Oral medications for MS present new treatment and cost-management challenges.
Abstract
The entry of oral medications for multiple sclerosis into the marketplace and the high cost of biologic drugs are presenting new challenges for stakeholders.
Three years ago, the mantra for managing multiple sclerosis (MS) was collaboration, highlighted in the first edition of the Multiple Sclerosis Trend Report, sponsored by Teva Neuroscience and the National Multiple Sclerosis Society (NMSS). Although collaboration among insurers, specialty pharmacies, and physicians is still critical, attention has now turned to the impact of oral medications, such as fingolimod (Gilenya), which hit the market last September; the predominance of coinsurance for biotech drugs; the rising costs of MS drugs; and targeting the right person with the right therapy.
The cost of managing MS is disproportionate to the number of persons with the disease, raising eyebrows among stakeholders. Jeff Januska, PharmD, director of pharmacy for CenCal Health, a California Medicaid plan, says that less than 1 percent of his plan members receive prescriptions for specialty pharmacy medications, including prescriptions for MS, but they account for 20 percent of the plan’s drug spend.
The second edition of the Multiple Sclerosis Trend Report explores trends in managing MS, including the impact of the new oral treatment options. The report also provides insight into the viewpoints of diverse stakeholders — MCOs, specialty pharmacies, physicians, and employers.
New oral therapies
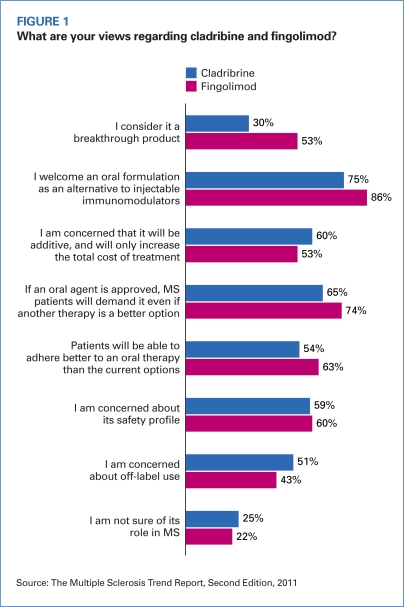
Of the 109 MCOs surveyed, 53 percent said they were aware of fingolimod, the first oral MS therapy to be approved by the U.S. Food and Drug Administration, and 86 percent indicated that they welcomed an oral formulation as an alternative to the injectable immunomodulators (Figure 1, page 22). The majority of respondents said they anticipate that patients will demand the oral formulation even if an immunomodulator is a better option, and 63 percent said they expect improved adherence to therapy with fingolimod because of its formulation. Cladribine, an oral contender from Merck KGaA, generated more concern among respondents because of its potential contribution to higher costs and off-label use. The FDA denied Merck KGaA’s marketing application for cladribine in March, asking the manufacturer for more data about the drug’s risk/benefit balance and safety.
FIGURE 1.
What are your views regarding cladribine and fingolimod?
Source: The Multiple Sclerosis Trend Report, Second Edition, 2011
Laquinimod, a once-daily oral immunomodulator from Teva Pharmaceuticals, is expected to be available in late 2012, based on positive results of a phase 3 trial. Biogen’s BG-12, a twice-daily immunomodulator, also is moving closer to FDA approval in light of its phase 3 trial.
Brendan O’Grady, vice president and head of managed markets for Teva North America Brand Pharmaceuticals, foresees a threefold impact of oral MS drugs: 1) the potential to expand the number of patients who can be treated for MS but who may have shied away from an injectable disease-modifying therapy (DMT); 2) an opportunity for concurrent drug use with an oral and an injectable DMT; and 3) potential payer challenges to premium pricing of oral products. Fingolimod’s cost is about 15 percent higher than its injectable counterparts.
Any one of these considerations has the potential to lead to increased treatment costs, O’Grady says. “With so much variability in patient response to MS drugs and with different manifestations of the disease, oral medications will definitely not phase out injectables.”
Mixed reactions
The attraction of the convenience and of the new oral drugs and their potential to improve adherence comes as no surprise. But the jury is still out on how quickly providers will prescribe fingolimod because of concerns about its safety profile.
Irene Girgis, PharmD, director of pharmacy services at Colorado Access, a not-for-profit, Denver-based health plan, is underwhelmed by the potential for uptake of orals, including dalfampridine (Ampyra), a non-DMT that improves the mobility of MS patients. If patients remain stable on their injectables, she says, they may not want to try an oral drug.
