Abstract
Head and neck squamous cell carcinoma (HNSCC) remains a significant public health problem, accounting for over 5% of all cancer-related deaths, and these deaths primarily result from metastatic disease. The molecular processes involved in HNSCC pathogenesis and progression are poorly understood, and here we present experimental evidence for a direct role of the cell surface receptor tyrosine kinase, TrkB, in HNSCC tumor progression. Using immunohistochemical analysis and transcriptional profiling of archival HNSCC tumor specimens, we found that TrkB and its secreted ligand, brain-derived neurotrophic factor (BDNF), are expresses in greater than 50% of human HNSCC tumors, but not in normal upper aerodigestive tract (UADT) epithelia. Studies with HNSCC cell lines reveal that in vitro stimulation with BDNF, the ligand for TrkB, upregulates the migration and invasion of HNSCC cells, and both transient and stable suppressions of TrkB result in significant abrogation of constitutive and ligand-mediated migration and invasion. Furthermore, enforced over-expression of TrkB results in altered expression of molecular mediators of epithelial-to-mesenchymal transition (EMT), including downregulation of E-cadherin and upregulation of Twist. Using an in vivo mouse model of HNSCC, we were able to show that downregulation of TrkB suppresses tumor growth. These results directly implicate TrkB in EMT and the invasive behavior of HNSCC, and correlate with the in vivo overexpression of TrkB in human HNSCC. Taken together, these data suggest that the TrkB receptor may be a critical component in the multi-step tumor progression of HNSCC, and may be an attractive target for much needed new therapies for this disease.
Keywords: TrkB, squamous cell carcinoma, metastasis
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a significant public health problem that affects approximately 45 000 Americans and results in over 15 000 deaths each year (Jemal et al., 2007). Worldwide, the impact of this disease is even greater, and is the sixth most common cancer diagnosis, with an estimated 650 000 newly diagnosed cases occurring yearly, and over 350 000 deaths (Parkin et al., 2005). Patients often succumb to local-regional recurrence of disease or distant metastasis. Progress in elucidating the fundamental mechanisms of carcinogenesis, progression and metastasis has yielded promising targets for treatment approaches in various cancers (Kupferman and Myers, 2006). The epidermal growth factor receptor seems to be a particularly promising target for therapy of HNSCC (Yigitbasi et al., 2004; Bonner et al., 2006; Karamouzis et al., 2007). However, only a select number of patients respond to epidermal growth factor receptor -targeted therapy, making it apparent that further delineation of the underlying molecular dysregulation in HNSCC holds promise for treating patients with this disease.
Several lines of evidence suggest that neurothrophin receptor B (TrkB), a 145-kDa receptor tyrosine kinase, is a key regulator of oncogenesis and tumor progression in human cancers. Further evidence suggests that this receptor tyrosine kinase may be co-opted by developing cancers to potentiate tumor progression. In particular, altered TrkB expression, signaling and mutations have been found to be important in various cancer types, including carcinomas of the pancreas, lung, colon and prostate, as well as neuroblastoma and multiple myeloma (Miknyoczki et al., 1999; Eggert et al., 2000, 2001; Bardelli et al., 2003; Ketterer et al., 2003; Pearse et al., 2005).
Mechanistic insights into the tumor-promoting potential of TrkB are based on several observations from multiple independent studies: (1) TrkB expression enhances the migratory capability and invasiveness of neurogenic tumor cell lines (Matsumoto et al., 1995; Martens et al., 2007); (2) cancer cell survival is enhanced by TrkB expression and activation (Ho et al., 2002; Pearse et al., 2005); (3) chemotherapy-induced apoptosis is inhibited by TrkB (Jaboin et al., 2002; Pearse et al., 2005); (4) TrkB positively regulates vascular endothelial growth factor expression and tumor-associated angiogenesis (Eggert et al., 2002; Nakamura et al., 2006). In addition, TrkB can transform normal cells and its expression can lead to the development of highly metastatic tumors in mouse models (Douma et al., 2004; Geiger and Peeper, 2007). These data suggest that the TrkB receptor may be a critical component of multistep tumor progression, and its involvement in key cancer-related pathways makes TrkB an attractive target for molecular targeted therapy. However, a direct oncogenic role for TrkB in human cancers of epithelial origin remains unexplored to date.
The epithelial-to-mesenchymal transition (EMT) has been considered to be a critical biological process in epithelial tumor invasion, progression and metastasis. One of the central mechanisms for EMT-associated tumor progression in human malignancies is transforming growth factor-β signaling through the Smad family of mediators (Hoot et al., 2008). The transcriptional activation of Snail and Twist, through AKT activation, induces profound alteration in epithelial cell polarity and morphology, resulting in a mesenchymal phenotype, mediated by the increased expression of mesenchymal molecular markers, with a reciprocal downregulation of epithelial marker expression. Recent data implicates TrkB as a regulator of EMT (Smit et al., 2009), but a link to human cancers has not been defined to date. Although EMT has been well described in squamous cell carcinomas, the precise molecular pathways responsible for initiating this complex process have yet to be delineated.
In this study, we describe a new link between TrkB and key regulators of EMT and HNSCC tumor progression. We first identified co-expression of TrkB and BDNF expression in human HNSCC, supporting the significance of TrkB in HNSCC human tumor biology. We then extended these findings to in vitro models of cellular migration and invasion, and elucidated the biological role of TrkB in these processes through genetic and pharmacological manipulation of TrkB function and expression. A direct association between TrkB function and EMT, as well as suppression of tumor progression, through inhibition of TrkB signaling, further substantiated the fundamental importance of TrkB in HNSCC pathophysiology. Our findings suggest that TrkB, functioning through AKT signaling and EMT, is a critical mediator of tumor progression in HNSCC.
Results
TrkB and BDNF are frequently coexpressed in HNSCC tumors
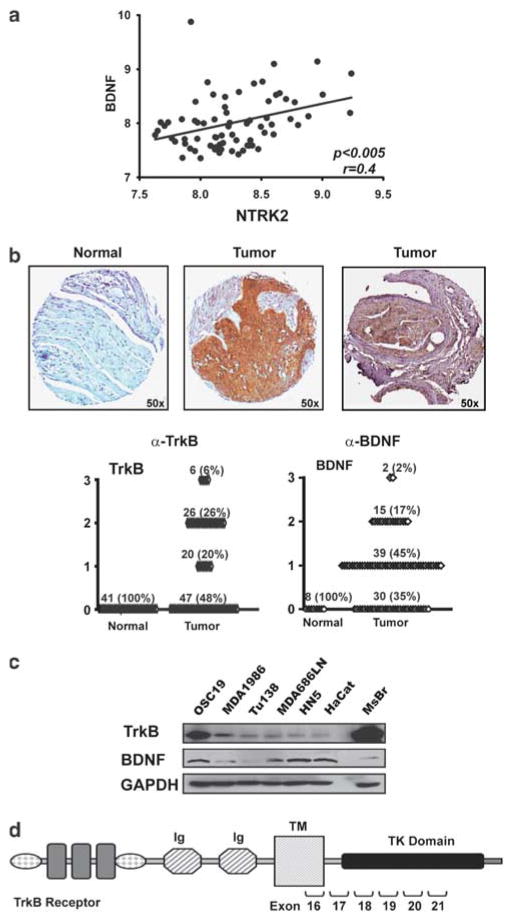
As our preliminary studies suggested upregulation of TrkB and BDNF expression in HNSCC, we used two high-throughput strategies to confirm this in a large cohort of patients. First, we analyzed the expression of TrkB and BDNF in 71 previously untreated tumors by complementary DNA microarray of snap-frozen HNSCC resection specimens using Affymetrix U133AGenechips (O’Donnell et al., 2005). A high correlation was noted (P<0.005) for messenger RNA coexpression of the ligand and receptor (Figure 1a), confirming our preliminary findings of receptor tyrosine kinase overexpression in tumor lysates and orthotopic tumors (Kupferman et al., 2009). We next extended these observations though immunohistochemical evaluation of a human HNSCC tissue microarray (Figure 1b) and identified significant upregulation of both TrkB (Figure 1b; middle, P<0.0001) and BDNF, the ligand for TrkB, (Figure 1b; right, P<0.001) in greater than 50% of tumor samples, compared with normal mucosa and normal lymph nodes.
