits that form the eight Fe2+ entry channels in the 24-subunit cage structure, all participate in protein-metal ion interactions in the channels, based on crystal structures (10, 29–31). We predict that E130A and D127A will have different effects during multiple catalytic turnovers.
Ferritin protein nanocages with E130A or D127A substitutions in each subunit were analyzed for differences in diferric peroxo formation, compared with wild type; DFP has a λmax at 650 nm (12, 33, 34). Fe2+ was added in multiple aliquots, each of which saturated the active sites, i.e. 48 Fe2+/ferritin nanocage (2 Fe2+/active site) to achieve distinguishable turnovers of the active site. Based on the NMR experiments (11), which determined where Fe3+O is, in the protein cage, four turnovers were required for [Fe3+O]n complexes (see Fig. 1C) to complete the transit through the nucleation channels to the cavity. In addition to the DFP complex, we also measured absorbance changes at 350 nm. Many Fe3+O species contribute to the absorbance in 300–450 nm range, which monotonically decreases with increasing wavelength (27, 28, 35, 36). We monitored changes in A350 nm because they can be observed for long time periods, during mineral growth, after DFP has decayed (Fig. 2B). During ferritin mineral formation, the many Fe3+O species absorbing in the 300–450 nm range (Figs. 1C and 2D) include DFP at the active sites (12, 13, 37), diferric oxo product (12, 25), and mineral nuclei that form as Fe3+O multimers moving through the channels to the cavity (11) (reaction scheme shown in Fig. 1C). Fig. 2D shows the absorbance versus wavelength spectrum of WT ferritin (48 Fe2+/cage) immediately after the addition of Fe2+ or 60 min later. Mineral growing in the protein cavity also contributes to the absorbance in this range. Assignment of specific spectral properties to each of the Fe3+O species that occur during iron oxidation and mineral formation is not possible without additional different spectroscopic information analyses related to kinetics of mineral formation; currently the mixed spectra of the Fe3+O species are unresolved. We use changes in A350 nm to monitor mineral formation because it is far enough away from spectral contributions due to aromatic amino acids and still high enough to provide sensitive measurements.
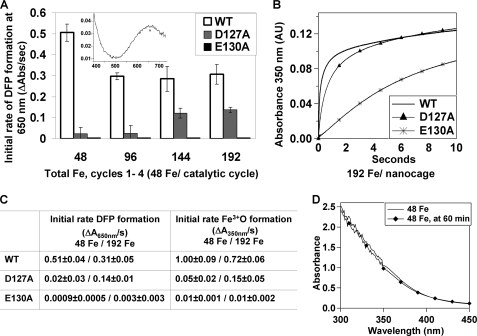
FIGURE 2.
Negatively charged residues in the 3-fold pore region are required for iron entry and active site access. For single turnover iron oxidation (uptake of 48 Fe2+/ferritin cage) (A–C), monitored by the change in A650 nm (DFP) or A350 nm (all Fe3+O) rapid mixing (<5 ms) protein concentrations were 2.08 μm ferritin cages (50 μm ferritin subunits) 100 μm ferrous sulfate solution in 100 mm MOPS, pH 7.0, 100 mm NaCl. Subsequent additions Fe2+ were 48 Fe nanocage (2/diiron active site) (see “Experimental Procedures”). For the absorbance spectra of Fe3+O in ferritin (D), the final protein concentration was 10 μm ferritin cages and 480 μm Fe2+ in 100 mm MOPS, pH 7.0, 100 mm NaCl. A, initial rates of DFP formation (ΔA650 nm/s) for the four catalytic cycles. Inset, DFP absorbance spectrum in WT frog-M ferritin, modified from Ref. 29. Progress curves at 650 nm, after each addition of iron, are illustrated in supplemental Fig. S1. B, Progress curves of A350 nm for the fourth (192 Fe2+/cage) catalytic cycle: wild type, solid line; D127A, ▴; and E130A, *. C, initial rates of single turnover for the formation of the DFP (ΔA650 nm/s) and iron oxidation (ΔA350 nm/s) for the first (48 Fe2+/cage) and fourth (192 Fe2+/cage) catalytic cycles. The numbers are averages from at least four to six experiments, each performed on at least two independent preparations of protein. D, spectra of Fe3+O species between 300 and 450 nm measured immediately (manual mixing) after Fe2+ addition and after 60 min.
