Abstract
The hexosamine biosynthesis pathway (HBP) regulates the post-translational modification of nuclear and cytoplasmic protein by O-linked N-acetylglucosamine (O-GlcNAc). Numerous studies have demonstrated increased flux through this pathway contributes to the development of β-cell dysfunction. The effect of decreased O-GlcNAc on the maintenance of normal β-cell function, however, is not well understood. We studied transgenic mice that over express β-N-acetylglucosaminidase (O-GlcNAcase), an enzyme that catalyzes the removal of O-GlcNAc from proteins, in the pancreatic β-cell under control of the rat insulin promoter. 3–4-Month-old O-GlcNAcase transgenic mice have higher glucose excursions with a concomitant decrease in circulating insulin levels, insulin mRNA levels, and total islet insulin content. In older (8–9-month-old) O-GlcNAcase transgenic mice glucose tolerance is no longer impaired. This is associated with increased serum insulin, islet insulin content, and insulin mRNA in the O-GlcNAcase transgenic mice. These improvements in β-cell function with aging are associated with increased angiogenesis and increased VEGF expression, with parallel increases in activation of Akt and expression of PGC1α. The biphasic effects as a function of age are consistent with published observations of mice with increased O-GlcNAc in islets and demonstrate that O-GlcNAc signaling exerts multiple effects on both insulin secretion and islet survival.
Keywords: Aging, Diabetes, Glycosylation, Insulin Secretion, Pancreatic Islets, O-GlcNAc
Introduction
In the hexosamine biosynthesis pathway (HBP),3 a small fraction of cellular fructose-6-phosphate is converted to glucosamine-6-phosphate by the first and rate-limiting enzyme glutamine:fructose-6-phosphate amidotransferase (GFA) (1). Subsequent steps metabolize glucosamine-6-phosphate to UDP-N-acetylglucosamine (UDP-GlcNAc) and other amino sugars used in the synthesis of glycoproteins and glycolipids. One pathway using UDP-GlcNAc as a donor substrate is the O-linked glycosylation of nuclear and cytosolic proteins on serine and/or threonine residues by the enzyme uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase (O-glycosyltransferase). Deglycosylation of these proteins is catalyzed by β-N-acetylglucosaminidase (O-GlcNAcase) (2, 3). Because O-glycosyl transferase is often limited by the availability of UDP-GlcNAc, and its levels are in turn rate-limited by hexose entry into the HBP, modification of proteins by O-GlcNAc serves a nutrient-sensing function. The HBP/O-GlcNAc pathway regulates metabolism in several tissues, exerting pleiotropic effects that are both acute (e.g. down-regulation of glucose uptake and glycogen synthase activity by direct modification of glycogen synthase and insulin signal transduction proteins) and long term (e.g. modification of transcription factors and epigenetic effects) (4–10).
Previous studies have demonstrated an important role for the HBP/O-GlcNAc pathway in regulating the function of the pancreatic β-cells. Acute increases in the levels of O-GlcNAc achieved by treating β-cells with inhibitors of O-GlcNAcase result in decreased glucose stimulated insulin secretion (11). Treatment of primary rat islets with glucosamine, which results in a large increase in O-GlcNAc by entering the HBP downstream of rate-limiting GFA, also decreases insulin secretion (12). The effects of O-GlcNAc are, however, dose- and time-dependent. Transgenic overexpression of GFA in β-cells, resulting in a more modest increase in UDP-GlcNAc and O-GlcNAc modification than the treatments above, has the opposite effect and leads to hyperinsulinemia and increased total insulin content in the pancreas of younger C57BL/6J mice (13). The latter results are consistent with the relatively acute nutrient-sensing function of the HBP wherein an apparent increase in glucose flux is sensed through the increased HBP activity and insulin secretory capacity is increased accordingly. As the same mice age, however, they exhibit decreased glucose stimulated insulin secretion, demonstrating the distinct effects of subacute versus chronic modulation of the HBP/O-GlcNAc pathway (14). Results such as these suggest that the pathway mediates aspects of both physiologic and adaptive nutrient-sensing in situations of normal glucose flux (15) and pathophysiologic, deleterious changes in β-cell function seen with chronic nutrient excess or diabetes (7, 8, 13).
The mechanisms of HBP/O-GlcNAc regulation of β-cell function are incompletely understood but have been shown to include regulation of the function, transcription, and translocation of transcription factors involved in β-cell development and insulin gene expression (16–18). Another pathway through which the HBP may regulate β-cell function on a more chronic basis is by altering angiogenesis. We recently reported that the HBP/O-GlcNAc pathway regulates angiogenesis in vascular endothelial cells (19), and angiogenesis is also crucial to islet function. To facilitate blood glucose sensing, pancreatic islets are richly vascularized, and β-cells are known to secrete the angiogenic VEGF (20). Deletion of VEGF in β-cells leads to significant loss of islet vessel density and results in glucose intolerance, impaired insulin production, and defective insulin secretion (21). This process is thought to be particularly critical in supporting the expansion of the β-cell mass in response to insulin resistance induced by aging or obesity.
The contribution of the HBP/O-GlcNAc pathway to overall β-cell function and insulin secretory capacity, particularly in situations of euglycemia and through the life of the organism and the full dynamic range of low to high O-GlcNAc, remains unclear. We therefore created transgenic mice overexpressing O-GlcNAcase in pancreatic β-cells under control of the rat insulin promoter. We report that these animals have decreased insulin synthesis and secretory capacity while young, but as they age, they compensate through a mechanism that involves increased angiogenesis. These studies demonstrate the multifaceted involvement of the HBP/O-GlcNAc pathway in supporting nutrient sensing functions through several mechanisms that result in changes that are both acute and chronic, adaptive and deleterious.
