Abstract
The present study investigated the contribution of lexico-semantic associations to impairments in establishing reference in schizophrenia. We examined event-related potentials as schizophrenia patients and healthy demographically-matched controls read five-sentence scenarios. Sentence 4 introduced a noun which referred back to three possible referents introduced in sentences 1–3. These referents were contextually appropriate, contextually inappropriate but lexico-semantically associated, and contextually inappropriate and lexico-semantically non-associated. In order to determine whether participants had correctly linked the anaphor to its referents, the final sentence reintroduced each referent and participants indicated whether the last two sentences referred to the same entity. Results indicated that between 300–400msec, patients, like healthy controls, used discourse context to link the noun with its preceding referent. However, between 400–500msec, neural activity in patients was modulated only by lexico-semantic associations rather than discourse context. Moreover, patients were also more likely than controls to incorrectly link the noun with contextually-inappropriate, but lexico-semantically associated, referents. Results suggest that at least some types of referential impairments may be driven by sustained activation of contextually inappropriate lexico-semantic associations.
Keywords: schizophrenia, language, ERPs, discourse, referential impairment, context, anaphor, comprehension
Introduction
Language production and comprehension require going beyond the meaning of the individual words that make up a text or an utterance to formulate a global discourse representation, or situation model (cf. Zwaan & Radvansky, 1998). Specifically, healthy adults establish causal relationships between events, link multiple references to characters and objects, and represent space and time information by using cues such as context and lexico-semantic associations to integrate relevant information and inhibit irrelevant information. Although building this rich discourse representation occurs quickly and seemingly effortlessly in healthy adults, patients with schizophrenia appear to have difficulty with such processes (for a review, see Ditman & Kuperberg, 2010).
Language disturbances in patients with schizophrenia are well-documented. They manifest at their most extreme in patients with thought disorder where speech is dominated by semantic associations at the expense of building overall message meaning. Bleuler (1911/1950), however, conceived of semantic associative disturbances as being a fundamental mechanism that can explain not only thought disorder itself but multiple aspects of cognitive dysfunction in schizophrenia. Even patients without clinically obvious thought disorder show evidence of illogical thinking which can lead to poor communication and social dysfunction. One well-documented aspect of such poor communication in schizophrenia is a failure to fully establish reference, i.e. a failure to link multiple mentions of the same character or object in discourse (see Ditman & Kuperberg, 2010, for a review; Docherty, DeRosa, & Andreasen, 1996; Rochester & Martin, 1979). The present study aimed to determine whether and how associative disturbances contribute to such failures to establish referential coherence in schizophrenia.
Most studies of associative disturbances in schizophrenia have examined relationships between single words using the semantic priming paradigm. The associative semantic priming effect describes the faster reaction time or attenuated electrophysiological response to target words that are preceded by semantically associated (versus non-associated) prime words. Under experimental conditions biasing towards automatic processing, most studies find normal priming effects in schizophrenia suggesting that patient’s implicit and automatic use of semantic associations is preserved (Barch, Cohen, Servan-Schreiber, Steingard, Steinhauer, & van Kammen, 1996; Blum & Freides, 1995; Ober, Vinogradov, & Shenaut, 1995; Vinogradov, Ober, & Shenaut, 1992); indeed, in patients with positive thought disorder, priming can even be increased suggesting some increase in automatic spreading activation between semantic associates in such patients (Kreher, Holcomb, Goff, & Kuperberg, 2008; Manschreck, Maher, Milavetz, Ames, Weisstein, & Schneyer, 1988; Moritz, Mersmann, Kloss, Jacobsen, Wilke, Andresen et al., 2001; Moritz, Woodward, Kuppers, Lausen, & Schickel, 2002; Spitzer, Weisker, Winter, Maier, Hermle, & Maher, 1994).
In contrast under experimental conditions biasing towards strategic controlled processing, such as the use of longer times between primes and targets or the use of tasks requiring explicit evaluations of the relationships between prime and targets, such as lexical decisions (Barch et al., 1996; Besche, Passerieux, Segui, Sarfati, Laurent, & Hardy-Bayle, 1997; Passerieux, Segui, Besche, Chevalier, Widlocher, & Hardy-Bayle, 1997; Kiang, Kutas, Light, & Braff, 2008) or explicit semantic judgments (Kreher, Goff, & Kuperberg, 2009), semantic priming effects are abnormally reduced in patients (reviewed by Kuperberg, Ditman, Kreher, & Goldberg, 2009, Minzenberg, Ober, & Vinogradov, 2002; Pomarol-Clotet, Oh, Laws, & McKenna, 2008). These priming deficits are thought to be driven by a failure of patients to engage different types of strategic semantic mechanisms. For example, patients may appropriately activate semantic associates to the prime but, when required to use such information to make a decision about the prime-target relationship, they may fail to select the word that matches the target and appropriately suppress or inhibit other activated semantic associates. This type of selection and suppression failure might even, under some circumstances, lead to increased activity to semantically associated versus non-associated targets. Such findings have been detected behaviorally in chronic patients who can show longer reaction times to associated (versus non-associated) targets (Maher, Manschrek, Redmond, & Beaudette, 1996), and in a recent fMRI study in which we reported increased neural activity to semantically associated relative to non-associated word-pairs in patients (Kuperberg, Deckersbach, Holt, Goff, & West, 2007; see also Han, Nestor, Hale-Spencer, Cohen, Niznikiewicz, McCarley et al., 2007).
During sentence processing, there are once again many situations in which schizophrenia patients are able to effectively use semantic associations between words to derive overall meaning (e.g., Miller & Phelan, 1980; Sitnikova, Salisbury, Kuperberg, & Holcomb, 2002; Kuperberg, Kreher, Goff, McGuire, & David, 2006). In other situations, however, processing breaks down. As with single-word priming paradigms, this is most likely to occur when there is a requirement to select the contextually appropriate meaning and to suppress irrelevant meanings of a word or its associates. For example, like healthy controls, patients with schizophrenia are able to use a sentence context to activate meanings of homonyms (words with more than one meaning). Unlike controls, however, they appear to be unable to suppress the contextually-inappropriate dominant meanings of these homonyms when the sentence context only moderately biases the subordinate meaning (Titone, Levy, & Holzman, 2000). Such a failure to suppress contextually inappropriate meanings can lead to processing being driven by semantic associates rather than overall sentence context. For example, Sitnikova et al. (2002) showed that, when critical words within sentences were associated with the dominant meaning of a preceding homonym but incongruous with the overall sentence context, patients, unlike controls, failed to detect the incongruity. However, when critical words were both semantically non-associated with individual preceding words and incongruous with the sentence context, incongruities were normally detected. Finally, we found that patients were insensitive to incongruous verbs within sentences when these verbs were semantically associated to words in the preceding context (Kuperberg, Sitnikova, Goff, & Holcomb, 2006; Kuperberg, Kreher, Goff, McGuire, & David, 2006). Once again, patients showed no problems in detecting incongruities when the violated verb was unrelated to preceding words in the context.
Most patients’ communication deficits manifest at the level of whole discourse rather than individual words or sentences (Andreasen, 1986; Rochester & Martin, 1979). Despite this, there have been very few studies examining how patients with schizophrenia build up meaning across sentence boundaries and only one study to date has examined the fast, online neural processes that are engaged as discourse unfolds word-by-word (Ditman & Kuperberg, 2007). In that study, participants read three-sentence scenarios in which final sentences varied in their causal relationship with their preceding context. Highly causally related and intermediately causally related scenarios were matched on pure lexico-semantic associations. Neural activity in patients did not differentiate between critical words in these two types of scenario. These findings suggested that processing was once again that driven by association rather than discourse context, even across sentence boundaries.
The present study examined another mechanism of building coherence in discourse – through the establishment of reference. To establish referential coherence, multiple references to the same character or object must be connected together, within and across clauses, to come up with a global representation of the described events, actions, and characters, known as a situation model (cf. Zwaan & Radvansky, 1998). As indicated above, referential impairments in patients with schizophrenia have been well documented (Rochester & Martin, 1979; Docherty, DeRosa, & Andreasen, 1996; Docherty, Strauss, Dinzeo, & St-Hilaire, 2006; Hoffman, Hogben, Smith, & Calhoun, 1985; Noel-Jorand, Reinert, Giudicelli, & Dassa, 1997). These impairments include confused references (e.g., a word or phrase that could refer to one of several possible referents), missing information references (e.g., referring to information that has not been previously mentioned), or vague references (e.g., using a word that lacks specificity) (Docherty et al., 1996; Docherty et al., 2006).
Although correlated with measures of positive thought disorder, referential failures are not unique to thought disordered patients and appear to be a stable across clinical state in patients with schizophrenia (Docherty, Cohen, Nienow, Dinzeo, & Dangelmaier, 2003; Docherty et al., 1988), suggesting that they may be trait markers of schizophrenia. In addition, they are also more common in first-degree relatives of patients with schizophrenia than in first-degree relatives of healthy controls (Docherty, Rhinewine, Labhart, & Gordinier, 1998; Docherty & Gottesman, 2000). Understanding the mechanisms contributing to referential disturbances in schizophrenia may therefore provide insight into the neural underpinnings of schizophrenia as a whole.