Glu130 is absolutely required for formation of the DFP catalytic intermediate of ferritin (Fe2+/O2 oxidoreductase activity) because insignificant amounts of DFP formed in E130A under any conditions tested (up to 8 Fe2+/active site or 192 Fe2+/cage) in stepwise Fe2+ addition experiments (Fig. 2A). In fact, the relative rates of Fe2+ oxidation are comparable with those for the animal-specific, L-type ferritin subunit that lacks a catalytic site (14) and where Fe2+ oxidation is thought to be facilitated by iron chelation at residues on the protein or on the caged, inorganic mineral (38). The initial rate of oxidation measured at 350 nm (ΔA350 nm/s) was ∼1% wild type.
Asp127 is required for DFP formation when the amount of Fe2+ added is low (Fig. 2, A and C); partial rescue occurs when six or more Fe2+ are added/active site (Fig. 2, A and C), likely due to saturation of competing, weaker Fe2+ sites that are inactive when Asp127 is present. Rates of DFP formation in D127A ferritin are 45% of WT (Fig. 2C) when an equivalent of six or more Fe2+/active site were added. When changes in A350 nm were analyzed at six or more Fe2+/active site, the initial rates were only 20% of the WT (Fig. 2, A and C). Such results emphasize contributions of Asp127 carboxylates around the Fe2+ channel exits (Fig. 3), to directing Fe2+ substrate to each of the three catalytic sites in the middle of the helix bundles that form the ion channels. With the addition of six or more Fe2+/active site, the inhibitory effect of D127A is partly reversed; DFP formation is restored and by 10 s after adding Fe2+, long after DFP decay, the mineral growth (A350 nm) reaches WT values (Fig. 2B). Such results suggest that nonspecific Fe2+ binding sites, compete with the active sites for binding in the absence of Asp127, but are saturated when enough Fe2+ is added. Apparently, Asp127 is too far away to alter reactions after oxidation and during mineral growth.
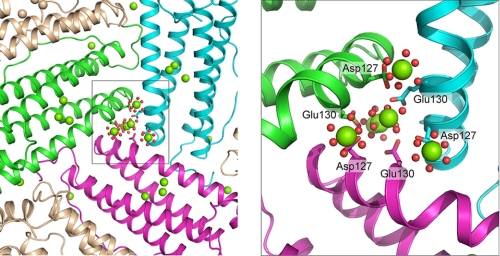
FIGURE 3.
Distribution of metal ion in ferritin protein cages to three active sites depends on the structure of the ion channels at the 3-fold cage axes. Three Mg2+ ions (hexahydrated) are observed bound to the Asp127 carboxylate and Glu130 carbonyl from each of the three frog-M ferritin near the ion channel exits into the ferritin cavity; the view is from inside the cavity into the ion channel toward the external pore opening in the exterior cage surface. In addition, in the center of the ion channel are three of the four hexahydrated Mg2+ in the line that stretches from the outside of the cage to the cavity entrance (10). The figure was made from Protein Data Bank ID code 3KA3; Mg, green spheres; coordinated water, red spheres; fragment of backbone of helix 3 of subunit forming the iron channel subunit 1, green; subunit 2, magenta; subunit 3, azure.
To show that the effects of altering pore residues Glu130 and Asp127 were selective for Fe2+ catalysis, we studied rates of mineral dissolution (NADH/FMN reduction + bipyridyl chelation of Fe2+ ions after dissolution and exit from the cage), because other conserved Fe2+ ion channel residues, e.g. Arg72, Leu110, Asp122, and Leu134, near the outside end of the channel, altered mineral reduction (20, 21). Once the hydrated ferric oxide mineral was formed in WT, D127A and E130A ferritins, the caged mineral was dissolved, and Fe2+ released at the same rates (supplemental Fig. S2) among all of the proteins. Such results emphasize the selectivity of Glu130 and Asp127 contributions to Fe2+ active site access and the reaction with dioxygen.