EXPERIMENTAL PROCEDURES
Experimental Animals and Generation of Rat Insulin Promoter-O-GlcNAcase Transgenic Mice
A bluescript SK+/− phagemid (Stratagene, La Jolla, CA), modified with the rat insulin promoter, human GFA gene and SV40 polyadenosine tail, had been used previously to generate an islet-specific GFA transgenic C57/BL6 mouse line (13). This plasmid was modified by removal of the human GFA gene and insertion of the human O-GlcNAcase gene, a gift of Gerald Hart (The Johns Hopkins University). A consensus Kozak sequence was introduced immediately adjacent to the initiation codon by site-directed mutagensis (Stratagene). The resulting plasmid was cut with Sap1 and BsaA1 enzymes and the rat insulin promoter/O-GlcNAcase/SV40 polA fragment was isolated, purified, and submitted to the Transgenic Core Facility (Department of Molecular Biology, Princeton University). Eight transgenic mouse lines were generated and selected based on combined human/mouse O-GlcNAcase mRNA levels in the islet. The mice were generated and maintained on the C57/BL6 background. The Institutional Animal Care and Use Committee of the University of Utah approved all experimental procedures.
Antibodies
Antibodies to Akt and phospho-Akt (Ser-473) were from Cell Signaling Technology (Danvers, MA). Anti-O-GlcNAc monoclonal IgM antibody (CTD 110.6) was a gift from Dr. Gerald Hart. GAPDH antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Islet Isolation
Islets were isolated using the intraductal Liberase RI digestion technique. Briefly, mice were killed by cervical dislocation, and 3 ml of Hanks' balanced salt solution containing 0.3 mg/ml Liberase RI Purified Enzyme Blend (Roche Applied Science) were injected into the pancreas through the bile duct. Pancreata were excised and incubated at 37 C for 12 min, briefly shaken, and filtered through a nylon mesh. The islets were further purified by handpicking under a dissecting microscope to eliminate any remaining exocrine tissue.
Islet Insulin Content
Size-matched islets (5–10/determination) from each group were sonicated in 1 ml of Hanks' balanced salt solution at setting 4 for 10 1-s bursts (Brinkmann Sonic Dismembrator 60, Fisher Scientific, Pittsburgh, PA). Insulin was measured using the Sensitive Rat Insulin RIA Kit (Linco Research, Inc.).
Intraperitoneal Glucose Tolerance Testing (IPGTT) and Acute Insulin Secretory Responses in Vivo
Experimental animals were fasted for 6 h, after which glucose (1 g/kg body weight) was administered intraperitoneally to nonsedated animals. Tail vein blood (3 ul) was sampled for glucose determination (Glucometer Elite, Bayer Corp., Tarrytown, NY) before and 5, 15, 30, 60, and 120 min after glucose administration. Tail vein blood (50 ul) was also collected for insulin determination before and 30 min after the start of the IPGTT. Values for homeostasis model assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-B) were calculated as described (22).
Intraperitoneal Insulin Tolerance Test
Human recombinant insulin (Eli Lilly & Co., Indianapolis, IN; 0.75 units/kg body weight) was administered i.p. to random-fed conscious mice. Tail vein blood (3 ul) was sampled for glucose determination (Glucometer Elite, Bayer Corp.) before and 15, 30, 60, 90, and 120 min after insulin administration.
Angiogenesis Assays
Mouse pancreata were dissected and fixed in 4% paraformaldehyde in PBS overnight. Cryosections were cut at 10 μm, collected on superfrost plus slides (Fisher), and stained with primary antibody CD31 (BD Biosciences), followed by Alexa Fluor 594 donkey anti-goat IgG secondary antibody (Invitrogen). DAPI was used to stain nuclei. The percentage of CD31-immunopositive area in islets was quantified from at least 30 sections from four to five animals in each group using NIH ImageJ software. Endothelial cell sprouting from cultured pancreatic islets was quantified as described previously (23). Briefly, mouse islets were isolated, embedded in Matrigel (BD Biosciences), and cultured in endothelial growth medium with 10% FBS. Vascular sprouting was monitored every 2 days under inverted microscopy and recorded at day 6. VEGF (10 ng/ml) was used to treat cultured WT islets as a positive control.
Cell Culture and Adenoviral-mediated Gene Transfer
INS-1 cells, a line derived from pancreatic β-cells, were maintained in RPMI medium (Invitrogen) with 10% FBS, 50 μm β-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin. INS-1 cells were infected with the adenovirus encoding O-GlcNAcase (a gift from Dr. Wolfgang Dillmann, University of California, San Diego) for 2 days. After removal of virus-containing medium, cells were maintained in RPMI with 10% FBS. A replication-defective vector expressing GFP was used as a control. Preliminary studies revealed that after 48 h of infection with control GFP, >90% of INS-1 cells expressed green fluorescent protein.
Western Blotting
Islets or cell pellets sonicated in lysis buffer (50 mm Hepes, pH 7.4, 100 mm NaCl, 5% glycerol, 1% Triton X-100, 1 mm EDTA, with protease inhibitor mixture (Roche Applied Science) and phosphatase inhibitor mixture (Sigma-Aldrich)). 20–60 μg of denatured protein were resolved on a 10% SDS-polyacrylamide gel and transferred onto Immobilon nitrocellulose membrane (Millipore, Billerica, MA). Blots were blocked with TBS-T (20 mm Tris, pH 7.4, 150 mm sodium chloride, and 0.5% Tween 20) containing 5% (w/v) nonfat dried milk for 1 h at room temperature. For detection with the CTD 110.6 antibody, blots were blocked with TBS-T containing 5% (w/v) bovine serum albumin. Blots were incubated with primary antibodies overnight at 4 °C and then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature.
