Abstract
Dopamine is an important catecholamine neurotransmitter modulating many physiological functions, and is linked to psychopathology of many diseases such as schizophrenia and drug addiction. Dopamine D1 and D2 receptors are the most abundant dopaminergic receptors in the striatum, and although a clear segregation between the pathways expressing these two receptors has been reported in certain subregions, the presence of D1-D2 receptor heteromers within a unique subset of neurons, forming a novel signaling transducing functional entity has been shown. Recently, significant progress has been made in elucidating the signaling pathways activated by the D1-D2 receptor heteromer and their potential physiological relevance.
Background
Dopamine plays a key role in the regulation of various physiological functions of normal brain including reward, locomotion, behavior, learning, and emotion. It is not then surprising that the dysregulation of the dopaminergic system has been linked to pathophysiology of many diseases, such as Alzheimer's disease, schizophrenia, Parkinson's disease, attention deficit hyperactivity disorder, depression and drug addiction [1-3], leading to the clinical use of drugs that target dopamine neurotransmission in the treatment of these disorders.
Five subtypes of dopamine receptors (D1R-D5R), belonging to the G-protein-coupled receptor (GPCR) superfamily have been cloned, through which dopamine transduces its various effects. Dopamine receptors are subdivided into D1-like (D1, D5) and D2-like (D2, D3, D4) receptor subclasses [1-3], with the D1 and D2 receptors being the major subtypes. The most studied dopamine signaling pathway is the modulation of cyclic AMP production, with D1-like receptors activating cyclic AMP production through Gs/olf, and D2-like receptors inhibiting adenylyl cyclase (AC) activity through Gi/o proteins [2]. This results in a bidirectional modulation of this pathway and related proteins, such as protein kinase A (PKA) and DARPP-32 (dopamine and cAMP regulated protein) [4]. Other important dopamine signaling pathways have also been reported, including the modulation of the Akt-GSK3 pathway [5] and the activation of the PAR4 signaling pathway [6].
For some actions of dopamine, such as the control of motor behavior [7] or dopamine-mediated reward processes in nucleus accumbens [8], a concomitant stimulation of D1 and D2 receptors is required, a phenomenon known as the "requisite" D1/D2 synergism [9]. In this type of synergism, D1 and D2 receptor-specific drugs potentiate the effect exerted by each other when delivered together, but are ineffective when administered separately [9]. The combined, but not separate, administration of a selective D1 and a selective D2 agonist was shown to be necessary for the dopamine-stimulated expression of immediate-early gene c-fos in striatal neurons [10] and in electro-physiological studies where both receptors were indeed responsible for GABA release in striatum [11]. The participation of both D1 and D2 receptors was also required for evoking neural and behavioral sensitization to cocaine [12] and for evoking the changes in behavior and basal ganglia output [13,14]. All these observations are other evidence for the presence of not only a synergism between dopamine D1 and D2 receptors, but an obligatory participation of both receptors to generate this synergism.
One explanation for how the well documented synergistic effects seen between D1 and D2 receptors [15,16] may be achieved is through the formation of heterooligomers between the two receptors, as it has been shown for many GPCRs [17-19]. Dopamine receptors, all subtypes included, in addition to their ability to exist as homomers, were shown to form different heteromeric complexes with other receptors (reviewed in 20). The presence of D1-D2 receptor heteromers with unique functional properties was first shown in transfected cells using different methods [21-24] as described below. Initially, the notion of heteromerization observed for many GPCRs and its functional relevance was not completely clear in physiological conditions and was in some cases regarded with a degree of skepticism, but at least for the D1-D2 receptor heteromer we have shown evidence of occurrence under physiological conditions in native tissues with emerging important functional relevance.