Figure 1.
Tyrosine kinase B (TrkB) is over-expressed in human head and neck squamous cell carcinoma (HNSCC) tumors. (a) Complementary DNA (cDNA) microarray analysis revealed that messenger RNA (mRNA) expression of NTRK2 correlated with expression of BDNF in 71 previously untreated human tumors (P<0.005) (b) Expression of TrkB and brain-derived neurotrophic factor (BDNF) in representative samples on a human HNSCC tissue array. Tumors were analyzed and graded for TrkB (P<0.0001) and BDNF (P<0.001) expression (scale: 0–3). (c) Protein lysates from subconfluent HNSCC or normal cell lines were separated by SDS–PAGE and assessed with the indicated antibodies. Mouse brain (MsBr) was used as a positive control for TrkB expression. (d) Schematic representation of the TrkB receptor. Exon numbers indicate regions of the receptor that were assessed for genomic mutational analysis (see Table 1). TM, transmembrane domain; TK, tyrosine kinase.
TrkB expression is differentially upregulated in HNSCC cells
To extend these findings to in vitro cell-based systems, the levels of TrkB and its ligand, BDNF, were studied in HNSCC tumor cell lines using both western blotting and RT–PCR methods. Initial studies demonstrated variable levels of TrkB and BDNF across cell lines. In addition, non-tumorigenic cell lines were evaluated for TrkB expression and did not express the receptor to a significant degree (Supplementary Figure 1), confirming that TrkB was selectively expressed in malignant cell lines. The OSC19, MDA1986 and Tu138 cell lines were chosen for further experiments, on the basis of their differential expression patterns; additionally, BDNF levels in these cells were assayed. Corresponding to their TrkB expression patterns, BDNF ligand was present in TrkB-overexpressing cell lines, but not in the low-expressing cell lines (Figure 1c). To determine whether mutations in NTRK2, the gene encoding TrkB, contribute to the biological behavior of tumor cell lines, we searched for somatic mutations in the gene. Sequencing of DNA revealed no evidence for genetic mutations in the intracellular domains, which encode the tyrosine kinase and shc-binding domains of the receptor (Figure 1d and Table 1). Taken together, these data suggested that TrkB is differentially expressed in aggressive tumors and may mediate unique biological phenotypes in HNSCC tumor cell lines (Bardelli et al., 2003).
Table 1.
Primers used for sequencing of the NTRK2 gene
| Forward Primer | Reverse Primer | Sequencing Primer | |
|---|---|---|---|
| Exon 16 | GGGGAGTGAGTGCTAACTGG | GCAGCAAATGGGACAATAAG | ACTACTCTGTGAATATACTAAAACCCAC |
| Exon 17 | GATGGACACCCAGCTTCTTC | TCATGGTTAATGAGACATTCTGG | GCCTGGAAGCTCAGGTACAG |
| Exon 18 | GCTTTGCATATGCCTAAGGAG | TCCACTCCTGAACCCTGAAG | GGCTGTTTTCTCATCTTTTGC |
| Exon 19 | GCATCTTTTAGCACCAGCAG | CCTCCAGAGCCATGAGAAAC | GTGGGGGTGAGGAGCTTAG |
| Exon 20 | TTGCCTTCTGTCTCTGTTGC | AGGCTACTTGGGAAGTGCTG | AAACAGTGTCCCCCAGCAG |
| Exon 21 | TTGCCTTCTGTCTCTGTTGC | AGGCTACTTGGGAAGTGCTG | AAACAGTGTCCCCCAGCAG |
Activation of TrkB by BDNF induces chemotaxis and invasion in HNSCC
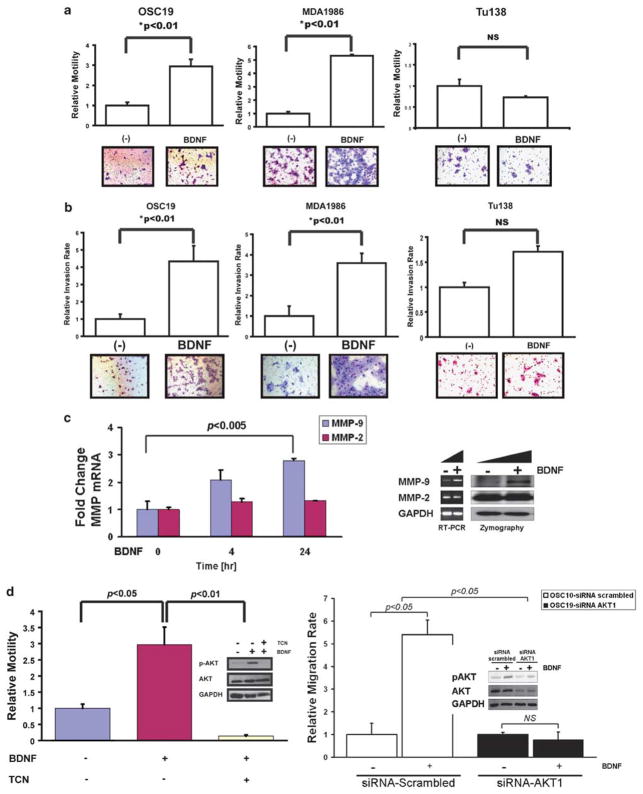
Studies with neuroblastoma cell lines have shown that BDNF stimulation induces TrkB-mediated induction of chemotaxis and invasion. To test whether this ligand–receptor system can transduce signals for cellular motility and invasion in HNSCC, migration and Matrigel experiments were performed under BDNF-stimulated conditions (Klein et al., 1992; Jaboin et al., 2002). When activated by a BDNF concentration gradient, significant upregulation of tumor cell motility was identified in the high TrkB expressing MDA1986 and OSC19 cell lines (Figure 2a). In contrast, Tu138, and HN5 (data not shown), which express low levels of TrkB, had a minimal increase in migration compared with the un-stimulated control. Similar results were noted when cells were analyzed in a Matrigel-coated migration chamber under a BDNF-chemotactic gradient (Figure 2b). Further, increased expression and functional activation of matrix metallopeptidase 9, but not matrix metallopeptidase 2, were noted under BDNF stimulation (Figure 2c). Collectively, these results suggested that the migratory and invasive properties of HNSCC may be mediated in part by a BDNF–TrkB signaling cascade.
Figure 2.