RT-PCR
RNA was isolated from mouse islets and INS-1 cells using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH), purified using an RNeasy mini kit (Qiagen, Valencia, CA), and reverse-transcribed into cDNA. cDNA was purified using a QIAquick PCR purification kit (Qiagen) prior to RT-PCR studies. mRNA levels were normalized to β-actin 5′-gtgggaatgggtcagaagca-3′ (forward) and 5′-ccctcgtagatgggcacagt-3′ (reverse). Other primers used for the studies were as follows: O-GlcNAcase, 5′-ccctcagcctggattactgct-3′ (forward) and 5′-agacaggaggcaagccatca-3′ (reverse); insulin-1, 5′-agcaagcaggtcattgttcc-3′ (forward) and 5′-gtagagggagcagatgctgg-3′ (reverse); insulin-2, 5′-cctgctggccctgctctt-3′ (forward) and 5′-ggctgggtagtggtgggtcta-3′ (reverse); PDX-1, 5′-tggatgaaatccaccaaagc-3′ (forward) and 5′-ttcaacatcactgccagctc-3′ (reverse); NeuroD1, 5′-caaagccacggatcaatctt-3′ (forward) and 5′-cccgggaatagtgaaactga-3′ (reverse); Maf-A, 5′-aggaggaggtcatccgactg-3′ (forward) and 5′-cttctcgctctccagaatgtg-3′ (reverse); Glut-2, 5′-gcctgtgtatgcaaccattg-3′ (forward) and 5′-gaagatggcagtcatgctca-3′ (reverse); VEGF-A, 5′-aaggaggagggcagaatcat-3′ (forward) and 5′-atctgcatggtgatgttgga-3′ (reverse); ANG-1, 5′-cttcaaggcttggtttctcg-3′ (forward) and 5′-tctgcacagtctcgaaatgg (reverse); TNFα, 5′-agcccccagtctgtatcctt-3′ (forward) and 5′-ctccctttgcagaactcagg-3′ (reverse); FGF1, 5′-ggacctggtgatgcaaagtt-3′ (forward) and 5′-atttgtgcggttctggtagg-3′ (reverse); ANG-2, 5′-gcatgtggtccttccaactt-3′ (forward) and 5′-tggtgtctctcagtgccttg-3′ (reverse); and PGC1α, 5′-atcaaggtccccaggcagtag-3′ (forward) and 5′-cgtgctctttgcggtattca-3′ (reverse).
Statistical Analysis
Results are presented as mean ± S.E. The unpaired Student's t test (two-tailed) was used to assess differences between experimental groups and controls. Probability values of <0.05 were considered statistically significant.
RESULTS
β-Cell Function Is Decreased in O-GlcNAcase Transgenic Mice
Transgenic mice exhibited a 6.4-fold increase (p < 0.01) in O-GlcNAcase expression in pancreatic islets (Fig. 1A). No increase was evident in liver, muscle, or fat (data not shown). O-GlcNAcase protein expression was also increased, and this resulted in a 130% decrease in overall protein O-linked glycosylation in islets, as demonstrated by Western blotting using a nonprotein-specific antibody to O-linked GlcNAc (Fig. 1B, p < 0.05). To limit variance in studies of these mice, all experiments were performed on female mice unless otherwise indicated, although selected critical results were replicated in males as indicated below.
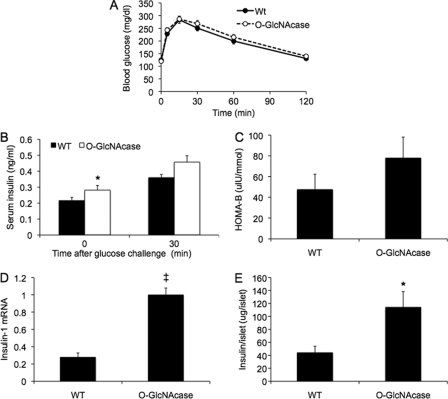
FIGURE 1.
Impaired glucose tolerance resulting from decreased insulin in 3–4-month-old O-GlcNAcase transgenic mice. A, islets of Langerhans were isolated from WT and O-GlcNAcase transgenic female mice (n = 4 each). The indicated mRNA levels in isolated islets were measured by quantitative PCR and normalized to β-actin. B, the indicated protein levels in isolated islets from WT and O-GlcNAcase transgenic (Tg) were measured by Western blot. CTD110.6 antibody was used for O-GlcNAc detection. C, experimental animals (3–4-month-old females) were fasted for 6 h followed by intraperitoneal glucose injection (1 g/kg body weight). At the indicated times, tail vein blood was sampled for glucose determination with a glucometer (n = 13–20/group). D, tail vein blood was also sampled for insulin measurement before and 30 min after challenge (n = 18–24/group). E, mice were injected with human recombinant insulin (0.75 units/kg body weight), and blood glucose levels were determined with a glucometer (n = 5/group). HOMA-IR (F)and HOMA-B (G) values were calculated as described under “Experimental Procedures” using the fasting glucose and insulin data obtained during the IPGTT in C (n = 13–20/group). *, p < 0.05; ‡, p < 0.01.
To determine the role of β-cell O-GlcNAcase in insulin secretion and glucose homeostasis, we performed an IPGTT on 3–4 month old mice fed normal chow and fasted for 6 h. O-GlcNAcase transgenic female mice displayed statistically significant (p < 0.01) increases in glucose levels at all time points compared with WT mice except at 120 min (Fig. 1C). Similar results were seen in male mice, with a 27 ± 3% increase in the area under the glucose curve after IPGTT in the transgenic compared with WT mice (p < 0.05, data not shown). The differences in glucose tolerance were not attributable to differences in weight between the groups (WT, 19.9 ± 0.2 g, O-GlcNAcase, 19.6 ± 0.2 g). Serum insulin levels of O-GlcNAcase mice were lower both in the fasted state and 30 min after glucose challenge (p < 0.05, Fig. 1D). To determine if the glucose intolerance might be related to decreased insulin responsiveness, we performed insulin tolerance testing. O-GlcNAcase transgenic and WT mice responded equally well to insulin, suggesting a primary defect in insulin secretion (Fig. 1E). Consistent with this, homeostasis model assessment of insulin resistance (HOMA-IR) (22) did not reveal increased insulin resistance in O-GlcNAcase mice (Fig. 1F). HOMA assessment of β-cell function (HOMA-B), by contrast, revealed a 30% decrease in β-cell function in the O-GlcNAcase transgenic mice (p < 0.05, Fig. 1G). These results demonstrate that O-GlcNAc signaling plays a role in maintaining normal insulin levels, even in normoglycemic, young, otherwise physiologically unstressed mice.
