Abstract
Background
The accuracy of gadolinium-enhanced magnetic resonance pulmonary angiography and magnetic resonance venography for diagnosing pulmonary embolism has not been determined conclusively.
Objective
To investigate performance characteristics of magnetic resonance angiography, with or without magnetic resonance venography, for diagnosing pulmonary embolism.
Design
Prospective, multicenter study from 10 April 2006 to 30 September 2008. (ClinicalTrials.gov registration number: NCT00241826)
Setting
7 hospitals and their emergency services.
Patients
371 adults with diagnosed or excluded pulmonary embolism.
Measurements
Sensitivity, specificity, and likelihood ratios were measured by comparing independently read magnetic resonance imaging with the reference standard for diagnosing pulmonary embolism. Reference standard diagnosis or exclusion was made by using various tests, including computed tomographic angiography and venography, ventilation–perfusion lung scan, venous ultra-sonography, D-dimer assay, and clinical assessment.
Results
Magnetic resonance angiography, averaged across centers, was technically inadequate in 25% of patients (92 of 371). The proportion of technically inadequate images ranged from 11% to 52% at various centers. Including patients with technically inadequate images, magnetic resonance angiography identified 57% (59 of 104) with pulmonary embolism. Technically adequate magnetic resonance angiography had a sensitivity of 78% and a specificity of 99%. Technically adequate magnetic resonance angiography and venography had a sensitivity of 92% and a specificity of 96%, but 52% of patients (194 of 370) had technically inadequate results.
Limitation
A high proportion of patients with suspected embolism was not eligible or declined to participate.
Conclusion
Magnetic resonance pulmonary angiography should be considered only at centers that routinely perform it well and only for patients for whom standard tests are contraindicated. Magnetic resonance pulmonary angiography and magnetic resonance venography combined have a higher sensitivity than magnetic resonance pulmonary angiography alone in patients with technically adequate images, but it is more difficult to obtain technically adequate images with the 2 procedures.
Primary Funding Source
National Heart, Lung, and Blood Institute.
We undertook this study to test whether magnetic resonance angiography in combination with venous phase-magnetic resonance venography might be useful for the diagnosis or exclusion of acute pulmonary embolism. The Agency for Healthcare Research and Quality identified the need for such a study (1). In an investigation of multidetector computed tomographic angiography for suspected acute pulmonary embolism (2), 24% of patients had 1 or more relative contraindications to ionizing radiation or iodinated contrast material. Gadolinium-enhanced magnetic resonance angiography, if shown to be accurate, would avoid these risks.
Methods
Design
PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) was a multicenter study designed to assess the sensitivity and specificity of magnetic resonance angiography, alone or with magnetic resonance venography, for diagnosing pulmonary embolism and venous thromboembolism. We made a reference standard diagnosis or exclusion by using various tests, including computed tomographic angiography and venography, ventilation–perfusion lung scan, venous ultrasonography, D-dimer assay, and clinical assessment. We recruited patients from 10 April 2006 through 30 September 2008. To eliminate evaluation of a disproportionate number of patients with negative test results, we used a computer-based system to randomly select patients with a negative reference test result by local interpretation to receive magnetic resonance angiography and venography. The proportion of patients selected varied by center and, during the recruitment period, by the prevalence of pulmonary embolism.
A data safety monitoring board appointed by the National Heart, Lung, and Blood Institute and the institutional review board of each center approved the protocol and consent forms. All recruited patients gave written informed consent.
Sites and Patients
The Appendix (available at www.annals.org) shows participating centers. Patients were eligible if they were 18 years or older and had been hospitalized or in the emergency department with diagnosed or excluded pulmonary embolism. We recruited patients consecutively during the nurse-coordinator’s working hours. Contraindications for receiving magnetic resonance angiography and venography included implanted ferromagnetic foreign bodies (3), dependency on an external electrical device, claustrophobia, inability to lie still for 30 minutes, and being pregnant or nursing. We have described additional exclusion criteria elsewhere (4). During recruitment, we changed the serum creatinine level and glomerular filtration rate at which we excluded patients (4) because of new information about the risks for nephrogenic systemic fibrosis (nephrogenic fibrosing dermopathy) (5, 6).
Magnetic Resonance Angiography and Venography
We performed magnetic resonance imaging within 48 hours of the reference test during the first 13 months of recruitment and within 72 hours during the subsequent 17 months. We used 1.5-T systems, except at the University of Michigan, where 73 patients were examined in a 3.0-T unit. We have described equipment characteristics and imaging parameters elsewhere (4).
Before recognition of the dangers of nephrogenic systemic fibrosis, we performed magnetic resonance angiography during intravenous infusion of gadodiamide, gadopentetate dimeglumine, or gadoversetamide, 0.2, mmol/kg (approximately 40 mL). In response to increased awareness of the risk for nephrogenic systemic fibrosis and nephrogenic fibrosing dermopathy, we changed our protocol to require gadobenate dimeglumine at a maximum dose of 0.1 mmol/kg of body weight (approximately 20 mL).
Our criteria for diagnosing acute pulmonary embolism with magnetic resonance angiography were a partially occlusive intraluminal filling defect or complete arterial occlusion with termination of the column of contrast material in a meniscus that outlined the trailing edge of the embolus. All magnetic images interpreted as negative showed adequate opacification of subsegmental branches. If pulmonary embolism was shown, we considered the image to be technically adequate regardless of the degree of opacification at the site of pulmonary embolism or elsewhere. Our criteria for diagnosing acute deep venous thrombosis were failure to opacify the entire lumen because of a central filling defect or a partial filling defect surrounded by opacification.
We required that both the magnetic resonance angiogram and venogram be technically adequate to exclude pulmonary embolism. We considered the combined result to be positive if either result was positive.
We defined wrap artifact (aliasing or back-folding) as anatomy extending beyond the prescribed field of view superimposed on the opposite side of the image and parallel imaging artifact as superimposed extraneous anatomy, often near the middle of the image.
We calculated the signal-to-noise ratio as signal in the main pulmonary artery divided by the SD of signal in the adjacent nonvascular lung. We calculated contrast-to-noise ratio as the ratio of signal in the main pulmonary artery minus signal in the adjacent nonvascular lung divided by the SD of signal in the adjacent nonvascular lung.
Diagnostic Reference Standard
Table 1 shows the reference standards for the diagnosis or exclusion of pulmonary embolism. We used the same diagnostic criteria and methods of computed tomographic angiography and venography as for PIOPED II (2, 7), but we permitted centers to modify the techniques. Centers used scanners with 16, 32, and 64 detectors.
Table 1.
