Abstract
Background
Prognostic models have been developed for HIV-1 infected patients who start combination antiretroviral therapy (ART) in high-income countries, but not for patients treated in sub-Saharan Africa.
Methods
We included 10,331 adult patients who started ART between 2004 and 2007 in ART scale-up programmes in Côte d’Ivoire, South Africa and Malawi. Data were analysed by intention-to-continue-treatment, ignoring treatment changes and interruptions. We used Weibull survival models to construct two prognostic models: one that included baseline CD4 count and one that did not, since in many African settings CD4 count is not routinely measured.
Findings
During the first year after starting ART, 912 (8.2%) patients died. Baseline CD4 cell count (adjusted hazard ratio 0.21 [95% CI 0.17–0.27] comparing ≥200 with <25 cells/μL), WHO clinical stage (3.45 [2.43–4.90] comparing stages III/IV with I/II), body weight (0.23 [0.18–0.30] comparing ≥60 with <45kg) and anaemia (0.27 [0.20–0.36] comparing none with severe) were strongly associated with mortality. Other independent risk factors were low total lymphocyte count, advanced age and male sex. The CD4 model included CD4 count, clinical stage, body weight, age and sex (160 risk strata). In the alternative model CD4 count was replaced by total lymphocyte count and degree of anaemia (288 risk strata). With the CD4 model the probability of death ranged from 0.9% (95% CI 0.6–1.4) in patients in the lowest risk stratum to 53% (44–62) in patients in the highest risk stratum. The corresponding probabilities for the total lymphocyte/haemoglobin model were 0.9% (0.5–1.4) and 60% (48–71).
Interpretation
Prognostic models based on the CD4 cell count, or total lymphocytes and haemoglobin provide similarly strong discrimination in predicting early mortality in patients starting ART in sub-Saharan Africa. These models are useful for counselling patients, planning health services and predicting outcomes at the population level.
Introduction
It is of obvious importance to HIV-1 infected patients and clinicians to know the likely prognosis when starting combination antiretroviral therapy (ART). Such information is also important for planning health service provision and to inform treatment guidelines. In industrialised countries the prognosis of patients starting ART has been modelled extensively.1–3 For example, a prognostic model developed by a collaboration of prospective studies from Europe and North America showed that the risk of death depended on CD4 count, HIV-1 viral load, clinical stage, history of injection drug use and age.1;2
ART has been scaled up in sub-Saharan Africa since 2004. The Word Health Organization (WHO) estimates that by the end of 2008, between 2.7 and 3.1 million patients had started ART in this region.4 In resource-limited settings mortality is higher than in industrialised countries, particularly in the first year of ART,5;6 but no prognostic models are currently available for sub-Saharan Africa. The CD4 cell count and HIV-1 viral load are important prognostic factors both in untreated patients and in patients on ART, but in many clinics in sub-Saharan Africa neither CD4 counts nor viral load are routinely available. In these settings WHO recommends using clinical stage, or clinical stage in conjunction with total lymphocyte counts (TLC), to assess eligibility for ART.7 Studies in high-income settings have also found haemoglobin to be an important predictor of mortality in patients starting ART,8;9 as did a small study in Durban, South Africa.10
We identified risk factors for death in patients starting ART in four large scale-up programmes in sub-Saharan Africa and developed two prognostic models, one including the CD4 count and an alternative model where CD4 count was replaced by TLC and haemoglobin.
Methods
Patients and cohorts
The present analysis included four large scale-up cohorts in sub-Saharan Africa that participate in the International epidemiological Databases to Evaluate AIDS (IeDEA): Gugulethu11 and Khayelitsha12 in Cape Town, South Africa, Lighthouse13 in Lilongwe, Malawi, and CEPREF (Centre de Prise en Charge de Recherches et de Formation)14 in Abidjan, Côte d’Ivoire. These programmes were chosen for their systematic efforts to trace patients lost to follow-up and ascertain deaths. Eligible patients were antiretroviral naïve before starting triple ART, aged 16 years and over, had a date of starting ART and a baseline CD4 count within the 6 months prior to treatment as well as sex and age recorded. Patients started ART between January 2004 and March 2007 and had at least one year of potential follow-up. In all sites patients with a CD4 count of <200cells/μL (irrespective of clinical stage) or WHO stage IV (irrespective of CD4 count) were eligible for ART. In Côte d’Ivoire and Malawi, but not in the Republic of South Africa, patients with WHO stage III disease and a CD4 count <350 cells/μL were also eligible for ART, in line with WHO guidelines.7 At all sites institutional review boards approved the collection of data for research.
Follow-up and ascertainment of deaths
The outcome was death from all causes in the first year of ART. Patients generally attended clinics monthly to obtain drug supplies, although scheduled medical check-ups were less frequent, typically 3-monthly during the first year of ART. The majority of deaths were reported by family members or hospital wards. Patients who did not return to the clinic were traced. In CEPREF patients who missed two successive visits were traced by social workers who telephoned patients or relatives, did home visits and read the newspaper obituaries. In Gugulethu patients were allocated a therapeutic counselor living in the same community who visited patients at home, providing ongoing counseling and adherence support. In Khayelitsha, patients lost to follow-up who could not be contacted telephonically were visited at home by a clinic nurse where possible, and additional information on vital status was available through linkage to the South African death registry. The Lighthouse clinic also used community support to trace patients, and additional efforts were made to trace all patients lost to follow-up from 2004 to March 2006 using visits to patients’ homes.