Islet Insulin Content and mRNA Are Decreased in O-GlcNAcase Transgenic Mice
To investigate the mechanism of the decreased fasting insulin level in the O-GlcNAcase transgenic mice, we studied isolated islets from the pancreata of 3–4-month-old mice. Transgenic islets exhibited a significantly lower expression of insulin-1 mRNA (Fig. 2A, p < 0.05). Insulin-2 mRNA also trended lower by 56% (p = 0.055). The transcription factors PDX-1 (pancreatic and duodenal homeobox-1), NeuroD1 (neurogenic differentiation 1), and Maf-A (V-maf musculoaponeurotic fibrosarcoma oncogene homologue A) act in a coordinated and synergistic manner to regulate insulin gene transcription (24–26). The activities or expression levels of all of these proteins have previously been shown to be regulated by the HBP/O-GlcNAc pathway (11, 16–18, 27). We observed no significant difference in the mRNA levels of these factors in the mice overexpressing O-GlcNAcase compared with WT (Fig. 2A). The mRNA level of the β-cell glucose transporter, GLUT-2, was also unaffected. Consistent with the changes in the mRNAs for insulin, insulin content per islet in 3–4-month-old transgenic mice, normalized to islet protein, was decreased by 44% (p < 0.05, Fig. 2B). To examine insulin secretion, isolated islets were exposed to different glucose concentrations and 30 mm KCl as a maximal pharmacologic stimulus. There were no differences in insulin secretion per islet between the two groups when treated with either 2.8 or 8 mm glucose (Fig. 2C). At 16 mm glucose, there was a trend toward decreased insulin secretion (27%) in islets from O-GlcNAcase transgenic mice but the result was not statistically significant (p = 0.16). Pharmacological treatment of islets with 30 mm KCl, however, resulted in a 46% decrease in insulin secretion in the O-GlcNAcase islets (p < 0.05, Fig. 2C). Thus, in islets of these younger mice, the decreased glucose tolerance seen with overexpression of O-GlcNAcase is associated with decreased insulin gene expression and decreased maximal insulin secretory capacity. We also investigated beta cell mass in the mice because of the reported effects of changes in O-GlcNAc on transcription factors that contribute to islet differentiation. There was no change in beta cell area per islet as determined by anti-insulin antibody staining in transgenic compared with WT islets, and the number of islets per pancreas actually trended to be higher in the transgenic mice (not shown). Thus, there is no evidence for an effect of O-GlcNAcase overexpression on the mass of beta cells, rather it is the case that the function and/or secretory capacity of the islets is the main determinant of the phenotype described in Fig. 1.
FIGURE 2.
Islet insulin mRNA, insulin content, and maximal insulin secretory capacity are decreased in O-GlcNAcase transgenic mice. A, mRNA was harvested from ∼100 islets per mouse, and transcript levels were normalized to β-actin (n = 6 mice/group). B, 10 islets were collected from the pancreas of each mouse, and insulin levels were measured after sonication (n = 3–4 mice/group). C, 10 islets were collected from the pancreas of each mouse, and insulin secretion was measured after static incubation in KRBH solution containing the indicated glucose concentrations or the pharmacological secretagogue, 30 mm KCl. (n = 4 mice/group). *, p < 0.05.
Glucose Tolerance Improves with Age in Mice with O-GlcNAcase Overexpression
Based on the observation that aging normally leads to increased O-GlcNAc levels (28, 29) and that elevated O-GlcNAc impairs β-cell function (11–14), we next examined the effect of O-GlcNAcase on β-cell function in aged mice. We performed IPGTT on 8–9 month old O-GlcNAcase mice, and in contrast to the younger mice, older O-GlcNAcase mice did not have impaired glucose tolerance compared with WT (Fig. 3A). In fact, O-GlcNAcase mice improved their glucose tolerance with aging, as assessed by a 17% decrease in the area under the glucose curve (AUCG) during IPGTT (p < 0.001, Table 1), whereas in the same age range, the WT mice did not change (5% decrease, p = 0.28). Older O-GlcNAcase transgenic mice exhibited 32% higher fasting insulin levels compared with WT controls (Fig. 3B, p < 0.05). There was a trend toward an increase in insulin levels 30 min post-glucose challenge (25%, Fig. 3B), but this was not statistically significant (p = 0.19).
FIGURE 3.
Older (9-month-old) O-GlcNAcase transgenic mice normalize glucose tolerance and exhibit superior β-cell function compared with WT mice. A, experimental animals (8–9-month-old females) were fasted for 6 h followed by intraperitoneal glucose injection (1 g/kg body weight). At the indicated times, tail vein blood was sampled for glucose determination with a glucometer (n = 6–8/group). B, tail vein blood was also sampled for insulin measurement at fasting and 30 min after glucose challenge (n = 6–8/group). C, HOMA-B values were calculated as described under “Experimental Procedures” using the fasting glucose and insulin data obtained during the IPGTT in A (n = 6–8/group). D, mRNA was harvested from ∼100 islets per mouse, and insulin-1 transcript levels were normalized to β-actin (n = 6 mice/group). E, 10 islets were collected from the pancreas of each mouse, and insulin levels were measured after sonication (n = 4–5 mice/group). *, p < 0.05; ‡, p < 0.01.
TABLE 1.
Areas under the glucose curve (AUCG) during glucose tolerance testing in younger and older wild type and O-GlcNAcase transgenic mice
The number of animals studied are in parentheses.
| AUCG (wild type) | AUCG (O-GlcNAcase) | |
|---|---|---|
| 3–4 Months | 25.4 ± 0.7 (20) | 31.1 ± 1.2 (13)a |
| 8–9 Months | 24.1 ± 1.0 (16) | 25.6 ± 0.8 (17) |
a p < 0.01 compared to all other groups.