Conserved Residues in the Fe3+ Nucleation Channel of Ferritin, Identified by NMR Spectroscopy, Affect Ferritin Function
Residues in the postoxidation channels, identified by NMR in the presence and absence of Fe3+ (11), influence Fe2+/O2 reaction rates at the active sites (Fig. 4, A and B). The nucleation channels, also called post-oxidation channels, are distal to the active sites, on the long axis of each subunit bundle; they open around the 4-fold axes on the inner surface of the cage. The Fe3+ paramagnetic effects indicated that the residues were ∼5 Å away from Fe2+ oxidized in situ (11). Note that the apparent absence of carboxylate residues in the Fe3+O channels relates in part to incomplete site-specific NMR assignments that underrepresented carboxylates due to large size and number of residues (480 kDa, 175 residues/subunit, 24 subunits), an unusually large number of carboxylate residues, the small 13C chemical shifts for Glu and Asp, the narrow dispersion between Asn/Asp or Gln/Glu, and the effects of α-helix bundle structure (low chemical shift dispersion). Thus, carboxylate ligands in the channels may simply remain among those residues for which assignments remain to be made. Alternatively, when Fe3+O moves through the channels, charge balance could be maintained, e.g. by proton loss from water coordinated to Fe3+ in the Fe3+O species.
FIGURE 4.
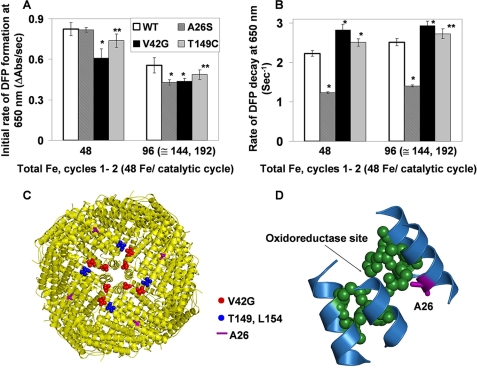
Ala26 near the active site destabilizes the DFP catalytic intermediates in ferritin. Single turnovers (48 Fe2+/nanocage; 2 Fe2+/oxidoreducatse site) by wild-type frog-M ferritin (WT) and site-directed amino acid substitutions variants were monitored, ΔA650 nm (DFP) and ΔA350 nm (Fe3+O, which includes DFP, diferric oxo, Fe3+O tetramers, and mineral), after rapidly (<5 ms) mixing equal volumes ferritin cage protein with ferrous sulfate solutions, described in Fig. 2 and under “Experimental Procedures.” The results are averages from two to three preparations of protein analyzed four to six times each, and the error is the S.D. Significantly different from WT, *, p < 0.0001 or **, p < 0.006 (t test). A, initial rates of DFP formation (ΔA650 nm/s). B, initial rates of DFP decay. C, frog-M ferritin nanocage structure viewed from the outside, with the 4-fold axis near the center (Protein Data Bank ID code 1MFR). The residues near the 4-fold pores are shown in red or blue spheres. D, part of a single subunit of the frog-M ferritin showing the ferroxidase center and the position of residue Ala26 nearby.
Residue Ala26 was identified as a part of the iron nucleation channel in the ferritin solution NMR study with and without Fe3+O species (11). In addition, we used covariation analysis, with the methods in Ref. 39 and analyzing ∼350 sequences of ferritins that were either catalytically active (His) or inactive (Leu). Residue 26 is part of a covariation network of 15 residues distributed among helices 1, 2, 3, and 4, coincident with catalytic activity. Ser26 occurs in wild-type, catalytically inactive, frog L ferritin, emphasizing the functional importance of Ala26 in ferroxidation and iron nucleation. For this reason and because of increased hydrophilicity and similar size of serine to alanine, the A26S substitution was studied in the well characterized frog-M, an H-type ferritin.
The position and orientation of Ala26 in the 4-helix bundle close to the active site at the entry of the nucleation channel suggest a role in catalysis and product release (Fig. 4D). Moreover, the A26S substitution had no effect on Fe2+ oxidation (DFP formation, A650 nm) in the first catalytic cycle. However, when more Fe2+ was added, inhibition of oxidation was significant (p < 0.0001) (Fig. 4A). In contrast to oxidation, DFP decay was sensitive to inhibition (p < 0.0001) even in the first catalytic turnover (Fig. 4B). Such observations show that Ala26 participates in product (Fe3+-O-Fe3+) release, which explains the stabilization of the DFP intermediate in A26S, even during the first catalytic cycle (Fig. 4B), and indicate slow movement of the Fe3+O product after the first turnover. The impact of abnormal product decay in A26S is further emphasized by examining the less specific A350 nm (Fig. 5). Fe3+O formation was inhibited 25% during the first catalytic turnover (supplemental Table S1), contrasting with DFP formation, which was only affected after the second addition of substrate (96 Fe2+/cage). The A350 nm was always lower in A26S than WT (Fig. 5) and even after 1 h had not reached the levels in WT protein, suggesting different properties of the Fe3+O nuclei. Thus, in addition to a role for Ala26 in the rates of product release from the Fe2+/O2 oxidoreductase center, the change in the entry to the nucleation channel changes the spectral properties of the growing mineral nuclei (11), possibly by altering hydration of the hydroxo bridges.