Reference Test Basis for Diagnosis or Exclusion of PE in Patients Who Had Magnetic Resonance Angiography
| Criteria | Patients, n (%) |
|---|---|
|
Diagnosis of PE
| |
| CT angiogram showing PE in a main or lobar pulmonary artery | 94 (90) |
|
| |
| CT angiogram showing PE in a segmental or subsegmental pulmonary artery in a patient with high clinical probability according to the Wells criteria (9) | 8 (8) |
|
| |
| High-probability VQ lung scan in a patient with no previous PE and high or intermediate probability according to the Wells criteria (9) | 2 (2) |
|
| |
| Total | 104 (100) |
|
Exclusion of PE
| |
| Normal D-dimer result by quantitative rapid enzyme-linked immunosorbent assay (D-dimer level 3500 ng/mL) in a patient with low or intermediate probability according to the Wells criteria (9); if whole blood or latex D-dimer was used, clinical assessment had to be low probability | 95 (36) |
|
| |
| Negative CT angiogram in a patient with low probability according to the Wells criteria (9) | 132 (49) |
|
| |
| Negative CT angiogram and CT venogram or venous ultrasonogram in a patient with intermediate probability according to the Wells criteria (9) | 34 (13) |
|
| |
| Normal VQ lung scan | 6 (2) |
|
| |
| Total | 267 (100) |
CT = computed tomography; PE = pulmonary embolism; VQ = ventilation–perfusion.
We used the same methods for ventilation–perfusion lung scans as in PIOPED II (7) and based our interpretations on the revised PIOPED criteria (8). Methods of venous ultrasonography (4) and the Wells clinical prediction rule (9) are described elsewhere.
Central Readers
Two study-certified readers, who had no knowledge of clinical information or results of other tests, interpreted all ventilation–perfusion lung scans, computed tomographic angiograms and venograms, and magnetic resonance angiograms and venograms. We selected readers randomly from available readers with no outstanding backlogs. PIOPED III had 13 central readers of magnetic resonance images. Readers did not read images from their own institution or reinterpret venous compression ultrasonograms. Images used for a reference test diagnosis (CT angiograms and ventilation–perfusion lung scans) were initially read locally to determine which patients had a negative reference test result and should be randomly selected to have magnetic resonance angiography. Central readers then reread these images, and we used the results of the central readings in our analysis.
If readers did not agree on a diagnosis of pulmonary embolism in at least 1 lobe or a negative diagnosis in all lobes, we sent the image to a third reader. Twenty-four percent (88 of 371) of magnetic resonance angiograms required consensus from 3 or more readers.
Follow-up Monitoring and Assessment of Adverse Events
We assessed adverse events at hospital discharge and on telephone calls after 3 months by using a questionnaire. We made additional calls at 6 months to patients with a glomerular filtration rate between 60 and 90 mL/min per 1.73 m2. In addition to death, we defined a serious adverse event as any event that was life-threatening, involved or prolonged hospitalization, resulted in disability, or required intervention to prevent permanent damage or disability. We asked patients whether they had had any such events and whether they had swelling, tightening of the skin, skin thickening, or reddened or darkened patches of skin on the arms or legs. If a patient reported an apparent event, we evaluated his or her records. An outcome committee assessed a narrative summary of the event, including duration, relationship to study procedures, and resolution, and we reported this information to the data safety and monitoring board.
Statistical Analysis
According to conventional analysis, we excluded technically inadequate studies from computation of sensitivity and specificity (10). We also calculated the proportion of patients in whom pulmonary embolism was diagnosed or excluded with technically inadequate images included in the calculations (11). We obtained exact 95% CIs for sensitivity and specificity from the binomial distribution by using SAS, version 9.1 (SAS Institute, Cary, North Carolina). We calculated likelihood ratios for a positive test result as sensitivity divided by (1 − specificity) and likelihood ratios for a negative result as (1 − sensitivity) divided by specificity (12, 13). We calculated CIs for likelihood ratios by using the normal distribution approximation of binomial distribution. We based our probabilities of pulmonary embolism with low, intermediate, and high probability objective clinical assessments on the average values observed with the Wells clinical prediction rule (9, 14, 15). We used the chi-square test to assess heterogeneity among clinics regarding proportion of technically inadequate tests, sensitivity, and specificity with magnetic resonance angiography and combined angiography and venography. We tested for mean differences in the contrast-to-noise ratio by using analysis of variance.
Role of the Funding Source
The National Heart, Lung, and Blood Institute had no role in study design, data collection and analysis, interpretation, or the decision to submit the manuscript for publication.
Results
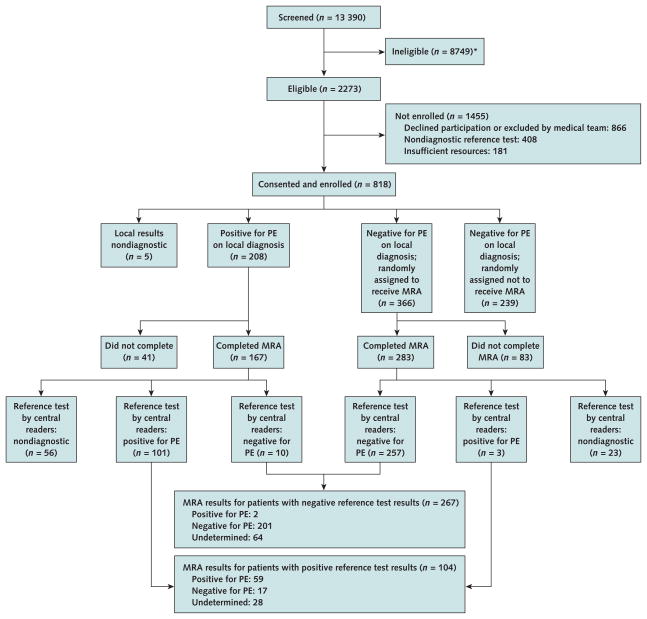
During 2.5 years of recruitment, we screened 13 390 patients; of 2273 eligible patients, we enrolled 818 (Figure). Among patients with no pulmonary embolism by local interpretation of the reference test, 60% (366 of 605) were randomly assigned to have magnetic resonance angiography. Among 371 patients who completed magnetic resonance angiography and had a diagnostic reference test, the reference test was positive in 104 and negative in 267 (Table 1). Table 2 shows demographic and clinical characteristics of these patients and of the 447 patients who were enrolled but not evaluated by magnetic resonance imaging.
Figure. Study flow diagram.
MRA = magnetic resonance angiography; PE = pulmonary embolism; PIOPED III = Prospective Investigation of Pulmonary Embolism Diagnosis III. * Exclusion criteria (number of patients): age <18 y (23), no suspected PE (23), no consent from medical team (438), no PIOPED III informed consent (829), could not complete MRA in ≤72 h (315), could not confirm not pregnant (171), contraindications to gadolinium MRA or magnetic resonance venography (2047), critically ill (1657), glomerular filtration rate <60 mL/min per 1.73 m2 (2922), receiving renal dialysis (418), hemodynamically unstable (552), shock or hypotension (167), receiving ventilatory support (779), ventricular fibrillation or tachycardia (90), received gadolinium in past 3 mo (693), expected to receive gadolinium within 3 mo (176), history of allergy to contrast agent (337), myocardial infarction in past month (437), current symptomatic asthma (184), pregnant (97), nursing mother (103), previously enrolled in PIOPED III (14), prisoner (17), mentally handicapped (262), institutionalized (179), could not give informed consent (1022), evidence of acute renal failure (771), not assessed (2368).