Statistical methods
Data were analysed by intention-to-continue-treatment, ignoring treatment changes and interruptions, using Kaplan-Meier curves and survival models based on the Weibull distribution. Time was measured from the date of starting ART to the earliest of: date of death; last follow-up visit; or one year of ART. The follow-up time of patients known to have transferred to another facility in the first year was censored at the last visit. Patients who were lost to follow-up in the first year were excluded from main analyses but included in a sensitivity analysis, with follow-up time censored at the last visit. A patient was considered lost to follow-up if the last visit was recorded during the first year of ART and the patient had at least six months of additional potential follow-up until the closing date of the database (defined as the most recent follow-up recorded in the database). Data on WHO disease stage, weight, haemoglobin and TLC were not available for all patients. We used multiple imputation to account for missing data.15 The main analysis was based on all patients using 25 imputed datasets. Further details of the imputation procedure are given in the Webappendix. In sensitivity analyses we included patients who had no missing data in any of the variables required for a particular analysis (complete case analysis). We used Stata Version 10 (Stata Corp., College Station, TX, USA) for all analyses and expressed results as hazard ratios (HR) with 95% confidence intervals (CIs).
Development and assessment of prognostic models
The development of the model followed methods used previously.1;2;16 We examined Kaplan-Meier survival curves for each prognostic factor in pre-specified categories: age (16–29, 30–39, 40–49, ≥50 years), sex, CD4 count, (<25, 25–49, 50–99, 100–199, ≥200 cells/μL) WHO disease stage (III or IV, I or II), weight (<45, 45–49, 50–59, ≥ 60 kg), anaemia (severe [haemoglobin <8 g/dl]; moderate [males 8– <11 g/dl, females 8– <10 g/dl]; mild [males 11–<13 g/dl, females 10–<12 g/dl]; none [males ≥13 g/dl, females ≥12 g/dl]) and TLC (<800, 800–1199, ≥1200 cells/μL). Weibull proportional hazards models were then used to explore crude and adjusted associations with mortality. Models were fitted on the pooled data with no modelling of cohort effect. We used spline smoothing of the baseline hazard that included indicators for the strata with the worse prognosis to model their steeper mortality in the first three months of treatment.17 Prognostic factors were considered for inclusion if the t statistic associated with their coefficient was >2.5 in the multivariable Weibull model. We considered different combinations of variables to develop a model that included CD4 count, and a second model where CD4 count was replaced by other variables.
The fit of models were compared using the Akaike information criterion (AIC), which penalises model complexity. We used a system of internal-external cross-validation that fits the model on three cohorts and tests predictions in the fourth cohort, rotating round the left out cohort.18 We used the D statistic (averaged across imputed datasets) to assess prognostic separation.19 We calculated the D statistic for the model fitted on the three cohorts and applied to the fourth cohort (Dtest). We also calculated the D statistic in the left out cohort after re-estimating the model coefficients on that cohort (Dr). The difference Dr − Dtest is a measure of the degradation in fit when the model is applied to independent data. We favoured models with low AIC, high Dtest and small Dr − Dtest. We assessed the discrimination of the final models using Harrell’s concordance (C) statistic16 and calculated the R2 measure of explained variation for survival models.19 Calibration was assessed by comparing Kaplan-Meier curves of observed mortality with curves estimated from the models.
Role of the funding source
Our funders had no involvement in the design of the study; the collection, analysis and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Results
The four cohorts included 11,153 eligible patients with 9,908 person years of follow-up within one year of starting ART. Table 1 shows numbers of patients, years of follow-up and outcomes at one year after starting ART by cohort and overall. There were 912 (8.2%) deaths within one year, 822 (7.4%) patients were lost to follow-up and 539 (4.8%) were transferred to other facilities. The median CD4 count at ART initiation was 111 cells/μL (interquartile range [IQR] 48–179) for all patients, 117 cells/μL (55–180) in those alive at one year, 50 cells/μL (16–124) in those who died, 98 cells/μL (35–186) in those lost to follow-up and 119 cells/μL (50–201) in those who transferred out. Of note, patients with CD4 counts >200 cells/μL were more likely to be in an advanced clinical stage (1575/1962, 80%) than patients with counts between 100 and 199 cells/μL (3088/4093, 75%). Data were missing in 345 (3%) patients for WHO clinical stage, 4691 (42%) for haemoglobin, 2328 (21%) for body weight and 4476 (60%) patients for TLC. A total of 10,331 (93%) patients remained in care during the first year.
Table 1.
