Abstract
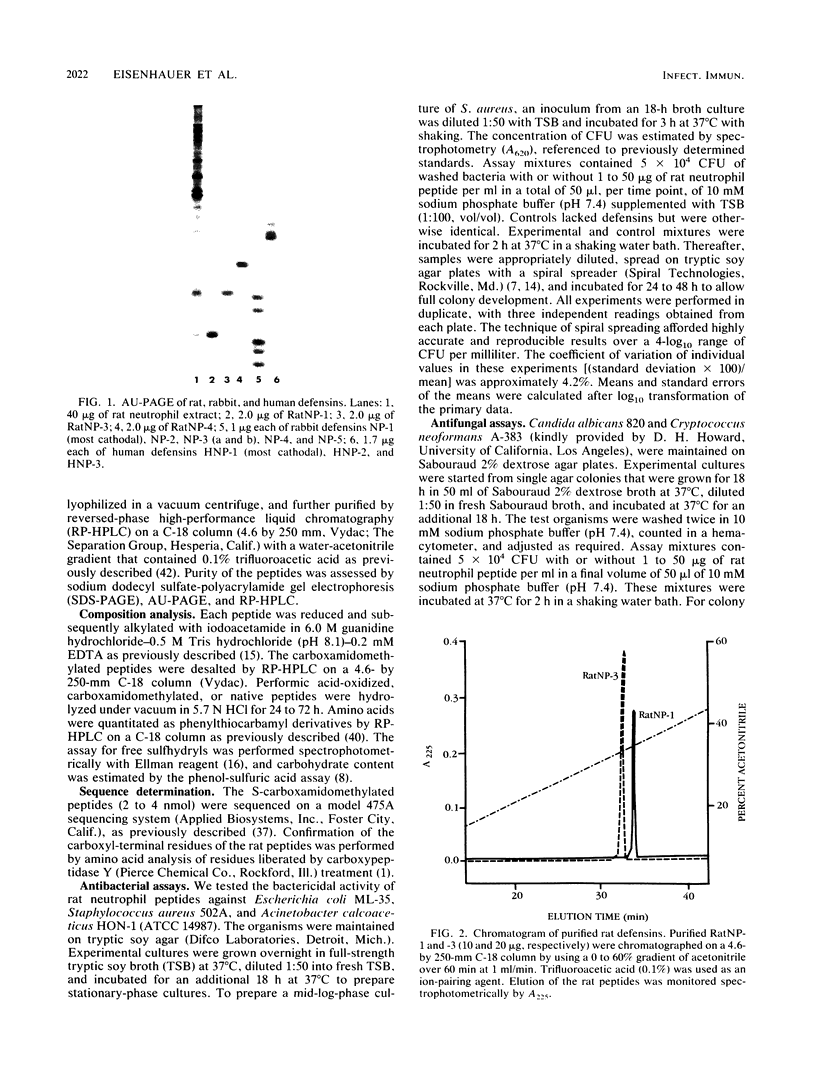
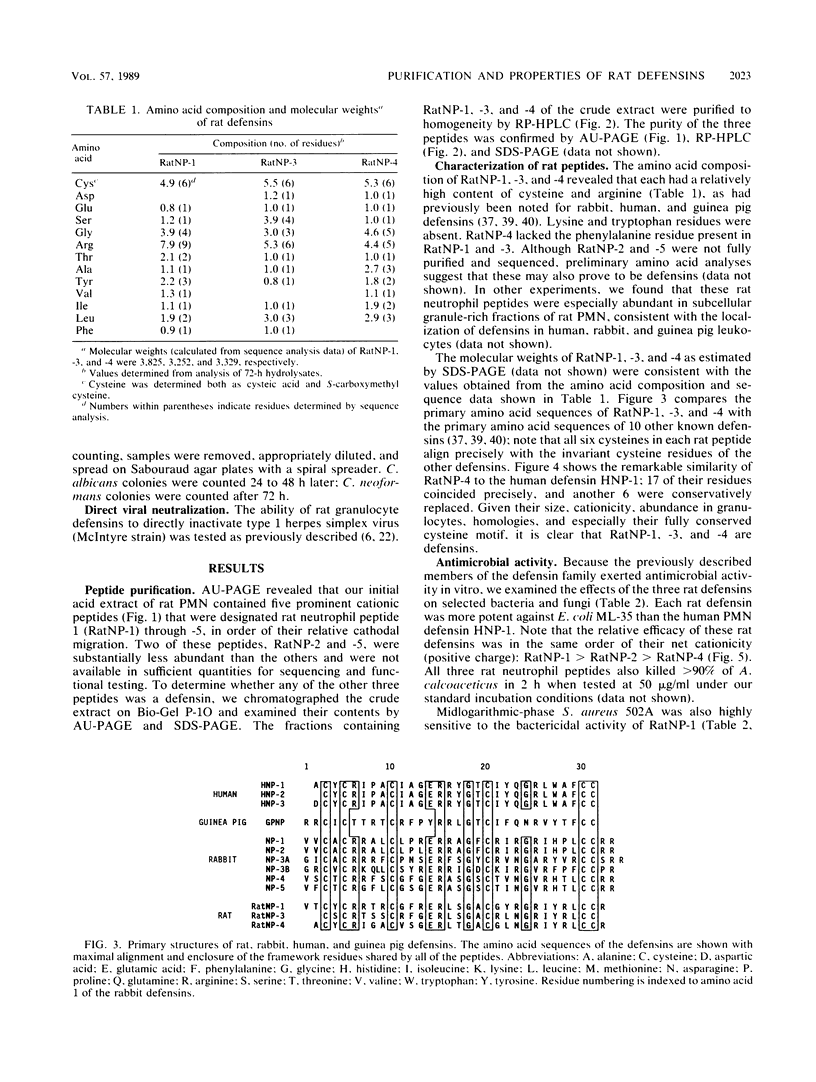
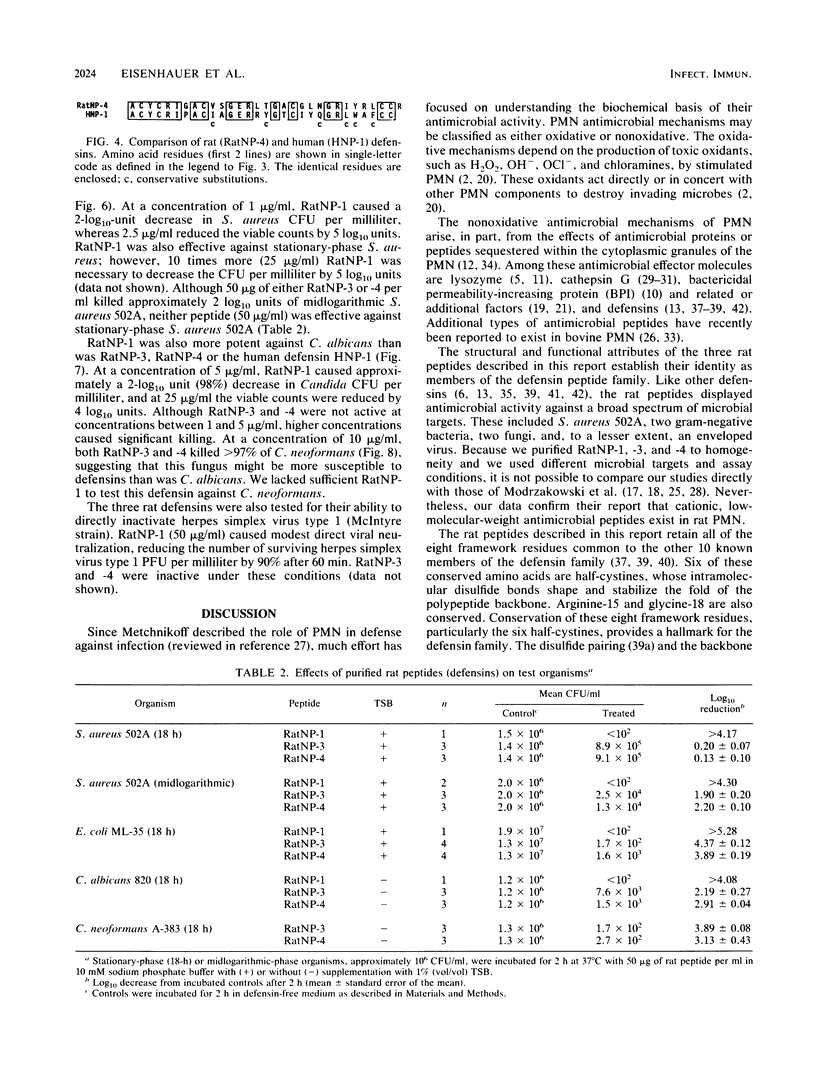
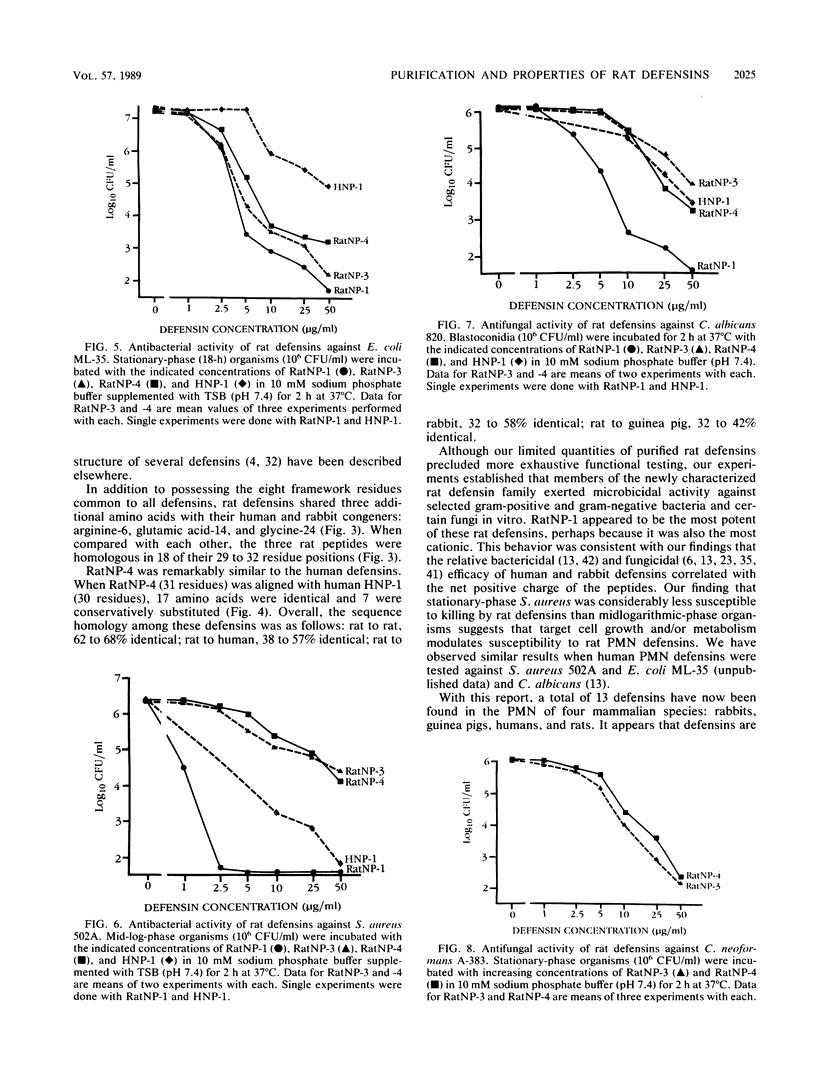
Three cysteine-rich cationic peptides, designated RatNP-1, RatNP-3, and RatNP-4, were purified from an acid extract of rat polymorphonuclear neutrophils, sequenced, and tested for antimicrobial activity. The peptides ranged from 29 to 32 amino acids in length (Mr, 3,252 to 3,825), and each contained all eight invariantly conserved "framework" residues that are characteristic of defensins. Each of the peptides killed Escherichia coli ML-35, Acinetobacter calcoaceticus HON-1, Staphylococcus aureus 502A, and Candida albicans 820 in vitro. RatNP-1, the most cationic rat defensin, was also the most potent. With this report, a total of 13 distinct defensins have been characterized in the polymorphonuclear leukocytes of four mammalian species. The existence of the defensin system in rats should facilitate investigations of the in vivo role of defensins in experimental infections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach A. C., 2nd, Selsted M. E., Pardi A. Two-dimensional NMR studies of the antimicrobial peptide NP-5. Biochemistry. 1987 Jul 14;26(14):4389–4397. doi: 10.1021/bi00388a030. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Pappagianis D. Inhibition by lysozyme of growth of the spherule phase of Coccidioides immitis in vitro. Infect Immun. 1974 Sep;10(3):616–623. doi: 10.1128/iai.10.3.616-623.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher K. A., Selsted M. E., Lehrer R. I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986 Dec;60(3):1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly C. B., Gilchrist J. E., Peeler J. T., Campbell J. E. Spiral plate count method for the examination of raw and pasteurized milk. Appl Environ Microbiol. 