Abstract
Animal studies have demonstrated that helminth vaccines which induce type 2 immune responses can be protective. To date, however, such vaccines have not been tested against repeated parasite challenges. Since repeated antigenic challenge of patients with allergic disease results in immunologic tolerance, we hypothesized that a helminth vaccine which induces type 2 immune responses may lose its protective efficacy in the setting of repeated parasite exposures (RPEs). To test this hypothesis, we examined whether RPEs induce immunological tolerance and reduce the effectiveness of a type 2 immune-inducing vaccine. BALB/c mice vaccinated against Litomosoides sigmodontis, a filarial nematode of rodents, were repeatedly exposed to irradiated larvae for two or eight weeks or to non-irradiated infectious larvae for three months.
Vaccination-induced parasite-specific IgE levels, parasite antigen-driven basophil interleukin 4 (IL-4) release, and Th2 skewing of the cellular immune response remained stable in the face of RPEs. Furthermore, RPEs in vaccinated mice did not augment immunoregulatory responses, as parasite antigen-driven cellular proliferation, production of IL-10, and frequencies of CD4+CD25+FoxP3+ regulatory T-cells were not altered by RPEs. Challenge infections with infectious L3-stage larvae resulted in lower worm burdens in vaccinated mice given RPEs than in vaccinated controls. These results demonstrate that vaccines which induce type 2 immune responses can maintain their efficacy in the setting of repeated parasite exposures.
Keywords: vaccine, helminth, IgE, type 2 immunity, IL-4, basophil, filarial, Litomosoides sigmodontis, immunological tolerance, desensitization, regulatory T-cells
1. Introduction
Helminths infect more than one billion people, primarily in developing regions in Sub-Saharan Africa, Asia and the Americas [1, 2]. Because of increasing appreciation for the substantial morbidity caused by these infections, including growth stunting, anemia, malnutrition, and impaired cognition [1, 3, 4], in the past several years there has been renewed interest in developing vaccines against human helminth infections.
Type 2 immune responses are characterized by the development of antigen-specific IgE, and production of type 2 cytokines such as IL-4 and IL-13. Research efforts have found that induction of type 2 immune responses and antigen-specific IgE may be desirable for the creation of effective helminth vaccines. Protection against helminth challenge after vaccination with irradiated parasites is associated with production of type 2 cytokines in animal models of hookworm and filaria infection [5, 6]. Similarly, high titers of parasite-specific IgE antibodies in people are associated with partial protection against reinfection with Schistosoma japonicum [7] and hookworm vaccine studies demonstrate that host IgE responses against hookworm antigen correlate with increased protection [8]. Additionally, IgE has been shown to be necessary for vaccine efficacy against Haemonchus contortus, a blood-feeding nematode of sheep [9], and for vaccine protection in the Onchocerca murine model of filariasis [10]. Because of these findings, consideration has been given towards developing helminth-specific vaccines which induce parasite-specific IgE responses [11].
To date, helminth vaccines that induce type 2 immunity and IgE responses have only been tested against a single challenge infection. A theoretical concern of an IgE-inducing vaccine, however, is that the protective efficacy of an IgE-driven vaccine response could possibly decrease in the setting of repeated parasite exposures in a manner akin to that observed in desensitization protocols of patients with allergen-specific IgE. In allergen-specific immunotherapy (SIT), repeated allergen exposures result in clinical tolerance towards allergen. Immunologic changes associated with SIT include decreases in allergen-specific IgE, increases in allergen-specific IgG4, an allergen-specific T-cell shift from Th2 to Th1, and peripheral T-cell tolerance towards allergen due to increased IL-10 production from antigen-specific and CD4+CD25+ regulatory T-cells [12, 13]. Thus, as Th2 responses and IgE have been shown to be involved in protection to filarial parasites [10, 14–19] and as induction of immunotolerance facilitates helminth survival [20, 21], we hypothesized that repeated parasite exposures (RPEs) may decrease the efficacy of a type 2 immune-inducing vaccine. Such a phenomenon would have important implications for helminth vaccine design since individuals in endemic areas are repeatedly exposed to parasites.
For our studies, we chose to use the Litomosoides sigmodontis murine model of filariasis [22, 23] in which a series of three vaccinations with irradiated larvae confers protection against challenge infection with infectious-stage L3 larvae [24]. This vaccination regimen induces a type 2 immune response with increases in type 2 cytokines such as IL-4 [5] and, as we show in this study, elevated levels of parasite-specific IgE. Testing of our hypothesis was done by evaluating whether repeated injections of either irradiated infectious-stage (L3) L. sigmodontis larvae for two or eight weeks or infectious L3 larvae for 3 months substantially alter the immune responses and protective efficacy of the type 2 immune-inducing vaccination regimen against L. sigmodontis.
2. Material and methods
2.1 Mice and parasites
Female BALB/c and C57BL/6 mice (NCI Mouse Repository, Frederick, MD) were maintained at the Uniformed Services University of the Health Sciences (USU) animal facility. Experiments were performed with 4–6-week old mice under a protocol approved by the USU Institutional Animal Care and Use Committee. Infectious-stage L. sigmodontis L3 larvae were isolated by lavage from the pleural cavity of four-day infected jirds (Meriones unguiculatus, obtained from TRS Laboratory Inc., Athens, GA) as described previously [25].