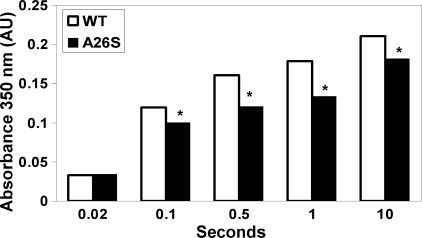
FIGURE 5.
Ala26, a residue near the entry of the Fe3+ nucleation channel, influences properties of growing ferritin mineral nuclei. Fe2+ was added in aliquot of 48 Fe2+/nanocage, under the conditions described in Fig. 2 and under “Experimental Procedures.” The absorbance between 300 and 450 nm (Fig. 2D), which increases during and after DFP decay (>0.1 s), is a combination of DFP, diferric oxo, and other ferric oxo multimers. Many studies analyze ferritin activity/mineralization at 305 or 320 nm (19, 20, 27, 28); we use A350 nm to avoid contributions from protein side chains. When DFP dominates the Fe3+O spectrum, Ala26 and WT are the same; only after DFP decays, when diferric oxo/hydroxo and larger multinuclear species begin to form, is the A350 nm absorbance difference detectable. The results are averages from two to three preparations of protein analyzed four to six times each, and the error is the S.D. Significantly different from WT, *, p < 0.0001 (Student's t test).
Thr149 in helix 4, and nearby Val42 in the loop connecting helices 1 and 2 of a neighboring subunit, are 15–20 Å from the active site residues Asp140 or Gln137 and near the Fe3+O channel exits into the cavity, around the 4-fold axes (Figs. 1B and 4C). Note that in the NMR experiment (11), Thr149 and Val42 were not affected by Fe3+ oxidized in situ until 144 Fe2+ were added (6 Fe2+/active site) with additional broadening of NMR signal when 192 Fe2+ were added (8 Fe2+/active site). Cysteine was chosen as the substitution for threonine because a Fe3+-O interaction, if it existed, would be changed by the substitution of sulfur for oxygen. Glycine was chosen as the substitution for valine to change the hydrophobicity in the loop around Val42. Compared with WT ferritin, rates of Fe2+ oxidation, monitored as ΔA650 nm or ΔA350 nm decreased significantly (p < 0.001) in V42G and T149C ferritins at all of the catalytic cycles analyzed (cycles 1–4), (Fig. 4A and supplemental Table S2); DFP stability also decreased compared with WT (p < 0.0001 for V42G and p < 0.006 for T149C) (Fig. 4B). In addition, in V42G the development of the broad absorbance band for Fe3+O (ΔA350 nm/s) lagged behind WT after mixing (supplemental Table S2). The threonine/cysteine substitution and valine/glycine substitution likely cause sufficient conformational changes throughout the channel from the active site to the channel exits to alter Fe3+O passage through the channel.
None of the amino acid substitutions studied, A26S, T149C, and V42G, like D127A and E130A, had any effect on mineral dissolution after adding reductant (FMN/NADH) with bipyridyl as an Fe2+ reporter. Such results indicate the separation of residue-specific protein-Fe interactions during iron entry and exit.
DISCUSSION
Ferritin ferric oxo minerals are built up from the diferric oxo products of catalysis in a multistep process that depends in part on the protein cage itself (Fig. 1C). The role of the protein in mineral buildup, beyond the catalytic reactions at the catalytic (oxidoreductase or ferroxidase) centers, has only recently been suggested by structural studies (11), which show that nucleation occurs inside the long (∼20 Å) Fe3+O nucleation channels (Fig. 1, A–C). However, negatively charged residues in the ion entry channels form an electrostatic environment favorable to moving Fe2+ ions to active sites (2–4). The carboxylate residues appear to have additional functions. For example, the three Asp127 residues from each subunit appear to also distribute Fe2+ substrate to each of the three active sites in the subunits that form the entry channels around the 3-fold axes (Fig. 3). Moreover, the three Glu130 residues, which form a constriction in the iron entry channel, may control not only metal ion access but also metal ion selectivity, blocking entry of large, divalent cations. Together, the structural results identified new potential functions or new amino acid-iron interactions with potential contributions to ferritin protein function (10, 11), that we studied here by making amino acid substitutions at conserved positions (Ala26, Val42, Asp127, Glu130, and Thr149) and by covariation between catalytically active (H) and inactive (L) ferritins (39).