The convenience of the new oral MS drugs is tempting. But with no long-term safety data to back them up, providers may decide to wait.
“Providers seem to be taking a conservative approach,” notes Girgis. She believes that the lack of long-term clinical studies and medical data to back use of oral MS drugs — not cost — is the main barrier to their adoption.
On the other hand, Brad Curtis, MD, vice president and medical director at Prescription Solutions, a pharmacy benefit manager headquartered in Irvine, Calif., anticipates a rapid uptake of the oral MS drugs. Curtis acknowledges there will be some initial caution about safety and side-effect profiles, as would be expected with the conservative prescribing approach many physicians take with the launch of a new therapeutic agent.
Nicholas G. LaRocca, PhD, vice president for health care delivery and policy research for NMSS, says it’s not clear how the new oral drugs will fit into the treatment landscape for MS. Their premium price, he says, may slow down acceptance, but there should be a gradual shift toward their use if their safety profiles hold up. Like many of his peers, LaRocca agrees that if a drug works for a particular patient, a prescriber may be reluctant to change to another medication.
Kent Rogers, vice president of managed markets at Hawthorn, N.Y.-based Acorda Therapeutics, which markets dalfampridine, says he is seeing payers apply traditional cost-containment approaches to MS, such as specialty tiers and high copayments, that may limit utilization. “Payers may not understand how improving the walking ability of MS patients can improve their lives,” Rogers says, calling for a “deeper dialogue” among payers, employers, and pharmaceutical companies to consider not only drug cost but also the quality of life of MS patients.
Neurologists surveyed also expressed concern expressed concern about the safety profiles of cladribine and fingolimod. One in five believe that both drugs have the potential to increase MS treatment costs without improving patient outcomes.
Sharing costs
As payers transition from co-payments to coinsurance for specialty drugs, O’Grady says there is concern about the impact of higher out-of-pocket costs on adherence to medications. “If patients don’t comply or drop off a drug, then payers aren’t getting the value from their investment in managing the condition,” he says.
Even for patients who have insurance, it has been estimated that the annual out-of-pocket expense for MS treatment is more than $3,000, according to one study (Joyce 2008).
Curtis concurs that higher out-of-pocket costs are likely to have an adverse effect on compliance. “There is certainly less value to MS drugs if they are not taken consistently,” he says. Prescription Solutions’ disease therapy management program, which combines disease self-management with medication therapy management, includes periodic telephone consultations, educational materials, and a personalized care plan for both members and their physicians, and will direct members toward financial assistance programs, if needed. According to the PBM’s own data, members who are enrolled in its structured, seven-month program remain significantly more adherent to therapy than those who fill their prescriptions at a retail pharmacy. A Prescription Solutions study demonstrated greater duration of therapy among enrollees than either those who filled prescriptions at a retail or a specialty pharmacy but did not participate in the program. Participants had a 33 percent lower rate of relapse, along with corresponding reductions in medical costs, compared with nonenrollees.
Januska, at CenCal Health, says his plan currently places DMTs on the fourth tier with a $100 copayment, but he anticipates the addition of a fifth tier for nonpreferred agents, with a 10 percent coinsurance attached.
Employers, too, are concerned about high out-of-pocket maximums and other cost-sharing devices that can challenge a worker’s ability to afford treatment, especially biologic drugs. Their worry is that inadequate treatment will affect productivity.
There is some relief at hand — at least for Medicare Part D beneficiaries. Health reform sets the stage for eliminating the “donut hole” by 2012. This provision will apply not only to primary therapies for MS, but also to medications for controlling secondary problems, such as fatigue, pain, and incontinence.
Prior authorization tops management tools
Health plans customarily put utilization management strategies in place to ensure that the right drugs get to patients at the right time. In the case of high-cost therapies like the MS drugs, the need for such policies is even more critical. As therapeutic options continue to expand, utilization strategies such as prior authorization (PA), restricted networks, step therapy, and therapeutic interchange are likely to evolve.
The MCOs surveyed use a variety of utilization- and cost-management tools for MS drugs. PA topped the list at 58 percent; 47 percent limit the use of immunomodulators to FDA-approved indications; 41 percent place dosage limits; and 38 percent set quantity limits (Table). Eighteen percent of plans use step therapy in MS, and therapeutic interchange is rare. Fourteen percent of plans have no restrictions for the six drugs used for relapsing or remitting MS — glatiramer acetate injection (Copaxone), natalizumab (Tysabri), and the interferons (Avonex, Extavia, Betaseron, and Rebif).