2.2 Repeated parasite exposures (RPEs) with irradiated, cryopreserved L3 larvae
BALB/c mice were vaccinated with three weekly subcutaneous injections of either 25 irradiated L3s (450 Gy, cobalt 60 irradiator) in media (RPMI-1640, Mediatech, Herndon, VA) or media alone. Two weeks after the third vaccination, mice were given RPEs by subcutaneous injection of five irradiated, cryopreserved L3s or media as control every other day for two weeks or three times a week for eight weeks. L3s were cryopreserved in RPMI-1640 containing 0.27M sucrose (Sigma, St. Louis, MO) and 6% DMSO (Sigma). Use of cryopreserved larvae for RPEs ensured a regular supply of worms available for injection every two to three days. Because irradiated larvae migrate through the tissues but do not develop into adult worms, use of irradiated, cryporeserved L3 larvae enabled us to easily distinguish worms acquired during RPEs from adult worms acquired by challenge infection.
Three days after the last RPE mice were sacrificed for immunological studies. Additionally, some BALB/c mice that received two or eight weeks of RPEs were challenged with 40 infectious L3s and euthanized 56 days later for enumeration of adult worms by pleural lavage.
2.3 Repeated parasite exposures with infectious L3 larvae
In an additional experiment BALB/c mice were vaccinated as described above and two weeks after the final vaccination repeatedly infected with 5 live, fully infectious L3 larvae (n=10) that were isolated from the pleural cavity of recently infected jirds or RPMI as control (n=9). Mice were repeatedly parasite exposed with infectious L3 larvae three times per month for 3 months with a total of 9 repeated parasite exposures. Two weeks after the last repeated parasite exposure mice were challenged with 40 infectious L3 larvae and euthanized 20 days after challenge. Numbers of fourth stage (L4) larvae in the pleural space at that timepoint represented worms from the final challenge infection, whereas any adult (L5) worms recovered at that timepoint were acquired by RPEs (34 days after the last RPE).
In a single experiment naïve C57BL/6 mice were infected with a total of 40 infectious L. sigmodontis L3 larvae by either a single challenge with 40 L3s (n=4) or 8 challenges of 5 L3s every other week (n=4). At day 62 after the last/single challenge, mice were sacrificed, worm burdens determined, and immunological studies performed.
2.4 Repeated administration of parasite antigen
In addition to testing RPEs with living worms, in a separate experiment we analyzed development of immunologic tolerance in BALB/c mice that were vaccinated with three weekly intra peritoneal injections of 100 μg of L. sigmodontis adult worm antigen (LsAg, prepared as described in 2.5) adsorbed to alum (Thermo Fisher Scientific Inc., Waltham, MA). Two weeks after the last vaccination mice were injected three times per week with 5 μg of LsAg (n=10) or PBS as control (n=10) for a total of eight weeks. Blood was collected from mice two weeks into the course of repeated LsAg/PBS injections and mice were euthanized after 8 weeks of LsAg/PBS injections to obtain blood and splenocytes for immunological studies.
2.5 L. sigmodontis adult worm antigen (LsAg)
Frozen adult L. sigmodontis worms were lyophilized, resuspended in PBS and stirred overnight at 4°C. After centrifugation (750×g, 10 min, 4°C) the supernatant was collected. The pellet was stirred again overnight, centrifuged, and the supernatant combined with the first supernatant. After a final centrifugation at 5300×g for 30 min at 4°C, supernatant was collected, sterile filtered, and the protein content measured with the BCA Protein Assay kit (Pierce, Rockford, IL).
2.6 Cellular proliferation
Three days after the last RPE, spleen, brachial and axillary lymph node cells were isolated and single cell suspensions processed. Red blood cell lysis was performed with spleen cells (ACK Lysing Buffer, Invitrogen Inc., Carlsbad, CA). Cells were plated as triplicates at 2×105 cells in 100 μl enriched media (Iscove’s Dulbecco modified medium (Mediatech) including 10% fetal calf serum (Valley Biomedical, Winchester, VA), 1% L-glutamine (Mediatech), 1% insulin-transferrin-selenium (Invitrogen Inc.) and 80 μg/ml gentamicin (Invitrogen Inc.)). Cells were stimulated with 20 μg/ml LsAg or 5 μg/ml anti-CD3 (eBioscience, San Diego, CA) and 2 μg/ml anti-CD28 (eBioscience) and cultured at 37°C, 5% CO2. After two days, BrdU was added and cells were cultured for an additional 16 hours. Cell proliferation was assessed using a BrdU chemiluminescent assay per the manufacturer’s instructions (Roche Diagnostics GmbH, Mannheim, Germany).
2.7 Flow cytometric detection of regulatory T-cells and intracellular cytokine production by T-cells and basophils
Three days after the last RPE, spleen cells were isolated and stimulated as described above, at a total of 1×107 cells in 5 ml enriched media. After two hours of incubation, BD GolgiStop (BD Biosciences, San Jose, CA) was added and cells were incubated for an additional four hours. Cells were prepared for flow as described previously [26]. Collected cells were blocked for one hour with PBS/1% BSA (Sigma), followed by fixation and permeabilization overnight in fix/perm-buffer (eBioscience). After two washing steps, cells were stained for flow cytometry with rat-anti-mouse CD4 PerCP (BD Biosciences), rat-anti-mouse FoxP3 FITC (eBioscience), rat-anti-mouse CD25 APC-Alexa Fluor 750 (eBioscience) and rat-anti-mouse IL-10 PE (eBioscience) or rat-anti-mouse CD4 PerCP (BD Biosciences), rat-anti-mouse IL-4 APC (BD Biosciences) and rat-anti-mouse gamma interferon (IFNγ) FITC (eBioscience).