β-cell function of the O-GlcNAcase mice assessed by HOMA-B only trended toward an increase compared with WT (Fig. 3C, 58%, p = 0.26). When comparing the HOMA-B values from the 3–4-month-old mice (Fig. 1G) with those of the 8–9-month-old mice (Fig. 3C), however, the older WT mice increased their HOMA-B values 90% over that of younger WT (p = 0.01), whereas the older O-GlcNAcase mice increased their HOMA-B values 344% over that of the younger transgenics (p < 0.001). The larger increase in HOMA-B in response to aging in the O-GlcNAcase mice compared with WT was statistically significant (p = 0.01). These changes were accompanied by reversal of the decreases in INS-1 mRNA and insulin content per islet that had been observed in the younger O-GlcNAcase mice (Fig. 2). In the 8–9-month-old transgenic mice, INS-1 mRNA normalized to islet protein was increased ∼3-fold compared with WT of the same age (Fig. 3D, p < 0.01), and insulin content per islet was increased ∼2.6-fold compared with WT (Fig. 3E, p < 0.05). The changes in islet insulin content in the older mice demonstrate an increased ability of the O-GlcNAcase transgenic mice to increase islet insulin as they age: older WT mice (Fig. 3E) increased their islet insulin content <4-fold over the levels observed in the younger mice (Fig. 2B), whereas older O-GlcNAcase mice increased their islet insulin content ∼16-fold.
Insulin secretion from isolated islets from the older O-GlcNAcase transgenic mice was also examined; there were no differences between the groups with glucose stimulation, but with KCl, there was no decrease in secretory capacity seen in the O-GlcNAcase mice as had been the case in the younger mice; in fact, the trend was now for higher response than in the WT (119% increase, p = 0.10, data not shown). HOMA-IR values did not differ between the groups (HOMA-IR = 1.5 ± 0.2 in WT, 1.9 ± 0.3 in the transgenics, p = 0.31). Thus, glucose tolerance in the O-GlcNAcase transgenic mice “catches up” with WT mice as they age, a change that is attributable to a reversal of the situation that had been observed in the younger mice, with the older transgenic mice now exhibiting increased fasting insulin levels, β-cell function, and islet insulin content.
Increased Angiogenesis in Islets from Older O-GlcNAcase Transgenic Mice
We next sought to determine possible mechanisms underlying the apparent protection afforded the O-GlcNAc transgenic mice from age-related changes in β-cell function. Islets express high levels of angiogenic factors and islet microvasculature network is essential for β-cell function of glucose sensing and insulin secretion (30). Recent evidence indicates that chronic activation of the HBP/O-GlcNAc pathway contributes to abnormal angiogenesis (19). We therefore examined the role of O-GlcNAc in angiogenesis in islets from the transgenic and WT mice. We initially examined islet morphology and vascularization by staining pancreatic sections from O-GlcNAcase transgenic mice (8–9-month-old) with antibodies against the endothelial marker CD31. A pronounced increase in blood vessel density was observed in O-GlcNAcase transgenic islets, as evidenced by increased CD31 staining compared with that in WT mice (Fig. 4A). Quantification of the CD31 staining demonstrated a 2.5-fold increase in the transgenic islets (Fig. 4B, p < 0.01). No significant increase in CD31 staining was observed in the younger transgenic mice compared with younger WT (Fig. 4C and D). These differences were further confirmed by an in vitro islet angiogenesis assay (Fig. 4E). Isolated islets were embedded into three-dimensional Matrigel, which allowed endothelial cells from islets to invade the surrounding matrix and form capillary-like structures. To quantify angiogenesis, the percentage of sprouting islets was determined, and transgenic islets showed a 50% increase in the percentage of sprouting islets compared with controls (Fig. 4F, p < 0.05).
FIGURE 4.
Angiogenesis in pancreatic islets. A, pancreata from female 8–10-month-old WT and O-GlcNAcase transgenic (Tg) female mice were fixed and 10 μm sections were stained using antibodies against the endothelial marker CD31 (red) and DAPI (blue). B, the percentage of CD31-immunopositive area in islets was quantified from at least 30 sections from four to five animals in each group using NIH ImageJ software. C and D, CD31 staining and quantification in islets of 2–4-month-old mice. E, endothelial cell sprouting from cultured pancreatic islets of 8–9-month-old mice was examined by isolating islets and embedding them in Matrigel. Islets were cultured in endothelial growth medium with 10% FBS, and vascular sprouting was recorded under inverted microscopy at day 6. VEGF (10 ng/ml) was used to treat cultured WT islets as positive control. F, the ratio of sprouting islets to total cultured islets was quantified from at least 20 islets/animal from four animals in each group. *, p < 0.05; ‡, p < 0.01.
Mechanism of Increased Angiogenesis in O-GlcNAcase Transgenic Islets
VEGF, a key angiogenic regulator, is highly expressed in pancreatic islets and is essential for normal islet vascularization and β-cell function (21, 30). O-GlcNAcase transgenic mice (10 months old) expressed 85% higher levels of VEGF mRNA (p < 0.05) in islets than WT (Fig. 5A). Expression of FGF1, which promotes endothelial cell migration and proliferation (31), and ANG2 (angiopoietin 2), which in the presence of VEGF facilitates new vessel sprouting from existing vessels (32), tended toward up-regulation in transgenic islets (p = 0.12 and 0.075, respectively). Expression of other angiogenic factors (TNFα, ANG1) did not change (Fig. 5A). We did not observe changes in expression of the above genes in islets of young (3–4-month-old) O-GlcNAcase transgenic mice: VEGF mRNA was 0.98 ± 0.15 in WT and 1.15 ± 0.35 in transgenic islets (p = 0.61). Likewise, FGF1 and ANG2 did not differ (data not shown). To confirm that the increased VEGF gene expression in the older islets has functional consequences, we next cultured islets for 48 h and then measured VEGF production in the media. Overexpression of O-GlcNAcase significantly increased VEGF production from islets (Fig. 5B, 61% increase, p = 0.05).