Antiretroviral treatment programmes with number of patients, characteristics at baseline, follow-up time, status at end of study and Kaplan-Meier estimates of mortality at one year.
| CEPREF (Abidjan, Côte d’Ivoire) | Gugulethu (Cape Town, South Africa) | Khayelitsha (Cape Town, South Africa) | Lighthouse (Lilongwe, Malawi) | All | |
|---|---|---|---|---|---|
| No. of patients | 2117 (19%) | 1611 (14%) | 4397 (39%) | 3028 (27%) | 11153 (100%) |
| Age (years) | 35 (30–42) | 33 (29–39) | 33 (28–39) | 36 (31–43) | 34 (29–41) |
| Sex | |||||
| Female | 1561 (74%) | 1099 (68%) | 3096 (70%) | 1798 (59%) | 7554 (68%) |
| Male | 556 (26%) | 512 (32%) | 1301 (30%) | 1230 (41%) | 3599 (32%) |
| WHO clinical stage | |||||
| No. with data | 2099 (99%) | 1611 (100%) | 4397 (100%) | 2701 (89%) | 10808 (97%) |
| Advanced | 1712 (82%) | 1251 (78%) | 3637 (83%) | 2599 (96%) | 9199 (85%) |
| Less advanced | 387 (18%) | 360 (22%) | 760 (17%) | 102 (4%) | 1609 (15%) |
| CD4 count (cells/μL) | 129 (50–218) | 101 (48–159) | 100 (45–161) | 127 (55–214) | 111 (48–179) |
| Plasma viral load (log copies/mL) | |||||
| No. with data | 5 (0.2%) | 1588 (99%) | 2732 (62%) | 3 (0.1%) | 4328 (39%) |
| Median (IQR) | - | 4.8 (4.4–5.3) | 5.1 (4.5–5.6) | - | 5.0 (4.5 (5.5) |
| Haemoglobin (g/dL) | |||||
| No. with data | 2111 (99%) | 1457 (90%) | 2879 (65%) | 15 (0.5%) | 6462 (58%) |
| Median (IQR) | 9.5 (8.3–10.7) | 11 (10–12) | 11 (10–12) | - | 10.4 (9–12) |
| Weight (kg) | |||||
| No. with data | 2055 (99%) | 0 | 3997 (91%) | 2773 (92%) | 8825 (79%) |
| Median (IQR) | 52 (46–59) | - | 59 (52–67) | 53 (47–60) | 55 (49–63) |
| Total lymphocyte count (cells/μL) | |||||
| No. with data | 2102 (99%) | 0 | 2374 (54%) | 0 | 4476 (40%) |
| Median (IQR) | 1512 (1040–2112) | - | 1310 (860–1870) | - | 1394 (930–1980) |
| Follow-up (years) | 1864 | 1478 | 4084 | 2482 | 9908 |
| No. transferred | Not recorded | 60 (3.7%) | 67 (1.5%) | 412 (13.6%) | 539 (4.8%) |
| No. lost to follow-up* | 244 (11.5%) | 83 (5.2%) | 121 (2.8%) | 374 (12.4%) | 822 (7.4%) |
| No. died | 185 (8.7%) | 124 (7.7%) | 332 (7.6%) | 271 (8.9%) | 912 (8.2%) |
| One-year mortality (%) | |||||
| Kaplan-Meier estimate (95% confidence interval) | 9.9 (8.6–11.3) | 8.2 (6.9–9.7) | 7.8 (7.0–8.6) | 10.5 (9.4–11.8) | 8.9 (8.4–9.5) |
CEPREF, Centre de Prise en Charge de Recherches et de Formation; WHO World Health Organization Medians (interquartile range, [IQR]), number of patients (%) or estimates (95% confidence interval) are shown.
Maximum follow-up per person is one year
Kaplan-Meier estimates of mortality relate to patients remaining in care.
See methods for definition of loss to follow-up
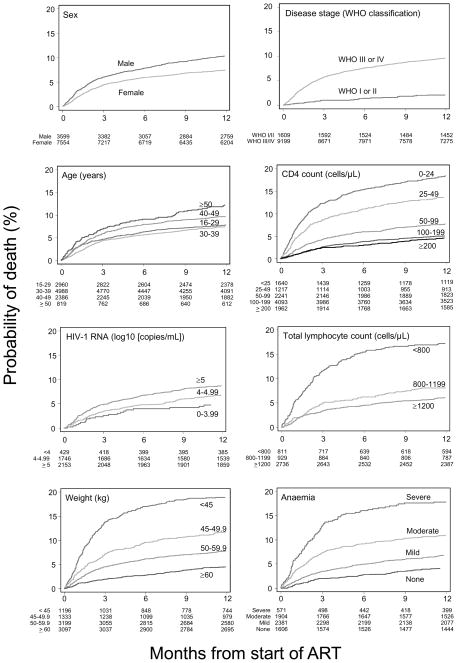
The estimated cumulative percentage of patients remaining in care who died within one year of starting ART was 8.9% (95% CI 8.4–9.5). Estimated mortality was lower (8.4%, 95% CI 7.9–8.9) when patients lost to follow-up were included. Figure 1 shows the Kaplan-Meier survival estimates by each of the prognostic factors examined. Patients who were male, aged ≥40 years, and patients with advanced disease had worse survival. CD4 count, weight, categories of anaemia and TLC were all prognostic with a graded relation with mortality.
Figure 1.
Kaplan-Meier curves of probability of death in patients starting antiretroviral therapy in sub-Saharan Africa 2004–2007, according to sex, age, baseline clinical stage, CD4 count, viral load, weight, degree of anaemia, and total lymphocyte count.