For D1 and D2 receptors, the presence of two anatomically segregated sets of neurons, forming the striatonigral D1-enriched direct pathway and the striatopallidal D2-enriched indirect pathway is commonly recognized, with D1R localizing to the dynorphin (DYN)-expressing neurons, and D2R localizing to the enkephalin (ENK)-expressing neurons [25,26]. Recent studies emanating from fluorophore-tagged promoter elements of D1R and D2R in bacterial artificial chromosome (BAC) transgenic mice [27] allowed an evaluation of the proportions of striatal neurons expressing D1R, D2R, or both [28-32]. There were, however, variations in the levels of expression of EGFP between one line and another [32], resulting in incomplete labeling of a significant proportion of striatal medium spiny neurons (MSNs) [28]. While this method supported the segregation between the D1-enriched direct pathway and the striatopallidal D2-enriched indirect pathway, a certain fraction of MSNs (~17%) expressing both receptors was predicted in the NAc shell, whereas only ~5-6% of MSNs were calculated to co-express both receptors in the dorsal striatum [30-32]. These BAC-calculated colocalization data are consistent with our data and the numerous other reports indicating a colocalization of D1R and D2R in neurons in culture or in situ with higher D1R and D2R co-localization observed in cultured striatal neurons (60 to 100%) than in the adult striatum [33-40].
Presence of dopamine D1-D2 receptor heteromers in brain
Several reports indicated the presence of a D1-like receptor activating IP3 production and/or increasing intracellular calcium in neurons in culture or slices from different brain regions, including striatum, hippocampus, and cortex [41-44]. However, the cloned D1R was devoid of such effects when expressed in different host cells (reviewed in 17 and 20) and persisted in a D1 receptor null mouse model [45]. We then demonstrated that dopamine D1 and D2 receptors form functional heterooligomeric complexes in cells and in vivo [21-23,40,46] and that the mobilization of intracellular calcium was in fact a unique signaling pathway resulting from the activation of this D1-D2 heteromeric receptor complex [21,23,40].
The presence of the D1-D2 receptor heteromer was demonstrated by different techniques including coimmunoprecipitating both receptors from rat striatum, as well as from cells coexpressing D1R and D2R [21,40], and by different methodologies using the fluorescence resonance energy transfer (FRET) technique in cells [22,24], in striatal neurons [40,47] and different brain regions [40,46].
Interestingly, in adult rat brain, coexpressed dopamine D1 and D2 receptors were present in a unique subset of neurons coexpressing both DYN and ENK neuropeptides in different brain regions, including nucleus accumbens (NAc), caudate-putamen (CP), ventral pallidum, globus pallidus (GP), and entopeduncular nucleus [46], with some inter-regional variation. The lowest proportion (~6-7%) of D1R-expressing neurons that coexpress D2R was shown in the CP [40,46], whereas the highest proportion (~59%) of D1R-expressing neurons that coexpress D2R was observed in GP [46]. A substantial number (~20-30%) of D1R neurons that coexpress D2R was also observed in NAc [40,46], consistent with the anatomical findings resulting from BAC transgenic mice [30-32].
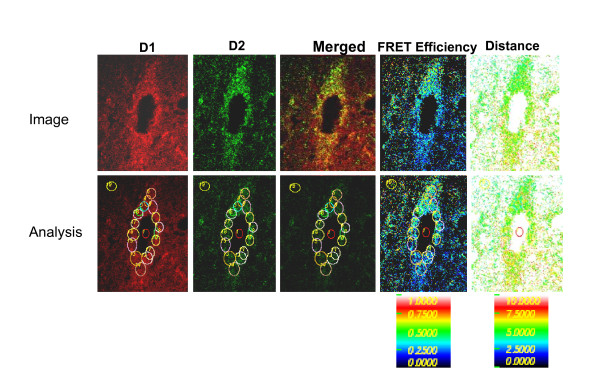
The direct interaction of D1R and D2R to form heteromers in brain was shown by confocal FRET technique using two methodologies [40,46,47]. The confocal FRET technique demonstrated clearly and directly the presence of the D1-D2 receptor heteromer in striatal neurons [40,47] and in brain in situ [40,46]. In NAc, acceptor photobleaching-based FRET showed a high FRET efficiency of ~21% [46], in the same range (~20%) as with a second quantitative confocal FRET, that further quantified the parameters of the interaction between D1R and D2R to calculate the FRET efficiency and the assessment of the distance separating both fluophore-tagged receptors [40,46]. In NAc, interactions between colocalized D1R and D2R (Figure 1) displayed high FRET efficiency (~20%) and a relative distance of 5-7 nm (50-70 Å) (Table 1), synonymous with a close proximity between D1 and D2 receptors and indicative of D1-D2 heteromer formation. In contrast, although an indication of D1-D2 heteromer formation in CP was observed, the parameters, FRET efficiency (~5%) and the relative distance of 8-9 nm (80-90 Å) between the receptors suggested that in CP either D1R-D2R interaction was weaker, or fewer D1-D2 receptor heteromers were formed, and/or lower order of D1-D2 oligomers than in the NAc was present [40,46].