Substitutions of Ala26, Val42, Asp127, Glu130, and Thr149 all changed ferritin function during the first four catalytic cycles when mineral nuclei are still in the protein cage and before mineral growth; in the case of Glu130 and Asp127, the effects were different (Fig. 2) as predicted using the new structural data (10). The functional effects fell into four categories: (i) Glu130, where all three carboxylates are close together and the channel is constricted, controls Fe2+ access to the active sites (DFP formation); (ii) three proximal carboxylate residues, Asp127, one from each subunit, regulate Fe2+ distribution to three active sites; (iii) Ala26 near the active site at the entry to the Fe3+ nucleation channel, regulates release of product, diferric oxo, and, thus, formation of the Fe3+O tetramer between two Fe3+O dimers; (iv) hydrophilic and hydrophobic residues in and around the Fe3+ nucleation channel influence Fe2+ oxidation and turnover. The specificity of the residues for mineral formation was shown by the absence of any effects on mineral dissolution in the ferritins with amino acid substitutions at Ala26, Val42, Asp127, Glu130, or Thr149.
Three Glu130 carboxylates form a constriction in the middle of the eight Fe2+ entry channels, one from each subunit that creates each channel and controls Fe2+ access to the active sites for DFP formation. Substitution of the negatively charged glutamate carboxylates with neutral alanine created a protein with no significant DFP formation (Fig. 2, A and C), as if the catalytic sites were inactive. E130A behaved like L ferritin, which has no catalytic sites and where mineral formation is attributed to chelating effects of conserved, clusters of carboxylate ligands on the inner surface of the protein (27, 28, 40). The diameter of the Fe2+ channels at the Glu130 constriction is ∼5.4 Å. Fe2+ hexahydrate has a diameter of ∼4.5 Å. Multiple small ions such as Mg2+ line up in the channels at and below the Glu130 constriction whereas a single, larger Co2+ ion is “stuck” at Glu130 in protein cocrystals (10). In addition to attracting the ions into the channel by the high concentration of negative charge, the close fit of Fe2+ at the Glu130 channel constriction suggests that Glu130 also exerts some metal ion selectivity.
The Asp127 residues in the Fe2+ entry channels, one from each ferritin subunit that form the channels, surround the channel exits into the mineral cavity (Figs. 1B and 3). Asp127 influences Fe2+ access to the three active sites because substitution with alanine prevented formation of the DFP intermediate, when only small numbers of Fe2+ were added. The amino acid substitutions D127A and E130A had no detectable effect on Fe release (supplemental Fig. S2).
However, when more Fe2+ was added (6 Fe2+/active site) DFP was detected (Fig. 2, A and C). The rescue of catalytic activity in D127A, by adding more Fe2+ substrate, contrasts with E130A and has several possible explanations, although in each case the electrostatic potential in the channels decreases with the substitution of alanine for each of the carboxylates. First, it may take at least 6 Fe2+ to eliminate competition from the carboxylate chelating residues on the inner surface (40). Second, with such a large number of Fe2+ ions, the dependence on subunit-subunit cooperativity around the channel may be overcome (Fe2+ binding at the active site, monitored by MCD/CD had a Hill coefficient of 3 (19) that could only be explained by subunit-subunit interactions). Third, in a recent structure of Mg2+-ferritin cocrystal, metal ions were bound to the three Asp127 carboxylates around each channel exit on the inner surface of the cage (Fig. 3), pointing toward the three active sites nearest each channel (10). A reasonable model that fits the available data is that Fe2+ after passing the Glu130 “filter” is directed by Asp127 to each of the three active sites in the subunits that make up each pore. Binding of Fe2+ at the active sites, thus, depends on: (i) the orientation of Fe2+ by Asp127 toward each of the three active sites in the subunits that form each pore, indicating the functional significance of cage assembly geometry (Fig. 3); (ii) conformational changes that enhance the coordinated filling of three active sites around each entry pore; and (iii) tight binding of Fe2+ at the active sites that outcompetes binding to clusters of carboxylate residues on the inner surface of the cavity (40).