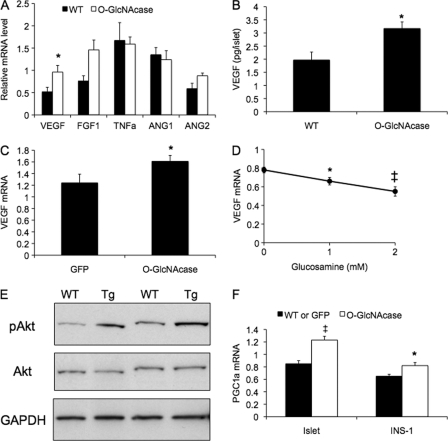
FIGURE 5.
Regulation of expression and secretion of VEGF by O-GlcNAc in islets from older O-GlcNAcase transgenic mice and INS-1 cells. A, islets were isolated from 10-month-old female WT and O-GlcNAcase transgenic (Tg) mice, and mRNA levels were measured by quantitative PCR and normalized to cyclophilin A (n = 3–4 mice for each group). B, size-matched islets were cultured for 48 h, and VEGF content in the medium was quantified (n = 3 for each group). C and D, INS-1 cells were infected with GFP or O-GlcNAcase virus for 2 days (C) or treated with different concentrations of glucosamine overnight (D). VEGF mRNA levels were measured by quantitative PCR and normalized to cyclophilin A. E, islets were isolated from female 10-month-old WT and O-GlcNAcase transgenic female mice. The indicated protein levels were measured by Western blot. F, PGC1α mRNA was measured in islets of 10-month-old female WT and O-GlcNAcase transgenic mice (left) and INS-1 cells infected with GFP or O-GlcNAcase adenovirus as described in C (right). *, p < 0.05; ‡, p < 0.01.
To further validate these results and to remove possible confounding effects of the complex variety of cells in isolated islets, we examined VEGF gene expression in an in vitro model of β-cells, cultured INS-1 cells. INS-1 cells infected with replication-deficient adenovirus encoding O-GlcNAcase exhibited significantly higher VEGF transcript levels compared with control GFP-infected cells (Fig. 5C, 30% increase, p = 0.03). Glucosamine, which increases HBP flux and protein O-GlcNAc modification, inhibited VEGF gene expression in a dose-dependent manner in INS-1 cells, with a 30% reduction in VEGF expression observed at 2 mm glucosamine (Fig. 5D, p = 0.007). These results demonstrate an inverse relationship between protein O-GlcNAc levels and VEGF expression in INS-1 cells and are consistent with the hypothesis that increased angiogenesis in the O-GlcNAcase islets are mediated by effects of O-GlcNAc on expression of VEGF and possibly other growth factors in β-cells.
We next examined possible mechanisms underlying the regulation of VEGF by O-GlcNAc in islets. Akt signaling plays an important role in the regulation of VEGF expression in various cells (33, 34). Furthermore, Akt activity is regulated by O-GlcNAc modification (6, 15, 35–37). Consistent with a possible role for Akt in O-GlcNAc-mediated VEGF expression in β-cells, O-GlcNAcase transgenic islets exhibited significantly higher Akt phosphorylation at Ser-473 as compared with the WT islets (p < 0.01, quantification not shown), with no change in total Akt levels (Fig. 5E). PGC1α (peroxisome proliferator receptor γ coactivator 1α), a nuclear metabolic regulator that promotes oxidative metabolism, has also recently been suggested to mediate the induction of VEGF gene expression in muscle (38). Gene expression of PGC1α in islets was up-regulated in 8–10-month-old transgenic mice (Fig. 5F, p < 0.01) but not younger mice (data not shown). Adenovirus mediated O-GlcNAcase expression also increased PGC1α expression in INS-1 cells compared with GFP-infected cells (Fig. 5F, p < 0.05).
DISCUSSION
O-GlcNAc modification of proteins serves a physiologic nutrient-sensing function through the normal range of tissue glucose fluxes and blood glucose concentrations (8, 10, 15, 39). In addition, excess nutrient intake is associated with increased O-GlcNAc modification of proteins and contributes to the adverse consequences of hyperglycemia through multiple tissue-specific mechanisms. An example of this mediation of both physiologic and pathologic effects by O-GlcNAc is seen in the liver. We have previously found that overexpression of the enzyme O-GlcNAcase in the liver of nondiabetic C57BL/6J mice, for example, reduces protein O-GlcNAc modification in the tissue and results in increased Akt signaling with a parallel decrease in transcription of gluconeogenic enzymes (15). In diabetic (db/db) mice, improvement of glucose tolerance has also been demonstrated with O-GlcNAcase overexpression in the liver (37). This, and previous findings demonstrating the deleterious effects of increased glycosylation in the β-cell (11–14), prompted us to investigate the effect of overexpression of O-GlcNAcase in the β-cells of nondiabetic mice without specific dietary or physiologic stressors.
In the present study, O-GlcNAcase overexpression in the younger 3–4-month-old mice led to higher glucose excursions after glucose challenge, with a concomitant decrease in circulating fasting insulin. The higher glucose excursions in the young O-GlcNAcase transgenic mice are accounted for by defects in β-cell function rather than peripheral insulin resistance. Expression of the mRNA for the insulin-1 gene and islet insulin content were both decreased. This is consistent with earlier results wherein increased O-GlcNAc through GFA overexpression in young mice had the opposite effect as O-GlcNAcase overexpression, namely increased insulin secretion (14). The phenotype of the younger mice is consistent with the nutrient sensing function of O-GlcNAc modification of proteins, with insulin secretion responding at least in part to the levels of O-GlcNAc modification in cells in addition to the ambient blood glucose levels. The mechanism for the down-regulation of insulin expression with increased O-GlcNAcase is not known. Although we did not observe any change in the transcript levels of several transcription factors known to regulate insulin gene expression, the decrease in islet insulin mRNA transcript levels might be explained through previous findings showing that activities of PDX-1 and NeuroD1 can be altered through O-GlcNAcylation (12, 16–18).