Figure 1.
Example of Confocal FRET analysis of D1 and D2 receptor interaction in a medium spiney neuron from the core region of rat nucleus accumbens. Anti-D2-Alexa 350 (green) and anti-D1-Alexa 488 (red) were used as donor and acceptor dipoles. The FRET signal was detected and measured in microdomains [regions of interest (ROIs)] within the neuron coexpressing D1 and D2 receptors. Analysis shows the FRET efficiency and the distance separating the dipoles.
Table 1.
Confocal FRET analysis of D1 and D2 receptor interaction
| ROI | Donor of FRET | Acceptor of FRET | PFRET | FRET Efficiency |
Distance between donor and acceptor (nm) |
|---|---|---|---|---|---|
| (1) Donor alone | 13.944 | 0 | 0 | 0 | 10 |
| 2 | 842.685 | 562.542 | 529.703 | 0.357 | 5.91 |
| 3 | 804.879 | 488.573 | 474.042 | 0.351 | 5.9 |
| 4 | 830.377 | 569.241 | 535.203 | 0.353 | 5.924 |
| 5 | 720.099 | 436.039 | 410.781 | 0.319 | 6.269 |
| 6 | 898.475 | 482.132 | 444.885 | 0.311 | 6.171 |
| 7 | 964.916 | 460.029 | 407.186 | 0.247 | 6.875 |
| 8 | 1116.854 | 399.85 | 384.365 | 0.234 | 6.632 |
| 9 | 951.224 | 324.177 | 314.284 | 0.206 | 7.145 |
| 10 | 1076.73 | 341.095 | 326.925 | 0.2 | 7.153 |
| 11 | 976.861 | 227.299 | 216.367 | 0.149 | 7.789 |
| 12 | 1201.314 | 363.612 | 336.45 | 0.191 | 7.121 |
| 13 | 998.373 | 283.121 | 269.621 | 0.187 | 7.197 |
| 14 | 1017.225 | 303.213 | 287.876 | 0.2 | 6.987 |
| 15 | 816.347 | 166.339 | 156.562 | 0.129 | 8.069 |
| 16 | 806.034 | 265.133 | 251.731 | 0.19 | 7.393 |
| 17 | 815.063 | 349.81 | 338.709 | 0.252 | 6.792 |
| 18 | 833.344 | 485.752 | 382.262 | 0.257 | 6.946 |
| (19)Non-Specific | 95.52 | 83.573 | 35.284 | 0.086 | 9.168 |
| Average | 921.8117 | 382.821 | 356.88 | 0.243117 | 6.83958 |
| SEM | 33.82434 | 29.9949 | 27.1577 | 0.018620 | 0.165392 |
Confocal FRET analysis of figure 1 shows the relative expression of the donor (D2-Alexa 350, green) and acceptor (D1-Alexa 488, red). The analysis also shows the processed FRET (pFRET), the FRET efficiency and the distances separating the two fluorophore-tagged receptors in each microdomain (ROI), with averages and SEM in the bottom of the table. A distance ~10 nm or higher indicates no FRET.
D1-D2 receptor heteromer-induced signaling pathway and its physiologic relevance
The specific activation of the D1-D2 receptor heteromer in postnatal striatal neurons [40], and from cells co-expressing D1R and D2R [21,23] resulted in the intracellular release of calcium from stores sensitive to activation of inositol triphosphate receptors (IP3-R). This rise in intracellular calcium was rapid, transient, independent of extracellular calcium influx, and involved the activation of Gq protein, and phospholipase C (PLC) [21,23,40]. This calcium signal resulted in an increase in the phosphorylated-activated form of CaMKIIα in postnatal striatal neurons [40] and rat striatum [23]. The use of dopamine D1-/-, D2-/- and D5-/- receptor null mice indicated clearly that the calcium-CaMKIIα signaling pathway exclusively involved both D1R and D2R within a functional complex [23,40], and was different from the calcium signal generated by the activation of D5R or the D2-D5 receptor heteromer [48,49].