We aimed to determine whether lexico-semantic associative disturbances contribute to impairments in establishing reference during discourse processing in schizophrenia. Healthy adults quickly use multiple sources of information to modulate the activation of current concepts during comprehension, consistent with theories of discourse comprehension such as Landscape model (Rapp & van den Broek, 2005; van den Broek, Young, Tzeng, & Linderholm, 1999). Most relevant to the present research, previous studies have demonstrated that both lexico-semantic associative and discourse-level global contextual information are used to establish reference in discourse (e.g., Ditman et al., 2007; cf. Rapp & van den Broek, 2005). To investigate the establishment of reference in patients with schizophrenia, we employed a rich five-sentence discourse context that included lexico-semantically associated distractors. In three introductory sentences, we introduced three potential referents. In a fourth sentence, a noun was presented that needed to be linked back to one of these referents. For example, in the scenario, “A suit is worn to work. A costume is worn during Halloween. A ring is worn on a finger. The night before work, Lisa ironed the outfit,” participants are required to link the noun “outfit” back to one of the three referents (“suit,” “costume,” “ring”). To make the correct link, i.e. link “outfit” back the word “suit,” we must use the discourse context to override or suppress the lexico-semantic association between “costume” and “outfit.” We predicted that schizophrenia patients would fail to suppress “costume,” leading to a failure to establish the correct reference to “suit”. However, we predicted that, like healthy controls, they would be able to appropriately select “suit” over a non-associated word, “ring.” In addition, we examined behavioral (accuracy and response time, RT) measures as participants indicated whether the last two sentences referred to the same entity.
To test these hypotheses, we measured ERPs in a final sentence that reintroduced one of the three referents. ERPs index neural activity with millisecond (msec) temporal resolution. Our focus was on the modulation of the N400, a negative deflection with a centroparietal scalp distribution that peaks at approximately 400 msec after word onset, and with larger negative-going amplitudes reflecting the ease of mapping the meaning of an word on to its preceding context and information stored within semantic memory (e.g. Holcomb 1993; reviewed by Kutas & Federmeier 2000). It is sensitive to semantic relationships between words (Bentin, McCarthy, & Wood, 1985; Rugg, 1984), stored knowledge within semantic memory (Federmeier & Kutas, 1999), real-world knowledge (Hagoort, Hald, Bastiaansen, & Petersson, 2004), and sentence-and discourse-level contexts (Kutas & Hillyard, 1984; Van Berkum, Hagoort, & Brown, 1999). Importantly, the N400 is modulated by referential processes across sentence boundaries (Ditman, Holcomb, & Kuperberg, 2007; Ditman, Holcomb, & Kuperberg, 2008.
Based on our previous study using this paradigm in young, healthy individuals (Ditman, Holcomb, & Kuperberg, 2007), we predicted that healthy controls would produce a smaller amplitude N400 to the reintroduced correct referent (“suit”) than to the lexico-semantically associated contextually incongruous referent (“costume”). We predicted that schizophrenia patients would fail to show this difference in ERP modulation and that, relative to controls, they would incorrectly judge scenarios containing these contextually incongruous words to be referentially coherent and would take longer to do so. This would provide evidence that patients’ failure to suppress the semantically associated distractor leads to their failure to establish reference. However, we expected that, like healthy controls, patients would show a smaller N400 to the reintroduced correct referent (“suit”) than to the lexico-semantically non-associated incorrect referent (“ring”) and that they would correctly judge scenarios containing these words to lack referential coherence and response times to be similar to scenarios with correct referents.
Methods
Stimuli Construction
Stimuli creation has been described in a previous paper (Ditman, Holcomb, & Kuperberg, 2007). Five-sentence scenarios were constructed from 252 triplets of words. Each word triplet constituted of a noun describing a category and two exemplars which would serve as referents to that noun (e.g., outfit: “suit”, “costume”). In each scenario, each of the first three sentences presented a potential referent, which was always the subject of the sentence. The fourth sentence introduced the category noun, termed the anaphor, always on its sentence-final word which referred back to one of these referents. Two of the potential referents in sentences 1–3 were exemplars of the categorical anaphor. The context of this fourth sentence biased towards one of the three referents as the correct interpretation of the anaphor. The fifth sentence, began with a reinstatement of one of the three referents introduced in the first three sentences (with the first word always being “The”). This gave rise to three conditions. In the first condition, the reinstated referent was the correct referent: it was both contextually-appropriate and lexico-semantically associated with the categorical anaphor (e.g., “suit”); in condition 2, the reinstated referent was contextually-inappropriate but was still associated with the categorical anaphor (e.g., “costume”); in condition 3, the reinstated referent was contextually-inappropriate and lexico-semantically non-associated to the categorical anaphor (e.g., “ring”).
Items were rotated through conditions so that on different lists they served as contextually-appropriate/associated, contextually-inappropriate/associated, and contextually-inappropriate/non-associated referents. This gave rise to four lists in total, each with 126 scenarios: 42 contextually-appropriate/associated, 42 contextually-inappropriate/associated, and 42 contextually-inappropriate/non-associated. The order of contextually-appropriate/associated, contextually-inappropriate/associated, and contextually-inappropriate/non-associated referent presentation was counterbalanced across scenarios such that each appeared in the first, second, and third sentences an equal number of times within each list. Each participant viewed one list.
Below is an example scenario from each of the four lists, demonstrating how items were rotated through each condition (although the examples below are shown with the correct referent presented first, the order in which these potential antecedents were presented was also rotated between-scenarios). Potential referents appear in bold, categorical anaphors appear in capital letters, and reinstated referents are italicized here (but not in the actual study):
A suit is worn to work. A costume is worn during Halloween. A ring is worn on a finger. The night before work, Lisa ironed the OUTFIT. The suitcontextually-appropriate/associated/costumecontextually-inappropriate/associated/ringcontextually-inappropriate/non-associated was fancy.
A costume is worn during Halloween. A suit is worn to work. A bracelet is worn on a wrist. The night before Halloween, Lisa ironed the OUTFIT. The costumecontextually-appropriate/associated/suitcontextually-inappropriate/associated/braceletcontextually-inappropriate/non-associated was fancy.
A ring is worn on a finger. A bracelet is worn on a wrist. A suit is worn to work. Lisa’s finger sparkled with the JEWELRY. The ringcontextually-appropriate/associated/braceletcontextually-inappropriate/associated/suitcontextually-inappropriate/non-associated was fancy.
A bracelet is worn on a wrist. A ring is worn on a finger. A costume is worn during Halloween. Lisa’s wrist sparkled with the JEWELRY. The braceletcontextually-appropriate/associated/ringcontextually-inappropriate/associated/costumecontextually-inappropriate/non-associated was fancy.
Participants
Sixteen patients meeting DSM-IV criteria for schizophrenia confirmed using the SCID (First, Spitzer, Miriam, & Williams, 2002a) and chart examination, all but one receiving stable doses of typical and/or atypical antipsychotic medication, were initially recruited from the Lindemann Mental Health Center, Boston. Sixteen demographically-matched volunteers on no medication and without histories of psychiatric disorders (First, Spitzer, Miriam, & Williams, 2002b) were recruited by advertisement. All participants were native, primarily monolingual English speakers who had not learned any other language before age five years. All were right-handed (Oldfield, 1971; White & Ashton, 1976), without histories of head trauma, neurological disorder, substance abuse within six months, or histories of substance dependence (as assessed using the DSM-IV). Written informed consent was obtained following the guidelines of the Massachusetts General Hospital and Tufts Medical Center Human Subjects Research Committees. Clinical assessments were carried out using the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1987), the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1989), the Positive and Negative Syndrome Scale (PANSS; Kay, Fiszbein, & Opler, 1987), and the Brief Psychiatric Rating Scale (BPRS; Lukoff, Neuchterlein, & Ventura, 1986) as a measure of overall psychopathology.
Demographic and psychopathological data of the patients and controls are summarized in Table 1. Patients and controls were matched closely on gender and race and there was no significant difference between the groups in age, years of education, parental socioeconomic status (SES) as assessed by the Hollingshead Index, or A-NART (Blair & Spreen, 1989) (all ps > .05).
Table 1.