Tyrosine kinase B (TrkB) expression is associated with differential chemotactic responsiveness to brain-derived neurotrophic factor (BDNF). (a) Head and neck squamous cell carcinoma (HNSCC) cell lines were exposed to BDNF (100 ng/ml) in Transwell migration plates and assessed for chemotactic cellular migration after 24 h. Cells were counted in five high-powered fields, analyzed for differential migration with ImagePro. Epidermal growth factor (EGF) (100 ng/ml) was added to a separate well as a positive control for migration experiments (data not shown). Columns, relative migration of cells in the presence or absence of BDNF; bars, s.e.m; NS, not significant. Representative experimental results from triplicate repeats. Magnification: ×100. (b) HNSCC cell lines were exposed to BDNF in Matrigel invasion plates and assessed for cellular invasion after 24 h. Cells were counted in five high-powered fields and analyzed for differential migration with ImagePro. EGF was added to a separate well as a positive control for invasion experiments (data not shown). Columns, relative invasion of cells in the presence or absence of BDNF; bars, s.e.m; NS, not significant. Magnification: ×100. (c) BDNF induces matrix metallopeptidase 9 (MMP-9) expression and activation in HNSCC. Quantitative RT–PCR (left) and gelatin zymography (right) were performed to determine the induction of MMP expression and function under the control of BDNF stimulation. (d) Cell lines were exposed to BDNF (100 ng/ml) with or without triciribine (5 μM) in migration plates and assessed for migration after 24 h (left). Cells were counted in five high-powered fields and analyzed for differential migration. In parallel, OSC19 cell lines were exposed to BDNF (100 ng/ml) with or without triciribine (5 μM) in 6-well plates after 24 h of serum starvation (left, inset). Cells were collected, lysed and proteins were separated by 10% SDS–PAGE and analyzed for the indicated antibodies. OSC19 cell lines were exposed to BDNF (100 ng/ml) after transfection with small interfering RNA (siRNA) targeting AKT1 or a scrambled sequence in 6-well plates after 24 h of serum starvation (right). Cells were collected, lysed and proteins were separated by 10% SDS–PAGE and analyzed for indicated antibodies.
AKT mediates TrkB-induced chemotaxis and invasion in HNSCC cells
Previous studies have demonstrated that the phosphoinositide-3 kinase–AKT and mitogen-activated protein kinase (MAPK) pathways are upregulated by TrkB activation in untransformed cells, leading to cellular migration. To explore the downstream signaling cascades that are activated by TrkB in HNSCC, we subjected tumor cells to the BDNF ligand and assessed the effects on distinct signaling pathways that are known to be active in HNSCC. Significant upregulation of STAT3 (Signal transducer and activator of transcription 3), AKT and MAPK was identified by increased intracellular phosphorylation of these molecules (Supplementary Figure 2). We next sought to determine whether AKT1 activation, through TrkB, affected cellular migration in HNSCC cells under BDNF-stimulating conditions. Direct inhibition of AKT activation in HNSCC cells with triciribine (Lu et al., 2007) or small interfering RNA (siRNA) targeting AKT1 resulted in significant suppression of BDNF-mediated cellular motility, but not cellular proliferation (Supplementary Figure 3). As demonstrated in Figure 2d, exposure to the AKT inhibitor (right) and AKT1 siRNA (left) not only suppressed BDNF-mediated AKT activation, but also decoupled the migratory phenotype from canonical ligand function. These data suggest that TrkB initiates key signaling changes that may mediate increased migration and invasion in HNSCC.
Transient knockdown of TrkB suppresses chemotaxis and invasion of HNSCC
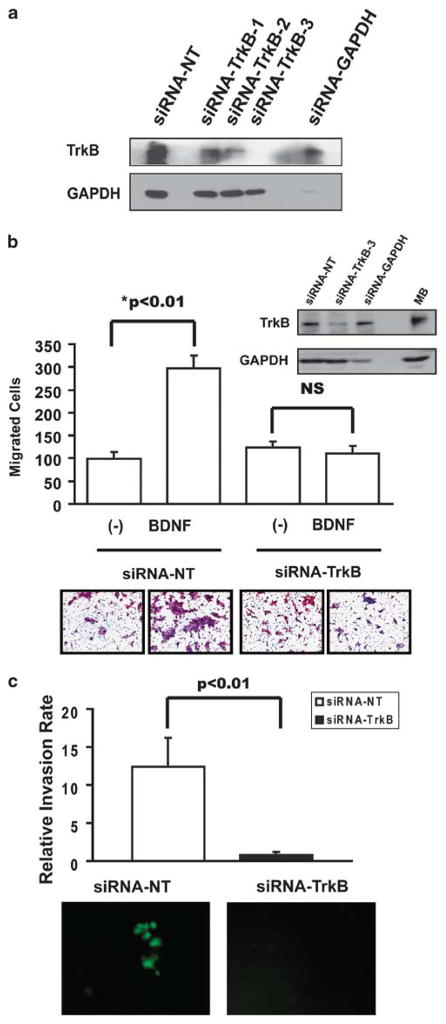
To directly link the function of TrkB to the phenotypic alterations seen in aggressive epithelial carcinomas, we first transiently inhibited TrkB expression with three different siRNA constructs targeting TrkB in HNSCC cell lines (Figure 3a). The most robust knockdown of TrkB expression in HNSCC cells was seen with siRNA construct 3, and on the basis of these findings, the effects of direct TrkB suppression on cellular motility were evaluated using this siRNA. TrkB knockdown (Figure 3b, inset) significantly inhibited BDNF-mediated HNSCC migration in vitro (Figure 3b). Marginal effects on chemotaxis and invasion were observed when TrkB expression was suppressed in cells with low levels of endogenous TrkB (data not shown). Further, transient TrkB suppression also inhibited cellular invasion in a Matrigel assay (Figure 3c). Notably, this siRNA inhibition did not abrogate cellular growth in vitro (data not shown). Taken together, these findings further highlight a direct role for TrkB in the phenotypic behavior of aggressive HNSCC cells under in vitro conditions, prompting us to investigate the role of TrkB in EMT.
Figure 3.
Transient manipulation of tyrosine kinase B (TrkB) in head and neck squamous cell carcinoma (HNSCC) alters ligand-mediated migration and invasion. (a) OSC19 cells were transiently transfected with siRNA constructs, and lysates from subconfluent cells were analyzed by SDS–PAGE with the indicated antibodies. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) knockdown was assayed as a positive control. (b) OSC19 cells were transiently transfected with small interfering RNA (siRNA) against TrkB (construct 3) or a non-targeted siRNA (inset), and seeded on Transwell migration plates for 24 h in the presence or absence of BDNF (100 ng/ml). Cells were counted in five high-powered fields and analyzed for differential migration with ImagePro. Experiments were performed in triplicate and repeated three times. Columns, number of migrated cells in the presence or absence of BDNF; bars, s.e.m.; NS, not significant; magnification: × 100. (c) OSC19-Luc cells were transiently transfected with siRNA against TrkB (construct 3) or a non-targeted siRNA, and seeded on Matrigel invasion plates for 24 h. Cells were counted with an inverted fluorescence microscope in five high-powered fields and analyzed for differential invasion with ImagePro. Experiments were performed in triplicate and repeated three times. Columns, relative invasion of cells; bars, s.e.m, magnification: × 100.