Basophil flow was performed on cultured spleen cells that were blocked, fixed, and permeabilized as above and then subsequently cryopreserved with PBS/10% DMSO (Sigma). After thawing, cells were washed and stained with rat-anti-mouse IgE FITC (BD Biosciences), rat-anti-mouse IL-4 APC (BD Biosciences), rat-anti-mouse CD4 PerCP (BD Biosciences), and rat-anti-mouse B220 PerCP (BD Biosciences). During flow cytometric analysis, basophils were identified as CD4-/B220-/IgE+ cells.
Detection of IL-4 production in basophils from peripheral whole blood samples was conducted as described previously [27]. Briefly, 100 μl of blood were diluted with 100 μl of RPMI and stimulated with LsAg or Media and incubated at 37°C with 5% CO2. After one hour GolgiStop (BD Bioscience) was added for an additional two hours of incubation. Cells were then fixed and red blood cells lysed using a whole blood lysing reagent kit (Immuno-Lyse, Beckman Coulter, Inc.). Cells were permeabilized with Perm/Wash buffer (BD Biosciences) and basophils stained for flow cytometric analysis as mentioned above.
Flow cytometry was performed using a BD LSRII system and subsequently analyzed with FACSDiva 6.0 software (BD Biosciences). All flow cytometry antibodies were individually titrated and, prior to each experiment, compensation conducted with BD CompBeads (BD Biosciences) bound to the flow cytometry antibodies used in that experiment. During analysis, cut-offs for cytokine and CD25-positivity were set using the fluorescence-minus-one approach.
2.8 Measurement of cytokines, LsAg-specific and total IgE by ELISA
Cytokine enzyme-linked immunosorbent assays (ELISAs) were performed on supernatants from 72 hour cultures of 2×106 splenocytes/ml stimulated as described above. IFNγ, IL-4, IL-5 and IL-10 were quantified according to manufacturer’s instructions (OptEIA™ Set Mouse, BD Biosciences). Spontaneous cytokine production of unstimulated cells was subtracted.
LsAg-specific and total IgE levels in mouse plasma were analyzed after two or eight weeks of RPEs. EIA/RIA-plates (Corning Inc., Corning, NY) were coated overnight at 4°C with 20 μg/ml of LsAg in PBS or 10 μg/ml of purified rat-anti-mouse IgE (BD Biosciences). Plates were then washed and samples incubated for 2 hours at RT. After another wash biotinylated anti-mouse IgE detection antibody (BD Biosciences) was added at 2 μg/ml for 2 hours at RT. After a final wash, alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was added and 4-nitrophenyl-phosphate-disodium-salt-hexahydrate (Sigma) was used as substrate. Absorbance was measured at 405 nm (Victor3V, PerkinElmer, Waltham, MA). For total IgE ELISAs, purified mouse IgE (BD Biosciences) was used for standard curves.
2.9 Statistics
Statistical analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA). Differences between multiple groups were tested for significance using the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. Differences between two unpaired groups were tested for significance with the Mann-Whitney-U-test and differences between paired groups with the Wilcoxon matched pairs test. P-values <0.05 were considered significant.
3. Results
3.1 Vaccination of BALB/c mice with irradiated L. sigmodontis larvae induces a protective type 2 immune response
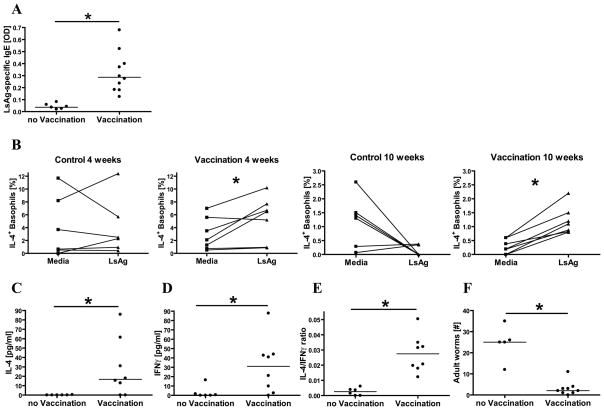
To confirm that vaccination with irradiated L. sigmodontis larvae induces a type 2 immune response, mice were vaccinated with irradiated L. sigmodontis larvae and assessed for both humoral and cellular parameters of immunity four weeks later. Whereas unvaccinated mice never developed detectable LsAg-specific IgE, vaccinated mice developed high quantities of circulating LsAg-specific IgE (Fig. 1A). This LsAg-specific IgE was shown to be functionally active, as flow cytometric studies demonstrated that stimulation of spleen cells with LsAg for 6 hours caused significant increases in the frequencies of basophils releasing IL-4 when compared to media stimulation in vaccinated mice but not in unvaccinated mice (Fig. 1B).
Fig. 1.
Comparisons of mice that were vaccinated against L. sigmodontis and non-vaccinated controls. (A) Plasma levels of L. sigmodontis-specific IgE (OD). (B) Percentages of basophils that stain positively for IL-4 by multicolor flow cytometry when cultured with media alone or with parasite antigen (LsAg). Shown are mice four or ten weeks after vaccination and the corresponding controls. (C) Splenic IL-4 and (D) IFNγ cytokine production in response to parasite antigen and (E) IL-4/IFNγ ratio in response to anti-CD3/anti-CD28 stimulation. (F) Worm recovery 8 weeks after challenge with 40 infectious L. sigmodontis larvae. Significant differences between unpaired groups were analyzed by Mann-Whitney-U-test and between paired groups by Wilcoxon matched pairs test (*p<0.05). Horizontal lines represent median values obtained from all experiments.