Table 2 shows crude and mutually adjusted HR with 95% CI from Weibull models using multiple imputation of missing values. The crude HR comparing HIV-1 RNA >5 log copies/ml with <4 log copies/ml was 2.34 (95% CI 1.69 to 3.24), but was markedly attenuated on adjustment to 1.30 (0.94 to 1.79), and viral load was not considered further. There was some indication that patients aged ≥50 years had worse survival than those aged 40–49 years, but there were few patients in the former age group and dichotomising age at 40 rather than 50 years resulted in models with better properties.
Table 2.
Characteristics and mortality of 10,331 treatment-naïve patients starting combination antiretroviral therapy and remaining in care in four treatment programmes in sub-Saharan Africa.
| No. of patients (%) | Person- years of follow-up | No. of deaths | Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI)* | |
|---|---|---|---|---|---|
| Age (years) | |||||
| 16–29 | 2723 (27) | 2504 | 228 | 1 | 1 |
| 30–39 | 4655 (45) | 4314 | 363 | 0.90 (0.76–1.06) | 0.83 (0.71–0.99) |
| 40–49 | 2203 (21) | 1995 | 224 | 1.17 (0.97–1.41) | 1.10 (0.91–1.33) |
| ≥50 | 750 (7) | 664 | 97 | 1.48 (1.17–1.89) | 1.48 (1.16–1.89) |
| Sex | |||||
| Male | 3279 (32) | 2949 | 360 | 1 | 1 |
| Female | 7052 (68) | 6528 | 552 | 0.71 (0.62–0.81) | 0.85 (0.74–0.98) |
| WHO clinical stage | |||||
| N=10040 (97%) | |||||
| Less advanced (I/II) | 1522 (15) | 1482 | 33 | 1 | 1 |
| Advanced (III/IV) | 8518 (85) | 7732 | 864 | 4.78 (3.37–6.77) | 3.45 (2.43–4.90) |
| CD4 count (cells/μL) | |||||
| <25 | 1489 (15) | 1231 | 293 | 1 | 1 |
| 25–49 | 1114 (11) | 977 | 162 | 0.73 (0.60–0.88) | 0.72 (0.60–0.88) |
| 50–99 | 2082 (20) | 1924 | 168 | 0.38 (0.32–0.46) | 0.40 (0.33–0.48) |
| 100–199 | 3862 (37) | 3663 | 203 | 0.25 (0.20–0.29) | 0.27 (0.23–0.33) |
| ≥200 | 1784 (17) | 1682 | 86 | 0.20 (0.15–0.25) | 0.21 (0.17–0.27) |
| Plasma viral load (log copies/mL) | |||||
| N=4187 (39%) | |||||
| <4 | 411 (10) | 395 | 20 | 1 | 1 |
| 4–4.99 | 1684 (40) | 1596 | 116 | 1.62 (1.15–2.27) | 1.17 (0.84–1.62) |
| ≥5 | 2092 (50) | 1943 | 187 | 2.34 (1.69–3.24) | 1.30 (0.94–1.79) |
| Anaemia† | |||||
| N=6056 (58%) | |||||
| Severe | 503 (8) | 422 | 100 | 1 | 1 |
| Moderate | 1751 (29) | 1589 | 207 | 0.60 (0.49–0.73) | 0.61 (0.50–0.73) |
| Mild | 2265 (38) | 2146 | 159 | 0.34 (0.28–0.43) | 0.41 (0.33–0.50) |
| None | 1537 (25) | 1484 | 64 | 0.20 (0.15–0.26) | 0.27 (0.20–0.36) |
| Weight (kg) | |||||
| N=8139 (79%) | |||||
| <45 | 1032 (13) | 824 | 219 | 1 | 1 |
| 45–49 | 1195 (15) | 1055 | 152 | 0.56 (0.43–0.72) | 0.58 (0.47–0.73) |
| 50–59 | 2958 (36) | 2723 | 244 | 0.35 (0.28–0.44) | 0.39 (0.32–0.48) |
| ≥60 | 2954 (36) | 2820 | 136 | 0.19 (0.14–0.25) | 0.23 (0.18–0.30) |
| Total lymphocyte count (cells/μL) | |||||
| N=4180 (40%) | |||||
| <800 | 738 (17) | 629 | 138 | 1 | 1 |
| 800–1199 | 871 (21) | 814 | 74 | 0.57 (0.38–0.85) | 0.74 (0.59–0.93) |
| ≥1200 | 2571 (62) | 2450 | 165 | 0.36 (0.26–0.51) | 0.69 (0.56–0.86) |
Results from Weibull models based on all 10,331 patients, with missing values imputed.