Intracellular calcium plays key roles in many neuronal functions including the regulation of synaptic transmission [50]. The intracellular calcium signaling pathway activated through the dopamine D1-D2 receptor heteromer resulted in CaMKIIα activation and BDNF production in striatal neurons in culture as well as in the nucleus accumbens of adult rats, leading ultimately in cultured postnatal striatal neurons to enhanced dendritic branching [40]. Both CaMKIIα and BDNF have been shown to be involved in synaptic plasticity. While evidence has indicated that CaMKIIα is a critical regulator of synaptic plasticity in neurons [51-54] with 50% of CaMKIIα-deficient mice presenting changes in behavior and learning [55], BDNF has been shown to modulate the branching and growth of axons, dendrites and spines (reviewed in 56). For example, BDNF was shown to be released from cell bodies and dendrites of cortical neurons and regulated the branching of dendrites in adjacent neurons [57]. The BDNF effect on the dendritic morphology and also on spine morphology (reviewed in 56) would be of great importance in the modulation of neuronal and synaptic function and plasticity [58]. The neurotrophin signaling transduced through BDNF receptor TrkB has been recently reported to be involved in the control of the size of the striatum by modulating the number of medium spiny neurons (MSNs), with deletion of the gene for the TrkB receptor in striatal progenitors leading to the loss of almost 50% of MSNs without affecting striatal interneurons [59]. Also, the BDNF signaling through TrkB was shown to be involved in the induction and the maintenance of synaptic plasticity, through its long-term potentiation (LTP) component [60]. The other component, long-term depression (LTD) was shown to involve BDNF signaling through the receptor p75 in hippocampal slices from p75-deficient mice [61]. BDNF plays also an important role in the modulation of neurotransmitter release, a key step in synaptic plasticity [56]. The release of glutamate for example involves PLC and BDNF through a mechanism involving a rise in intracellular calcium via a release from IP3 receptor-sensitive stores [62,63]. It is very interesting to draw the parallel between these mechanisms by which CaMKII and BDNF modulate synaptic plasticity and the signaling pathway revealed with the activation of dopamine D1-D2 receptor heteromer in the striatum [40], which also involves PLC, the intracellular calcium release from IP3 receptor-sensitive stores, CaMKII activation and BDNF production. This suggests that the D1-D2 receptor heteromer-mediated signaling pathway may play an essential role in synaptic plasticity, notably in its LTP component [20,40,49], the dysregulation of which may lead to alterations in cognition, learning, and memory that contribute to the pathophysiology of dopamine-related disorders such as schizophrenia or drug addiction [20,40,46,49].
Further, we showed that in rat striatum amphetamine administration significantly increased the affinity of SKF 83959, a specific D1-D2 receptor heteromer agonist [64], by 10-fold for the D1-D2 receptor heteromer and increased the proportion of the D1-D2 heteromer in the agonist-detected high affinity state [46]. GTPγS binding studies indicated that the D1-D2 heteromer was functionally supersensitive in response to repeated increases in dopamine transmission following amphetamine administration [46]. In addition to increasing the activity and sensitivity of D1-D2 receptor heteromers, amphetamine also increased the D1-D2 receptor heteromer density in the NAc as assessed by FRET technique [46].
Interestingly, the increase in the proportion of D1-D2 heteromers in the high affinity state was also detected in schizophrenia globus pallidus (GP) [46]. Amphetamine treatment leading to increased dopamine transmission and behavioral sensitization has been used as an animal model for schizophrenia [65], since schizophrenia has been linked to increased dopamine transmission [66]. Moreover, the different components of calcium signaling, including Gq proteins, PLC, and CaMKII were shown to be affected in the brains of schizophrenia patients [67]. Given these facts, the findings showing an increase in the proportion of D1-D2 heteromers in high affinity state in both schizophrenia and chronic amphetamine treatment may indicate a preponderant role of the D1-D2 receptor heteromer-mediated calcium-CaMKII-BDNF signaling pathway in both drug addiction and schizophrenia.