Table 2.
Demographic Characteristics, Coexisting Illnesses, Presenting Signs and Symptoms, and Clinical Probability of PE
| Variable | Patients Enrolled (n = 447)* | Reference Test Result (n = 371)
|
MRA Result (n = 371)
|
|||
|---|---|---|---|---|---|---|
| Positive for PE (n = 104) | Negative for PE (n = 267) | Technically Inadequate (n = 92) | Positive for PE (n = 61) | Negative for PE (n = 218) | ||
|
Site, n/N (%)
| ||||||
| Outpatient | 404/447 (91) | 98/104 (94) | 247/267 (93) | 88/92 (96) | 58/61 (95) | 199/218 (92) |
|
| ||||||
| Nursing home or rehabilitation center | 1/447 (0) | 1/104 (1) | 3/267 (1) | 0/92 (0) | 1/61 (2) | 3/218 (1) |
|
| ||||||
| Hospital | 29/447 (6) | 3/104 (3) | 16/267 (6) | 3/92 (3) | 1/61 (2) | 15/218 (7) |
|
| ||||||
| Intensive care unit | 0/447 (0) | 0/104 (0) | 0/267 (0) | 0/92 (0) | 0/61 (0) | 0/218 (0) |
|
| ||||||
| Unknown | 13/447 (3) | 2/104 (2) | 1/267 (0.3) | 1/92 (1) | 1/61 (2) | 1/218 (0.5) |
|
| ||||||
| Demographic characteristic | ||||||
| Women, n/N (%) | 262/447 (59) | 43/104 (41) | 163/267 (61) | 52/92 (57) | 25/61 (41) | 129/218 (59) |
|
| ||||||
| Mean age (SD), y | 49 (15) | 50 (16) | 49 (15) | 47 (14) | 51 (17) | 49 (15) |
|
| ||||||
| Race, n/N (%) | ||||||
| White | 284/447 (64) | 73/104 (70) | 179/267 (67) | 58/92 (63) | 46/61 (75) | 148/218 (68) |
|
| ||||||
| Black | 139/447 (31) | 25/104 (24) | 78/267 (29) | 32/92 (35) | 10/61 (16) | 61/218 (28) |
|
| ||||||
| Other | 24/447 (5) | 6/104 (6) | 10/267 (4) | 2/92 (2) | 5/61 (8) | 9/218 (4) |
|
| ||||||
| Mean body weight (SD), kg | 86.2 (22.4) | 91.2 (21.5) | 83.5 (20.1) | 87.0 (20.3) | 89.4 (22) | 84.1 (20.4) |
|
| ||||||
| Mean body mass index (SD), kg/m2 | 29.8 (7.7) | 29.8 (6.9) | 29.5 (7.3) | 30.1 (7.3) | 29.1 (7.1) | 29.5 (7.2) |
|
Coexisting condition, n/N (%)
| ||||||
| Smoking history | 216/435 (50) | 46/104 (44) | 136/267 (51) | 45/92 (49) | 21/61 (34) | 116/218 (53) |
|
| ||||||
| Congestive heart failure | 33/437 (8) | 7/104 (7) | 17/267 (6) | 3/92 (3) | 3/61 (5) | 18/218 (8) |
|
| ||||||
| Current asthma | 54/438 (12) | 15/104 (14) | 36/267 (13) | 19/92 (21) | 7/61 (11) | 25/218 (11) |
|
| ||||||
| Chronic obstructive pulmonary disease | 40/438 (9) | 9/104 (9) | 26/266 (10) | 11/92 (12) | 5/61 (8) | 19/217 (9) |
|
| ||||||
| Current pneumonia | 76/434 (18) | 17/104 (16) | 40/267 (15) | 16/92 (17) | 8/61 (13) | 33/218 (15) |
|
| ||||||
| Surgery in the past 3 mo | 46/438 (11) | 21/104 (20) | 23/267 (9) | 10/92 (11) | 11/61 (18) | 23/218 (11) |
|
| ||||||
| Cancer | 67/438 (15) | 24/104 (23) | 36/267 (13) | 13/92 (14) | 14/61 (23) | 33/218 (15) |
|
| ||||||
| Central venous instrumentation | 16/439 (4) | 7/104 (7) | 7/267 (3) | 5/92 (5) | 2/61 (3) | 7/218 (3) |
|
Symptom, n/N (%)
| ||||||
| Dyspnea | 137/434 (32) | 48/104 (46) | 70/267 (26) | 32/92 (35) | 28/61 (46) | 58/218 (27) |
|
| ||||||
| Pleuritic pain | 34/434 (8) | 13/104 (13) | 32/267 (12) | 16/92 (17) | 9/61 (15) | 20/218 (9) |
|
| ||||||
| Cough | 183/435 (42) | 44/104 (42) | 90/265 (34) | 42/92 (46) | 21/61 (34) | 71/216 (33) |
|
| ||||||
| Calf pain | 95/431 (22) | 34/104 (33) | 37/267 (14) | 15/92 (16) | 20/61 (33) | 36/218 (17) |
|
Sign, n/N (%)
| ||||||
| Hemoptysis | 21/443 (5) | 10/104 (10) | 6/267 (2) | 5/92 (5) | 3/61 (5) | 8/218 (4) |
|
| ||||||
| Tachypnea (≥20 breaths/min) | 152/431 (35) | 38/103 (37) | 77/266 (29) | 28/92 (30) | 22/60 (37) | 65/217 (30) |
|
| ||||||
| Crackles | 60/443 (14) | 22/104 (21) | 34/265 (13) | 15/92 (16) | 12/61 (20) | 29/216 (13) |
|
| ||||||
| Tachycardia (>100 beats/min) | 69/432 (16) | 26/104 (25) | 28/267 (10) | 14/92 (15) | 15/61 (25) | 25/218 (11) |
|
| ||||||
| Swollen calf (>1 cm) | 73/415 (18) | 32/98 (33) | 49/259 (19) | 26/87 (30) | 17/59 (29) | 38/211 (18) |
|
| ||||||
| Clinical probability, n/N (%)† | ||||||
| Low | 270/447 (60) | 8/104 (8) | 218/267 (82) | 57/92 (62) | 8/61 (13) | 161/218 (74) |
|
| ||||||
| Intermediate | 161/447 (36) | 73/104 (70) | 49/267 (18) | 28/92 (30) | 41/61 (67) | 53/218 (24) |
|
| ||||||
| High | 16/447 (4) | 23/104 (22) | 0/267 (0) | 7/92 (8) | 12/61 (20) | 4/218 (2) |
MRA = magnetic resonance angiography; PE = pulmonary embolism.
239 patients for whom PE was excluded and who were randomly selected not to have MRA, 84 with a nondiagnostic reference test result, and 124 in whom MRA was not completed.
On the basis of the Wells objective clinical assessment (9).