Stable downregulation of TrkB compromises cellular motility and differentiation
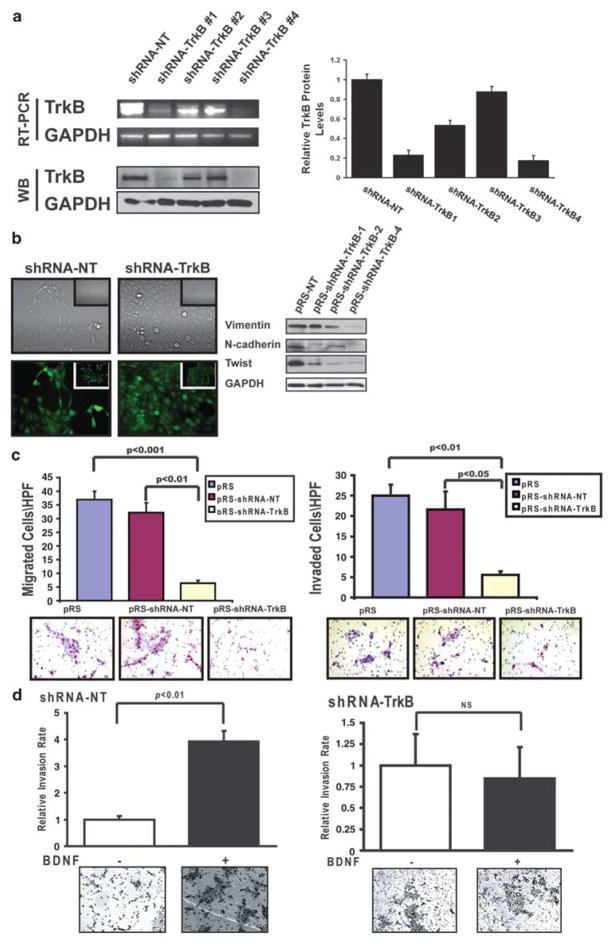
To further characterize the effects of TrkB on the oncogenic behavior of epithelial tumor cells, we stably expressed retroviral vectors containing a short hairpin (shRNA) sequence that targeted TrkB (OSC19-shRNA-TrkB), or a non-targeting sequence (OSC19-shRNA-NT), in TrkB-overexpressing cells (Figure 4a and Table 2). The profound suppression noted in the shRNA-TrkB-1 cells prompted further experimental analysis for this cell line. Selective suppression of TrkB, among members of the Trk receptor family, was also confirmed (Supplementary Figure 4). These cells were notably rounded and compact in culture, with few of the lamellipodial protrusions typically associated with aggressive tumor cells. The rounded and compact nature of the TrkB-suppressed cells reflected a transition to a more differentiated keratinocyte-like morphology, suggestive of a phenotypic transition from a mesenchymal morphology to an epithelial one (Figure 4b, left). This was in contrast to the shRNA-NT cells that maintained their characteristic spindle-like morphology. Corresponding alterations in the protein levels of N-cadherin and Twist revealed an altered transcriptional program that mirrored the observed morphologic alteration (Figure 4b, right). Functionally, the shRNA-TrkB cells displayed a significant quantitative decrement in migration (Figure 4c, left) and invasion through Matrigel-coated membranes (Figure 4c, right) and in a wound-scratch assay (Supplementary Figure 5). Moreover, responsiveness to BDNF-mediated invasion was maintained in the shRNA-NT cells (Figure 4d, left), whereas the shRNA-TrkB cells had minimal alteration under Matrigel-engagement conditions (Figure 4d, right). Taken together, these findings demonstrate a molecular association between TrkB and EMT, further supporting the hypothesis that TrkB mediates mechanisms of tumor progression in HNSCC.
Figure 4.
Morphological and functional alterations induced by targeted reduction of tyrosine kinase B (TrkB). (a) Messenger RNA (mRNA) (top) and protein (bottom) levels of TrkB after stable transfection of OSC19-Luc cells with vectors targeting an irrelevant sequence (shRNA-NT) or TrkB (shRNA-TrkB). Quantification with ImagePro was performed to determine the relative change in TrkB protein expression among the various constructs (right). (b) Cellular morphology of stably transfected cells ( ×200, inset ×40) under both light (top) and fluorescence (bottom) microscopy. Deregulation of epithelial-to-mesenchymal transition (EMT) markers in transfected cells was determined by 10% SDS–PAGE and assayed with the indicated antibodies (right). (c) Constitutive migration (left) and invasion (right) of parental (pRS), shRNA-NT or shRNA-TrkB transfected cells toward a 10% fetal bovine serum (FBS) gradient. Experiments were performed in triplicate and repeated three times. Columns, relative migration or invasion of cells; bars, s.e.m.; magnification: ± 100. (d) brain-derived neurotrophic factor (BDNF)-mediated chemotactic invasion of shRNA-NT (left) and shRNA- TrkB (right) cells was determined as above. Columns, relative invasion of cells (expressed as a ratio of invaded cellsBDNF/invaded cellscontrol); bars, s.e.m.; magnification: × 100. NT, non targeting.
Table 2.
Oligonucleotide sequences used for creation of the shRNA constructs targeting TrkB
| Construct | shRNA Sequence |
|---|---|
| shRNA-TrkB-1 | 5′-ATCGTGGCATTTCCGAGATTGGAGCCTAA-3′ |
| shRNA-TrkB-2 | 5′-GCTGTCAAACAATGAGGTGATAGAGTGTA-3′ |
| shRNA-TrkB-3 | 5′-GGAGGAACGGTTCATCTTAGAGACTAATT-3′ |
| shRNA-TrkB-4 | 5′-GCTGCTCTCCTTCACTCTGACAGTATTAA-3′ |
Abbreviations: shRNA, short hairpin RNA; TrkB, tyrosine kinase B.
Targeted reduction of TrkB abrogates ligand-induced migration and invasion through AKT-dependent mechanisms
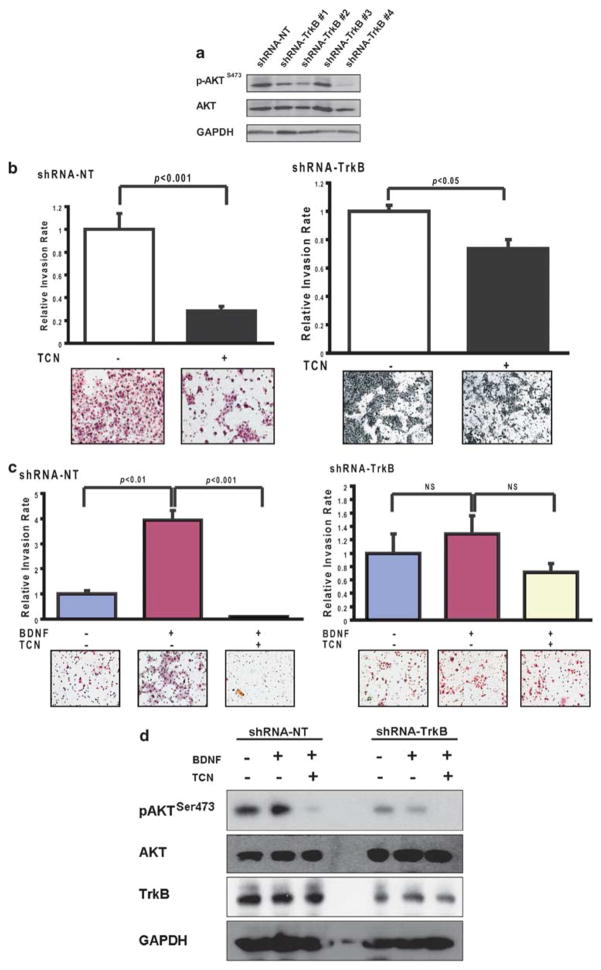
To further investigate the potential molecular mechanisms of altered TrkB downregulation, we analyzed AKT activation in these cells and found significant suppression of AKT phosphorylation in the TrkB-downregulated cells (Figure 5a). When exposed to a selective AKT inhibitor under constitutive conditions, both the shRNA-NT and shRNA-TrkB cells had compromised invasiveness, although the degree of suppression was far more profound in shRNA-NT cells (Figure 5b). This suggested that AKT has a direct role in the invasive potential of HNSCC cells. To directly link this to the BDNF–TrkB signaling cascade, transfected cells were assessed for BDNF responsiveness under conditions of AKT inhibition. Pharmacological inhibition of AKT marginally affected mobility in shRNA-TrkB cells. In contrast, the OSC19-shRNA-NT cells, with high levels of TrkB, were responsive to BDNF-mediated invasion; moreover, AKT inhibition markedly abrogated these effects (Figure 5c). A parallel analysis of AKT activation confirmed the suppression of AKT phosphorylation (Figure 5d). Previous investigators have shown that TrkB-mediated AKT activation facilitates migration and invasion, and our data provide further experimental support for the direct role of this signaling axis in cellular motility.