To determine whether this vaccination protocol also resulted in type 2 skewing of the cellular immune response, cytokine production from splenocytes cultured with media, LsAg, or anti-CD3/anti-CD28 was assessed in vaccinated and unvaccinated mice. Splenocytes from vaccinated mice produced significantly greater amounts of IL-4 in response to LsAg than splenocytes from unvaccinated mice (Fig. 1C). While vaccination also resulted in the production of LsAg-specific IFNγ (Fig. 1D), as a whole the cellular immune responses of vaccinated animals were skewed towards type 2 phenotypes as the IL-4/IFNγ ratio measured after stimulation of splenocytes with anti-CD3/anti-CD28 was significantly greater in vaccinated than control mice (median 0.0261 vs. 0.0026, Fig. 1E).
Protective efficacy of this vaccine regimen was clearly demonstrated as challenge infection with 40 infectious-stage L3 larvae resulted in an average protective efficacy of 88% (median of 25 adult worms in control mice vs. 2 in vaccinated mice, Fig. 1F).
3.2 Vaccination-induced LsAg-specific IgE levels remain high and functional despite repeated parasite exposures with irradiated larvae
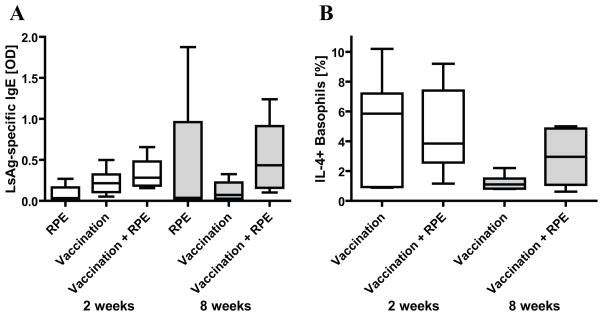
Since repeated allergen exposures in specific immunotherapy decrease allergen-specific IgE levels [12, 28], we investigated if RPEs decrease the amount of circulating parasite-specific IgE in vaccinated mice. Beginning two weeks after final vaccination, mice were injected with irradiated larvae every other day for two weeks or 3x/week for 8 weeks and then assessed for circulating LsAg-specific IgE and for basophil IL-4 production in response to LsAg. Two weeks of RPEs did not significantly alter the amount of LsAg-specific IgE present in the serum of vaccinated mice, and 8 weeks of RPEs resulted in higher levels of LsAg-specific IgE compared to vaccinated controls, though this difference was not statistically significant (Fig. 2A). Circulating LsAg-specific IgE levels remained at similar levels in vaccinated controls four and ten weeks after final vaccination.
Fig. 2.
(A) L. sigmodontis-specific plasma IgE levels (OD) from mice that were vaccinated against L. sigmodontis and/or given repeated parasite exposures (RPE) with irradiated larvae for two (○) or eight weeks (●). OD levels from unvaccinated, media-exposed controls were set to zero. (B) Percentages of IL-4 positive basophils in response to parasite antigen from mice that were vaccinated and/or repeatedly parasite exposed with irradiate larvae for 2 or 8 weeks, as determined by multicolor flow cytometry. Significant differences between groups were analyzed by the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons (*p<0.05). Timepoints listed are duration of RPEs. As RPEs started 2 weeks after final vaccination, mice in the 2 week group were tested 4 weeks after final vaccination and those in the 8 week group tested 10 weeks after final vaccination. Shown are medians from two independent experiments (2 week timepoint: RPE n=5, Vaccination n=8, Vaccination + RPE n=8; 8 week timepoint: RPE n=4, Vaccination n=7, Vaccination + RPE n=8).
Flow cytometric studies evaluating basophil IL-4 release paralleled those of circulating LsAg-specific IgE levels. Percentages of basophils that released IL-4 in response to LsAg were the same for vaccinated mice that received 2 weeks of RPEs as for those that did not (Fig. 2B). In contrast, the percentages of basophils that stained positively for IL-4 in response to LsAg was slightly greater in vaccinated mice that had received 8 weeks of RPEs compared to vaccinated mice without RPEs (Fig. 2B).
3.3 Repeated administration of irradiated larvae to vaccinated mice does not decrease parasite antigen-driven spleen or lymph node cell proliferation
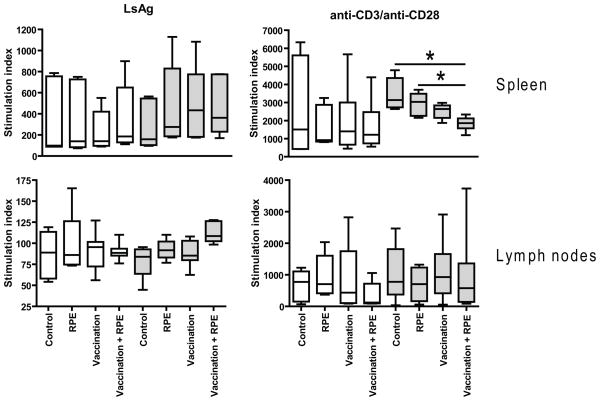
SIT is associated with induction of T-cell tolerance characterized by decreased allergen-specific cellular proliferation [13, 29]. Thus, we assessed the proliferative responses of spleen and lymph node cells to parasite antigen from mice that were vaccinated alone or vaccinated and administered RPEs with irradiated larvae for two or eight weeks.