Most patients (84% [315 of 370; data were missing for 1]) received gadobenate dimeglumine. We administered gadopentetate dimeglumine to 14% (51 of 370). Only 4 patients received gadodiamide or gadoversetamide, and the doses ranged from 30 to 45 mL. Mean examination time (from first scout view through last image) was 26 minutes.
Results of Magnetic Resonance Angiography
Magnetic resonance angiography was technically inadequate in 25% of patients (92 of 371) who had pulmonary artery imaging (Table 3). Among these, 28 patients had pulmonary embolism according to the reference test and 64 did not. Inadequate image quality was ascribed to more than 1 cause in 72% of patients (66 of 92) with technically inadequate images. Causes of technically inadequate magnetic resonance angiograms were poor arterial opacification (67%), motion artifact (36%), wraparound artifact (4%), and parallel imaging artifact (2%).
Table 3.
Results of MRA and Combined MRA and MRV, by Reference Test
| Test Result | Reference Test Result, n |
Total, n | |
|---|---|---|---|
| Positive for PE | Negative for PE | ||
|
MRA result
| |||
| Positive | 59 | 2 | 61 |
|
| |||
| Negative | 17 | 201 | 218 |
|
| |||
| Technically inadequate | 28 | 64 | 92 |
|
| |||
| Total | 104 | 267 | 371 |
|
MRA and MRV result*
| |||
| Positive | 65 | 4 | 69 |
|
| |||
| Negative | 6 | 101 | 107 |
|
| |||
| Technically inadequate | 33 | 161 | 194 |
|
| |||
| Total† | 104 | 266 | 370 |
MRA = magnetic resonance angiography; MRV = magnetic resonance venography; PE = pulmonary embolism.
We considered the combined MRA and MRV result to be positive if either the MRA or MRV result was positive and to be negative only if both the MRA and MRV results were negative. If the MRA or MRV result was negative but the accompanying MRV or MRA result for that patient was technically inadequate, we classified the combination of images as technically inadequate.
One patient who had MRA did not have MRV.
Of the 104 patients with pulmonary embolism according to the reference test, magnetic resonance angiography identified pulmonary embolism in 59 (57% [95% CI, 47% to 66%]), including those with technically inadequate images. Among the 267 patients who did not have pulmonary embolism according to the reference test, angiography excluded pulmonary embolism in 201 (75% [CI, 70% to 80%]), including those with technically inadequate images.
Among patients with technically adequate images, magnetic resonance angiography had a sensitivity of 78% (CI, 67% to 86%) (59 of 76) in patients with pulmonary embolism established by the reference test and a specificity of 99% (CI, 96% to 100%) (201 of 203) in patients with pulmonary embolism excluded by the reference test. The likelihood ratio was 78.8 (CI, 19.7 to 315) for a positive test result and 0.23 (CI, 0.15 to 0.34) for a negative result. With an assumed prevalence of 11% to 50% for pulmonary embolism, the estimated positive predictive value would range from 91% to 98% and the estimated negative predictive value would range from 97% to 82%.
Magnetic resonance angiography had a sensitivity of 79% (50 of 63) for detecting pulmonary embolism in a main or lobar pulmonary artery. Angiography rarely identified pulmonary embolism when the largest embolism was in a segmental or subsegmental branch. Sensitivity was 50% (2 of 4) for detecting emboli in segmental arteries and 0% (0 of 1) for detecting emboli in subsegmental arteries. Specificity was 98% to 100%, regardless of the order of the vessel. The likelihood ratio was 49.6 (CI, 12.5 to 197) for a positive test result for pulmonary embolism in a main or lobar pulmonary artery and 0.2 (CI, 0.13 to 0.34) for a negative result.
Arterial phase opacification had to be of good or fair quality to be adequate for interpretation. Opacification was adequate in 91% of patients (338 of 371) for the main or lobar pulmonary arteries, 87% of patients (323 of 371) for the segmental pulmonary arteries, and 73% of patients (271 of 371) for the subsegmental branches; however, some of these images may have had artifacts that made them technically inadequate. The proportion of magnetic resonance angiograms with inadequate opacification and the sensitivity and specificity did not change over tertiles of study time.
Arterial phase opacification was similar for 1.5-T and 3.0-T systems. However, the data were from multiple sites, software, and hardware platforms. A subset analysis from 2 sites that had a common vendor and receiver coil design showed a 24% higher signal-to-noise ratio in the main pulmonary artery with the 3.0-T system (P = 0.030).
Both the signal-to-noise ratio and contrast-to-noise ratio were higher in the main pulmonary artery with gadobenate dimeglumine, 0.1 mmol/kg of body weight (approximately 20 mL), than with gadopentetate dimeglumine, 0.2 mmol/kg (approximately 40 mL) (P = 0.01 and 0.008, respectively).
Results of Magnetic Resonance Angiography and Venography
Combined magnetic resonance angiography and venography was technically inadequate in 52% of patients (194 of 370) who had imaging of the pulmonary arteries and lower extremities. Of these patients, the reference test established pulmonary embolism in 33 and excluded it in 161 (Table 3). One patient who had magnetic resonance angiography did not have magnetic resonance venography. The combination was more frequently technically inadequate than magnetic resonance angiography alone because both tests had to be technically adequate to rule out venous thromboembolism. Venous opacification was inadequate in 32% to 47% of patients, depending on the vein. Severe wrap artifact occurred in 3%. No severe motion artifacts occurred.
Combined magnetic resonance angiography and venography identified venous thromboembolism in 65 of 104 patients (63% [CI, 53% to 72%]) with pulmonary embolism established by the reference test, including those with technically inadequate images. The combined techniques excluded 101 of 266 patients (38% [CI, 32% to 44%]) for whom pulmonary embolism was excluded by the reference test, including those with technically inadequate images (Table 3).
Among patients with technically adequate images, combined magnetic resonance angiography and venography had a sensitivity of 92% (CI, 83% to 97%) (65 of 71) in patients with pulmonary embolism established by the reference test and a specificity of 96% (CI, 91% to 99%) (101 of 105) in patients with pulmonary embolism excluded by the reference test (Table 3). The likelihood ratio was 24.0 (CI, 9.2 to 63.0) for a positive test result and 0.09 (CI, 0.04 to 0.18) for a negative result. With a prevalence of pulmonary embolism ranging from 11% to 50%, the positive predictive value would range from 74% to 98% and the negative predictive value would range from 99% to 92%.
Results by Center
The proportion of technically inadequate magnetic resonance angiograms varied by clinical center (P < 0.001) and ranged from 11% to 51% (Table 4). Sensitivity of magnetic resonance angiograms ranged from 45% to 100% among centers, and specificity ranged from 95% to 100% (Table 4). Inadequate opacification occurred in 3% to 22% of images of main or lobar pulmonary arteries, 3% to 31% of images of segmental arteries, and 13% to 43% of images of subsegmental arteries.
Table 4.