Figure 5.
Tyrosine kinase B (TrkB)-mediated cellular invasion is AKT dependant. (a) Differential phosphorylation of AKT was determined with 10% SDS–PAGE. (b) Short hairpin RNA (shRNA)-transfected OSC19-Luc cells were seeded onto Matrigel-coated wells, with 10% fetal bovine serum (FBS) with or without triciribine (5 μM) in the lower chamber. Experiments were performed in triplicate and repeated three times. Columns, relative invasion of cells (expressed as a ratio of invaded cellsTCN/invaded cellscontrol); bars, s.e.m.; statistical significance was determined using Student’s t-test. Magnification: × 100. (c) Brain-derived neurotrophic factor (BDNF)-mediated chemotactic invasion of shRNA-NT (left) and shRNA-TrkB (right) cells was determined in the presence or absence of triciribine (TCN), as above. Columns, relative invasion of cells; bars, s.e.m.; magnification: × 100. Experiments were performed in triplicate and repeated three times. (d), TCN abrogates BDNF-mediated AKT phospharylation. Serum-starved shRNA-NT or shRNA-TrkB cells were stimulated with BDNF after pre-treatment with TCN. BDNF-mediated AKT phosphorylation is suppressed with AKT inhibition only in the shRNA-NT cells (left). BDNF-mediated phosphorylation is completely abrogated in the shRNA-TrkB cells, although TCN is an effective inhibitor of basal AKT activity (right). NT, non targeting.
Suppression of TrkB abrogates tumor growth in HNSCC
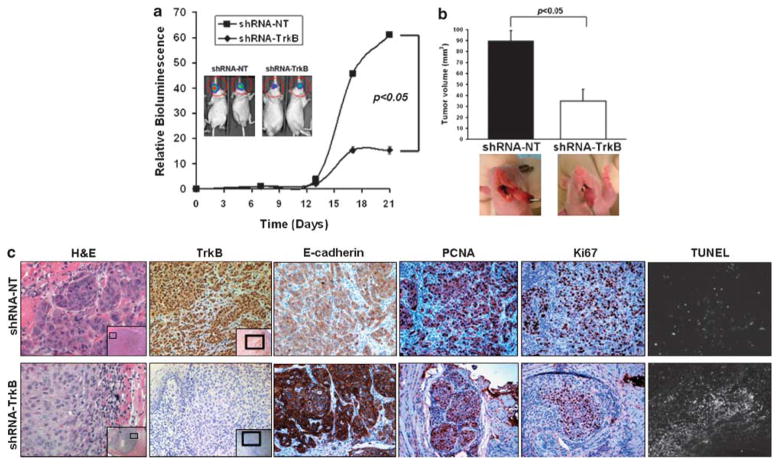
To test the hypothesis that TrkB potentiates the malignant progression of HNSCC, we used a well-characterized murine model for HNSCC and evaluated how TrkB affects tumor growth in vivo. Othotopically injected tongue tumors expressing the non-targeting construct (shRNA-NT) or the TrkB-suppressing construct (shRNA-TrkB) were serially observed with both direct measurements and with bioluminescence imaging (Figures 6a and b). Analysis of the temporal development of orthotopic tongue carcinomas revealed a significant abrogation of tumor growth (P<0.05) in tumors with downregulated TrkB (shRNA-TrkB) compared with those harboring a non-targeting vector (shRNA-NT). Further, upregulation of E-cadherin was noted in cells the shRNA-TrkB tumors, recapitulating in vivo the EMT transcriptional shift (Figure 6c). Further, we noted a suppressed proliferative index in the shRNA-TrkB tumors, as revealed by proliferating cell nuclear antigen and Ki67 staining, but not in the shRNA-NT tumors (Figure 6c, Supplementary Figure 6). Conversely, apoptosis was increased in the shRNA-TrkB tumors compared with the shRNA-NT ones, suggesting an important role for TrkB in regulating tumor viability and tumor growth in HNSCC.
Figure 6.
Downregulation of tyrosine kinase B (TrkB) suppresses tumor growth in an orthotopic model of head and neck squamous cell carcinoma (HNSCC). (a) In vivo bioluminescence reveals suppression of tumor growth in orthotopically implanted cells harboring short hairpin RNA (shRNA) targeting TrkB (OSC19-shRNA-TrkB; n = 8) or a non-targeting construct (OSC19-shRNA-NT; n = 9). Results are representative of three independent experiments. (b) Tumor size is inhibited by 62% in tumors with TrkB knockdown. (c), Hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) analysis of tumors. IHC confirmed knockdown of TrkB in the shRNA-TrkB primary tumors compared with shRNA-NT tumors ( ×200, inset: ×50). Alterations in E-cadherin, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling), Ki67 and proliferating cell nuclear antigen (PCNA) were confirmed by IHC. NT, non targeting.