As with specific IgE levels, LsAg-driven proliferative responses of spleen and lymph nodes did not decrease when vaccinated mice were given RPEs with irradiated larvae for two or eight weeks (Fig. 3).
Fig. 3.
Proliferation of murine spleen and lymph node cells to L. sigmodontis antigen (LsAg) and anti-CD3/anti-CD28. Shown is the stimulation index (OD of stimulated cells/baseline)*100. Mice were vaccinated with irradiated L. sigmodontis L3s and/or given repeated parasite exposures (RPE) with irradiated larvae for two (open bars) or eight weeks (grey bars). Control groups were vaccinated with media and then given repeated media injections. Significant differences between groups were analyzed by the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons (*p<0.05). Timepoints listed are duration of RPEs. Shown are results from two independent experiments (2 week timepoint: RPE n=5, Vaccination n=8, Vaccination + RPE n=8; 8 week timepoint: RPE n=4, Vaccination n=7, Vaccination + RPE n=8).
Whereas none of the groups tested after the two week RPE period showed increased proliferation of spleen or lymph node cells to LsAg as compared to unvaccinated mice given sham parasite exposures (control group), all mice that had received L3s, either by RPEs, vaccination, or both, showed slightly increased splenocyte and lymph node cell proliferation to LsAg compared to controls after the eight week RPE period (Fig. 3). Importantly, there was no difference in LsAg-specific spleen cell proliferation between vaccinated mice that had received RPEs compared to vaccinated mice that had not. LsAg-specific lymph node cell proliferation was highest in the group that received vaccination and eight weeks of RPE, although it did not reach statistical significance compared to the other treatment groups.
There were no differences in spleen cell proliferation to anti-CD3/anti-CD28 stimulation between any of the conditions studied at two weeks after vaccination. In contrast, lymph node cells from vaccinated mice given RPEs for two weeks showed the lowest proliferation rate to anti-CD3/anti-CD28 compared to other groups tested after the two week RPE period (Fig. 3). Interestingly, spleen cells from mice that were vaccinated and given RPEs for eight weeks exhibited less proliferation in response to anti-CD3/anti-CD28 than spleen cells from mice that had received either vaccination alone, eight weeks of RPEs alone, or were sham treated, though the difference between the vaccine + RPEs and vaccine only groups was not statistically significant (Fig. 3).
3.4 Vaccination-induced type 2 cytokine skewing is not diminished by RPEs
As a shift from Th2 to Th1 immune responses is observed during allergy desensitization [13], we compared by flow cytometry and ELISA the production of Th1 and Th2 cytokines by spleen cells from vaccinated mice that were and were not given RPEs with irradiated larvae.
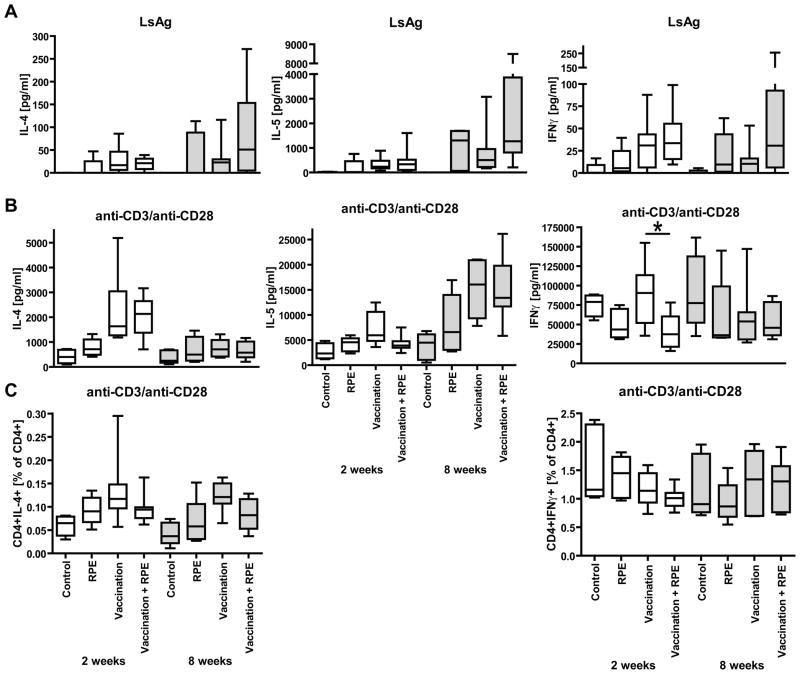
Neither two nor eight weeks of RPEs with irradiated larvae decreased vaccination-induced type 2 cytokine skewing. There were no differences in LsAg-driven IL-4, IL-5 or IFNγ cytokine production from splenocytes of vaccinated mice that were given RPEs for two weeks and those that were not (Fig. 4A). As with LsAg-specific IgE levels, mice administered eight weeks of RPEs had greater splenocyte production of IL-4, IL-5 and IFNγ in response to LsAg than mice given vaccination alone, though these differences were not statistically significant.
Fig. 4.