This D1-D2 receptor heteromer-calcium signal may represent a first common biochemical bridge between the dopaminergic system-CaMKII-BDNF, synaptic plasticity and the occurrence of drug addiction and schizophrenia. The finding that the activation of CaMKIIα was necessary for the induction of behavioral sensitization to drugs [68], a physiological phenomenon that also requires the coactivation of D1 and D2 dopamine receptors [14], provides additional evidence of the important role of dopamine D1-D2 receptor heteromer-calcium signal in drug addiction.
After years of some skepticism surrounding the physiological presence and relevance of GPCR homo- and hetero-oligomers, there is ample evidence for the presence in the brain of a unique entity, the D1-D2 receptor heteromer, with a unique signaling pathway different from the signals generated by each receptor homomer, with a physiological relevance and high importance in at least two major pathologies, schizophrenia and drug addiction, making the D1-D2 receptor an interesting therapeutic target for these disorders.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors read and approved the final manuscript
Contributor Information
Ahmed Hasbi, Email: a.hasbi@utoronto.ca.
Brian F O'Dowd, Email: brian.odowd@utoronto.ca.
Susan R George, Email: s.george@utoronto.ca.
Acknowledgements
One of a series of four reviews on G protein-coupled receptors published in memory of Hubert H. M. Van Tol (1959-2006), formerly Head of Molecular Biology at the Centre for Addiction and Mental Health, and a Professor in the Departments of Psychiatry and Pharmacology at the University of Toronto. Hubert's contributions to G protein-coupled receptor research and neuroscience are numerous and are best remembered by his central role in the cloning of the dopamine receptor family. His many achievements were recognized through awards such as the John Dewan award, The Prix Galien, and the Joey & Toby Tanenbaum Distinguished Scientist Award for Schizophrenia Research.
SRG is the holder of a Tier 1 Canada Research Chair in Molecular Neuroscience. This work was supported by a grant from the NIH National Institute of Drug Abuse.
References
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ. Novel insights in dopamine receptor physiology. Eur J Endocrinol. 2007. pp. S13–S21. [Erratum in: Eur J Endocrinol 157:543] [DOI] [PubMed]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/S0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Park SK, Nguyen MD, Fischer A, Luke MP, Affar el B, Dieffenbach PB, Tseng HC, Shi Y, Tsai LH. Par-4 links dopamine signaling and depression. Cell. 2005;122:275–287. doi: 10.1016/j.cell.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Mailman RB, Schultz DW, Lewis MH, Staples L, Rollemar H, Dehaven DL. SCH23390: a selective D1 dopamine antagonist with potent D2 behavioral actions. Eur J Pharmacol. 1984;101:159–160. doi: 10.1016/0014-2999(84)90044-X. [DOI] [PubMed] [Google Scholar]
- White FJ, Bednarz LM, Wachtel SR, Hjorth S, Brooderson RJ. Is stimulation of both D1 and D2 receptors necessary for the expression of dopamine-mediated behaviors? Pharmacol Biochem Behav. 1988;30:189–193. doi: 10.1016/0091-3057(88)90442-X. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M. Brain dopamine receptors - research perspectives and potential sites of regulation. Pol J Pharmacol. 2004;56:659–671. [PubMed] [Google Scholar]
- La Hoste GJ, Yu J, Marshall JF. Striatal Fos expression is indicative of dopamine D1/D2 synergism and receptor supersensitivity. Proc Natl Acad Sci USA. 1993;90:7451–7455. doi: 10.1073/pnas.90.16.7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsing LG Jr, Zigmond MJ. Influence of dopamine on GABA release in striatum: Evidence for D1-D2 interactions and nonsynaptic influences. Neuroscience. 1997;77:419–429. doi: 10.1016/S0306-4522(96)00475-7. [DOI] [PubMed] [Google Scholar]
- Capper-Loup C, Canales JJ, Kadaba N, Graybiel AM. Concurrent activation of dopamine D1 and D2 receptors is required to evoke neural and behavioral phenotypes of cocaine sensitization. J Neurosci. 2002;22:6218–6227. doi: 10.1523/JNEUROSCI.22-14-06218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters JR, Bergstrom DA, Carlson JM, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science. 1987;236:719–722. doi: 10.1126/science.2953072. [DOI] [PubMed] [Google Scholar]