Overexpression of TrkB potentiates in vitro tumor migration and invasion
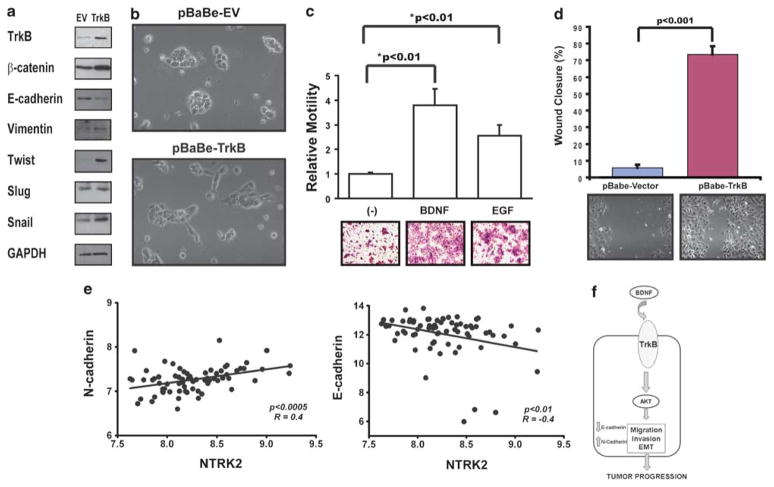
Our initial experimental evidence indicated a possible role for TrkB in EMT and as a direct mediator of in vitro chemomigration. To further establish a role for TrkB in the EMT dynamics, we overexpressed TrkB in Tu138 cells to determine whether enhancing the expression of this receptor may contribute to a more aggressive phenotype in HNSCC cells (Figure 7a). Forced expression of TrkB resulted in a concomitant upregulation of mesenchymal markers that was accompanied by upregulation of Twist and Snail, molecular alterations frequently seen in cell undergoing EMT. In cells harboring the TrkB expression vector, a morphological alteration suggestive of an alteration toward a mesenchymal phenotype was noted (Figure 7b). The cells that stably overexpress TrkB demonstrated markedly increased migratory capability under BDNF-stimulated conditions (Figure 7c). These pro-migratory findings were further confirmed in TrkB-expressing NIH3T3 cells (data not shown). In corresponding wound scratch assays, a marked increase in cellular migration of TrkB-transfected cells over that seen in control transfected cells was observed (Figure 7d). These results are concordant with clinical data from human HNSCC tumors, which revealed a significant correlation between TrkB expression and markers of mesenchymal differentiation (Figure 7e). Collectively, these studies suggested that TrkB contributes to the invasive behavior of HNSCC, which may be due, in part, to the activation of an EMT transcriptional program.
Figure 7.
Tyrosine kinase B (TrkB) overexpression induces epithelial-to-mesenchymal transition (EMT) and head and neck squamous cell carcinoma (HNSCC) migration and invasion. (a) Tu138 cells were infected with a lentiviral vector containing full-length TrkB, grown to confluency, and analyzed by 7% SDS–PAGE with the indicated antibodies. (b) Morphological alterations of Tu138 cells after stable infection with either an empty vector or TrkB-containing vector in 10% fetal bovine serum (FBS; magnification: × 200). (c) Mock or TrkB-transfected cells were seeded onto Transwell migration plates in the presence of either BDNF or epidermal growth factor (EGF) (positive control) and then analyzed for migration after 24 h. Experiments were performed in triplicate and repeated three times. Columns, relative migration of cells; bars, s.e.m; magnification: × 100. (d) Mock or TrkB-infected cells were seeded onto 6-well plates, grown to confluency and assessed for haptotaxis after a scratch wound was made. Images were taken at 0 and 8h, and degree of wound closure was determined with ImagePro. Experiments were performed in triplicate and repeated three times. Columns, percentage of wound closure from 0 h; bars, s.e.m; magnification ×100. (e) Expression of TrkB positively correlated with N-cadherin (P<0.0005) but negatively correlated with E-cadherin (P<0.01) in human HNSCC tumors. (f) Hypothetical model of the role of TrkB in HNSCC pathobiology.
Discussion
In this study, we describe a new role for the TrkB receptor in the progression of HNSCC and as a mediator of EMT in this disease. First, TrkB and its stimulatory ligand, BDNF, were found to be expressed in human tumor specimens and not in normal tissues, suggesting its role in tumorigenesis. The functional significance of the BDNF–TrkB cascade was defined through genetic suppression of TrkB expression in HNSCC cell lines, and our results show that activation of TrkB directly induces cellular migration and invasion, a process that is partially dependant on downstream AKT signaling mechanisms. Moreover, inhibition of TrkB expression leads to suppression of tumor progression in a mouse model of HNSCC. Furthermore, a mesenchymal expression profile was induced through induction of TrkB gene expression, implicating this receptor in EMT. In this study, we describe, to the best of our knowledge, for the first time a mechanistic link between the neurotrophin receptor TrkB and tumor invasion through EMT in HNSCC. Our data support the idea that this receptor has a critical function in mediating tumor progression in this disease and may represent a viable target for future therapies in squamous cancers.
It has been long recognized that TrkB is oncogenic in tumors of neurogenic origin, such as neuroblastoma, and is linked phenotypically to chemotherapeutic resistance, cellular motility and the hypoxic response. However, the unique embryogenic origin of neurogenic tumors has highlighted mechanisms of carcinogenesis and progression that are distinct from squamous histologies. Consequently, little has been known of the importance of TrkB in carcinomas, and using HNSCC as a model, we describe the first direct link of TrkB to EMT and human epithelial tumor progression. Selected studies have focused on correlative studies of neurotrophin receptor expression in the lung, prostate and pancreatic tumors, but a mechanistic understanding has not been defined to date. There is limited evidence to support a role for gene amplification or activating mutations (Ding et al., 2008). Although constitutive activation of the tyrosine kinase domain is oncogenic in mouse models, this phenomenon has not been identified in human tumors. Our data suggest that ligand-directed TrkB activation is sufficient to increase tumor invasion and migration in HNSCC, a finding that is supported by the demonstration of elevated TrkB levels in human cancer specimens.
Key oncogenic signaling pathways have been linked to TrkB receptor activation (Huang and Reichardt, 2003; Chao and Lee, 2004) including the upregulation of the phosphoinositide-3 kinase–AKT cascade, which is associated with TrkB phosphorylation and enhanced anoikis resistance (Douma et al., 2004). Similar to its intracellular effects on neurons, BDNF-induced TrkB activation leads to MAPK p42/44 and AKT phosphor-ylation, resulting in cyclic AMP response element binding protein activation (Li et al., 2007). A recently reported link between phosphoinositide-3 kinase–AKT signaling and cellular motility in non-tumorigenic cells (Luikart et al., 2008) led us to focus our studies on the interaction of TrkB and AKT, hypothesizing that TrkB mediates cellular migration and invasion in HNSCC through the AKT pathway. Our experimental evidence revealed new findings suggesting that the pro-migratory and invasive effects of TrkB activation were least partially dependant on AKT, thus linking this receptor to the canonical intracellular mechanisms of tumor invasion in epithelial neoplasms (see model; Figure 7f). It remains to be seen whether complete phosphoinosi-tide-3 kinase–AKT suppression is necessary to abrogate the effects of TrkB on this process.
A critical component of the invasive process, particularly in squamous cell carcinoma, has been ascribed to EMT (Arias, 2001). The link between TrkB and EMT is substantiated here by the previously unknown finding of increased expression of TrkB in the more pathologically aggressive head and neck cancers. Distinct molecular alterations are noted in EMT (Thiery, 2002), and ultimately, cellular morphology, adhesion, motility and invasiveness are all radically altered, contributing to cancer invasion and metastasis (Christofori, 2006). Abundant data support the role of EMT in squamous cell carcinoma tumor progression (Han et al., 2005; Yang et al., 2006). Our results suggest that TrkB is a new molecular mediator of these alterations in HNSCC. We further confirmed that TrkB expression positively correlated with mesenchymal markers, and its decreased expression correlated with the expression of epithelial markers, highlighting the importance of TrkB in EMT and tumor progression, as has been demonstrated recently in mouse models (Smit et al., 2009). Interestingly, although Twist was recently shown to upregulate AKT2 in EMT (Cheng et al., 2007), we found that the genetic suppression of TrkB indirectly downregulated Twist, but paradoxically upregulated AKT2. This suggests that alternative positive transcriptional regulation at AKT2, independent of TrkB, can occur. The precise mechanism for this alteration warrants further exploration.