IL-4, IL-5, and IFNγ cytokine production from mice given L. sigmodontis vaccination, RPEs with irradiated larvae for two (open bars) or eight weeks (grey bars), both, or sham-vaccination and sham-exposures (Controls). (A) Supernatant measurements of splenocyte production of IL-4, IL-5, and IFNγ in response to parasite antigen (LsAg) or (B) anti-CD3/anti-CD28. (C) Percentages of CD4+IL-4+ and CD4+IFNγ+ spleen cells in response to anti-CD3/anti-CD28. Statistical significant differences between groups were analyzed by the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons. Only statistical significant differences between vaccinated and vaccinated + RPE mice are shown (*p<0.05). Timepoints listed are duration of RPEs. Shown are results from two independent experiments (2 week timepoint: RPE n=5, Vaccination n=8, Vaccination + RPE n=8; 8 week timepoint: RPE n=4, Vaccination n=7, Vaccination + RPE n=8).
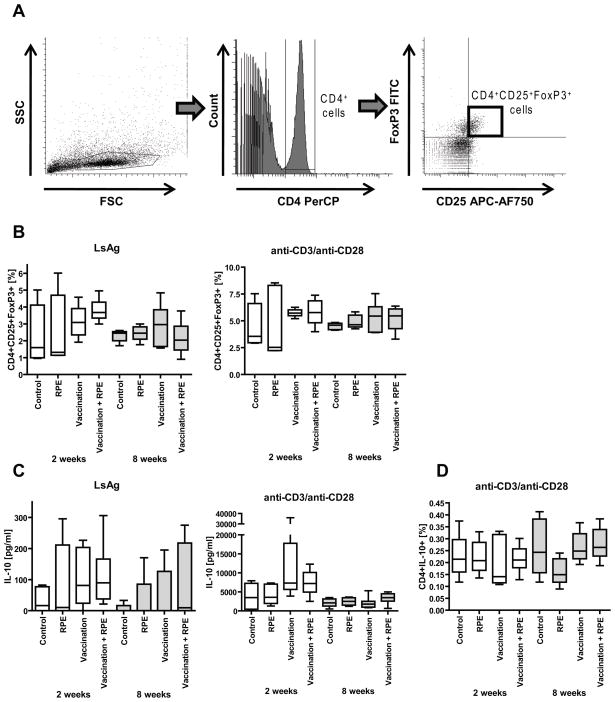
The total capacity of splenocytes from vaccinated mice to produce either IL-4, IL-5 or IFNγ in response to anti-CD3/anti-CD28 stimulation appeared to be diminished by two weeks of RPEs as IL-5 (p>0.05) and IFNγ (p<0.05) cytokine levels in cell culture supernatants and flow cytometry frequencies of CD4+IL-4+ T-cells (p>0.05) were lower in mice that had received vaccination and two weeks of RPEs than in mice that had received vaccination alone (Fig. 4B, C). Except for a slightly lower frequency of CD4+IL-4+ T-cells in the vaccination + RPE group, cytokine differences in response to anti-CD3/anti-CD28 stimulation were no longer present between the vaccination and the vaccinatioin +RPE groups after eight weeks of RPEs (Fig. 4B, C).
3.5 RPEs with irradiated larvae increase neither frequencies of regulatory T-cells nor IL-10 production
Since the peripheral T-cell tolerance that develops in SIT is believed to be due to increased IL-10 production and increased numbers of regulatory T-cells [12, 13, 29, 30], we analyzed the frequencies of splenic CD4+CD25+FoxP3+ regulatory and CD4+IL-10+ T-cells as well as IL-10 production from spleen cells of vaccinated mice given RPEs with irradiated larvae or media injections to determine if RPEs induce these immunoregulatory mechanisms in mice vaccinated against L. sigmodontis.
Frequencies of CD4+CD25+FoxP3+ regulatory spleen cells were determined using flow cytometry (Fig. 5A). Neither two nor eight weeks of RPEs with irradiated larvae increased the frequencies of splenic CD4+CD25+FoxP3+ regulatory T-cells after in vitro incubation with LsAg or anti-CD3/anti-CD28 compared to vaccinated and unvaccinated controls (Fig. 5B).
Fig. 5.
(A) Gating strategy for identification of CD4+CD25+FoxP3+ regulatory T-cells by flow cytometry. Lymphocytes were gated by forward (FSC) and sidescatter (SSC) characteristics (left panel). CD4 PerCP positive lymphocytes were then gated (middle panel) and analyzed for CD25 APC-AF750 and FoxP3 FITC positivity (right panel).
(B) Percentages of splenic CD4+ cells that are CD4+CD25+FoxP3+ after in vitro stimulation of splenocytes with parasite antigen (LsAg) or anti-CD3/anti-CD28. (C) IL-10 cytokine release from spleen cell culture supernatants in response to LsAg or anti-CD3/anti-CD28. (D) Percentages of splenic CD4+ cells that are CD4+IL-10+ in response to anti-CD3/anti-CD28. Shown are cells from mice that were vaccinated with L. sigmodontis L3s (Vaccination) and/or given repeated parasite exposures (RPE) with irradiated larvae for two (open bars) or eight weeks (grey bars). Control groups were vaccinated with media and then given repeated media injections. Significant differences between groups were analyzed by the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons (*p<0.05). Timepoints listed are duration of RPEs. Shown are results from two independent experiments (2 week timepoint: RPE n=5, Vaccination n=8, Vaccination + RPE n=8; 8 week timepoint: RPE n=4, Vaccination n=7, Vaccination + RPE n=8).