1976 Jul;32(1):21–27. doi: 10.1128/aem.32.1.21-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. A reevaluation of the roles of the O2-dependent and O2-independent microbicidal systems of phagocytes. Rev Infect Dis. 1983 Sep-Oct;5(5):843–853. doi: 10.1093/clinids/5.5.843. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Gadebusch H. H., Johnson A. G. Natural host resistance to infection with Cryptococcus neoformans. IV. The effect of some cationic proteins on the experimental disease. J Infect Dis. 1966 Dec;116(5):551–565. doi: 10.1093/infdis/116.5.551. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Antimicrobial activity of phagocyte granule proteins. Semin Respir Infect. 1986 Jun;1(2):107–117. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist J. E., Donnelly C. B., Peeler J. T., Campbell J. E. Collaborative study comparing the spiral plate and aerobic plate count methods. J Assoc Off Anal Chem. 1977 Jul;60(4):807–812. [PubMed] [Google Scholar]
- Hodinka R. L., Modrzakowski M. C. Bactericidal activity of granule contents from rat polymorphonuclear leukocytes. Infect Immun. 1983 Apr;40(1):139–146. doi: 10.1128/iai.40.1.139-146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Modrzakowski M. C. Granule contents from rat polymorphonuclear leukocytes: antimicrobial properties and characterization. Can J Microbiol. 1986 Jun;32(6):498–504. doi: 10.1139/m86-091. [DOI] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Association of lactoferrin with lysozyme in granules of human polymorphonuclear leukocytes. Infect Immun. 1972 Nov;6(5):761–765. doi: 10.1128/iai.6.5.761-765.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Daher K., Ganz T., Selsted M. E. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985 May;54(2):467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E., Babior B. M., Curnutte J. T. Neutrophils and host defense. Ann Intern Med. 1988 Jul 15;109(2):127–142. doi: 10.7326/0003-4819-109-2-127. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Ganz T., Selsted M. E. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985 Jul;49(1):207–211. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M. J., Modrzakowski M. C. Antimicrobial mechanisms against Acinetobacter calcoaceticus of rat polymorphonuclear leukocyte granule extract. Infect Immun. 1988 Mar;56(3):552–556. doi: 10.1128/iai.56.3.552-556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffelholz M. J., Modrzakowski M. C. Isolation of cationic peptides from rat polymorphonuclear leukocyte granule contents using fast protein liquid chromatography. Anal Biochem. 1986 Nov 1;158(2):377–381. doi: 10.1016/0003-2697(86)90564-6. [DOI] [PubMed] [Google Scholar]
- Marzari R., Scaggiante B., Skerlavaj B., Bittolo M., Gennaro R., Romeo D. Small, antibacterial and large, inactive peptides of neutrophil granules share immunoreactivity to a monoclonal antibody. Infect Immun. 1988 Aug;56(8):2193–2197. doi: 10.1128/iai.56.8.2193-2197.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrzakowski M. C., Dosch-Meier D., Hodinka R. L. Effect of rat polymorphonuclear leukocyte granule components on the growth and survival of Pseudomonas aeruginosa and Salmonella typhimurium. Can J Microbiol. 1983 Oct;29(10):1339–1343. doi: 10.1139/m83-208. [DOI] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Antibacterial activity of cationic proteins from human granulocytes. J Clin Invest. 