Recruited Patients and Technical Adequacy, Sensitivity, and Specificity of Magnetic Resonance Angiography, by Clinical Center*
| Clinical Center | Patients, n | Technically Inadequate [95% CI], n/N (%) | Sensitivity [95% CI], n/N (%) | Specificity [95% CI], n/N (%) |
|---|---|---|---|---|
| 1 | 68 | 20/68 (29 [19–40]) | 5/11 (45 [17–77]) | 35/37 (95 [82–99]) |
|
| ||||
| 2 | 41 | 13/41 (32 [17–46]) | 6/6 (100 [54–100]) | 22/22 (100 [85–100]) |
|
| ||||
| 3 | 59 | 11/59 (19 [9–29]) | 19/22 (86 [65–97]) | 26/26 (100 [87–100]) |
|
| ||||
| 4 | 78 | 14/78 (18 [9–26]) | 1/1 (100 [3–100]) | 63/63 (100 [94–100]) |
|
| ||||
| 5 | 53 | 12/53 (23 [11–34]) | 11/18 (61 [36–83]) | 23/23 (100 [85–100]) |
|
| ||||
| 6 | 37 | 4/37 (11 [1–21]) | 12/12 (100 [74–100]) | 21/21 (100 [84–100]) |
|
| ||||
| 7 | 35 | 18/35 (51 [35–68]) | 5/6 (83 [36–100]) | 11/11 (100 [72–100]) |
|
| ||||
| Total | 371 | 92/371 (25 [20–29]) | 59/76 (78 [67–86]) | 201/203 (99 [96–100]) |
We do not know why test adequacy and sensitivity differed among clinics; causes may include vendor, age of equipment, software, experience of technicians, and level of supervision by radiologists.
The quality of magnetic resonance angiography combined with venography also varied by clinic (P < 0.001). Technically inadequate images ranged from 27% to 74% at the clinical centers (Table 5). Sensitivity ranged from 82% to 100%, and specificity ranged from 89% to 100% (Table 5).
Table 5.
Recruited Patients and Technical Adequacy, Sensitivity, and Specificity of Combined Magnetic Resonance Angiography and Venography, by Clinical Center
| Clinical Center | Recruited Patients, n | Technically Inadequate [95% CI], n/N (%) | Sensitivity [95% CI], n/N (%) | Specificity [95% CI], n/N (%) |
|---|---|---|---|---|
| 1 | 68 | 43/68 (63 [52–75]) | 5/6 (83 [36–100]) | 17/19 (89 [67–99]) |
|
| ||||
| 2 | 41 | 26/41 (63 [49–78]) | 6/6 (100 [54–100]) | 9/9 (100 [66–100]) |
|
| ||||
| 3 | 59 | 21/59 (36 [23–49]) | 21/22 (95 [77–100]) | 15/16 (94 [70–100]) |
|
| ||||
| 4 | 77* | 43/77 (56 [45–67]) | 1/1 (100 [3–100]) | 32/33 (97 [84–100]) |
|
| ||||
| 5 | 53 | 25/53 (47 [34–60]) | 14/17 (82 [57–96]) | 11/11 (100 [72–100]) |
|
| ||||
| 6 | 37 | 10/37 (27 [13–41]) | 13/13 (100 [75–100]) | 14/14 (100 [77–100]) |
|
| ||||
| 7 | 35 | 26/35 (74 [60–89]) | 5/6 (83 [36–100]) | 3/3 (100 [29–100]) |
| Total | 370 | 194/370 (52 [47–58]) | 65/71 (92 [83–97]) | 101/105 (96 [91–99]) |
One patient had magnetic resonance angiography but did not have magnetic resonance venography.
Adverse Events
No serious adverse events occurred that were related to magnetic resonance angiography and venography or any other tests used for evaluating possible pulmonary embolism. Follow-up was complete for 93% of patients in 3 months, 84% in 6 months, and 61% in 12 to 15 months.
Discussion
Magnetic resonance angiography was technically inadequate because of poor quality in 25% of patients. However, rates varied by center, with 1 center providing technically inadequate images for only 11% of patients. We do not know why the proportion of technically adequate images differed among clinics, but causes may include vendor, age of equipment, software, experience of technicians, and level of supervision by radiologists. When images were technically adequate, the sensitivity of magnetic resonance angiography was 78% and the specificity was 99%. Combined magnetic resonance angiography and venography had a higher sensitivity (92%) and similar specificity (96%), but technically adequate images were harder to obtain in both the lungs and lower extremities.
For both magnetic resonance angiography alone and combined magnetic resonance angiography and venography, a positive result combined with a high or intermediate objective clinical assessment by the Wells clinical prediction rule (9) indicated a 91% to 99% probability of pulmonary embolism. However, a positive test result with a discordantly low-probability objective clinical assessment resulted in a probability of pulmonary embolism of 84% with magnetic resonance angiography and 62% with combined magnetic resonance angiography and venography. Positive likelihood ratios greater than 10 or negative likelihood ratios less than 0.1, when combined with a concordant clinical probability, usually provide strong evidence to rule in or rule out diagnoses (12).
Because of the presumed importance of showing most pulmonary artery branches, we excluded pulmonary embolism in a patient only if pulmonary artery opacification was adequate through the subsegmental branches. Whether it is important to diagnose pulmonary embolism in subsegmental branches has been debated (16). Regardless of its importance, pulmonary embolism limited to subsegmental branches was diagnosed in only 1% (1 of 102) of patients who had computed tomographic angiography. Pulmonary embolism limited to subsegmental branches was found in 6% (22 of 375) of patients in PIOPED I (17) and 5% (8 of 175) of patients in PIOPED II (2). Oser and colleagues (18) found pulmonary embolism limited to subsegmental branches in 30% (23 of 76) of their patients.
The inability of magnetic resonance angiography to reliably show pulmonary embolism in subsegmental branches is not unique. The positive predictive value of apparent pulmonary embolism limited to subsegmental branches was only 25% (2 of 8) when using mostly 4-detector computed tomographic angiography (2), and readers of conventional pulmonary angiograms could agree on only 2 of 15 cases of pulmonary embolism limited to subsegmental branches (19).
We searched PubMed in all languages through September 2009 to identify published trials that showed the accuracy of magnetic resonance angiography for pulmonary embolism. Most previous investigations of magnetic resonance angiography (20 –25) had fewer than 20 patients with pulmonary embolism. Several investigators defined an adequate quality magnetic resonance angiography as adequate opacification through segmental vessels (22, 23, 26, 27), although some evaluated both subsegmental branches and larger branches (21, 25, 28). Investigators reported adequate visualization of main to segmental pulmonary arteries in 94% to 100% of patients (22–24, 26, 28). Images were adequate in main or lobar pulmonary arteries in 91% of patients and in segmental branches in 87% of patients.
Other studies have also reported a higher sensitivity in larger-order vessels. Previous investigations of magnetic resonance angiography (21, 23, 25, 28) showed sensitivities of 77% to 100% for pulmonary embolism in main or lobar pulmonary arteries. In segmental branches, sensitivity ranged from 68% to 84% (21, 24, 25, 28). In PIOPED III, sensitivity for detecting pulmonary embolism in main or lobar arteries was 79%. Data were too sparse (4 patients) to estimate sensitivity in patients in whom the largest pulmonary embolism was in a segmental branch. Most showed 0% to 40% sensitivity for detecting pulmonary embolism in subsegmental branches or isolated subsegmental branches (21, 22, 24, 28), although 1 investigation showed 60% sensitivity (25). Specificities with blinded readings, regardless of vessel order, were greater than 92% in all but 1 study (20–25, 28).