- Pan HS, Engber TM, Chase TN, Walters JR. The effect of striatal lesion on turning behavior and globus pallidus single unit response to dopamine agonist administration. Life Sci. 1990;46:73–80. doi: 10.1016/0024-3205(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Robertson HA. Synergistic interactions of D1- and D2-selective dopamine gonists in animal models for Parkinson's disease: sites of action and mplications for the pathogenesis of dyskinesias. Can J Neurol Sci. 1992;19:147–152. [PubMed] [Google Scholar]
- Braun AR, Laruelle M, Mouradian MM. Interactions between D1 and D2 dopamine receptor family agonists and antagonists: the effects of chronic exposure on behavior and receptor binding in rats and their clinical implications. J Neural Transm. 1997;104:341–362. doi: 10.1007/BF01277656. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G-protein-coupled transmitter receptors. Nat Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Milligan G, White JH. Protein-protein interactions at G-protein-coupled receptors. Trends Pharmacol Sci. 2001;22:513–518. doi: 10.1016/S0165-6147(00)01801-0. [DOI] [PubMed] [Google Scholar]
- George SR, O'Dowd BF. A novel dopamine receptor signaling unit in brain: heterooligomers of D1 and D2 dopamine receptors. Sci World J. 2007;7:58–63. doi: 10.1100/tsw.2007.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR. In: G Protein-Coupled Receptors: Structure, Signaling, and Physiology. Sandra Siehler and Graeme Milligan, editor. Cambridge University Press; 2011. Signaling of dopamine receptor homo- and hetero-oligomers. Eds. [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor coactivation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CH, Varghese G, Curley KJ, Kong MM, Alijaniaram M, Ji X, Nguyen T, O'Dowd BF, George SR. D1 and D2 dopamine receptors form heterooligomers and co-internalize after selective activation of either receptor. Mol Pharmacol. 2005;68:568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- Dziedzicka-Wasylewska M, Faron-Górecka A, Andrecka J, Polit A, Kuśmider M, Wasylewski Z. Fluorescence studies reveal heterodimerization of dopamine D1 and D2 receptors in the plasma membrane. Biochemistry. 2006;45:8751–8759. doi: 10.1021/bi060702m. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. In: The Rat Nervous System. Paxinos G, editor. Academic, New York; 2004. The basal ganglia; pp. 455–508. ed. [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in rat striatum: Sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous systembased on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- Shuen JA, Chen M, Gloss B, Calakos N. Drd1a-tdTomato BAC transgenic mice for simultaneous visualization of medium spiny neurons in the direct and indirect pathways of the basal ganglia. J Neurosci. 2008;28:2681–2685. doi: 10.1523/JNEUROSCI.5492-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Hervé D, Girault JA, Valjent E. What is the Degree of Segregation between Striatonigral and Striatopallidal Projections? Front Neuroanat. 2010;4(pii):136. doi: 10.3389/fnana.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman O, Brismar H, Uhlé n P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Shetreat ME, Lin L, Wong AC, Rayport S. Visualization of D1 dopamine receptors on living nucleus accumbens neurons and their colocalization with D2 receptors. J Neurochem. 1996;66:1475–1482. doi: 10.1046/j.1471-4159.1996.66041475.x. [DOI] [PubMed] [Google Scholar]
- Wong AC, Shetreat ME, Clarke JO, Rayport S. D1- and D2-like dopamine receptors are colocalized on the presynaptic varicosities of striatal and nucleus accumbens neurons in vitro. Neuroscience. 1999;89:221–233. doi: 10.1016/S0306-4522(98)00284-X. [DOI] [PubMed] [Google Scholar]