TABLE.
For the following immunomodulators, which restrictions are currently in place for a majority of your members?
| Avonex | Betaseron | Copaxone | Extavia | Rebif | Tysabri | |
|---|---|---|---|---|---|---|
| Prior authorization | 61% | 58% | 56% | 53% | 56% | 66% |
| Limit use to FDA-approved indications | 51% | 47% | 48% | 40% | 47% | 48% |
| Quantity limits | 42% | 40% | 42% | 37% | 39% | 29% |
| Dosage limits | 47% | 46% | 46% | 35% | 42% | 30% |
| Restricted pharmacy network | 31% | 29% | 30% | 27% | 30% | 25% |
| Prescribing restricted to specialist | 23% | 24% | 25% | 23% | 24% | 31% |
| No restrictions | 16% | 16% | 20% | 11% | 15% | 6% |
| Step therapy | 12% | 19% | 9% | 19% | 11% | 19% |
| Not covered | 5% | 6% | 2% | 17% | 6% | 11% |
| Therapeutic interchange | 2% | 6% | 2% | 6% | 2% | 2% |
| Not applicable | 2% | 1% | 2% | 4% | 4% | 8% |
Trade names: Avonex (interferon beta-1a intramuscular injection); Betaseron (interferon beta-1b subcutaneous injection); Copaxone (glatiramer acetate injection); Extavia (interferon beta-1b subcutaneous injection); Rebif (interferon beta-1a subcutaneous injection); Tysabri (natalizumab). Source: The Multiple Sclerosis Trend Report, Second Edition, 2011
Specialty pharmacies and PBMs see PA as the most widely used strategy for controlling drug costs and usage, while quantity limits and a restricted pharmacy network are also commonly used.
“We see the use of step therapy as another emerging trend, especially as the market basket of MS drugs grows,” says Teva’s O’Grady. PA, he says, is the first stage of step therapy, which can help ensure that the right patient receives the right diagnosis and gets the right drug. “In the past, payers have been reluctant to force patients to use one drug over another and, outside of formulary tiering, have left that choice to physicians and their patients. We see that beginning to change.”
Girgis says that Colorado Access has phased out PA as a management tool because there is little potential for abuse of MS drugs; however, the plan does put quantity limits in place to prevent inappropriate use. “We want to cover the most optimal product, not necessarily the least expensive,” she says. “If a patient responds well to a specific drug, its use would ideally decrease costs in the long run by preventing adverse episodes that would require attention and money.”
CenCal places step therapy only on natalizumab but subjects all DMTs to PA with periodic renewal criteria. “We want to review a member’s use of a drug to ensure it is still effective,” Januska says. The health plan also utilizes quantity limits (a one-month supply) so that “we have a contact point with members to review persistence and adherence.”
At Anthem Blue Cross of California, fingolimod falls on the third or fourth tier (specialty tier) and requires PA, as do all first-line DMTs.
Using specialty pharmacy
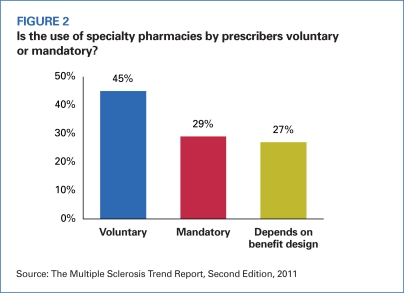
Based on responses from MCOs, 74 percent of health plans use specialty pharmacies (SPs) to distribute and manage MS drugs. Among those that use a specialty pharmacy provider, 39 percent mandate SP use for MS therapies; 61 percent place their use on a voluntary basis (Figure 2). MCOs that mandate the use of an SP say their reasons for doing so are improved economic and clinical outcomes, comprehensive fulfillment, disease management services, better contracts, and clinical recommendations.
FIGURE 2.
Is the use of specialty pharmacies by prescribers voluntary or mandatory?
Source: The Multiple Sclerosis Trend Report, Second Edition, 2011
From the SP and PBM perspectives, respondents said that 7 out of 10 of their contracted MCOs require the use of an SP for MS immunomodulators. About one fifth of their MCO clients simply offer an SP option.
O’Grady sees an increased push toward mandating the use of SPs, which he feels provide important services such as sophisticated quality-measurement tools, back-end monitoring, fulfillment down to a science, 90-day compliance calls, and clinical assistance — all leading to better outcomes and cost savings.
“Payers are afraid to force members into a particular channel of distribution,” he says, “but they will start to mandate use if not enough members are doing it.”