Demographic and psychopathological characteristics of healthy controls and schizophrenia patients.
| Controls | Patients | |
|---|---|---|
| N | 16 | 16 |
| Male/Female | 11M/5F | 10M/6F |
| Age | 42.31 (8.11) | 40.13 (10.07) |
| Years of Education | 14.63 (2.68) | 12.94 (2.14) |
| Premorbid IQ | 112.74 (8.79) | 109.79 (11.33) |
| Parental SES | 3.56 (0.96) | 2.73 (1.44) |
| CPZ Equivalent | --- | 494.44 (440.12) |
| PANSS | --- | 64.63 (17.17) |
| SANS | --- | 39.33 (16.31) |
| SAPS | --- | 38.50 (24.23) |
Means are shown with standard deviations in parentheses. CPZ = chlorpromazine; PANSS: Positive and Negative Syndrome Scale (Kay, Fiszbein, & Opler, 1987); SANS: Scale for the Assessment of Negative Symptoms (Andreasen, 1989); SAPS: Scale for the Assessment of Positive Symptoms (Andreasen, 1987); SES: social economic status.
Stimulus presentation and task
Each trial began with a 400 msec fixation with an interstimulus interval (ISI) of 100 msec. The first three sentences were presented self-paced. The fourth and fifth sentences were presented word by word for 400 msec per word and a 100 msec ISI with the exception of the sentence-final word. This rate of presentation was comparable to that used in a previous ERP discourse comprehension study in patients with schizophrenia (Ditman & Kuperberg, 2007). It is also in keeping with patients’ natural speed of reading sentences presented word by word, as indicated in a previous self-paced reading study (Kuperberg, Kreher et al., 2006). The sentence-final word appeared with a period and was followed by a 750 msec blank-screen interval and then a “?”. This cued participants to indicate whether the final two sentences were related to one another by pressing a “yes” button or a “no” button on a gamepad (counterbalanced across participants). This delayed response reduced contamination of the ERP waveform by response sensitive components such as the P300 (Donchin & Coles, 1988) and triggered the onset of the next trial. Participants completed six to twelve practice trials at the start of the experiment to ensure task comprehension. All patients reported being able to comfortably read the words on the computer screen following the practice session.
Electrophysiological Recording
Twenty-nine active tin electrodes were held in place on the scalp by an elastic cap (Electro-Cap International, Inc., Eaton, OH), see Figure 1. Electrodes were also placed below the left eye and at the outer canthus of the right eye to monitor vertical and horizontal eye movements, and on the left and right mastoids. The EEG signal was amplified by an Isolated Bioelectric Amplifier System Model HandW-32/BA (SA Instrumentation Co., San Diego, CA) with a bandpass of 0.01 to 40 Hz and was continuously sampled at 200 Hz by an analogue-to-digital converter. The stimuli and behavioral responses were simultaneously monitored by a digitizing computer.
Figure 1.
Electrode montage.
Behavioral Data Analysis
A 3 (Referential condition: contextually-appropriate/associated, contextually-inappropriate/associated, contextually-inappropriate/non-associated) × 2 (Group: controls, patients) mixed model ANOVA, with Referential condition as a within-subjects variable and Group as a between-subjects factor, was performed on the accuracy data and response times (RTs) to correctly answered trials. Planned comparisons were conducted to follow up significant effects.
ERP Data Analysis
Analyses were conducted on the mean amplitudes of ERPs evoked by critical words (using a 100 msec prestimulus baseline), regardless of participants' ratings. We characterized the N400 across two 100-ms time windows: 300–400 msec and 400–500 msec. This yielded a fine-grained analysis of the time-course of the N400, capturing its upslope and onset as well as its downslope and offset (see Kreher et al., 2008; De Grauwe, Swain, Holcomb, Ditman, & Kuperberg, 2010) and captured the differences observed in Figures 3 and 4a/b. Two repeated measures ANOVAs – one at midline sites and one at non-midline sites – were conducted at each time window in order to yield statistical information about differences in the distribution of effects. Both midline and non-midline ANOVAs included the within-subject factor of Referential condition (contextually-appropriate/associated, contextually-inappropriate/associated, contextually-inappropriate/non-associated). In addition to this factor, the midline ANOVA included anterior, central, and posterior sites along the midline electrode column (Anterior-to-Posterior, AP, Distribution: Fz, Cz, Pz) to examine the anterior to posterior distribution of the effect, and the non-midline ANOVA included factors of Hemisphere (left, right), AP Distribution (anterior, central, posterior), and Column (peripheral, lateral, and medial), resulting in six regions covering the non-midline sites: left-peripheral (F7, T3, T5), right-peripheral (F8, T4, T6), left-lateral (FC5, CP5, P3), right-lateral (FC6, CP6, P4), left-medial (FC1, C3, CP1), and right-medial (FC2, C4, CP2) (see Figure 1). A Greenhouse-Geisser correction was applied to analyses with more than one degree of freedom in the numerator. We report original degrees of freedom with corrected p values. Significant interactions were further examined with simple effects tests and planned comparisons.
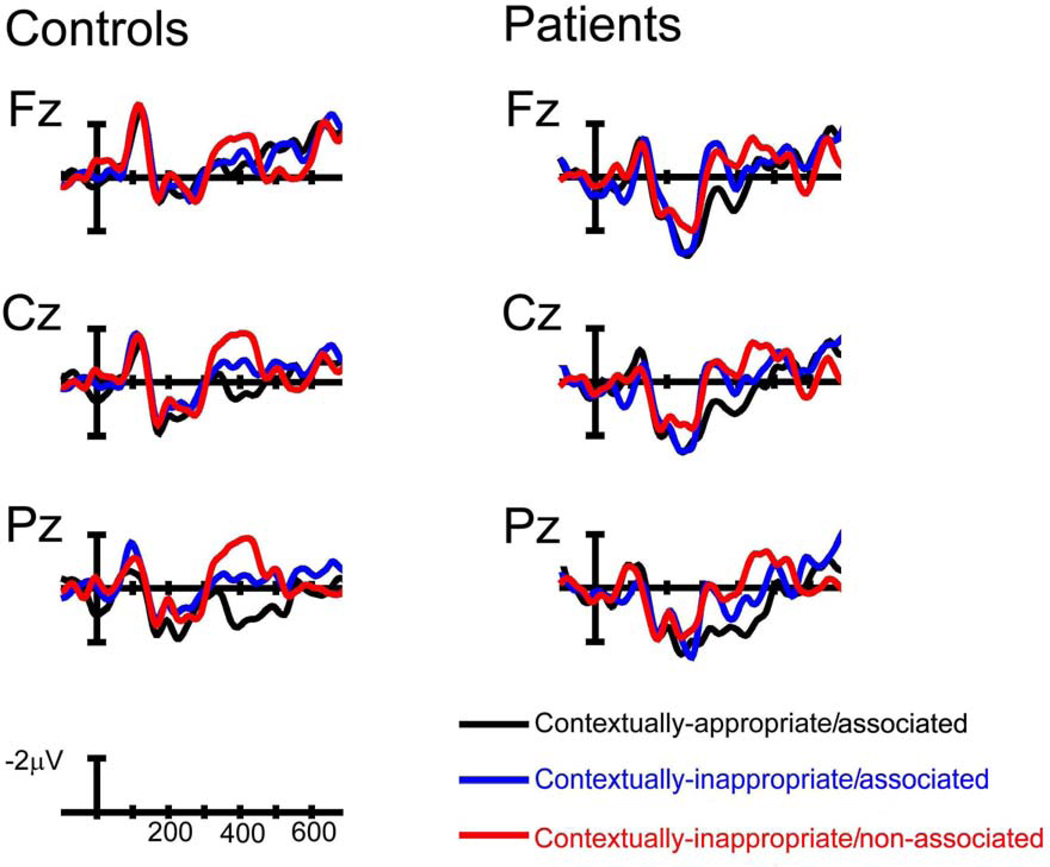
Figure 3.
ERPs in healthy adults and patients with schizophrenia at the midline electrode sites.
