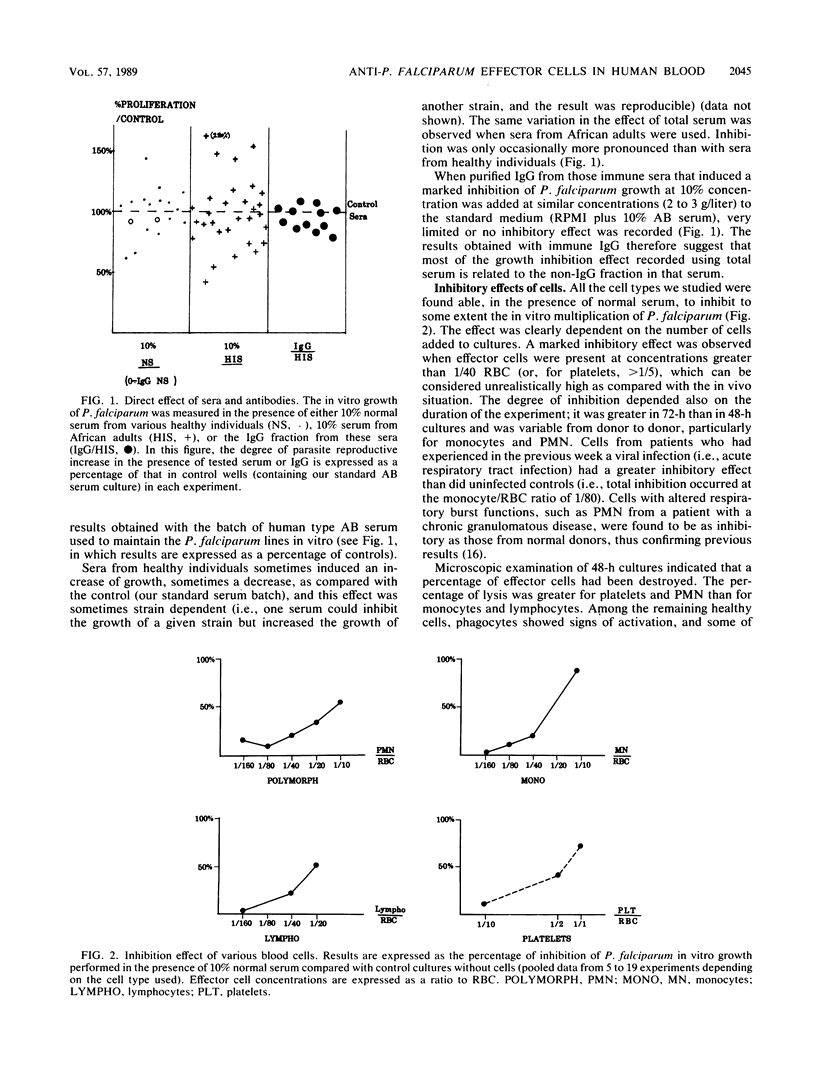
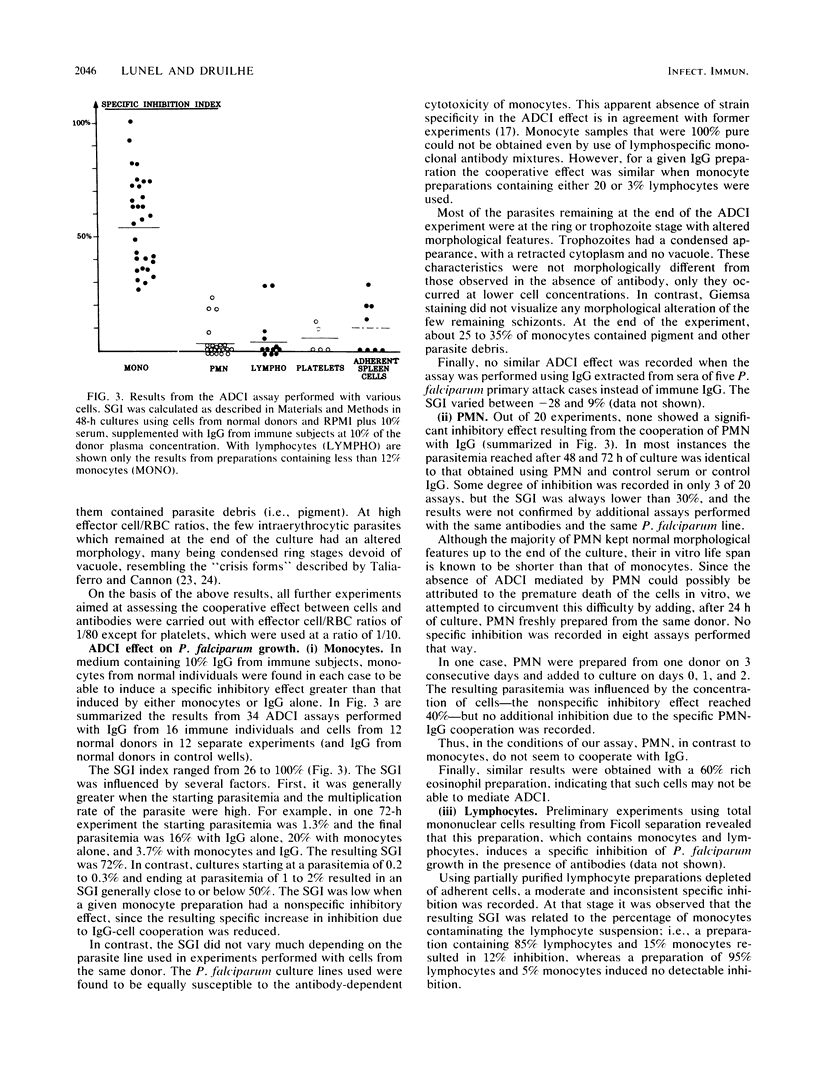
Abstract
We have evaluated in in vitro conditions the possible cooperative effect of antimalarial antibodies with several human blood cell types. When used alone, immunoglobulin G from African adults who had reached a state of premunition against malaria was found to have no or very limited direct effect on invasion and multiplication of P. falciparum asexual blood stages. In contrast, these antibodies induced a marked specific inhibition of parasite growth in the presence of normal blood monocytes, and the inhibition did not appear to be strain dependent. No similar antibody-dependent cellular inhibitory effect was found using human blood polymorphonuclear leukocytes, lymphocytes, platelets, or adherent spleen cells. However, these cells could all exert in vitro some non-antibody-dependent inhibitory effect when present at high effector/target cell ratios.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Brown G. V., Anders R. F., Knowles G. Differential effect of immunoglobulin on the in vitro growth of several isolates of Plasmodium falciparum. Infect Immun. 1983 Mar;39(3):1228–1235. doi: 10.1128/iai.39.3.1228-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., Greenwood B. M., Terry R. J. Cellular mechanisms involved in recovery from acute malaria in Gambian children. Parasite Immunol. 1986 Nov;8(6):551–564. doi: 10.1111/j.1365-3024.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Brown J., Smalley M. E. Inhibition of the in vitro growth of Plasmodium falciparum by human polymorphonuclear neutrophil leucocytes. Clin Exp Immunol. 1981 Oct;46(1):106–109. [PMC free article] [PubMed] [Google Scholar]
- Brown J., Smalley M. E. Specific antibody-dependent cellular cytotoxicity in human malaria. Clin Exp Immunol. 1980 Sep;41(3):423–429. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Chulay J. D., Aikawa M., Diggs C., Haynes J. D. Inhibitory effects of immune monkey serum on synchronized Plasmodium falciparum cultures. Am J Trop Med Hyg. 1981 Jan;30(1):12–19. doi: 10.4269/ajtmh.1981.30.12. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guguen-Guillouzo C., Campion J. P., Brissot P., Glaise D., Launois B., Bourel M., Guillouzo A. High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol Int Rep. 1982 Jun;6(6):625–628. doi: 10.1016/0309-1651(82)90187-4. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Hoffman S. L., Boland M. T., Akood M. A., Laughlin L. W., Kurniawan L., Marwoto H. A. Comparison of immunity to malaria in Sudan and Indonesia: crisis-form versus merozoite-invasion inhibition. Proc Natl Acad Sci U S A. 1984 Feb;81(3):922–925. doi: 10.1073/pnas.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Jepsen S. Enhanced inhibition of in vitro multiplication of Plasmodium falciparum by stimulated human polymorphonuclear leucocytes. Clin Exp Immunol. 1984 Aug;57(2):287–292. [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A., Jepsen S., Valerius N. H. Polymorphonuclear leucocytes defective in oxidative metabolism inhibit in vitro growth of Plasmodium falciparum. Evidence against an oxygen-dependent mechanism. Scand J Immunol. 1984 Jul;20(1):93–96. doi: 10.1111/j.1365-3083.1984.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P. Cooperation between antibodies and monocytes that inhibit in vitro proliferation of Plasmodium falciparum. Infect Immun. 1983 Jul;41(1):219–223. doi: 10.1128/iai.41.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khusmith S., Druilhe P., Gentilini M. Enhanced Plasmodium falciparum merozoite phagocytosis by monocytes from immune individuals. Infect Immun. 1982 Mar;35(3):874–879. doi: 10.1128/iai.35.3.874-879.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGREGOR I. A. THE PASSIVE TRANSFER OF HUMAN MALARIAL IMMUNITY. Am J Trop Med Hyg. 1964 Jan;13:SUPPL–239. doi: 10.4269/ajtmh.1964.13.237. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Reese R. T., Motyl M. R. Inhibition of the in vitro growth of Plasmodium falciparum. I. The effects of immune serum and purified immunoglobulin from owl monkeys. J Immunol. 1979 Oct;123(4):1894–1899. [PubMed] [Google Scholar]
- Vande Waa J. A., Jensen J. B., Akood M. A., Bayoumi R. Longitudinal study on the in vitro immune response to Plasmodium falciparum in Sudan. Infect Immun. 1984 Aug;45(2):505–510. doi: 10.1128/iai.45.2.505-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