All mice that had received L3s, either by RPEs, vaccination, or both showed increased IL-10 production from spleen cells in response to LsAg, but there were no differences between vaccinated mice that received RPEs and vaccinated mice that did not (Fig. 5C). The total capacity of spleen cells to produce IL-10 was similar between vaccinated mice given 2 or 8 weeks of RPEs compared to vaccinated mice not given RPEs (Fig. 5C). The frequencies of splenic CD4+IL-10+ T-cells in response to anti-CD3/anti-CD28 were similar in all studied groups (Fig. 5D).
3.6 Repeated parasite exposures with either irradiated or infectious larvae do not diminish the protective efficacy of helminth vaccination
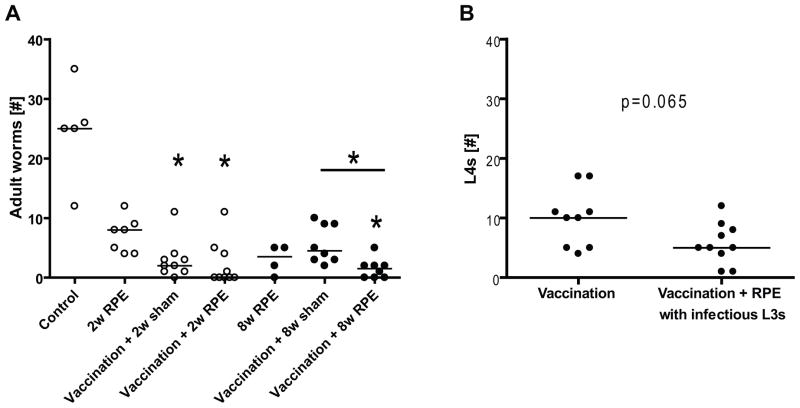
RPEs with irradiated larvae clearly did not decrease vaccine efficacy. Vaccinated mice given eight weeks of RPEs with irradiated larvae had significantly fewer worms recovered than mice given vaccination and sham exposures for eight weeks (median 1.5, range 0–5 vs median 4.5, range 2–10, p<0.05, Fig. 6A). Similarly, challenge of vaccinated mice that had received two weeks of RPEs with irradiated larvae resulted in fewer worms recovered (median 0, range 0–11) than challenge of vaccinated controls (median 2, range 0–11), though this difference was not statistically significant (Fig. 6A). Two and eight weeks of RPEs with irradiated larvae in the absence of initial vaccination resulted in partial protection of mice, with a median of 8 and 3.5 worms recovered, respectively (range 4–12 and 0–5, Fig. 6A).
Fig. 6.
(A) Worm recovery 56 days after challenge infection of mice with 40 infectious L. sigmodontis larvae. Mice were vaccinated against L. sigmodontis and/or given repeated parasite exposures (RPE) with irradiated larvae for two (○) or eight weeks (●) or were given sham vaccination and sham exposures (Control). Significant differences between groups were analyzed by the Kruskal-Wallis test, followed by Dunn’s post-hoc multiple comparisons, single stars show significant differences compared to the Control group (*p<0.05). (B) Worm recovery 20 days after challenge infection with 40 infectious L3 larvae. Mice were vaccinated against L. sigmodontis and given RPEs with infectious L3 larvae or RPMI as control for three months. Significant differences were analyzed by Mann-Whitney-U-test (*p<0.05).
As irradiated, cryopreserved L3s are not fully functional, we evaluated whether repeated administration of fully infectious live L3s alters the protective efficacy of helminth vaccination. To accomoplish this, vaccinated BALB/c mice were given a total of 9 subcutaneous injections of 5 non-irradiated infectious L3 larvae or media as control over 3 months. Two weeks after the last repeated parasite exposure mice were challenged with 40 infectious L3s and the worm burdens obtained from the pleural cavity determined 20d later. This timing enabled us to distinguish worms from the challenge infection (which at 20d p.i. were still at the L4 stage) from worms acquired during the RPEs (adult worms).
RPEs with infectious L3 larvae did not decrease vaccine efficacy as vaccinated mice given RPEs had fewer fourth stage larvae (p=0.065, median 5, range 1–12) than vaccinated control mice (median 10, range 4–17, Fig. 6B). Of note, the median number of adult worms acquired from the repeated infectious parasite exposures was 1 (range 0–6).
3.7 Repeated parasite exposures with infectious L3 larvae do not cause a resistant host to become susceptible to L. sigmodontis infection
In addition to determining whether repeated parasite exposures alter the efficacy of a type 2 immune-inducing vaccine, we also sought to test whether repeated challenges with infectious L3 larave cause immunologic tolerance to develop in a resistant host. To determine this, we tested whether repeated infections with non-irradiated infectious L. sigmodontis L3 larvae increase the susceptibility of C57BL/6 mice to L. sigmodontis. In the C57BL/6 mouse strain, single challenge infections of L. sigmodontis are able to develop into adult worms over the first month of infection but are then cleared prior to establishment of a chronic patent infection [22].
C57BL/6 mice were infected with 5 non-irradiated infectious L3 larvae every other week for a total of eight challenges and final worm burdens compared to C57BL/6 mice that were given a single challenge with 40 L3 larvae. Mice were euthanized 62d after the final or single challenge, a timepoint at which susceptible strains of mice still harbor adult worms and are microfilariae positive. At study endpoint, microfilaraemia and worm burdens as well as parasite-antigen driven splenocyte proliferation and production of Th1 and Th2 cytokines were investigated.