Ala26, close to the active site at the beginning of the Fe3+ nucleation channel (Fig. 4D), enhances reaction/product release because rates of oxidation and decay were decreased in A26S ferritin cages, except for the first catalytic cycle when DFP formation was normal but decay was still slower. The importance of conserved Ala26 was indicated by proximity to the diferric oxo dimer (<5 Å) in the NMR experiment when 48 Fe2+/cage were added (2 Fe2+/active site) (11), as well as covariation analysis (39) of catalytically active (H-type) and inactive (L-type) ferritin subunits. In addition, when the A350 nm is measured after the DFP intermediate has been converted to the diferric oxo precursor (<1 s), contributions of A26S to slower reactions such as mineral growth itself are observed (Fig. 5). Ala26 may also influence Fe3+O tetramer formation in the channels, observed by magnetic susceptibility (11), because the full effect of A26S is not observed until 96 Fe2+ ions (4 Fe3+O/channel) are present (data not shown). Possibly the steric resistance or potential hydrogen bond interaction of the serine, which replaces alanine in the A26S ferritin, not only inhibits diferric oxo product release from the catalytic sites but also inhibits the reaction of two Fe3+O dimers to form Fe3+oxo tetramer in the nucleation channel, possibly by interfering with the correct orientation of Fe3+O dimers, after they leave the active sites.
Val42 and Thr149, situated near each other and near the exits of the Fe3+ nucleation channels, at the cavity entrance, are ∼25 Å from Fe2+ entry channel exits into the cavity. They most likely influence Fe2+/O2 oxidoreductase catalysis and Fe3+O mineral nucleation through effects on subunit conformations along the long axes of each subunit bundle and at subunit-subunit interfaces, because the Val42 close to [Fe3+O]n is in another subunit adjacent to the one with Thr149 (Fig. 1B). A role for uncharged residues in moving ions through channel proteins has recently been demonstrated in a Kv 2.1 channel (41). Because the Val42 and Thr149 are in different subunits (11), interactions between pairs of subunits may be key in moving Fe3+O through ferritin nucleation channels. Such an idea is supported by the inhibitory effects of subunit cross-links between pairs of ferritin subunits on Fe3+O/mineral formation (42).
An active role of H ferritin subunits in ferritin mineral nucleation and ordered iron mineral growth (11) provides an explanation for the physiologically significant and different combinations of catalytically active (H) and inactive (L) ferritin subunits in animal ferritins and the coincident variations in iron mineral order (43, 44). Ordered/more crystalline ferritin iron minerals will dissolve more slowly (release iron more slowly) than disordered ferritin minerals, which explains why increased L ferritin subunits increased cell proliferation: iron was released faster for critical iron proteins such as ribonucleotide reductase, which is rate-limiting for DNA synthesis. Decreased L subunits deceased cell proliferation (43). Moreover, heart ferritin, which functions in highly oxygenated tissue, has more H subunits, more antioxidant activity, and more crystalline (slow iron release) mineral (44). However, liver ferritin, which must release iron for other tissues, has a large number of L subunits and less ordered mineral. The disordered growth of ferritin iron minerals with large numbers of L subunits such as that from liver, is attributable to protein-independent, inorganic reactions (45).
Conserved amino acids, both hydrophilic and hydrophobic, along the entire length of each ferritin four α-helix subunit bundle contribute to function in ferritin protein nanocages, based on the new data in this report, and contrasting with earlier studies that have focused on the functions of localized clusters of conserved amino acids related, e.g. to catalysis or mineralization (5, 38). Previously, conserved amino acids in ferritin that were not in localized clusters such as the oxidoreductase sites were assigned roles in folding and stabilizing the unique ferritin cage structure, with the exception of those around the 3-fold channels shown to regulate Fe2+ release from the mineral and the cage (5) or those theoretically shown to create local electrostatic gradients for Fe2+ entry through the ion channels (32). The amino acids studied here, which all have functional effects on iron oxidation and mineralization, are distributed over a distance of ∼50 Å. From the Fe2+ entry channels, formed by three subunits at the 3-fold cage axes, to the nucleation channel exits, at the other end of the subunit 4-helix bundles around the 4-fold cage axes, where mineral nuclei emerge, conserved, ferritin subunit amino acid residues influence the process of iron biomineralization. How these studies relate to the ferritins with different active sites and/or cage locations as in bacteria and archaea is not known (46, 47). However, the results here suggest that the unusually high cage symmetry in ferritins, especially in the more highly evolved eukaryotic ferritins, contributes to function emphasizing the role of cage geometry itself in function.