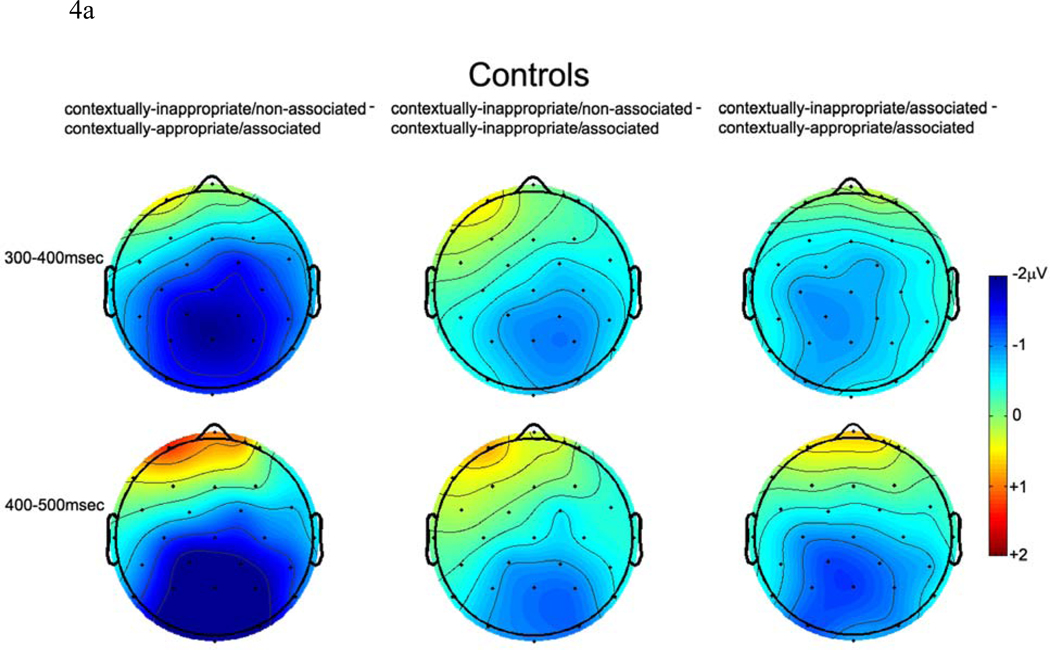
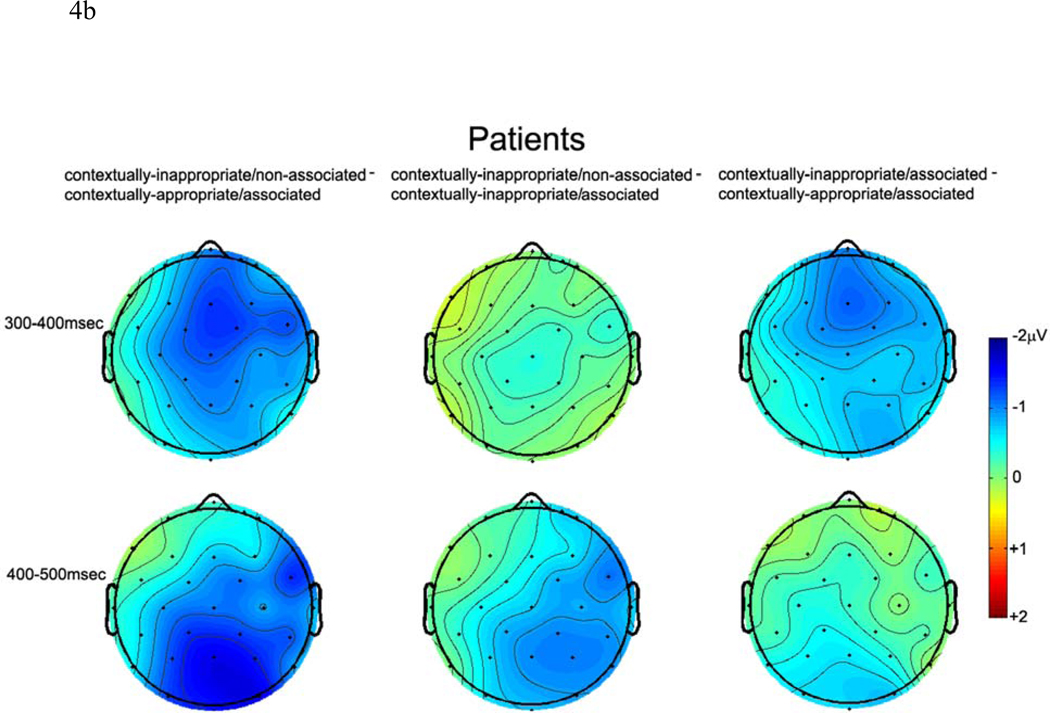
Figure 4.
a. Voltage maps depicting the distribution of neural activity across the scalp in healthy controls.
b. Voltage maps depicting the distribution of neural activity across the scalp in patients with schizophrenia.
In addition, correlations were conducted between (a) difference scores between each of the pairwise comparisons in the two time windows and (b) total SAPS, SANS, positive thought disorder, delusions, and hallucinations as assessed by the SAPS, and chlorpromazine equivalents. Alpha was set to p < 0.05.
Results
Behavioral Data
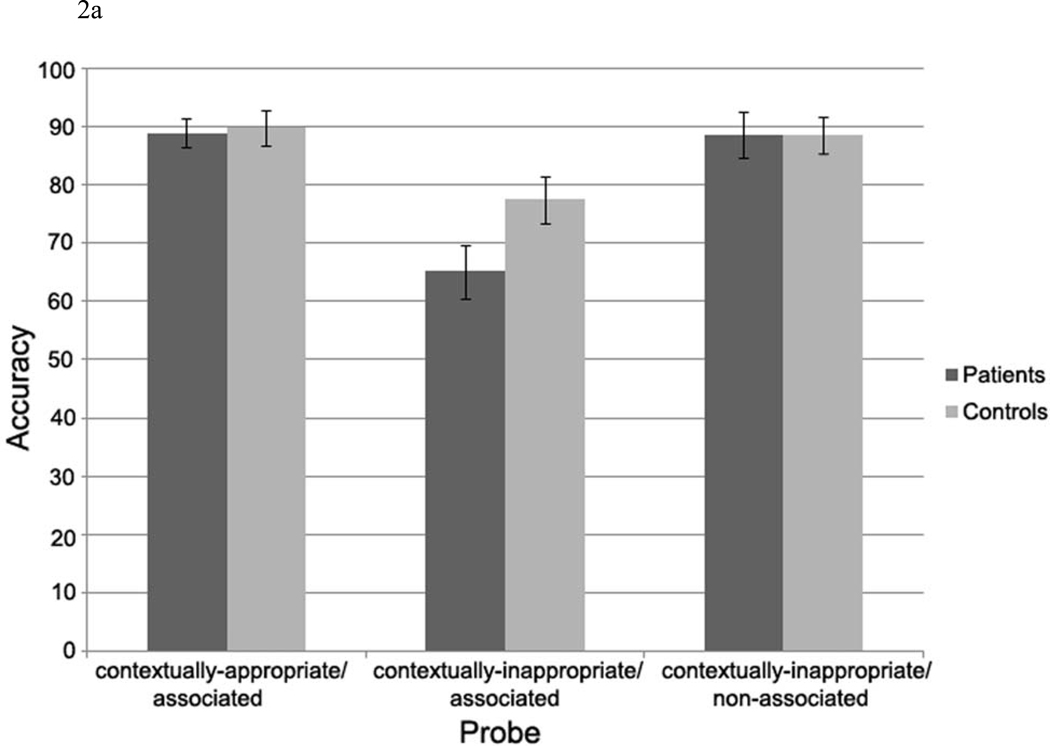
Accuracy (see Figure 2a)
Figure 2.
a. Accuracy data.
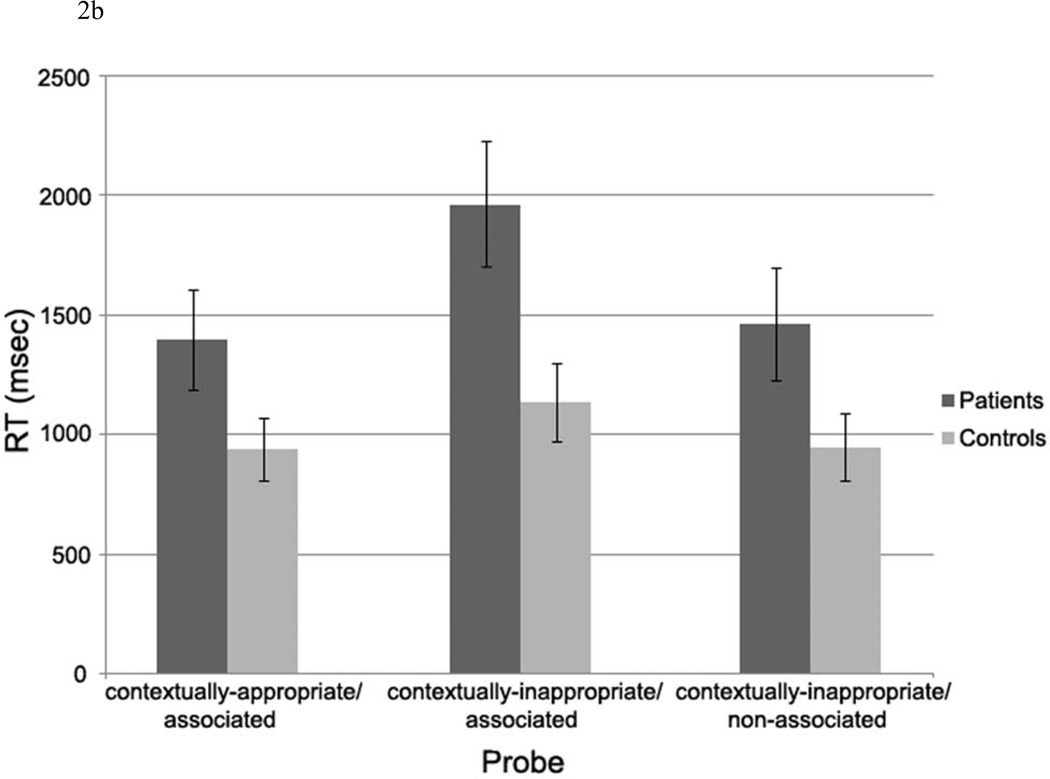
b. Response (RT) data.