Adjusted for age, sex, clinical stage and CD4 group
Severe=haemoglobin <8 g/dL, moderate=haemoglobin 8–10 g/dL in females and 8–11 g/dL in males, mild=haemoglobin 10–12 g/dL in females and 11–13 g/dL in males; none=haemoglobin >12 g/dL in females and >13 g/dL in males
We found little evidence of between-cohort heterogeneity in either covariate effects or baseline hazard and therefore fitted a model across all cohorts using the internal-external cross-validation system. Also, baseline survival (adjusted for covariates) from the complete case analyses were consistent with those from the analyses of imputed datasets. The first of the two final prognostic models included CD4 count, age, gender, WHO clinical stage and body weight (“CD4 model”). In the alternative model CD4 count was replaced by TLC and haemoglobin (“TLC/Hb model”). Table 3 shows the categories of prognostic variables and corresponding HR for the two models. We combined mild and no anaemia categories because there were few deaths in patients with no anaemia. Associations were consistent across models based on all patients and imputed datasets, the subgroup of patients with complete data, and including or excluding patients lost to follow-up (Webtable 1). Statistics for the CD4 and TLC/Hb models were 0.730 and 0.721 (C statistic), 1.30 and 1.26 (D statistic) and 28.8% and 27.5% (R2), respectively.
Table 3.
Adjusted hazard ratios for death used in the two prognostic models: CD4 model and total lymphocyte count/haemoglobin (TLC/Hb) model.
| CD4 model | TLC/Hb model | |
|---|---|---|
| Age ≥ 40 years (v. < 40 years) | ||
| <40 (reference) | 1 | 1 |
| ≥40 | 1.43 (1.23–1.66) | 1.36 (1.12–1.64) |
| Sex | ||
| Male (reference) | 1 | 1 |
| Female | 0.68 (0.58–0.79) | 0.60 (0.50–0.73) |
| Clinical stage* | ||
| Less advanced (reference) | 1 | 1 |
| Advanced | 2.72 (1.87–3.95) | 2.96 (1.83–4.78) |
| CD4 count (cells/μL) | ||
| <25 (reference) | 1 | |
| 25–49 | 0.76 (0.62–0.94) | |
| 50–99 | 0.46 (0.38–0.57) | |
| 100–199 | 0.35 (0.28–0.42) | |
| ≥200 | 0.29 (0.22–0.38) | |
| Weight (kg) | ||
| <45 (reference) | 1 | 1 |
| 45–49 | 0.59 (0.48–0.72) | 0.61 (0.47–0.79) |
| 50–59 | 0.40 (0.33–0.48) | 0.40 (0.32–0.51) |
| ≥60 | 0.24 (0.19–0.30) | 0.25 (0.19–0.33) |
| Total lymphocyte count (cells/μL) | ||
| 0–799 (reference) | 1 | |
| 800–1199 | 0.71 (0.55–0.91) | |
| ≥1200 | 0.53 (0.43–0.65) | |
| Categories of anaemia† | ||
| Severe (reference) | 1 | |
| Moderate | 0.71 (0.56–0.91) | |
| Mild or none | 0.47 (0.37–0.61) | |
Results from Weibull models adjusted for all variables shown, based on 10,331 patients remaining in care, with missing values imputed.
Less advanced=WHO stage I or II, advanced=WHO stage III or IV
Severe=haemoglobin <8 g/dL, moderate=haemoglobin 8–10 g/dL in females and 8–11 g/dL in males, mild=haemoglobin 10–12 g/dL in females and 11–13 g/dL in males; none=haemoglobin >12 g/dL in females and >13 g/dL in males
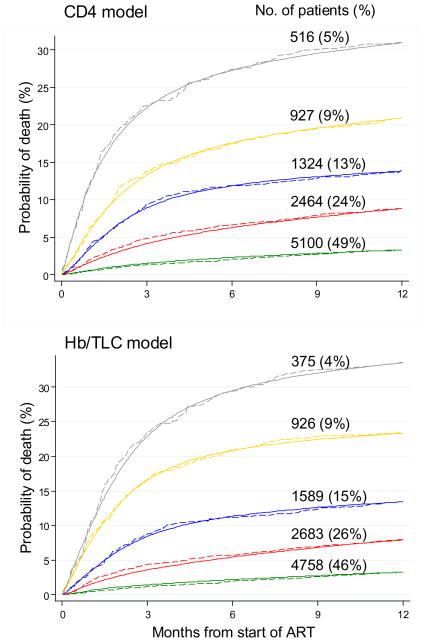
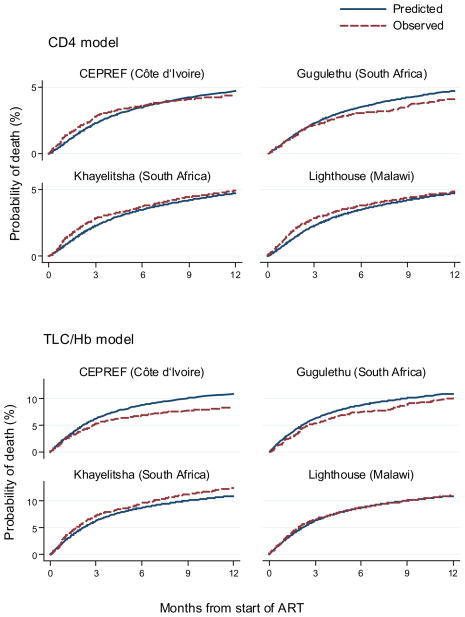
Figure 2 compares survival predicted by the prognostic models with Kaplan-Meier estimates of observed survival, for five prognostic groups defined by levels of risk, such that approximately 20% of the deaths occur in each group. The number (%) of patients is also shown. Each model produced well separated survival curves and a good fit to the observed data. Figure 3 compares survival predicted by the prognostic models with corresponding Kaplan-Meier curves separately for each cohort for a group of patients at medium risk (female, age<40, advanced disease stage, weight 50–59 kg and CD4 100–199 cells/μL or severe anaemia and TLC 800–1199/μL). For these patient groups the CD4 model slightly over estimates mortality in Gugulethu and the TLC/Hb model slightly over estimates mortality in CEPREF and Gugulethu and under estimates in Khayelitsha.