Supplementary Material
Acknowledgment
We thank Dr. Tamara Lawson for cloning V42G and T149C.
This work was supported, in whole or in part, by National Institutes of Health Grant DK-20251 (to L. E. B., S. H., T. T., and E. C. T.). This work was also supported by grants from The Netherlands Organization for Scientific Research (to L. E. B.) and the Japan Society for the Promotion of Science (to T. T.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text, Tables S1 and S2, Figs. S1 and S2, and an additional reference.
- DFP
- diferric peroxo
- MCD
- magnetic circular dichroism
- bis-Tris
- bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane.
REFERENCES
- 1. Theil E. C. (2011) Curr. Opin. Chem. Biol. 15, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiancone E., Ceci P. (2010) Biochim. Biophys. Acta 1800, 798–805 [DOI] [PubMed] [Google Scholar]
- 3. Bou-Abdallah F. (2010) Biochim. Biophys. Acta 1800, 719–731 [DOI] [PubMed] [Google Scholar]
- 4. Arosio P., Ingrassia R., Cavadini P. (2009) Biochim. Biophys. Acta 1790, 589–599 [DOI] [PubMed] [Google Scholar]
- 5. Liu X., Theil E. C. (2005) Acc. Chem. Res. 38, 167–175 [DOI] [PubMed] [Google Scholar]
- 6. Theil E. C., Goss D. J. (2009) Chem. Rev. 109, 4568–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shanklin J., Guy J. E., Mishra G., Lindqvist Y. (2009) J. Biol. Chem. 284, 18559–18563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaelin W. G., Jr., Ratcliffe P. J. (2008) Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 9. Ferreira C., Bucchini D., Martin M. E., Levi S., Arosio P., Grandchamp B., Beaumont C. (2000) J. Biol. Chem. 275, 3021–3024 [DOI] [PubMed] [Google Scholar]
- 10. Tosha T., Ng H. L., Bhattasali O., Alber T., Theil E. C. (2010) J. Am. Chem. Soc. 132, 14562–14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turano P., Lalli D., Felli I. C., Theil E. C., Bertini I. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pereira A. S., Small W., Krebs C., Tavares P., Edmondson D. E., Theil E. C., Huynh B. H. (1998) Biochemistry 37, 9871–9876 [DOI] [PubMed] [Google Scholar]
- 13. Bou-Abdallah F., Papaefthymiou G. C., Scheswohl D. M., Stanga S. D., Arosio P., Chasteen N. D. (2002) Biochem. J. 364, 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu X., Theil E. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 8557–8562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao G., Bou-Abdallah F., Arosio P., Levi S., Janus-Chandler C., Chasteen N. D. (2003) Biochemistry 42, 3142–3150 [DOI] [PubMed] [Google Scholar]
- 16. Levi S., Luzzago A., Cesareni G., Cozzi A., Franceschinelli F., Albertini A., Arosio P. (1988) J. Biol. Chem. 263, 18086–18092 [PubMed] [Google Scholar]
- 17. Bauminger E. R., Harrison P. M., Hechel D., Hodson N. W., Nowik I., Treffry A., Yewdall S. J. (1993) Biochem. J. 296, 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tosha T., Hasan M. R., Theil E. C. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 18182–18187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz J. K., Liu X. S., Tosha T., Theil E. C., Solomon E. I. (2008) J. Am. Chem. Soc. 130, 9441–9450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takagi H., Shi D., Ha Y., Allewell N. M., Theil E. C. (1998) J. Biol. Chem. 273, 18685–18688 [DOI] [PubMed] [Google Scholar]
- 21. Jin W., Takagi H., Pancorbo B., Theil E. C. (2001) Biochemistry 40, 7525–7532 [DOI] [PubMed] [Google Scholar]