As the O-GlcNAcase transgenic mice aged, their glucose tolerance normalized, and this was associated with reversal of the defects in serum insulin levels, islet insulin content, and insulin gene expression that had been observed in the younger mice. Islet insulin content, for example, increased much more with aging in the O-GclNAcase mice (∼16-fold when comparing Fig. 2B with 3E) than in the WT (∼4-fold). These results are in good agreement with a converse model, namely increased O-GlcNAc in islets that was achieved by overexpression of GFA, wherein the older animals became diabetic and exhibited decreased insulin secretion (13, 14). These results are also consistent with the observation in multiple models and multiple tissues that although changes in metabolism induced by increased O-GlcNAc in younger mice are consistent with a physiological glucose-sensing function of O-GlcNAc, chronically increased O-GlcNAc in older mice mimics many of the phenotypes of type 2 diabetes, including increased hepatic glucose production and decreased insulin sensitivity in muscle (for example, Refs. 37 and 41–43). Mice with increased O-GlcNAc targeted to liver, for example, are relatively hypoglycemic compared with controls, whereas older mice become hyperglycemic (41). We therefore speculate that the biphasic nature of the effects of O-GlcNAcase overexpression in β-cells is the result of decreased nutrient signaling in the younger animals, i.e. less of a perceived need for insulin and therefore worsening of glucose tolerance. In the older animals, by contrast, the O-GlcNAcase overexpression serves to counteract the pathophysiologic increases in O-GlcNAc that are seen in aging (28, 29), therefore resulting in the relative improvement in glucose tolerance.
To understand possible mechanisms underlying the protection from age-related declines in β-cell function, we took advantage of the recent demonstration by our group of the deleterious effect of excess O-GlcNAc signaling on angiogenesis (19). Specifically, elevated O-GlcNAc levels lead to impaired angiogenesis in aortae from diabetic mice, and removal of O-GlcNAc improves angiogenic properties of endothelial cells. Accumulating evidence shows that secretion of VEGF by β-cells promotes islet angiogenesis. Deletion of VEGF in either β-cells or the entire pancreas of mice results in fewer islet capillaries, impaired insulin production, and defective insulin secretion. Conversely, enhanced production of VEGF in β-cells increases islet angiogenesis and β-cell function. All of these indicate that angiogenesis is important to maintain β-cell function (21, 30). We show herein that O-GlcNAc regulates VEGF expression and angiogenesis in intact aged animals, isolated islets, and cultured β-cell-derived lines. Many pathways and factors have been shown to regulate VEGF; we chose to examine the possible role of Akt both because of its role in VEGF regulation (33, 34) and the demonstration that O-GlcNAc and the HBP regulate Akt signaling (6, 15, 35–37). Our previous studies of endothelial cells also tied O-GlcNAc signaling to Akt and angiogenic signaling (19). Our current results showing increased activation of Akt with decreased O-GlcNAc are in agreement with all of these prior observations. Our results do not suggest that this is the sole mechanism for VEGF regulation by O-GlcNAc, however. For example, VEGF is also regulated by the transcriptional coactivator PGC-1α (38), and it, too, is up-regulated in the islets of the O-GlcNAcase transgenic mice. PGC-1α activity, in turn, is regulated by the O-GlcNAc/HBP pathway (44). All of these data are consistent with complex, pleiotropic, and integrated effects of O-GlcNAc on angiogenesis. Interestingly, overexpression of O-GlcNAcase in β-cells significantly increased angiogenesis in islets from old but not young mice. We suspect that no change in angiogenesis from young islets might be due to either of the following: 1) O-GlcNAcase substrate limitation (i.e. O-GlcNAc modifications are less prevalent in young mice), or 2) the more highly adaptive nature of β-cells in young mice.
In sum, the current results demonstrate a central role for O-GlcNAc modification of proteins in several aspects β-cell and islet function. The results point to the importance of the O-GlcNAc/HBP pathway to glucose homeostasis and the overall regulation of metabolism and hormone responsiveness. The complexity of the effects of O-GlcNAc in normal physiologic nutrient signaling, acute and chronic, as well as in the mediation of adverse effects of chronic nutrient excess and aging, suggest that attempts to target the pathway pharmacologically are likely to have complicated and mixed results.
This work was supported, in whole or in part, by National Institutes of Health Grants DK-43526, UL1-RR025764, and C06-RR11234 and the Research Service of the Veterans Administration. This work was also supported by the Robinson Family Foundation.
- HBP
- hexosamine biosynthesis pathway
- GFA
- glutamine:fructose-6-phosphate amidotransferase
- IPGTT
- intraperitoneal glucose tolerance testing
- HOMA-IR
- homeostasis model assessment of insulin resistance
- HOMA-B
- HOMA assessment of β-cell function
- O-GlcNAcase
- β-N-acetylglucosaminidase.