Both patients and controls were fairly accurate in their responses to the probe when it followed contextually-appropriate/associated scenarios and contextually-inappropriate/non-associated scenarios, but less accurate when the probe followed contextually-inappropriate/associated scenarios. A 2 (Group: patients, controls) × 3 (Referential condition: contextually-appropriate/associated, contextually-inappropriate/associated, contextually-inappropriate/non-associated) repeated measures ANOVA was performed on the accuracy data, revealing a main effect of Referential condition, F (2, 60) = 24.91, p < .001, and a marginal Group × Referential condition interaction, F (2, 60) = 3.05, p < .07. There was no main effect of Group, F (1, 30) = 1.97, p > .10.
Examination of each group separately indicated that both patients and controls were least accurate in the contextually-inappropriate/associated sentences but this decrement in accuracy was larger in patients than controls (Main effect of Referential condition in controls: F (2, 30) = 6.99, p < .001, with planned comparisons: p < .05 for contextually-inappropriate/associated relative to other conditions; Main effect of Referential condition in patients: F (2, 30) = 18.53, p < .001, with planned comparisons: p < .001 for contextually-inappropriate/associated relative to other conditions). Follow ups comparing patients and controls for each condition separately indicated that patients performed less accurately than controls to contextually-inappropriate/associated probes (t (30) = 2.23, p < .05), but there were no between-group differences in responses to contextually-appropriate/associated probes (t (30) = .80, p > .10) or contextually-inappropriate/non-associated probes (t (30) = .18, p > .10).
Response Times (see Figure 2b)
A 2 (Group: patients, controls) × 3 (Referential condition: contextually-appropriate/associated, contextually-inappropriate/associated, contextually-inappropriate/non-associated) repeated measures ANOVA was performed on the response time data. This revealed a similar pattern of results, with a main effect of Referential condition, F (2, 60) = 16.50, p < .001, and a Group × Referential condition interaction, F (2, 60) = 3.63, p < .05. In addition, patients were overall slower in responding (main effect of Group: F (1, 30) = 5.17, p < .05).
As above, in order to further examine the interaction, we performed simple effects ANOVAs for patients and controls separately. Both patients and controls were slower to respond to probes following contextually-inappropriate/associated scenarios relative to both other conditions, but the relative difference in reaction times was larger in patients than in controls (Main effect of Referential condition in controls: F (2, 30) = 4.17, p < .05; planned comparisons: p = .05 for contextually-inappropriate/associated vs. contextually-appropriate/associated, p < .01 for contextually-inappropriate/associated vs. contextually-inappropriate/non-associated; Main effect of Referential condition in patients: F (2, 30) = 12.36, p < .001; planned comparisons: p < .01 for contextually-inappropriate/associated relative to both other conditions). In addition, pairwise comparisons comparing groups to each condition separately indicated that patients and controls only differed significantly in response times to contextually-inappropriate/associated probes (t (30) = 2.67, p < .05), although there were marginally significant differences between the groups in response times to contextually-appropriate/associated (t (30) = 1.87, p = .07) and contextually-inappropriate/non-associated (t (30) = 1.89, p = .07) probes.
ERP Data
Artifact contamination from eye movement or amplifier blocking led to the rejection of 14.24% of the trials in controls and 15.24% of the trials in patients. A 2 (Group: patients, controls) × 3 (Referential condition: contextually-appropriate/associated, contextually-inappropriate/associated, contextually-inappropriate/non-associated) repeated measures ANOVA was performed to ensure that artifact rejection did not differ between the conditions and/or participant groups. There was no main effect of Group or Referential condition and no interaction between these variables (all ps > .10).
Early N400: 300–400 msec
As can be seen in Figures 3, 4a/b, and 5, both patients and controls were sensitive to differences between the Referential conditions although neural activity was modulated differentially by these conditions in patients and controls, as evidenced by Group × Referential condition × A-P Distribution interactions (midline: F (4, 120) = 3.91, p < .05; non-midline: F (4, 120) = 3.14, p < .05). To follow-up these interactions, separate ANOVAs were conducted in controls and patients. In controls, main effects of Referential condition (midline: F (2, 30) = 10.52, p < .001; non-midline: F (2, 30) = 12.70, p < .001) demonstrated that the reintroduced referents in the contextually-appropriate/associated condition evoked the smallest amplitude N400, followed by a medium-sized amplitude N400 to contextually-inappropriate/associated referents, with the largest amplitude N400 evoked to contextually-inappropriate/non-associated referents. This effect was seen primarily at centroparietal sites, as demonstrated by Referential condition × A-P Distribution interactions (midline: F (4, 60) = 3.97, p < .05; non-midline: F (4, 60) = 3.84, p < .05), consistent with the scalp distribution of the N400 component.
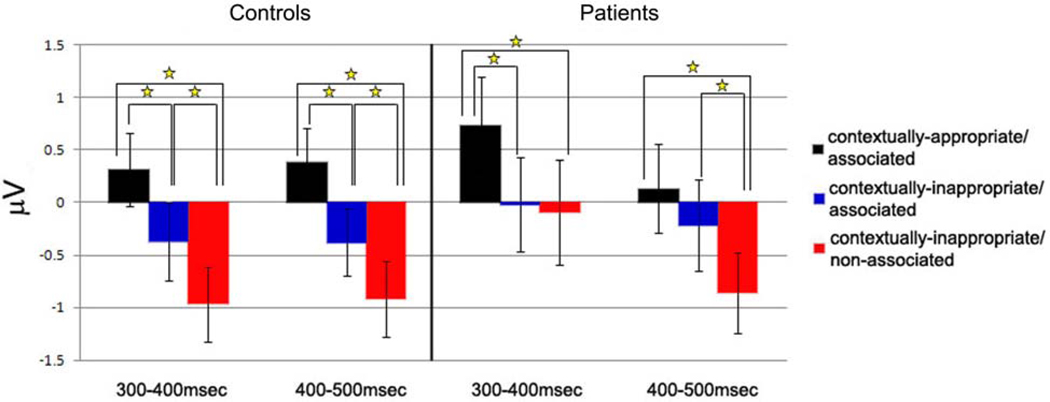
Figure 5.
Mean amplitude (in µV) averaged across all electrode sites for controls (left side) and patients (right side), with error bars depicting the standard error of the mean. Significant differences between conditions are denoted with stars.
In contrast, N400 amplitude in patients did not differentiate between the two contextually inappropriate conditions (contextually-inappropriate/associated and contextually-inappropriate/non-associated) although both conditions evoked larger amplitude N400s than discourse appropriate referents (contextually-appropriate/associated condition) as evidenced by main effects of Referential condition (midline: F (2, 30) = 4.32, p < .05; non-midline: F (2, 30) = 3.37, p = .05). A Referential condition × Column interaction at non-midline electrode sites (F (4, 60) = 3.72, p < .05) indicated that this effect was maximal at medial and lateral columns. No other interactions with Referential condition were statistically significant (all ps > .10).
Late N400: 400–500 msec
Again, within this later time window, neural activity in both controls and patients differentiated between the Referential conditions but the pattern of neural activity was not the same across both groups, as evidenced by Group × Referential condition × A-P Distribution interactions in both midline and non-midline ANOVAs (midline: F (4, 120) = 3.76, p < .05; non-midline: F (4, 120) = 3.13, p < .05). To follow-up these interactions, separate ANOVAs were conducted to examine control and patient data. As in the 300–400 time window, neural activity in controls continued to discriminate between the three Referential conditions, as evidenced by main effects of Referential condition (midline: F (2, 30) = 7.31, p < .01; non-midline: F (2, 30) = 10.94, p < .001). Consistent with the scalp distribution of the N400, this effect was largest at centro-parietal sites, as evidenced by Referential condition × A-P Distribution interactions (midline: F (4, 60) = 14.08, p < .001; non-midline: F (4, 60) = 13.64, p < .001). Pairwise t-tests at centroparietal electrode sites demonstrated that N400 amplitude differences between the three conditions (ps < .05).
In patients, unlike in the previous time window, neural activity was modulated by lexico-semantic associations rather than contextual appropriateness. Main effects of Referential condition confirmed differences between the three conditions (midline: F (2, 30) = 3.98, p < .05; non-midline: F (2, 30) = 4.46, p < .05), which were largest at posterior sites (Referential condition × A-P Distribution interactions: midline: F (4, 60) = 3.46, p < .05; non-midline: F (4, 60) = 5.59, p < .01). Pairwise t-tests at centroparietal electrode sites demonstrated that both related conditions (contextually-appropriate/associated and contextually-inappropriate/associated conditions) evoked equal but smaller N400 amplitudes than the non-associated condition (ps < .05).