- 22. Liu X., Jin W., Theil E. C. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3653–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rohrer J. S., Joo M. S., Dartyge E., Sayers D. E., Fontaine A., Theil E. C. (1987) J. Biol. Chem. 262, 13385–13387 [PubMed] [Google Scholar]
- 24. Rohrer J. S., Frankel R. B., Papaefthymiou G. C., Theil E. C. (1989) Inorg. Chem. 28, 3393–3395 [Google Scholar]
- 25. Jameson G. N.., Jin W., Krebs C., Perreira A. S., Tavares P., Liu X., Theil E. C., Huynh B. H. (2002) Biochemistry 41, 13435–13443 [DOI] [PubMed] [Google Scholar]
- 26. Jones T., Spencer R., Walsh C. (1978) Biochemistry 17, 4011–4017 [DOI] [PubMed] [Google Scholar]
- 27. Levi S., Santambrogio P., Corsi B., Cozzi A., Arosio P. (1996) Biochem. J. 317, 467–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Treffry A., Bauminger E. R., Hechel D., Hodson N. W., Nowik I., Yewdall S. J., Harrison P. M. (1993) Biochem. J. 296, 721–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lawson D. M., Artymiuk P. J., Yewdall S. J., Smith J. M., Livingstone J. C., Treffry A., Luzzago A., Levi S., Arosio P., Cesareni G. (1991) Nature 349, 541–544 [DOI] [PubMed] [Google Scholar]
- 30. Ha Y., Shi D., Small G. W., Theil E. C., Allewell N. M. (1999) J. Biol. Inorg. Chem. 4, 243–256 [DOI] [PubMed] [Google Scholar]
- 31. Gallois B., Langlois d'Estaintot B., Michaux M. A., Dautant A., Grainer T., Precigoux G., Soruco J. A., Roland F., Chavas-Alba O., Herbas A., Crichton R. R. (1997) J. Biol. Inorg. Chem. 2, 360–367 [Google Scholar]
- 32. Takahashi T., Kuyucak S. (2003) Biophys. J. 84, 2256–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moënne-Loccoz P., Krebs C., Herlihy K., Edmondson D. E., Theil E. C., Huynh B. H., Loehr T. M. (1999) Biochemistry 38, 5290–5295 [DOI] [PubMed] [Google Scholar]
- 34. Hwang J., Krebs C., Huynh B. H., Edmondson D. E., Theil E. C., Penner-Hahn J. E. (2000) Science 287, 122–125 [DOI] [PubMed] [Google Scholar]
- 35. Honarmand Ebrahimi K., Hagedoorn P. L., Jongejan J. A., Hagen W. R. (2009) J. Biol. Inorg. Chem. 14, 1265–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li C., Fu X., Qi X., Hu X., Chasteen N. D., Zhao G. (2009) J. Biol. Chem. 284, 16743–16751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fetter J., Cohen J., Danger D., Sanders-Loehr J., Theil E. C. (1997) J. Biol. Inorg. Chem. 2, 652–661 [Google Scholar]
- 38. Chasteen N. D., Harrison P. M. (1999) J. Struct. Biol. 126, 182–194 [DOI] [PubMed] [Google Scholar]
- 39. Süel G. M., Lockless S. W., Wall M. A., Ranganathan R. (2003) Nat. Struct. Biol. 10, 59–69 [DOI] [PubMed] [Google Scholar]
- 40. Bou-Abdallah F., Biasiotto G., Arosio P., Chasteen N. D. (2004) Biochemistry 43, 4332–4337 [DOI] [PubMed] [Google Scholar]
- 41. Tao X., Lee A., Limapichat W., Dougherty D. A., MacKinnon R. (2010) Science 328, 67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mertz J. R., Theil E. C. (1983) J. Biol. Chem. 258, 11719–11726 [PubMed] [Google Scholar]
- 43. Cozzi A., Corsi B., Levi S., Santambrogio P., Biasiotto G., Arosio P. (2004) Blood 103, 2377–2383 [DOI] [PubMed] [Google Scholar]
- 44. St Pierre T. G., Tran K. C., Webb J., Macey D. J., Heywood B. R., Sparks N. H., Wade V. J., Mann S., Pootrakul P. (1991) Biol. Met. 4, 162–165 [DOI] [PubMed] [Google Scholar]
- 45. Yang X., Chen-Barrett Y., Arosio P., Chasteen N. D. (1998) Biochemistry 37, 9743–9750 [DOI] [PubMed] [Google Scholar]
- 46. Le Brun N. E., Crow A., Murphy M. E., Mauk A. G., Moore G. R. (2010) Biochim. Biophys. Acta 1800, 732–744 [DOI] [PubMed] [Google Scholar]
- 47. Chiancone E., Ceci P. (2010) Front. Biosci. 15, 122–131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.