Figure 2.
Cumulative mortality predicted from prognostic models compared to Kaplan-Meier curves of observed mortality for five prognostic groups of patients starting antiretroviral therapy in sub-Saharan Africa. Upper panel shows comparison with CD4 count model, lower panel with total lymphocyte count (TLC)/haemoglobin (Hb) model.
Figure 3.
Cumulative mortality predicted from prognostic models compared to Kaplan-Meier curves of observed mortality for a group of patients at medium risk starting combination antiretroviral treatment in four programmes in sub-Saharan Africa. The upper panel shows the comparison with CD4 count model, the lower panel with total lymphocyte count/haemoglobin (TLC/Hb) model.
Patients had the following characteristics: female, age<40, advanced disease stage, weight 50–59 kg and CD4 100–199 cells/μL or severe anaemia and TLC 800–1199/μL.
Webtable 2 shows estimates of cumulative mortality at 3, 6 and 12 months after starting ART from the CD4 model, for 160 risk groups defined by sex, CD4 count, age, clinical stage and weight. Webtable 3 shows estimates from the TLC/Hb model, for 288 risk groups defined by sex, degree of anaemia, TLC, age, disease stage and weight. The CD4 model estimates 1-year cumulative mortality ranging from 0.9% (95% CI 0.6 to 1.4) for women aged <40 with less advanced disease, weight ≥60 kg and CD4 ≥200 cells/μL to 52.5% (43.8 to 61.7) for men aged ≥40 years with advanced disease, weight <45 kg and CD4 counts <25 cells/μL. Similarly, the TLC/Hb model estimates range from 0.9% (0.5 to 1.4) for women aged <40 years with less advanced disease, weight ≥60 kg, TLC ≥1200 cells/μL and mild or no anaemia to 59.6% (48.2 to 71.4) for men aged ≥40 years with advanced disease, weight <45 kg, TLC <800 cells/μL and severe anaemia.
Sensitivity analyses in which the prognostic models were fitted using all patients, censoring follow-up time in patients lost to follow-up produced lower estimates of cumulative mortality than in the main analysis that excludes patients lost to follow-up, particularly in the higher risk strata. For example, for the group at highest risk described above, 1-year cumulative mortality was 45.8% rather than 52.5% with the CD4 model and 53.8% rather than 59.6% with the TLC/Hb model.
Discussion
We developed two prognostic models with strong discriminatory power, one including the CD4 count and one where CD4 count was replaced by TLC and haemoglobin. The CD4 count is the single most important prognostic factor in HIV infection, but many ART programmes in sub-Saharan Africa do not have the resources to determine it. Our study shows that for prognostic purposes CD4 counts can be replaced by haemoglobin and TLC. The hazard of mortality was highest in the first three months of ART, particularly for patients with worse prognosis. Finally, the models confirm that mortality in patients from sub-Saharan Africa is substantially higher than in industrialized countries. For example, African men aged <40 years with advanced disease and a CD4 count of 50–99 cells/μL have an estimated risk of death at one year of 9% to 33%, depending on their body weight. The estimated mortality in such men in Europe and North America is between 2% and 4%, depending on whether or not the patient has a history of intravenous drug use.1
We found that mortality was higher in men compared to women and therefore produced gender-specific estimates of cumulative mortality. Women were younger and started treatment with less advanced disease, in line with earlier analyses.20;21 This may be explained by differences in health seeking behaviours and women’s more timely access to ART through antenatal care. Mortality differences persisted when adjusting for age, CD4 cell count and clinical stage. Better adherence to ART in women and differences in mortality from other causes, particularly violence, may therefore also have played a role. Gender might not be prognostic in other settings, for example where women and men have equal access to ART, or differences may be reversed where men are more likely to access ART, for example in India or Latin America.20
Several studies, mainly from North America and Europe,8;9;22;23 showed that anaemia in HIV-infected patients is associated with higher rates of disease progression and death, independently of the CD4 count and other prognostic factors. Anaemia in HIV-infection is related to anaemia of chronic disease, infections of the bone marrow and myelosuppressive drugs.24;25 A study in Malawi found that most non-pregnant adults admitted to hospital with severe anaemia had HIV infection.26 The CD4 model would have been improved by additionally including anaemia, but its applicability would have been reduced, because haemoglobin is often not measured routinely in programmes with CD4 cell count monitoring. Whereas haemoglobin is prognostic for mortality independent of CD4 count, the prognostic value of TLC is explained by its correlation with CD4 count. Weight was also prognostic independent of CD4 count: weight changes mirror levels of viral replication27 and weight is also affected by severe opportunistic infections and malignancies.28
The accuracy of prognostic models tends to decrease when they are applied to independent data.29 We aimed to minimise this effect by choosing the model that generalised best to cohorts omitted from the estimation procedure.18 We included data from four public sector programmes in three countries, including two cohorts from South African townships. The range of patients included men and women, from teenagers to elderly people. The majority of patients had evidence of clinical disease, but all CD4 count categories were well represented. We were interested in prognosis during the scale-up of ART and therefore limited analyses to patients starting ART after 2004. All programmes provided ART free of charge: a previous analysis showed that free access to treatment was associated with lower mortality.1 We acknowledge that the programmes included in this study reflect best practice in urban settings and are not representative for all treatment programmes in sub-Saharan Africa. We analysed mortality from all causes and estimates for patients with less advanced infection might not be applicable to settings that differ in terms of non-HIV related mortality. Nevertheless, the results from these models should be applicable to many patients treated in public scale-up clinics in sub-Saharan Africa.