In summary, we report here the role of TrkB as a critical regulator of migration, invasion and EMT in HNSCC. Our data suggest that a functional TrkB neurotrophin receptor signaling axis may mediate multiple aspects of the phenotypic behavior of HNSCC cells. Through genetically engineered modifications of TrkB expression, we demonstrate the profound effects that TrkB induces on molecular mediators of EMT. The direct negative effects of TrkB abrogation on the in vitro and in vivo invasive potential of tumor cells reveal new roles for this receptor in tumor progression. Our findings provide further insights into biological mechanisms of HNSCC and establish TrkB as a potential target for future therapies for this disease.
Materials and methods
Cell lines and reagents
The head and neck squamous cell carcinoma cell lines OSC19, MDA1986, Tu138, MDA686LN, HN5, JMAR, HaCat, 293 and NIH3T3 cells were maintained as described previously (Nakashima et al., 2000; Zhou et al., 2008). The OSC19-Luc cell line is a stable transfectant constitutively expressing luciferase and green fluorescent protein (J Myers lab). Recombinant human BDNF and nerve growth factor were obtained from Peprotech (Rocky Hill, NJ, USA). Triciribine was obtained from Berry Associates (Dexter, MI, USA). Following antibodies were used: TrkA (sc-14024, Santa Cruz Biotechnology, Santa Cruz, CA, USA), TrkB (sc-8316), TrkC (sc-117), nerve growth factor (sc-548), BDNF (sc-546), vimentin (sc-51719), glyceraldehyde-3-phosphate dehydrogen-ase, MAPK (9107, Cell Signaling Technologies, Danvers, MA, USA), phospho-MAPK (9101), STAT3 (9132), phospho-STAT3 (9145), AKT (9272), phospho-AKTser473 (3787), Twist (4119), E-cadherin (4065), N-cadherin (4061), β-catenin (9562), proliferating cell nuclear antigen (2586), Slug and Snail.
DNA sequencing
Genomic DNA was extracted according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA) from cell lines and sequenced as previously described. Sequence alignment with the full-length human NTRK2 gene (NM_006180) was performed with Lasergene 7.2 (DNAStar, Madison, WI, USA) (Bardelli et al., 2003).
siRNA and plasmid transfections
Small interfering RNA targeting TrkB, glyceraldehyde-3-phosphate dehydrogenase (Ambion, Austin, TX, USA) and AKT1 (Dharmacon, Lafayette, CO, USA) were transfected into cells according to the manufacturer’s protocol. Confirmation of target gene downregulation was confirmed after 48 h. For TrkB transfection experiments, following constructs were used: pBabe-TrkB and pBabe (kind gift from J Myers), and the transfections were performed as previously described (Zhou et al., 2008). Short-hairpin RNA constructs targeting TrkB were purchased from Origene (Rockville, MD, USA) (TR320436) and were introduced into cells through retroviral infection according to the manufacturer’s protocol and were selected with puromycin (1 μg/ml).
Western blotting (WB)
Cells were grown to 80% confluency, washed with phosphate buffered saline and lysed for 30 min on ice (50 mM Tris–HCl, 100 mM NaCl, 1% Triton-X 100, 0.5% deoxycholute, 10 mM MgCl2, 1 mM NaVO3, 50 mM NaF, 1 mM phenylmethanesul-phonylfluoride, protease inhibitor in phosphate buffered saline). The SDS–PAGE analysis was performed and membranes were incubated overnight at 4° with antibodies directed against the indicated proteins. Membranes were washed, incubated with the appropriate secondary antibodies and exposed with the ECL chemiluminescent substrate kit (Pierce, Rockford, IL, USA). Images were analyzed with ImagePro (Media Cybernetics, Bethesda, MD, USA) and Prism (Graph-Pad Software, La Jolla, CA, USA). Densitometry data were analyzed by using either conventional Student’s t-test or analysis of variance followed by post hoc comparisons on the basis of modified Newman–Keuls–Student procedure, where appropriate. Results are reported as mean±s.e.m. A P-value <0.05 was considered significant and all were two-tailed.
Measurement of apoptosis
Cells were seeded in 6-well plates at a density of 3 × 105 per well and treated the next day with either 5 uM AKT Inhibitor, triciribine, 100 nM AKT1 siRNA, 100 nM scrambled siRNA or 250 nM of TrkB inhibitor, K252a. After 24 h post-treatment, both detached and attached cells were collected and washed once in phosphate buffered saline. Cells were centrifuged at 200 g for 5 min and the pellet was resuspended in annexin-binding buffer and incubated in annexin V for 15 min in the dark according to the recommendation of the manufacturer (EMD Chemicals, Gibbstown, NJ, USA). Propidium iodide flow cytometric analyses were performed on Gallios (Beckman Coulter, Brea, CA, USA).
Gelatin zymography
Cells were seeded in 10-cm dishes and grown to 70% confluency. The next day, cells were washed twice with serum-free Dulbecco’s modified Eagle medium and cultured with serum-free medium containing BDNF (100 ng/ml) for 24 h. Supernatants were collected and centrifuged to remove cellular debris and protein was concentrated with the Centricon 3 system (Millipore, Billerica, MA, USA).
Human tumor analysis and immunohistochemistry
Fresh-frozen and paraffin-embedded HNSCC tumors were obtained from the MD Anderson Cancer Center’s Head and Neck Tumor Tissue Repository under an Institutional Review Board-approved protocol. The HNSCC tissue array was designed as previously described (Armistead et al., 2007). Reactivity to TrkB or BDNF was assessed immunohistochemically on the basis of the percentage of positive cells in 10 consecutive high-power fields (1000 cells) and the intensity of staining graded from 0 to 3 + (0 = < 10% cells with weak staining, 1 = 10–25% cells with weak to intermediate staining, 2 = 25–50% cells with intermediate staining, 3 = 51–100% cells with intermediate to strong staining). Positive (normal mouse brain) and negative (normal mucosa) controls were run in parallel for all experiments. The results were analyzed with Fisher’s exact test to determine the statistical significance of staining and tumor differentiation across experimental groups. A P-value <0.05 was considered significant. The TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling) (Roche, Madison, WI, USA), proliferating cell nuclear antigen, Ki67 (Dakocytomation, Carpinteria, CA, USA, #7240) and E-cadherin staining and analyses were performed as previously described (Zhou et al., 2008; Kupferman et al., 2009).
For microarray experiments, human HNSCC tumors were obtained from the MD Anderson Cancer Center’s Head and Neck Tumor Tissue Repository under an Institutional Review Board approved protocol. After extraction, messenger RNA was hybridized to the Affymetrix U133A Genechip (Affymetrix, Santa Clara, CA, USA). The microarrays were evaluated as described by Affymetrix using a GeneArray 2500 confocal scanner (Affymetrix). The average signal from two sequential scans was calculated for each microarray feature. Data were normalized and analyzed by Pearson correlation with Prism (GraphPad Software). A P-value <0.05 was considered significant.