- Iwatsubo K, Suzuki S, Li C, Tsunematsu T, Nakamura F, Okumura S, Sato M, Minamisawa S, Toya Y, Umemura S, Ishikawa Y. Dopamine induces apoptosis in young, but not in neonatal, neurons via Ca2+-dependent signal. Am J Physiol Cell Physiol. 2007;293:C1498–1508. doi: 10.1152/ajpcell.00088.2007. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. et al. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Nat Acad Sci USA. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, George SR. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA. 2009;106:21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Lezcano N, Bergson C. D1/D5 dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol. 2002;87:2167–2175. doi: 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
- Tang TS, Bezprozvanny I. Dopamine receptor-mediated Ca2+ signaling in striatal medium spiny neurons. J Biol Chem. 2004;279:42082–42094. doi: 10.1074/jbc.M407389200. [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol Pharmacol. 1997;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Hasbi A, O'Dowd BF, George SR. Dopamine D1-D2 receptor Heteromer-mediated calcium release is desensitized by D1 receptor occupancy with or without signal activation: dual functional regulation by G protein-coupled receptor kinase 2. J Biol Chem. 2010;285:35092–35103. doi: 10.1074/jbc.M109.088625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So CH, Verma V, Alijaniaram M, Cheng R, Rashid AJ, O'Dowd BF, George SR. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor heterooligomers. Mol Pharmacol. 2009;75:843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O'Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Current Opinion in Pharmacology. 2010;10:93–99. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/S0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;3:344–353. doi: 10.1038/nn2054. [Erratum in: Nat Neurosci 5: 617] [DOI] [PubMed] [Google Scholar]
- Lowetha JA, Bakerb LK, Guptaab T, Guillorya AM, Vezina P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci Lett. 2008;444:157–160. doi: 10.1016/j.neulet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Noda A, Nakamura T, Tokura T, Yura Y, Nitta A, Furukawa H, Nabeshima T. Involvement of a dysfunctional dopamine-D1/N-methyl-D-aspartate-NR1 and Ca2+/calmodulin-dependent protein kinase II pathway in the impairment of latent learning in a model of schizophrenia induced by phencyclidine. Mol Pharmacol. 2007;71:1598–1609. doi: 10.1124/mol.106.032961. [DOI] [PubMed] [Google Scholar]
- Blaeser F, Sanders MJ, Truong N, Ko S, Wu LJ, Wozniak DF, Fanselow MS, Zhuo M, Chatila TA. Long-term memory deficits in Pavlovian fear conditioning in Ca2+/calmodulin kinase kinase alpha-deficient mice. Mol Cell Biol. 2006;26:9105–9115. doi: 10.1128/MCB.01452-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Baydyuk M, Russell T, Liao G-Y, Zang K, An JJ, Reichardt LF, Xu B. TrkB receptor controls striatal formation by regulating the number of newborn striatal neurons. Proc Natl Acad Sci USA. 2011;108:1669–674. doi: 10.1073/pnas.1004744108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. 2008;89:312–323. doi: 10.1016/j.nlm.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösch H, Schweigreiter R, Bonhoeffer T, Barde YA, Korte M. The neurotrophin receptor p75NTR modulates long-term depression and regulates the expression of AMPA receptor subunits in the hippocampus. Proc Natl Acad Sci USA. 2005;102:7362–7367. doi: 10.1073/pnas.0502460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Matsumoto T, Adachi N, Yokomaku D, Kojima M, Takei N, Hatanaka H. Brain-derived neurotrophic factor triggers a rapid glutamate release through increase of intracellular Ca(2+) and Na(+) in cultured cerebellar neurons. J Neurosci Res. 2001;66:96–108. doi: 10.1002/jnr.1201. [DOI] [PubMed] [Google Scholar]
- Numakawa T, Yamagishi S, Adachi N, Matsumoto T, Yokomaku D, Yamada M, Hatanaka H. Brain-derived neurotrophic factor-induced potentiation of Ca(2+) oscillations in developing cortical neurons. J Biol Chem. 2002;277:6520–9. doi: 10.1074/jbc.M109139200. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, O'Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci. 2007;28:551–555. doi: 10.1016/j.tips.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitizedstate as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III- the final common pathway. Schizophr Bull. 2009;35:549–62. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, Ma L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]