Correlations with psychopathological variables within the patient group
No correlations reached statistical significance (all ps > .05).
Discussion
The present study examined the use of lexico-semantic associations to establish referential links in discourse context. Schizophrenia patients and healthy adults read scenarios which required them to link a noun back to one of three referents. To determine whether these nouns had been linked to the correct referent, i.e. whether referential coherence had been correctly established, each of the three possible referents was reinstated in the fifth sentence and neural activity was measured. We also measured response times and accuracy as participants explicitly judged reference coherence: whether the last two sentences referred to the same entity. Neural activity in both patients and controls showed a centroparietal scalp distribution between 300–500msec, consistent with the timing and distribution of the N400 component. ERP findings in the healthy controls indicated that they were able to use both lexico-semantic associations and global contextual information to establish reference during online processing: the smallest N400 amplitude was evoked to contextually-appropriate/associated referents, a medium-sized N400 amplitude was evoked to contextually-inappropriate/associated referents, and the largest N400 amplitude was evoked to contextually-inappropriate/non-associated referents during the entire 300–500 msec N400 time window. The behavioral data indicated that these lexico-semantic associations remained partially active, leading to later costs in evaluating referential coherence in scenarios with lexico-semantically associated distractor referents: accuracy to the contextually incongruous but lexico-semantically associated scenarios was impaired relative to the other two conditions and participants took relatively longer to make these decisions than to the other conditions.
Patients with schizophrenia showed a different pattern of findings. In the early 300–400 msec time window, there was no differential effect of lexico-semantic association at all: both contextually-inappropriate referents (contextually-inappropriate/associated and contextually-inappropriate/non-associated) evoked larger N400s than the contextually-appropriate referent (contextually-appropriate/associated). However, by the later 400–500ms window, patients appeared to be inappropriately dependent on lexico-semantic associations such that these fully overrode discourse context: there was no difference in the N400 to referents that were lexico-semantically associated with the anaphor, regardless of whether they were contextually appropriate or contextually inappropriate; only the contextually-inappropriate/non-associated referents continued to evoke a larger amplitude N400. Moreover, lexico-semantic associations continued to inappropriately influence behavioral judgments: patients were less accurate and slower than healthy controls in judging contextually-inappropriate scenarios with lexico-semantically associated distractor referents. This impairment was selective as patients and controls performed similarly when responding to the other two conditions.
Taken together, these findings suggest that lexico-semantic associations may be more slowly activated in patients than in healthy adults but, once activated, have a long-lasting influence on discourse comprehension at the expense of global meaning. These results will be discussed in more detail below.
Our ERP findings in healthy adult controls replicate those of college-aged healthy adults examined in a previous study (Ditman et al., 2007). Healthy adults quickly used both lexico-semantic associative and discourse-level global contextual information to establish reference in discourse. These findings are consistent with theories of discourse comprehension, such as the Landscape model (Rapp & van den Broek, 2005; van den Broek, Young, Tzeng, & Linderholm, 1999), which postulate that activation of current concepts during comprehension is influenced by multiple sources of information, including associations from the text to concepts in semantic memory, and prior and current information from the text. They also converge with other ERP studies in demonstrating that these pieces of information can influence lexical activation in parallel (e.g., Ditman et al., 2007; cf. Rapp & van den Broek, 2005). Behaviorally, decreased accuracy and longer response times to discourse incongruous lexico-semantically associated distractor referents suggests that these distractor referents may have remained activated at the point of the probe, leading to increased difficulty evaluating referential coherence of scenarios containing these items. This remaining activation may be due to a failure to actively suppress information that is inconsistent with the discourse model in the older adults examined in the present study (cf. Gernsbacher, 1991) or the result of a normally-functioning relatively long decay process for concepts that are not reinforced (cf. Linderholm, Virtue, Tzeng, & van den Broek, 2004).
Patients’ failure to show a three-way distinction in electrophysiological modulation across the three conditions in either the early or late N400 time-windows indicates that they were unable to use both discourse-level and semantic associative information in parallel to establish referential coherence. During the early N400 time window (300–400ms), the absence of any modulation to contextually incongruous lexico-semantically associated relative to contextually incongruous lexico-semantically non-associated referents suggests that patients were initially unable to use semantic associations at all to facilitate the establishment of referential coherence. This reduced effect of semantic associations is consistent with the reduced semantic priming effects shown by patients at relatively long SOAs in patients relative to controls: in the present study, the associated referents and the anaphor were in different sentences and the SOA was 500msec. It is important to note, however, that patients’ initial ability to use some discourse context is encouraging and suggests that when such context is highly explicit and reinforces real-world knowledge, patients are able to use such information appropriately.
During the late N400 time window (400–500 msec), the attenuation of the waveform to contextually incongruous semantically associated relative to contextually incongruous semantically non-associated referents indicates that patients were able to later use such semantic associations to establish referential coherence. However, at this later stage of processing, the N400 amplitude was influenced solely by semantic association at the expense of context: lexico-semantic associations effectively completely overrode discourse-level context such that the amplitude of the N400 to contextually-inappropriate/associated was equal to that evoked by contextually-appropriate/associated words. These results are consistent with previous electrophysiological findings at the sentence and discourse levels which have also demonstrated that, when pitted directly against one another, close lexico-semantic associations will override an incongruous context (Sitnikova et al., 2002; Kuperberg, Sitnikova et al., 2006; Kuperberg, Kreher et al., 2006; Ditman & Kuperberg, 2007). Importantly, as in these previous studies, the abnormality was quite specific: patients showed the same differentiation as controls between referents in contextually incongruous and congruous discourse when the referent was not associated. The current findings extend these previous findings to show that an overdependence on semantic associations may lead to abnormalities in establishing referential coherence at the discourse level, an impairment that is widely observed in patients with schizophrenia.
These ERP findings strongly argue against a generalized performance deficit in patients for the following reasons. Firstly, the N400 was differentially modulated by lexico-semantically associated lures in the early and late time windows – a unique pattern of activity that can only be accounted for by the specificity of the manipulation. In the early time window, the N400 was not decreased by lexico-semantically associated lures relative to non-associated lures. This finding indicates that at this point patients were able to use the discourse context to activate only the appropriate referent and not semantic associates. However, in the late time window, the N400 was modulated by lexico-semantically associated lures relative to non-associated lures. The finding that the patients did not activate semantic associates initially and then, once activated, these lures influenced discourse comprehension for a sustained time suggests a specific impairment in both the initial activation and then the later inhibition, or passive decay of, these associations. Secondly, N400 amplitude was appropriately modulated by the contextually-appropriate/associated and contextually-inappropriate/non-associated conditions in both early and late N400 time windows. Thus, patients were able to use discourse context to modulate neural activity under these conditions. Had patients been unable to perform the task, then we would have expected to observe impaired performance in all conditions. However, this was not the case; the specificity of the manipulation (i.e., the addition of lexico-semantic associations to a discourse incongruent context) is what led to differential neural modulation in response to the contextually-inappropriate/associated condition.
The behavioral findings give further insights into the observed referential impairment. Like the controls, patients’ behavioral performance was worse in the presence of a lexico-semantically associated distractor referent, which continued to exert an influence on participants’ actual judgments about these referential relationships. However, this relative deficit was particularly marked in patients. Patients and controls did not differ in accuracy or response times to probes following contextually congruous scenarios or scenarios with contextually incongruous and lexico-semantically non-associated critical words; rather, patients only performed less accurately and responded more slowly when contextually incongruous words were lexico-semantically associated to the anaphor. This finding suggests that patients were able to use the global context to appropriately evaluate scenarios that were contextually congruous or contextually incongruous without lexico-semantic associations but were specifically impaired at evaluating the coherence of the scenarios when they contained lexico-semantically associated lures. Whether this prolonged activation is the result of an inability to actively suppress globally incongruous information (cf. Gernsbacher, 1991) or the result of a potentially longer, more passive decay process for information that is not reinforced (cf. Linderholm et al., 2004) is unclear. However, these results unequivocally demonstrate that these contextually incongruous lexico-semantic associations remain activated to a greater extent, and thus impair comprehension and, specifically, the ability to establish referential coherence more severely, in patients with schizophrenia than in healthy adults.
Slow and steady: Mechanisms of semantic associative abnormalities in schizophrenia
The combination of online neural and behavioral measures in this study sheds some light on the interaction between the automatic activation of lexico-semantic associates and controlled semantic selection and suppression mechanisms in schizophrenia. There is now fairly consistent evidence that patients are able to use context to appropriately activate lexico-semantically associative information. Most problems occur when there are increased demands to select the most appropriate lexical item and suppress or deactivate contextually inappropriate distractors. Here we used the precise temporal resolution of ERPs to demonstrate again that patients are able to use lexico-semantic associations during language processing (albeit with slight delay), but that they later fail to suppress or deactivate these associations, manifest a few milliseconds later in the ERP response and at the end of the discourse in the behavioral response. This provides evidence for the inappropriately sustained effect of lexico-semantic associations.