Although the Centers for Medicare and Medicaid Services allows Medicare beneficiaries to buy specialty products from any pharmacy, Girgis says that Colorado Access encourages members to take advantage of specialty pharmacies, which also provide support services.
Concurrent treatment
Fifty-eight percent of respondents said their organizations allow concurrent treatment with more than one FDA-approved immunomodulating drug. In addition, 55 percent of respondents said they impose no restrictions on choices, while 12 percent agreed that clinical guidelines, FDA approval, and mainstream literature may influence restrictions. Twenty-one percent of respondents said they designate certain combinations that may be used, while other respondents said they consider requests on a case-by-case basis, or rely on PA or on the judgment of a neurologist.
Many survey participants agreed that there is insufficient evidence to support concurrent therapies. Anthem Blue Cross of California does not approve of combination treatment with FDA-approved immunomodulating drugs for MS.
Employers debate MS concern
While Larry Boress does not speak for all employers, he is wired into the needs of the 100+ members of the Midwest Business Group on Health, whose participating companies purchase benefits for more than 3 million lives. Not mincing words, Boress, the group’s president and CEO, says that MS is not a top concern of most employers, although he acknowledges that its effect on older adults is pressing.
The Multiple Sclerosis Trend Report highlights the results of an employer roundtable in which Boress participated. The seven panelists agreed that investing in an employee with MS is worth it if it keeps a skilled worker on the job and off disability rather than paying the cost of recruiting, hiring, and training a replacement. The panelists also agreed that if the use of combination therapy for MS becomes more prevalent, health plans will be compelled to evaluate the efficacy of this approach with more precision. Panelists also expressed interest in learning how concurrent medications can influence productivity, absenteeism, and medical costs at the workplace as their use increases.
Of the MCOs surveyed, respondents said that plan sponsors have various concerns with regard to MS in the workplace: increased medical expenses as the disease progresses (57%); loss of productivity (40%); absenteeism (38%); and prevention of early disability (36%).
“Employers with a heightened appreciation and understanding of the impact of MS on employees are the ones who make the connection between managing the disease and increasing productivity,” says Jon Congleton, senior vice president and general manager at Teva Neuroscience.
Lack of providers
Some 136 practicing neurologists or MS specialists responded to the survey. Although most of the participants were general neurologists, management of MS is clearly a central concern for neurologists across a broad range of subspecialties and practice settings.
Respondents identified significant challenges in the treatment of MS, including the tolerability of medications, the achievement of treatment goals, and payment and reimbursement issues. Nearly two thirds of the respondents said payers implement procedures to restrict the use of a DMT, and 15 percent said they always or nearly always have difficulty obtaining reimbursement for infused DMTs, such as natalizumab.
“One of the big concerns we have is that it’s getting harder and harder to treat MS, and fewer young doctors seem to be going into the field,” says David Brandes, MD, director of the Hope MS Center, in Knoxville, Tenn. He cites two significant barriers to entry for younger doctors: 1) difficulty in treating MS because there are so many different manifestations of the disease that need to be treated and medications from which to choose and 2) relatively poor reimbursement for the amount of time needed to properly evaluate and treat MS patients.
Only 33 percent of neurologists surveyed said that more than 75 percent of their patients attained the therapeutic goal of preventing relapses after one year, and only 22 percent noted that disease progression was slowed in three quarters of their patients.
LaRocca concurs that not only is there a limited number of neurologists but that only a small number specialize in MS. “It is difficult for neurologists to bill for the time spent managing a complex disorder like MS compared with, for example, a cardiologist, who can bill for procedures such as an EKG. It is important for our industry to understand what will motivate physicians to go into neurology.”
It will be interesting to see how quickly oral MS drugs make their way into the marketplace and how readily they will be accepted by prescribers and covered by health plans under their formularies. What is clear, though, is the impact that expensive specialty drugs — whether oral, injected, or infused — and increased cost-sharing will have on patients’ pocketbooks and on their adherence to medications.
Although step therapy is on the increase as an effective management tool for MS drugs, prior authorization heads the list of the most used methods to control cost and utilization. The new oral offerings may change the landscape.
Footnotes
For a copy of The Multiple Sclerosis Trend Report: Perspectives from Managed Care, Providers and Employers, visit www.mstrendreport.com.
Reference
- Joyce GF, Goldman DP, Karaca-Mandic P, Lawless GD. Impact of specialty drugs on the use of other medical services. Am J Manag Care. 2008;14:821–828. [PMC free article] [PubMed] [Google Scholar]