1975 Nov;56(5):1118–1124. doi: 10.1172/JCI108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeberg H., Olsson I. Mechanisms for the microbicidal activity of cationic proteins of human granulocytes. Infect Immun. 1976 Dec;14(6):1269–1275. doi: 10.1128/iai.14.6.1269-1275.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I., Venge P. Cationic proteins of human granulocytes. II. Separation of the cationic proteins of the granules of leukemic myeloid cells. Blood. 1974 Aug;44(2):235–246. [PubMed] [Google Scholar]
- Pardi A., Hare D. R., Selsted M. E., Morrison R. D., Bassolino D. A., Bach A. C., 2nd Solution structures of the rabbit neutrophil defensin NP-5. J Mol Biol. 1988 Jun 5;201(3):625–636. doi: 10.1016/0022-2836(88)90643-2. [DOI] [PubMed] [Google Scholar]
- Romeo D., Skerlavaj B., Bolognesi M., Gennaro R. Structure and bactericidal activity of an antibiotic dodecapeptide purified from bovine neutrophils. J Biol Chem. 1988 Jul 15;263(20):9573–9575. [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Lehrer R. I. Primary structures of MCP-1 and MCP-2, natural peptide antibiotics of rabbit lung macrophages. J Biol Chem. 1983 Dec 10;258(23):14485–14489. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide. J Biol Chem. 1989 Mar 5;264(7):4003–4007. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S. Purification, primary structure, and antimicrobial activities of a guinea pig neutrophil defensin. Infect Immun. 1987 Sep;55(9):2281–2286. doi: 10.1128/iai.55.9.2281-2286.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Ganz T., Lehrer R. I. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985 Jul;49(1):202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Lehrer R. I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun. 1984 Jul;45(1):150–154. doi: 10.1128/iai.45.1.150-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]