Our study has several limitations. Data on haemoglobin and TLC were missing in a substantial proportion of patients. We imputed missing data so that we could include all patients, avoid selection bias and increase applicability.15;30 Of note, the associations with prognostic factors from the complete-case analyses were consistent with those from the analyses using imputed datasets. Nevertheless, the somewhat superior discrimination of the CD4 model may be due to the inclusion of CD4 count, or because of the larger amount of data imputed in the TLC/Hb model.
Data on symptomatic disease, tuberculosis and other opportunistic infections were not available, but are likely to affect prognosis.11 We used weight, rather than BMI, as many clinics do not measure height. Also, we considered weight at the start of ART only, but weight loss before and weight gain after ART initiation have been shown to affect survival.28;31 Patients with CD4 count above 200 cells/μL appeared to have similar prognosis to those with CD4 between 100 and 200 cells/μL. This result is probably explained by confounding by indication: the WHO guidelines recommend initiating ART below 200 CD4 cells/μL unless the patients have advanced or very advanced disease, in whom treatment is recommended below 350 cells/μL, or irrespective of the CD4 count.7 Indeed, patients with CD4 counts above 200 cells/μL were more likely to be in an advanced clinical stage than patients with counts between 100 and 199 cells/μL.
We excluded patients lost to follow-up in order to reduce bias due to under-ascertainment of death. Mortality in patients lost but subsequently traced was found to be 40% to 50% in public ART programmes in sub-Saharan Africa,32 considerably higher than the mortality of patients remaining in care (9% at one year in this study). Previous analyses generally censored follow-up time at the last visit in those lost to follow-up. Mortality was reduced in a sensitivity analysis that included patients lost to follow-up because additional follow-up time, but no deaths, was added to the data. Differences in estimated mortality, compared with the model excluding patients lost to follow-up, were modest overall but greater in the groups at higher risk of death. We stress that excluding patients lost to follow-up will not have completely removed bias, because patients lost to follow-up may have died in care, i.e. before they met the definition for loss to follow-up. Furthermore, mortality rates might have been higher in patients whose follow-up was censored (but who did not meet criteria for loss to follow-up) than in otherwise identical patients whose follow-up time was not censored (“informative censoring”).
The Development of AntiRetroviral Therapy in Africa (DART) study, a randomised trial comparing clinical monitoring with clinical plus laboratory monitoring (haematology, biochemistry and CD4-cell counts) concluded that there was a role for routine CD4 counts only from the second year of ART, to guide the switch to second-line treatment.33 CD4 counts may thus neither be required at the time of starting ART, nor during the first year of ART. Furthermore, it is possible that haemoglobin and TLC could replace the CD4 count for monitoring patients in the second year of ART, and of patients not yet eligible for ART.34 Further research is required to clarify the role of different laboratory examinations in the scale-up of ART in resource-limited settings, including the role of the point-of-care tests for viral load that are in development. Finally, although our study cannot determine the CD4 count when ART should be started, it is clear that the prognosis of many patients would be improved with more timely initiation of ART. Further expansion of public health strategies to allow earlier access to ART in sub-Saharan Africa is urgently needed.
Supplementary Material
Acknowledgments
We are grateful to all patients, doctors, and study nurses who were involved in the participating cohort studies. This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID, grants 1 U01 AI069924-03 and 5 U01 AI069919-03), the Office of AIDS Research (OAR) of the National Institutes of Health and the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS).
Footnotes
Contributors
M May and M Egger designed and coordinated the current analyses. M May did statistical analyses and developed and validated prognostic models. J Sterne and O Keiser advised on statistical analyses. M Egger and M May wrote the first draft of the paper. A Boulle, S Phiri, E Messou, L Myer and R Wood assisted in implementation, fieldwork, and data collection at study sites. All authors contributed to the final text. M Egger and A Boulle (Southern Africa) and F Dabis (West Africa) are the principle investigators of the IeDEA regions involved in the study.