REFERENCES
- 1. Marshall S., Bacote V., Traxinger R. R. (1991) J. Biol. Chem. 266, 4706–4712 [PubMed] [Google Scholar]
- 2. Gao Y., Wells L., Comer F. I., Parker G. J., Hart G. W. (2001) J. Biol. Chem. 276, 9838–9845 [DOI] [PubMed] [Google Scholar]
- 3. Kreppel L. K., Blomberg M. A., Hart G. W. (1997) J. Biol. Chem. 272, 9308–9315 [DOI] [PubMed] [Google Scholar]
- 4. Parker G., Taylor R., Jones D., McClain D. (2004) J. Biol. Chem. 279, 20636–20642 [DOI] [PubMed] [Google Scholar]
- 5. Parker G. J., Lund K. C., Taylor R. P., McClain D. A. (2003) J. Biol. Chem. 278, 10022–10027 [DOI] [PubMed] [Google Scholar]
- 6. Park S. Y., Ryu J., Lee W. (2005) Exp. Mol. Med. 37, 220–229 [DOI] [PubMed] [Google Scholar]
- 7. McClain D. A., Crook E. D. (1996) Diabetes 45, 1003–1009 [DOI] [PubMed] [Google Scholar]
- 8. Hart G. W., Housley M. P., Slawson C. (2007) Nature 446, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 9. Hanover J. A. (2010) Chem. Biol. 17, 1272–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Love D. C., Krause M. W., Hanover J. A. (2010) Semin. Cell Dev. Biol. 21, 646–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akimoto Y., Hart G. W., Wells L., Vosseller K., Yamamoto K., Munetomo E., Ohara-Imaizumi M., Nishiwaki C., Nagamatsu S., Hirano H., Kawakami H. (2007) Glycobiology 17, 127–140 [DOI] [PubMed] [Google Scholar]
- 12. Kaneto H., Xu G., Song K. H., Suzuma K., Bonner-Weir S., Sharma A., Weir G. C. (2001) J. Biol. Chem. 276, 31099–31104 [DOI] [PubMed] [Google Scholar]
- 13. Tang J., Neidigh J. L., Cooksey R. C., McClain D. A. (2000) Diabetes 49, 1492–1499 [DOI] [PubMed] [Google Scholar]
- 14. Cooksey R. C., Pusuluri S., Hazel M., McClain D. A. (2006) Am. J. Physiol. Endocrinol. Metab. 290, E334–340 [DOI] [PubMed] [Google Scholar]
- 15. Soesanto Y. A., Luo B., Jones D., Taylor R., Gabrielsen J. S., Parker G., McClain D. A. (2008) Am. J. Physiol. Endocrinol. Metab. 295, E974–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andrali S. S., Qian Q., Ozcan S. (2007) J. Biol. Chem. 282, 15589–15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrali S. S., Sampley M. L., Vanderford N. L., Ozcan S. (2008) Biochem. J. 415, 1–10 [DOI] [PubMed] [Google Scholar]
- 18. Gao Y., Miyazaki J., Hart G. W. (2003) Arch. Biochem. Biophys. 415, 155–163 [DOI] [PubMed] [Google Scholar]
- 19. Luo B., Soesanto Y., McClain D. A. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuroda M., Oka T., Oka Y., Yamochi T., Ohtsubo K., Mori S., Watanabe T., Machinami R., Ohnishi S. (1995) J. Clin. Endocrinol. Metab. 80, 3196–3200 [DOI] [PubMed] [Google Scholar]
- 21. Lammert E., Gu G., McLaughlin M., Brown D., Brekken R., Murtaugh L. C., Gerber H. P., Ferrara N., Melton D. A. (2003) Curr. Biol. 13, 1070–1074 [DOI] [PubMed] [Google Scholar]
- 22. Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985) Diabetologia 28, 412–419 [DOI] [PubMed] [Google Scholar]
- 23. Kidszun A., Schneider D., Erb D., Hertl G., Schmidt V., Eckhard M., Preissner K. T., Breier G., Bretzel R. G., Linn T. (2006) Cell Transplant. 15, 489–497 [DOI] [PubMed] [Google Scholar]
- 24. Matsuoka T. A., Kaneto H., Stein R., Miyatsuka T., Kawamori D., Henderson E., Kojima I., Matsuhisa M., Hori M., Yamasaki Y. (2007) Mol. Endocrinol. 21, 2764–2774 [DOI] [PubMed] [Google Scholar]
- 25. Naya F. J., Stellrecht C. M., Tsai M. J. (1995) Genes Dev. 9, 1009–1019 [DOI] [PubMed] [Google Scholar]
- 26. Ohneda K., Mirmira R. G., Wang J., Johnson J. D., German M. S. (2000) Mol. Cell. Biol. 20, 900–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vanderford N. L., Andrali S. S., Ozcan S. (2007) J. Biol. Chem. 282, 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vosseller K. (2010) Aging 2, 749–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chatham J. C., Marchase R. B. (2010) Biochim. Biophys. Acta. 1800, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watada H. (2010) Endocr. J. 57, 185–191 [DOI] [PubMed] [Google Scholar]
- 31. Augustin-Voss H. G., Voss A. K., Pauli B. U. (1993) J. Cell. Physiol. 157, 279–288 [DOI] [PubMed] [Google Scholar]
- 32. Lobov I. B., Brooks P. C., Lang R. A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang B. H., Zheng J. Z., Aoki M., Vogt P. K. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 1749–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mazure N. M., Chen E. Y., Laderoute K. R., Giaccia A. J. (1997) Blood 90, 3322–3331 [PubMed] [Google Scholar]
- 35. Vosseller K., Wells L., Lane M. D., Hart G. W. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gandy J. C., Rountree A. E., Bijur G. N. (2006) FEBS. Lett. 580, 3051–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dentin R., Hedrick S., Xie J., Yates J., 3rd, Montminy M. (2008) Science 319, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 38. Arany Z., Foo S. Y., Ma Y., Ruas J. L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S. M., Baek K. H., Rosenzweig A., Spiegelman B. M. (2008) Nature 451, 1008–1012 [DOI] [PubMed] [Google Scholar]
- 39. Taylor R. P., Parker G. J., Hazel M. W., Soesanto Y., Fuller W., Yazzie M. J., McClain D. A. (2008) J. Biol. Chem. 283, 6050–6057 [DOI] [PubMed] [Google Scholar]
- 40. Deleted in proof.
- 41. Veerababu G., Tang J., Hoffman R. T., Daniels M. C., Hebert L. F., Jr., Crook E. D., Cooksey R. C., McClain D. A. (2000) Diabetes 49, 2070–2078 [DOI] [PubMed] [Google Scholar]
- 42. Cooksey R. C., Hebert L. F., Jr., Zhu J. H., Wofford P., Garvey W. T., McClain D. A. (1999) Endocrinology 140, 1151–1157 [DOI] [PubMed] [Google Scholar]
- 43. Hebert L. F., Jr., Daniels M. C., Zhou J., Crook E. D., Turner R. L., Simmons S. T., Neidigh J. L., Zhu J. S., Baron A. D., McClain D. A. (1996) J. Clin. Invest. 98, 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Housley M. P., Udeshi N. D., Rodgers J. T., Shabanowitz J., Puigserver P., Hunt D. F., Hart G. W. (2009) J. Biol. Chem. 284, 5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]