Migration, wound scratch and invasion assays
Cells were serum-starved overnight and then 5 × 104 cells were plated in cell culture insert wells (BD Falcon) or Matrigel-coated wells (BD Biosciences, San Jose, CA, USA) under described conditions. After 24 or 48 h, the unmigrated cells on the upper chamber were removed and inserts were fixed and stained with Diff-Quik. Images were captured (Cool-Pix digital camera, Nikon, Melville, NY, USA) and the degree of migration was determined by the average number of migrated cells in five × 100 fields (ImagePro, Media Cybernetics). Experiments were performed in triplicate and repeated three times. Wound scratch assays were performed as previously described (Liang et al., 2007). Measurements were taken at indicated time points and were quantified with ImagePro. Differences between groups were analyzed by using either conventional Student’s t-test or analysis of variance followed by post hoc comparisons based on modified Newman–Keuls–Student procedure, where appropriate. Results are reported as mean±s.e.m. A P-value <0.05 was considered significant and all were two-tailed.
Mouse model and imaging
Male athymic nude mice (NCI-nu), aged 8–12 weeks, were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD, USA). The mice were used in accordance with Animal Care and Use Guidelines of The University of Texas MD Anderson Cancer Center under a protocol approved by the Institutional Animal Care and Use Committee. For establishment of orthotopic HNSCC tumors, cells were injected into the tongues of athymic nude mice, as described previously. Mice were imaged weekly with the IVIS Imaging System (Xenogen, Alameda, CA, USA) after intraperitoneal administration of D-luciferin (Xenogen; Zhou et al., 2008). Differences between tumor volumes were evaluated by the non-parametric Mann–Whitney test. Results from the in vivo luciferase assays were evaluated by the non-parametric Mann–Whitney test. Results are reported as mean±s.e.m. A P-value <0.05 was considered significant and all were two-tailed.
Supplementary Material
Acknowledgments
We thank S Jasser, MS, for technical assistance, T Astin for administrative assistance and X Wu, PhD, for critical review of the paper. This study was supported by the following funding sources: American Head and Neck Society; Young Investigator Award (MEK); Head and Neck SPORE Program-Career Development Award, MDACC (MEK); and the MD Anderson Cancer Center Physician-Scientist Program (MEK). NIH Cancer Center Support Grant CA16672 (MDACC) and Grant K08 DE019185 from the NIDCR (MEK).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Arias AM. Epithelial mesenchymal interactions in cancer and development. Cell. 2001;105:425–431. doi: 10.1016/s0092-8674(01)00365-8. [DOI] [PubMed] [Google Scholar]
- Armistead P, Salganick J, Roh J, Steinert D, Patel S, Munsell M, et al. Expression of receptor tyrosine kinases and apoptotic molecules in rhabdomyosarcoma. Cancer. 2007;110:2293–2303. doi: 10.1002/cncr.23038. [DOI] [PubMed] [Google Scholar]
- Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S, et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science. 2003;300:949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Chao MV, Lee FS. Neurotrophin survival signaling mechanisms. J Alzheimers Dis. 2004;6:S7–S11. doi: 10.3233/jad-2004-6s611. [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang L-H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–1808. [PubMed] [Google Scholar]
- Eggert A, Grotzer MA, Ikegaki N, Zhao H, Cnaan A, Brodeur GM, et al. Expression of the neurotrophin receptor TrkB is associated with unfavorable outcome in Wilms’ tumor. J Clin Oncol. 2001;19:689–696. doi: 10.1200/JCO.2001.19.3.689. [DOI] [PubMed] [Google Scholar]
- Eggert A, Ikegaki N, Liu XG, Brodeur GM. Prognostic and biological role of neurotrophin-receptor TrkA and TrkB in neuroblastoma. Klin Padiatr. 2000;212:200–205. doi: 10.1055/s-2000-9677. [DOI] [PubMed] [Google Scholar]
- Geiger TR, Peeper DS. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 2007;67:6221–6229. doi: 10.1158/0008-5472.CAN-07-0121. [DOI] [PubMed] [Google Scholar]
- Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6466. [PubMed] [Google Scholar]
- Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial–mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jaboin J, Kim CJ, Kaplan DR, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from chemotherapy-induced apoptosis via phosphatidylinositol 3′-kinase pathway. Cancer Res. 2002;62:6756–6763. [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA. 2007;298:70–82. doi: 10.1001/jama.298.1.70. [DOI] [PubMed] [Google Scholar]
- Ketterer K, Rao S, Friess H, Weiss J, Buchler MW, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9:5127–5136. [PubMed] [Google Scholar]
- Klein R, Lamballe F, Bryant S, Barbacid M. The trkB tyrosine protein kinase is a receptor for neurotrophin-4. Neuron. 1992;8:947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- Kupferman ME, Jayakumar A, Zhou G, Xie T, Dakak-Yazici Y, Zhao M, et al. Therapeutic suppression of constitutive and inducible JAK/STAT activation in head and neck squamous cell carcinoma. J Exp Ther Oncol. 2009;8:117–127. [PubMed] [Google Scholar]
- Kupferman ME, Myers JN. Molecular biology of oral cavity squamous cell carcinoma. Otolaryngol Clin North Am. 2006;39:229–247. doi: 10.1016/j.otc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang J, Liu Z, Woo CW, Thiele CJ. Downregulation of Bim by brain-derived neurotrophic factor activation of TrkB protects neuroblastoma cells from paclitaxel but not etoposide or cisplatin-induced cell death. Cell Death Differ. 2007;14:318–326. doi: 10.1038/sj.cdd.4401983. [DOI] [PubMed] [Google Scholar]
- Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- Lu C-H, Wyszomierski SL, Tseng L-M, Sun M-H, Lan K-H, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- Luikart BW, Zhang W, Wayman GA, Kwon CH, Westbrook GL, Parada LF. Neurotrophin-dependent dendritic filopodial motility: a convergence on PI3K signaling. J Neurosci. 2008;28:7006–7012. doi: 10.1523/JNEUROSCI.0195-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J Biol Chem. 2007;282:14379–14388. doi: 10.1074/jbc.M609857200. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55:1798–1806. [PubMed] [Google Scholar]
- Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Pak SC, Silverman GA, Spring PM, Frederick MJ, Clayman GL. Genomic cloning, mapping, structure and promoter analysis of HEADPIN, a serpin which is down-regulated in head and neck cancer cells. Biochim Biophys Acta. 2000;1492:441–446. doi: 10.1016/s0167-4781(00)00100-7. [DOI] [PubMed] [Google Scholar]
- O’Donnell RK, Kupferman M, Wei SJ, Singhal S, Weber R, O’Malley B, et al. Gene expression signature predicts lymphatic metastasis in squamous cell carcinoma of the oral cavity. Oncogene. 2005;24:1244–1251. doi: 10.1038/sj.onc.1208285. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429–4436. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- Smit MA, Geiger TR, Song J-Y, Gitelman I, Peeper DS. A Twist–Snail axis critical for TrkB-induced EMT-like transformation, anoikis resistance and metastasis. Mol Cell Biol. 2009;29:3722–3737. doi: 10.1128/MCB.01164-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Yang MH, Chang SY, Chiou SH, Liu CJ, Chi CW, Chen PM, et al. Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene. 2006;26:1459–1467. doi: 10.1038/sj.onc.1209929. [DOI] [PubMed] [Google Scholar]
- Yigitbasi OG, Younes MN, Doan D, Jasser SA, Schiff BA, Bucana CD, et al. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004;64:7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- Zhou G, Xie TX, Zhao M, Jasser SA, Younes MN, Sano D, et al. Reciprocal negative regulation between S100A7/psoriasin and beta-catenin signaling plays an important role in tumor progression of squamous cell carcinoma of oral cavity. Oncogene. 2008;27:3527–3538. doi: 10.1038/sj.onc.1211015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.