This inappropriately sustained effect of lexico-semantic associations in schizophrenia may be most likely to manifest during sentence and discourse processing where there are increased demands to integrate or combine different types of representations to construct an overall meaning: within sentences there are demands to integrate and combine semantic and syntactic information to determine ‘who does what to whom’ (Kuperberg, Sitnikova et al., 2006) and, across sentences, coherence must be built through the establishment of referential and other types of discourse relationships (Ditman & Kuperberg, 2010). Patients’ impairments in combination and integration may leave associative semantic memory-based activity relatively unchecked; thus, as previously argued, abnormalities in integrative and associative activity may actually reflect two sides of the same coin (see Kuperberg, 2007, for a theoretical framework in healthy individuals, and Kuperberg, 2010, for a discussion of how this may play out in schizophrenia). An open question is whether such an imbalance between integrative/combinatorial and associative activity during the build-up of meaning can be explained by more general working memory and executive function mechanisms (including selection and suppression) which are also known to be impaired in schizophrenia (Barch & Smith 2008; Kerns, Nuechterlein, Braver, & Barch, 2008; Lee & Park, 2005).
Relationship with thought and communication failures in schizophrenia
Nonetheless, this raises the question of how thought disorder (disorganized speech) actually arises. In previous work, we have argued that thought disorder reflects an extreme of an imbalance between integrative and associative activity (Kuperberg, 2010), suggesting that an additional bottom-up, automatic associative abnormality (e.g. Kreher et al., 2008; Spitzer et al., 1994) shifts the system into further imbalance, leading to the intrusion of semantic associates on to speech output. The present study does not provide direct evidence for this hypothesis: we saw no correlation between neural patterns of activity and clinical measures of thought disorder within the patient group. However, the relatively small number of participants in the present experimental study may have limited our ability to detect a correlation.1
Open questions
As with all studies in patients with chronic schizophrenia, it is important to consider the possible confound of medication effects. We did not observe any correlation with medication dosage with neural or behavioral measures in schizophrenia patients in the present study. However, it will be important for future studies to examine these types of processes in patients in their first episode of illness as well as to investigate whether these impairments are present in first-degree relatives of schizophrenia patients, in whom referential disturbances have been previously reported (Docherty et al., 1998; Docherty & Gottesman, 2000).
It is also unclear from the present study what the neuroanatomical basis is of the abnormally sustained effect of lexico-semantic associations we observed in the schizophrenia patients. ERPs have excellent temporal resolution but poor spatial resolution, and we can therefore deduce little about the sources underlying the patterns of ERP activity observed at the surface of the scalp. Previous intracranial electrode studies (e.g. Nobre & McCarthy, 1995), fMRI studies using N400 paradigms (e.g. Kuperberg, Holcomb, Sitnikova, Greve, Dale, & Caplan, 2003), and studies using source localization algorithms in conjunction with MEG and fMRI (e.g., Dale, Liu, Fischl, Buckner, Belliveau, Lewine, & Halgren, 2000) have indicated that the N400 component has multiple neuroanatomical sources, with lexico-semantic activity likely localized within the left posterior lateral temporal cortex, but influenced by both bottom-up automatic activity from the inferior temporal cortex and top-down activity from prefrontal cortices (reviewed by Lau, Poeppel, & Phillips, 2008). Integrative activity during language processing is thought to be mediated primarily within the left inferior prefrontal cortex and more superior dorsolateral prefrontal cortices (cf. Hagoort, 2005; e. g., Kuperberg, Sitnikova, & Lakshmanan, 2008). In previous work, we have hypothesized that an imbalance between integrative and lexico-semantic associative activity is linked to an imbalance in activity across temporal and prefrontal cortices, and some evidence for this idea comes from a study of language processing at the sentence level in schizophrenia (Kuperberg, West, Lakshmanan, & Goff, 2008). Future studies using fMRI in conjunction with ERPs and MEG will determine whether a similar imbalance of activity is seen when, as in the current paradigm, semantic associations are pitted against referential coherence, during the build-up of meaning across whole discourse.
Conclusions
In sum, the present study used a novel psycholinguistic paradigm to investigate the influence of discourse context and semantic associations in establishing discourse coherence in patients with schizophrenia. We found that when a strong context was provided, patients were able to initially use this to modulate neural activation. However, ultimately, when this context was pitted against conflicting semantic associations, patients were unable to maintain the influence of context and activation was instead modulated by these lexico-semantic associations, leading to referential failure. These findings are the first to link associative disturbances to failures of reference in schizophrenia. More generally, they show clearly that associative abnormalities in schizophrenia are not specific to clinical thought disorder, but may, as Bleuler hypothesized, reflect a fundamental mechanism that contributes to other types of thought and communication deficits in schizophrenia.
Acknowledgments
Research was supported by a NARSAD Young Investigator Award to TD (with the Sidney Baer Trust), and NIMH (R01 MH071635) and NARSAD (with the Sidney Baer Trust) to GRK. We are grateful to Phillip J. Holcomb for providing insights regarding the design and methodology of this study, Sarah Choi and Donna Kreher for assistance with data collection, and Kaila Norman and Daphne Holt for assistance with patient recruitment.
Footnotes
Previous studies that have found an association between positive thought disorder and referential communication disturbances have used at least twice as many participants as in the present study. In addition, positive thought disorder and referential communication impairments are more likely to correlate in severely ill patients (Docherty et al., 2003) and we employed a relatively stable outpatient sample in the present study.
References
- Andreasen NC. Scale for the assessment of thought, language, and communication (TLC) Schizophrenia Bulletin. 1986;12(3):473–482. doi: 10.1093/schbul/12.3.473. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The comprehensive assessment of symptoms and history. Iowa City: University of Iowa College of Medicine; 1987. [Google Scholar]
- Andreasen NC. Scale for the assessment of negative symptoms (SANS) British Journal of Psychiatry. 1989;155(S):53–58. [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: Relevance to CNTRICS and schizophrenia. Biological Psychiatry. 2008;64(1):11–17. doi: 10.1016/j.biopsych.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Cohen JD, Servan-Schreiber D, Steingard S, Steinhauer S, van Kammen D. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. Journal of Abnormal Psychology. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- Bentin S, McCarthy G, Wood CC. Event-related potentials, lexical decision and semantic priming. Electroencephalography and Clinical Neurophysiology. 1985;60:343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Besche C, Passerieux C, Segui J, Sarfati Y, Laurent J-P, Hardy-Bayle M-C. Syntactic and semantic processing in schizophrenic patients evaluated by lexical-decision tasks. Neuropsychology. 1997;11:498–505. doi: 10.1037//0894-4105.11.4.498. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bleuler E. Dementia Praecox, or the Group of Schizophrenias. New York: International Universities Press; 1911/1950. [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic Statistical Parametric Mapping: Combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;22:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- De Grauwe S, Swain A, Holcomb PJ, Ditman T, Kuperberg GR. Electrophysiological insights into the processing of nominal metaphors. Neuropsychologia. 2010;48(7):1965–1984. doi: 10.1016/j.neuropsychologia.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditman T, Holcomb PJ, Kuperberg GR. The contributions of lexico-semantic and discourse information to the resolution of ambiguous categorical anaphors. Language & Cognitive Processes. 2007;22:793–827. [Google Scholar]
- Ditman T, Kuperberg GR. The time course of building discourse coherence in schizophrenia: an ERP investigation. Psychophysiology. 2007;44:991–1001. doi: 10.1111/j.1469-8986.2007.00565.x. [DOI] [PubMed] [Google Scholar]
- Ditman T, Kuperberg GR. Building coherence: A framework for exploring the breakdown of links across clause boundaries in schizophrenia. Journal of Neurolinguistics. 2010;23(3):254–269. doi: 10.1016/j.jneuroling.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty NM, DeRosa M, Andreasen NC. Communication disturbances in schizophrenia and mania. Archives of General Psychiatry. 1996;53(4):358–364. doi: 10.1001/archpsyc.1996.01830040094014. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Cohen AS, Nienow TM, Dinzeo TJ, Dangelmaier RE. Stability of formal thought disorder and referential communication disturbances in schizophrenia. Journal of Abnormal Psychology. 2003;112(3):469–475. doi: 10.1037/0021-843X.112.3.469. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Gottesman II. A twin study of communication disturbances in schizophrenia. Journal of Nervous and Mental Disease. 2000;188(7):395–401. doi: 10.1097/00005053-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Rhinewine JP, Labhart RP, Gordinier SW. Communication disturbances and family psychiatric history in parents of schizophrenic patients. Journal of Nervous and Mental Disease. 1998;186(12):761–768. doi: 10.1097/00005053-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Docherty N, Schnur M, Harvey PD. Reference performance and positive and negative though disorder: A follow-up study of manic and schizophrenic patients. Journal of Abnormal Psychology. 1988;97:437–442. doi: 10.1037//0021-843x.97.4.437. [DOI] [PubMed] [Google Scholar]