On the basis of pooled data from previous studies (29), magnetic resonance venography has a sensitivity of 91.5% and a specificity of 94.8% for diagnosis of deep venous thrombosis. Combined magnetic resonance angiography and venography identified 17% more cases of venous thromboembolism than magnetic resonance angiography alone (30).
No consensus on the percentages required for a “technically adequate” test has been achieved. Ascending contrast venography, a reference standard for trials of drugs for preventing deep venous thrombosis, was technically inadequate in 1 trial (31) in 497 of 1551 patients (32%). Others (32–34) reported lower proportions of technically inadequate tests that ranged from 2% to 8%. Ventilation–perfusion lung scans, although of technically adequate quality, are now thought to be nondiagnostic (not indicative of a definitive diagnosis) in 21% of patients, which is considered a low enough value to use the test (35).
Had we not randomly selected patients with a negative reference test result, 239 additional patients with a negative result would have had magnetic resonance angiography. The random sampling aspect of our design made the investigation more economical, avoided unnecessarily subjecting additional patients with a negative result to magnetic resonance angiography, and still resulted in a representative group.
Our study’s strengths include incorporation of all of the STARD (Standards for Reporting Diagnostic Accuracy) criteria (36, 37). Its limitations include the high proportion of patients who declined or were ineligible, mostly because of concerns about nephrogenic systemic fibrosis. In addition, selection bias is possible because some outpatients who did not want to return for additional tests declined to participate. Finally, imaging results may not apply to pregnant women; patients with renal failure; or patients who are critically ill, in shock, or on ventilatory support.
Future developments in technology may affect the detection of pulmonary embolism by magnetic resonance angiography. An intravascular magnetic resonance contrast agent, such as gadofosveset trisodium (recently approved by the U.S. Food and Drug Administration), remains in the blood pool in sufficiently high concentrations to continue imaging for 1 hour (38). This should reduce the number of studies rendered technically inadequate because of difficulties in timing the bolus and would allow repeated imaging if the patient moved during the scan. Non–contrast-enhanced magnetic resonance angiography imaging methods, such as steady-state free precession techniques coupled to respiratory navigators (which allow free breathing during imaging [39]), have been used to scan the aorta and coronary arteries, but no one has reported using them to detect pulmonary embolism. This technique may be useful in patients who cannot hold their breath or have renal insufficiency.
In conclusion, magnetic resonance angiography often resulted in technically inadequate images, and the rate of technically inadequate images varied considerably among centers. Magnetic resonance pulmonary angiography should therefore be considered only at centers that routinely perform it well and only for patients for whom standard tests are contraindicated. Magnetic resonance pulmonary angiography and magnetic resonance venography combined have a higher sensitivity than magnetic resonance pulmonary angiography alone in patients with technically adequate images, but it is harder to obtain technically adequate images with the combination of procedures. The posttest probability of a correct diagnosis with the imaging techniques is high when concordant with an objective clinical assessment, but additional testing is necessary when objective clinical assessment is inconsistent with imaging results.
Acknowledgments
Grant Support: By the U.S. Department of Health and Human Service, Public Health Service, National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland (grants HL081593, HL177150, HL077149, HL077151, HL077154, HL081594, HL077358, HL077155, and HL077153).
Appendix: The PIOPED III Investigators
Emory University, Atlanta, Georgia: K. Leeper (principal investigator), A. Thron, S. Sadler, S. Clemens, E. Berkowitz, D. Fluker, B. Hatfield, D. Shaz, E. Sherling, A. Silverstein, R. Woodcock, M. Wright.
Massachusetts General Hospital, Boston, Massachusetts: C. Hales (principal investigator), J. Cahill, M. Hegarty, P. Currier, S. Abbara, A. Greenfield, C. Kabrhel, E. Palmer, D. Peak, D. Quinn, J. Scott, A. Syrkina, A. Waltman, C. Wittram.
New York University, New York, New York: D. Naidich (principal investigator), S. Chow, W. Goldberg, E. Hecht, V. Lee, F. Ponzo, E. Suan.
University of Calgary, Calgary, Alberta, Canada: R. Hull (principal investigator), S. McGuire, J. Wright, G. Brunet, P. Burrowes, S. Curtis, D. Elliot, R. Kloiber, T. Lenko, J. Mac-Gregor, H. Mhallati, C. Molnar, E. Raber, B. So.
University of Michigan, Ann Arbor, Michigan: J. Weg (principal investigator), L. Sawyer, T. Chenevert, K. Cho, P. Cronin, B. Desjardins, F. Londy, M. Lowell, G. Mueller, S. Patel, T. Wakefield, K. Zebari.
St. Joseph-Mercy Oakland Hospital, Pontiac, Michigan: D. Sak (principal investigator), J. Chapp, O. Yaekoub, P. Stein, I. Alesh, M. Alnas, A. Badshah, A. Beemath, L. Billingsley, J. Denier, T. Elam, S. Foust, L. Gerych, F. Kayali, C. Lamphere, R. Malik, M. Malafa, K. McKlusky, A. Munaco, R. Najjar, D. Reigle, S. Sharp, M. Sioma, E. Skaf, S. Zuska.
Washington University, St. Louis, Missouri: P. Woodard (principal investigator), M. Mohrman, S. Bhalla, D. Brown, L. Gallagher, J. Heiken, C. Javidan-Nejad, D. Lesniak, L. Lewis, J. Liu, V. Narra, H. Royal, B. Rubin, B. Siegel, J. Wade, R. Yusen, J. Zheng.
Consultants: L. Goodman, Medical College of Wisconsin; A. Gottschalk, Michigan State University; E. Kanal, University of Pittsburgh; H. Sostman, Methodist Hospital Houston and Cornell University; V. Tapson, Duke University.
Data Coordinating Center—George Washington University, Rockville, Maryland: S. Fowler (principal investigator), J. Bamdad, A. Gottlieb, K. Jablonski, A. Sapzhnikova, M. Yau.
Administrative Centers—Michigan State University College of Osteopathic Medicine, East Lansing, Michigan, and St. Joseph Mercy Oakland Hospital, Pontiac, Michigan: P. Stein (principal investigator), F. Matta, A. Yaekoub.
Project Office—National Heart, Lung, and Blood Institute, Bethesda, Maryland: J. Kiley, E. Denholm.
Data Safety Monitoring Board: J. Dalen (Chair), University of Arizona; T. Grist, University of Wisconsin; P. Lachenbruch, Oregon State University; F. Miller, University of California, San Diego; H. Peavy (executive secretary), National Heart, Lung, and Blood Institute.
Steering Committee: P. Stein (Chair), S. Fowler, L. Goodman, A. Gottschalk, C. Hales, R. Hull, K. Leeper, D. Naidich, D. Sak, H. Sostman, V. Tapson, J. Weg, P. Woodard.