Conflict of interest statement
We declare that we have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.May M, Sterne JA, Sabin C, Costagliola D, Justice AC, Thiebaut R, et al. Prognosis of HIV-1-infected patients up to 5 years after initiation of HAART: collaborative analysis of prospective studies. AIDS. 2007;21(9):1185–1197. doi: 10.1097/QAD.0b013e328133f285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundgren JD, Mocroft A, Gatell JM, Ledergerber B, D’Arminio MA, Hermans P, et al. A clinically prognostic scoring system for patients receiving highly active antiretroviral therapy: results from the EuroSIDA study. J Infect Dis. 2002;185(2):178–187. doi: 10.1086/338267. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Towards universal access. Scaling up priority HIV/AIDS interventions in the health sector. Progress Report. 2009 Available from: URL: http://www.who.int/hiv/pub/2009progressreport/en/index.html.
- 5.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 6.Keiser O, Orrell C, Egger M, Wood R, Brinkhof MW, Furrer H, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5(7):e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach. Geneva: World Health Organisation; 2004. 2003 Revision. [Google Scholar]
- 8.Mocroft A, Kirk O, Barton SE, Dietrich M, Proenca R, Colebunders R, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13(8):943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 9.Harris RJ, Sterne JA, Abgrall S, Dabis F, Reiss P, Saag M, et al. Prognostic importance of anaemia in HIV type-1-infected patients starting antiretroviral therapy: collaborative analysis of prospective cohort studies. Antivir Ther. 2008;13(8):959–967. [PMC free article] [PubMed] [Google Scholar]
- 10.Ojikutu BO, Zheng H, Walensky RP, Lu Z, Losina E, Giddy J, et al. Predictors of mortality in patients initiating antiretroviral therapy in Durban, South Africa. S Afr Med J. 2008;98(3):204–208. [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24(4):563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 13.Beadles WI, Jahn A, Weigel R, Clutterbuck D. Peripheral neuropathy in HIV-positive patients at an antiretroviral clinic in Lilongwe, Malawi. Trop Doct. 2009;39(2):78–80. doi: 10.1258/td.2008.080213. [DOI] [PubMed] [Google Scholar]
- 14.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22(7):873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol. 2003;56(1):28–37. doi: 10.1016/s0895-4356(02)00539-5. [DOI] [PubMed] [Google Scholar]
- 16.May M, Royston P, Egger M, Justice AC, Sterne JA. Development and validation of a prognostic model for survival time data: application to prognosis of HIV positive patients treated with antiretroviral therapy. Stat Med. 2004;23(15):2375–2398. doi: 10.1002/sim.1825. [DOI] [PubMed] [Google Scholar]
- 17.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 18.Royston P, Parmar MK, Sylvester R. Construction and validation of a prognostic model across several studies, with an application in superficial bladder cancer. Stat Med. 2004;30:907–926. doi: 10.1002/sim.1691. [DOI] [PubMed] [Google Scholar]
- 19.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23(5):723–748. doi: 10.1002/sim.1621. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Boulle A, Nash D, Brinkhof MW, Dabis F, Laurent C, et al. Gender and the use of antiretroviral treatment in resource-constrained settings: findings from a multicenter collaboration. J Womens Health (Larchmt ) 2008;17(1):47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 21.Cornell M, Myer L, Kaplan R, Bekker LG, Wood R. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14(7):722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Semba RD, Shah N, Klein RS, Mayer KH, Schuman P, Vlahov D. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis. 2002;34(2):260–266. doi: 10.1086/338151. [DOI] [PubMed] [Google Scholar]
- 24.Moses A, Nelson J, Bagby GC., Jr The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91(5):1479–1495. [PubMed] [Google Scholar]
- 25.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38(10):1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 26.Lewis DK, Whitty CJ, Walsh AL, Epino H, Broek NR, Letsky EA, et al. Treatable factors associated with severe anaemia in adults admitted to medical wards in Blantyre, Malawi, an area of high HIV seroprevalence. Trans R Soc Trop Med Hyg. 2005;99(8):561–567. doi: 10.1016/j.trstmh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Mwamburi DM, Wilson IB, Jacobson DL, Spiegelman D, Gorbach SL, Knox TA, et al. Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(1):167–173. doi: 10.1086/426591. [DOI] [PubMed] [Google Scholar]
- 28.Mangili A, Murman DH, Zampini AM, Wanke CA. Nutrition and HIV infection: review of weight loss and wasting in the era of highly active antiretroviral therapy from the nutrition for healthy living cohort. Clin Infect Dis. 2006;42(6):836–842. doi: 10.1086/500398. [DOI] [PubMed] [Google Scholar]
- 29.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130(6):515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Br Med J. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madec Y, Szumilin E, Genevier C, Ferradini L, Balkan S, Pujades M, et al. Weight gain at 3 months of antiretroviral therapy is strongly associated with survival: evidence from two developing countries. AIDS. 2009;27(7):853–861. doi: 10.1097/QAD.0b013e32832913ee. [DOI] [PubMed] [Google Scholar]
- 32.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugyenyi P, Walker AS, Hakim J, Munderi P, Gibb DM, Kityo C, et al. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet. 2010;375(9709):123–131. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lau B, Gange SJ, Phair JP, Riddler SA, Detels R, Margolick JB. Use of total lymphocyte count and hemoglobin concentration for monitoring progression of HIV infection. J Acquir Immune Defic Syndr. 2005;39(5):620–625. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.