- Docherty NM, Strauss ME, Dinzeo TJ, St-Hilaire A. The Cognitive Origins of Specific Types of Schizophrenic Speech Disturbances. American Journal of Psychiatry. 2006;163:2111–2118. doi: 10.1176/ajp.2006.163.12.2111. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:355–372. [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2002a. [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York: New York State Psychiatric Institute, Biometrics Research; 2002b. [Google Scholar]
- Gernsbacher MA. Cognitive processes and mechanisms in language comprehension: The structure building framework. In: Bower GH, editor. The psychology of learning and motivation. New York: Academic Press; 1991. pp. 217–263. [Google Scholar]
- Hagoort P. On Broca, brain, and binding: A new framework. Trends in Cognitive Sciences. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Han SD, Nestor PG, Hale-Spencer M, Cohen A, Niznikiewicz M, McCarley RW, Wible CG. Functional neuroimaging of word priming in males with chronic schizophrenia. Neuroimage. 2007;35(1):273–282. doi: 10.1016/j.neuroimage.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PJ. Semantic priming and stimulus degradation: Implications for the role of the N400 in language processing. Psychophysiology. 1993;30:47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Hogben GL, Smith H, Calhoun WF. Message disruptions during syntactic processing in schizophrenia. Journal of Communication Disorders. 1985;18(3):183–202. doi: 10.1016/0021-9924(85)90020-6. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biological Psychiatry. 2008;64(1):26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kiang M, Kutas M, Light GA, Braff DL. An event-related brain potential study of direct and indirect semantic priming in schizophrenia. American Journal of Psychiatry. 2008;165(1):74–81. doi: 10.1176/appi.ajp.2007.07050763. [DOI] [PubMed] [Google Scholar]
- Kreher DA, Goff DC, Kuperberg GR. Why all the confusion? Experimental task explains discrepant semantic priming effects in schizophrenia under "automatic" conditions: Evidence from Event-Related Potentials. Schizophrenia Research. 2009;111:174–181. doi: 10.1016/j.schres.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher DA, Holcomb PJ, Goff DC, Kuperberg GR. Neural evidence for faster and further automatic spreading activation in schizophrenic thought disorder. Schizophrenia Bulletin. 2008;34:473–482. doi: 10.1093/schbul/sbm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR. Neural mechanisms of language comprehension: Challenges to syntax. Brain Research. 2007;1146:23–49. doi: 10.1016/j.brainres.2006.12.063. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR. Language in schizophrenia Part 2: What can psycholinguistics bring to the study of schizophrenia and vice versa? Language and Linguistic Compass. 2010;4:590–604. doi: 10.1111/j.1749-818X.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Deckersbach T, Holt DJ, Goff DC, West WC. Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Archives of General Psychiatry. 2007;64:138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Ditman T, Kreher D, Goldberg T. Approaches to Understanding language dysfunction in neuropsychiatric disorders: Insights from the study of schizophrenia. In: Wood S, Allen N, Pantelis C, editors. Handbook of Neuropsychology of Mental Disorders. Cambridge University Press; 2009. pp. 68–95. [Google Scholar]
- Kuperberg GR, Holcomb PJ, Sitnikova T, Greve D, Dale AM, Caplan D. Distinct patterns of neural modulation during the processing of conceptual and syntactic anomalies. Journal of Cognitive Neuroscience. 2003;15:272–293. doi: 10.1162/089892903321208204. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Kreher DA, Goff D, McGuire PK, David AS. Building up linguistic context in schizophrenia: evidence from self-paced reading. Neuropsychology. 2006;20(4):442–452. doi: 10.1037/0894-4105.20.4.442. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: Electrophysiological evidence for abnormal interactions between semantic and syntactic processing. Journal of Abnormal Psychology. 2006;115(2):243–256. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: Evidence from functional Magnetic Resonance Imaging. NeuroImage. 2008;40:367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, West WC, Lakshmanan BM, Goff DC. fMRI reveals neuroanatomical dissociations during semantic integration in schizophrenia. Biological Psychiatry. 2008;64:407–418. doi: 10.1016/j.biopsych.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4:463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic anomalies. Nature. 1984;307:161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Lau EF, Poeppel D, Phillips C. A cortical network for semantics: (de)constructing the N400. Nature Reviews Neuroscience. 2008;9:920–932. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Linderholm T, Virtue S, Tzeng Y, van den Broek P. Fluctuations in the availability of information during reading: Capturing cognitive processes using the Landscape Model. Discourse Processes. 2004;37:165–186. [Google Scholar]
- Lukoff D, Neuchterlein KH, Ventura J. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophrenia Bulletin. 1986;12:578–602. doi: 10.1093/schbul/12.4.578. [DOI] [PubMed] [Google Scholar]
- Maher BA, Manschreck TC, Redmond D, Beaudette S. Length of illness and the gradient from positive to negative semantic priming in schizophrenic patients. Schizophrenia Research. 1996;22:127–132. doi: 10.1016/s0920-9964(96)00066-7. [DOI] [PubMed] [Google Scholar]
- Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought disordered schizophrenic patients. Schizophrenia Research. 1988;1:61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Miller WK, Phelan JG. Comparison of adult schizophrenics with matched normal native speakers of English as to "acceptability" of English sentences. Journal of Psycholinguistic Research. 1980;9(6):579–593. doi: 10.1007/BF01068118. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming in schizophrenia: A review and synthesis. Journal of the International Neuropsychological Society. 2002;8(5):699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- Moritz S, Mersmann K, Kloss M, Jacobsen D, Wilke U, Andresen B, Naber D, Pawlik K. 'Hyper-priming' in thought-disordered schizophrenic patients. Psychological Medicine. 2001;31(2):221–229. doi: 10.1017/s0033291701003105. [DOI] [PubMed] [Google Scholar]
- Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophrenia Research. 2002;59:181–186. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. Journal of Neuroscience. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel-Jorand MC, Reinert M, Giudicelli S, Dassa D. A new approach to discourse analysis in psychiatry, applied to a schizophrenic patient's speech. Schizophrenia Research. 1997;25(3):183–198. doi: 10.1016/s0920-9964(97)00022-4. [DOI] [PubMed] [Google Scholar]
- Ober BA, Vinogradov S, Shenaut GK. Semantic priming of category relations in schizophrenia. Neuropsychology. 1995;9(2):220–228. [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Passerieux C, Segui J, Besche C, Chevalier JF, Widlocher D, Hardy-Bayle M-C. Heterogeneity in cognitive functioning of schizophrenic patients evaluated by a lexical decision task. Psychological Medicine. 1997;27(6):1295–1302. doi: 10.1017/s003329179700562x. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Oh TM, Laws KR, McKenna PJ. Semantic priming in schizophrenia: systematic review and meta-analysis. British Journal of Psychiatry. 2008;192(2):92–97. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- Rapp DN, Van den Broek P. Dynamic text comprehension: An integrative view of reading. Current Directions in Psychological Science. 2005;14:276–279. [Google Scholar]
- Rochester S, Martin JR. Crazy Talk: A study of the discourse of schizophrenic speakers. New York: Plenum Press; 1979. [Google Scholar]
- Sitnikova T, Salisbury DF, Kuperberg G, Holcomb PI. Electrophysiological insights into language processing in schizophrenia. Psychophysiology. 2002;39(6):851–860. doi: 10.1111/1469-8986.3960851. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher BA. Semantic and phonological priming in schizophrenia. Journal of Abnormal Psychology. 1994;103:485–494. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- Titone D, Levy DL, Holzman PS. Contextual insensitivity in schizophrenic language processing: evidence from lexical ambiguity. Journal of Abnormal Psychology. 2000;109(4):761–767. doi: 10.1037//0021-843x.109.4.761. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Van Berkum JJA, Hagoort P, Brown CM. Semantic integration in sentences and discourse: Evidence from the N400. Journal of Cognitive Neuroscience. 1999;11(6):657–671. doi: 10.1162/089892999563724. [DOI] [PubMed] [Google Scholar]
- van den Broek P, Young M, Tzeng Y, Linderholm T. The landscape model of reading: Inferences and the on-line construction of a memory representation. In: van Oostendorp H, Goldman SR, editors. The construction of mental representations during reading. Mahwah, NJ: Erlbaum; 1999. pp. 71–98. [Google Scholar]
- Vinogradov S, Ober BA, Shenaut GK. Semantic priming of word pronunciation and lexical decision in schizophrenia. Schizophrenia Research. 1992;8:171–181. doi: 10.1016/0920-9964(92)90033-2. [DOI] [PubMed] [Google Scholar]
- Zwaan RA, Radvansky GA. Situation models in language comprehension and memory. Psychological Bulletin. 1998;123:162–185. doi: 10.1037/0033-2909.123.2.162. [DOI] [PubMed] [Google Scholar]