Operations Committee: P. Stein (Chair), J. Bamdad, P. Currier, S. Fowler, C. Hales, R. Hull, K. Jablonski, D. Naidich, H. Sostman, J. Weg, P. Woodard, M. Yau.
Outcome Committee: J. Weg (Chair), V. Tapson.
Ethics Committee: J. Weg (Chair), S. Fowler.
Ancillary Studies Committee: S. Fowler (Chair), P. Stein.
Magnetic Resonance Imaging Working Group: P. Woodard (Chair), S. Abbara, T. Chenevert, P. Cronin, B. Hatfield, E. Hecht, J. Liu, G. Mueller, A. Munaco, D. Naidich, V. Narra, H. Sostman, R. Woodcock.
Computed Tomography Working Group: L. Goodman (Chair), S. Bhalla, P. Burrowes, B. Hatfield, J. Heiken, J. Ko, J. MacGregor, S. Patel, M. Shiau, J. Vlahos, C. Wittram, P. Woodard, R. Woodcock.
Ventilation–Perfusion Scan Working Group: A. Gottschalk (Chair), E. Palmer, H. Royal, J. Scott, H. Sostman.
Clinical Science Working Group: J. Weg (Chair), C. Hales, R. Hull, K. Leeper, D. Quinn, D. Sak, P. Stein, V. Tapson, R. Yusen.
Footnotes
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M09-1652.
Reproducible Research Statement: Study protocol and data set: Will be released to the NHLBI in May 2010. Interested parties should contact the NHLBI (www.nhlbi.nih.gov/resources/deca/directry.htm) after this date to obtain copies. Statistical code: Not available.
Author Contributions: Conception and design: P.D. Stein, S.E. Fowler, L.R. Goodman, A. Gottschalk, C.A. Hales, R.D. Hull, K.A. Jablonski, H.D. Sostman, V.F. Tapson, J.G. Weg, P.K. Woodard.
Analysis and interpretation of the data: P.D. Stein, S.E. Fowler, L.R. Goodman, A. Gottschalk, C.A. Hales, R.D. Hull, K.A. Jablonski, K.V. Leeper, D.P. Naidich, D.J. Sak, H.D. Sostman, V.F. Tapson, J.G. Weg, P.K. Woodard.
Drafting of the article: P.D. Stein, T.L. Chenevert, S.E. Fowler, C.A. Hales, R.D. Hull, K.A. Jablonski, H.D. Sostman, J.G. Weg, P.K. Woodard.
Critical revision of the article for important intellectual content: P.D. Stein, T.L. Chenevert, S.E. Fowler, L.R. Goodman, A. Gottschalk, C.A. Hales, R.D. Hull, K.A. Jablonski, K.V. Leeper, H.D. Sostman, V.F. Tapson, J.G. Weg, P.K. Woodard.
Final approval of the article: P.D. Stein, S.E. Fowler, L.R. Goodman, A. Gottschalk, C.A. Hales, R.D. Hull, K.A. Jablonski, K.V. Leeper, D.P. Naidich, D.J. Sak, H.D. Sostman, V.F. Tapson, J.G. Weg, P.K. Woodard.
Provision of study materials or patients: C.A. Hales, R.D. Hull, K.V. Leeper, D.P. Naidich, J.G. Weg, P.K. Woodard.
Statistical expertise: S.E. Fowler, R.D. Hull, K.A. Jablonski, H.D. Sostman.
Obtaining of funding: P.D. Stein, C.A. Hales, R.D. Hull, J.G. Weg, P.K. Woodard.
Administrative, technical, or logistic support: P.D. Stein, T.L. Chenevert, R.D. Hull, K.A. Jablonski.
Collection and assembly of data: P.D. Stein, T.L. Chenevert, S.E. Fowler, C.A. Hales, R.D. Hull, K.A. Jablonski, K.V. Leeper, D.P. Naidich, D.J. Sak, J.G. Weg, P.K. Woodard.
References
- 1.Agency for Healthcare Research and Quality. Evidence Report/Technology Assessment No. 68. Rockville, MD: Agency for Healthcare Research and Quality; 2003. [18 February 2010]. Diagnosis and Treatment of Deep Venous Thrombosis and Pulmonary Embolism. Accessed at www.ahrq.gov/clinic/epcsums/dvtsum.htm on. [Google Scholar]
- 2.Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–27. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 3.Shellock FG. Manual for Magnetic Resonance Safety Implants and Devices. Los Angeles: Biomedical Research Publishing; 2004. [Google Scholar]
- 4.Stein PD, Gottschalk A, Sostman HD, Chenevert TL, Fowler SE, Goodman LR, et al. Methods of Prospective Investigation of Pulmonary Embolism Diagnosis III (PIOPED III) Semin Nucl Med. 2008;38:462–70. doi: 10.1053/j.semnuclmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration. Information for Healthcare Professionals: Gadolinium-Based Contrast Agents for Magnetic Resonance Imaging (marketed as Magnevist, MultiHance, Omniscan, OptiMARK, ProHance) Silver Spring, MD: U.S. Food and Drug Administration; 2006. [18 February 2010]. Accessed at www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142884.htm on. [Google Scholar]
- 6.Cowper SE. [18 February 2010];Nephrogenic fibrosing dermopathy. 2001–2009 doi: 10.1097/00000372-200110000-00001. Accessed at www.icnsfr.org on. [DOI] [PubMed]
- 7.Gottschalk A, Stein PD, Goodman LR, Sostman HD. Overview of Prospective Investigation of Pulmonary Embolism Diagnosis II. Semin Nucl Med. 2002;32:173–82. doi: 10.1053/snuc.2002.124177. [DOI] [PubMed] [Google Scholar]
- 8.Gottschalk A, Sostman HD, Coleman RE, Juni JE, Thrall J, McKusick KA, et al. Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. J Nucl Med. 1993;34:1119–26. [PubMed] [Google Scholar]
- 9.Wells PS, Ginsberg JS, Anderson DR, Kearon C, Gent M, Turpie AG, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Fisher L, van Belle G. Biostatistics: A Methodology for the Health Sciences. New York: J Wiley; 1993. p. 206. [Google Scholar]
- 11.Simel DL, Feussner JR, DeLong ER, Matchar DB. Intermediate, indeterminate, and uninterpretable diagnostic test results. Med Decis Making. 1987;7:107–14. doi: 10.1177/0272989X8700700208. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–7. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 13.Sox HC. Commentary: D-Dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:602. doi: 10.7326/0003-4819-140-8-200404200-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 15.Sanson BJ, Lijmer JG, MacGillavry MR, Turkstra F, Prins MH, Büller HR. Comparison of a clinical probability estimate and two clinical models in patients with suspected pulmonary embolism. ANTELOPE-Study Group. Thromb Haemost. 2000;83:199–203. [PubMed] [Google Scholar]
- 16.Goodman LR. Small pulmonary emboli: what do we know? [Editorial] Radiology. 2005;234:654–8. doi: 10.1148/radiol.2343041326. [DOI] [PubMed] [Google Scholar]
- 17.Stein PD, Henry JW. Prevalence of acute pulmonary embolism in central and subsegmental pulmonary arteries and relation to probability interpretation of ventilation/perfusion lung scans. Chest. 1997;111:1246–8. doi: 10.1378/chest.111.5.1246. [DOI] [PubMed] [Google Scholar]
- 18.Oser RF, Zuckerman DA, Gutierrez FR, Brink JA. Anatomic distribution of pulmonary emboli at pulmonary angiography: implications for cross-sectional imaging. Radiology. 1996;199:31–5. doi: 10.1148/radiology.199.1.8633168. [DOI] [PubMed] [Google Scholar]
- 19.Quinn MF, Lundell CJ, Klotz TA, Finck EJ, Pentecost M, McGehee WG, et al. Reliability of selective pulmonary arteriography in the diagnosis of pulmonary embolism. AJR Am J Roentgenol. 1987;149:469–71. doi: 10.2214/ajr.149.3.469. [DOI] [PubMed] [Google Scholar]
- 20.Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR. Diagnosis of pulmonary embolism with magnetic resonance angiography. N Engl J Med. 1997;336:1422–7. doi: 10.1056/NEJM199705153362004. [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Frazer CK, Ferguson JM, Kumar AB, Davis SJ, Fallon MJ, et al. Acute pulmonary embolism: diagnosis with MR angiography. Radiology. 1999;210:353–9. doi: 10.1148/radiology.210.2.r99fe53353. [DOI] [PubMed] [Google Scholar]
- 22.Loubeyre P, Revel D, Douek P, Delignette A, Baldy C, Genin G, et al. Dynamic contrast-enhanced MR angiography of pulmonary embolism: comparison with pulmonary angiography. AJR Am J Roentgenol. 1994;162:1035–9. doi: 10.2214/ajr.162.5.8165977. [DOI] [PubMed] [Google Scholar]
- 23.Ohno Y, Higashino T, Takenaka D, Sugimoto K, Yoshikawa T, Kawai H, et al. MR angiography with sensitivity encoding (SENSE) for suspected pulmonary embolism: comparison with MDCT and ventilation-perfusion scintigraphy. AJR Am J Roentgenol. 2004;183:91–8. doi: 10.2214/ajr.183.1.1830091. [DOI] [PubMed] [Google Scholar]
- 24.Pleszewski B, Chartrand-Lefebvre C, Qanadli SD, Déry R, Perreault P, Oliva VL, et al. Gadolinium-enhanced pulmonary magnetic resonance angiography in the diagnosis of acute pulmonary embolism: a prospective study on 48 patients. Clin Imaging. 2006;30:166–72. doi: 10.1016/j.clinimag.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Kluge A, Luboldt W, Bachmann G. Acute pulmonary embolism to the subsegmental level: diagnostic accuracy of three MRI techniques compared with 16-MDCT. AJR Am J Roentgenol. 2006;187:W7–14. doi: 10.2214/AJR.04.1814. [DOI] [PubMed] [Google Scholar]
- 26.Ersoy H, Goldhaber SZ, Cai T, Luu T, Rosebrook J, Mulkern R, et al. Time-resolved MR angiography: a primary screening examination of patients with suspected pulmonary embolism and contraindications to administration of iodinated contrast material. AJR Am J Roentgenol. 2007;188:1246–54. doi: 10.2214/AJR.06.0901. [DOI] [PubMed] [Google Scholar]
- 27.Blum A, Bellou A, Guillemin F, Douek P, Laprévote-Heully MC, Wahl D GENEPI study group. Performance of magnetic resonance angiography in suspected acute pulmonary embolism. Thromb Haemost. 2005;93:503–11. doi: 10.1160/TH04-08-0495. [DOI] [PubMed] [Google Scholar]
- 28.Oudkerk M, van Beek EJ, Wielopolski P, van Ooijen PM, Brouwers-Kuyper EM, Bongaerts AH, et al. Comparison of contrast-enhanced magnetic resonance angiography and conventional pulmonary angiography for the diagnosis of pulmonary embolism: a prospective study. Lancet. 2002;359:1643–7. doi: 10.1016/S0140-6736(02)08596-3. [DOI] [PubMed] [Google Scholar]
- 29.Sampson FC, Goodacre SW, Thomas SM, van Beek EJ. The accuracy of MRI in diagnosis of suspected deep vein thrombosis: systematic review and meta-analysis. Eur Radiol. 2007;17:175–81. doi: 10.1007/s00330-006-0178-5. [DOI] [PubMed] [Google Scholar]
- 30.Kluge A, Mueller C, Strunk J, Lange U, Bachmann G. Experience in 207 combined MRI examinations for acute pulmonary embolism and deep vein thrombosis. AJR Am J Roentgenol. 2006;186:1686–96. doi: 10.2214/AJR.05.0756. [DOI] [PubMed] [Google Scholar]
- 31.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–80. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 32.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. RE-NOVATE Study Group Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 34.Planes A, Vochelle N, Darmon JY, Fagola M, Bellaud M, Huet Y. Risk of deep-venous thrombosis after hospital discharge in patients having undergone total hip replacement: double-blind randomised comparison of enoxaparin versus placebo. Lancet. 1996;348:224–8. doi: 10.1016/s0140-6736(96)01453-5. [DOI] [PubMed] [Google Scholar]
- 35.Sostman HD, Miniati M, Gottschalk A, Matta F, Stein PD, Pistolesi M. Sensitivity and specificity of perfusion scintigraphy combined with chest radiography for acute pulmonary embolism in PIOPED II. J Nucl Med. 2008;49:1741–8. doi: 10.2967/jnumed.108.052217. [DOI] [PubMed] [Google Scholar]
- 36.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Standards for Reporting of Diagnostic Accuracy. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med. 2003;138:W1–12. doi: 10.7326/0003-4819-138-1-200301070-00012-w1. [DOI] [PubMed] [Google Scholar]
- 37.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. STARD group. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Ann Clin Biochem. 2003;40:357–63. doi: 10.1258/000456303766476986. [DOI] [PubMed] [Google Scholar]
- 38.Hadizadeh DR, Gieseke J, Lohmaier SH, Wilhelm K, Boschewitz J, Verrel F, et al. Peripheral MR angiography with blood pool contrast agent: prospective intraindividual comparative study of high-spatial-resolution steady-state MR angiography versus standard-resolution first-pass MR angiography and DSA. Radiology. 2008;249:701–11. doi: 10.1148/radiol.2492072033. [DOI] [PubMed] [Google Scholar]
- 39.Krishnam MS, Tomasian A, Deshpande V, Tran L, Laub G, Finn JP, et al. Noncontrast 3D steady-state free-precession magnetic resonance angiography of the whole chest using nonselective radiofrequency excitation over a large field of view: comparison with single-phase 3D contrast-enhanced magnetic resonance angiography. Invest Radiol. 2008;43:411–20. doi: 10.1097/RLI.0b013e3181690179. [DOI] [PubMed] [Google Scholar]