Worm recovery revealed that repeated parasite infections did not increase the final worm burden, as no worms were found in the repeatedly infected group and only one worm was found in one out of four mice in the single challenge group (Tab. 1). In addition, none of the mice developed a patent infection with microfilaraemia (data not shown).
Tab. 1.
Repeated L. sigmodontis challenges in resistant C57BL/6 mice do not increase the susceptibility to infection compared to a single challenge.
| Worm # | Proliferation | IL-4 | IL-5 | IFNγ | IL-10 | |
|---|---|---|---|---|---|---|
| Single challenge | 1 | 461 ± 89 | 1.4 ± 2.8 | 1121 ± 585 | 8.2± 1.6 | 187 ± 46 |
| Repeated challenges | 0 | 448 ± 54 | 10.6 ± 9.2 | 1377 ± 684 | 15.8 ± 7.8 | 89 ± 10 |
C57BL/6 mice received a single challenge with 40 infectious L3 larvae (n=4) or 8 challenges of 5 L3s every other week (n=4). Total adult worm numbers per group and immunological studies were examined 62 days after the last infection. Proliferation of spleen cells (stimulation index) and cytokine release (pg/ml) in response to parasite antigen are shown. Shown are means ± standard deviation.
Repeated challenges in mice did not decrease or alter splenocyte proliferation to parasite antigen (Tab. 1) or anti-CD3/anti-CD28 (data not shown) compared to administration of a single challenge. Additionally, there were no statistically significant differences in IL-4, IL-5, IL-10, or IFNγ production in spleen cell cultures stimulated with either LsAg (Tab. 1) or anti-CD3/anti-CD28 (data not shown).
3.8 Repeated LsAg injections upregulate regulatory T-cell numbers and decrease antigen-specific splenocyte proliferation and cytokine production, but do not suppress IgE responses in LsAg-sensitized mice
To evaluate whether the protocols we utilized for RPEs were of sufficient duration and frequency to enable the development of immune tolerance in BALB/c mice such as that which occurs in specific immunotherapy when an antigen is repeatedly administered to an allergic subject, we tested whether whole worm antigen administration can induce immunologic tolerance in worm antigen-sensitized BALB/c mice. To test this, BALB/c mice were first sensitized to LsAg by administration of three weekly i.p. injections of 100 μg LsAg adsorbed to alum and then given 5μg LsAg injections three times per week for eight weeks.
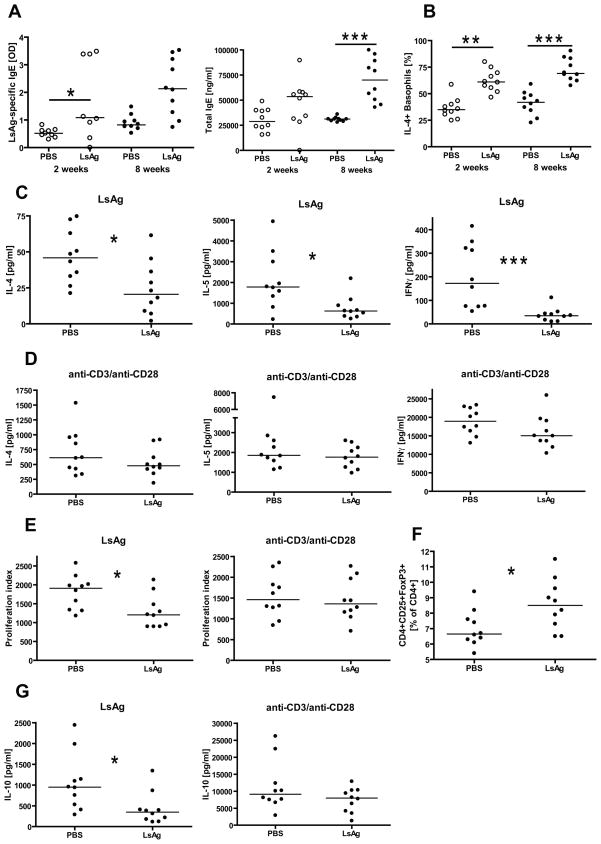
Blood samples were drawn after two and eight weeks of repeated LsAg administration and then tested for IgE levels and for basophil IL-4 positivity in response to LsAg. Mice that were repeatedly LsAg injected for two and eight weeks had higher levels of LsAg-specific and total IgE compared to repeatedly PBS injected controls (Fig. 7A). These differences were significant for LsAg-specific IgE after 2 weeks of repeated exposures and for total IgE after eight weeks of repeated injections. Similarly, mice that were repeatedly LsAg injected for two weeks had significantly higher frequencies of spontaneous (data not shown) and LsAg-stimulated IL-4+ basophils than vaccinated controls (Fig. 7B). Eight weeks of repeated LsAg injections resulted in significantly increased frequencies of LsAg-stimulated IL-4+ basophils compared to vaccinated controls (Fig. 7B), although no difference was observed in the frequency of unstimulated IL-4+ basophils between both groups (data not shown).
Fig. 7.
Immunological studies from BALB/c mice that were vaccinated against parasite antigen (LsAg) and repeatedly injected with LsAg or PBS for two (○) or eight weeks (●). (A) LsAg-specific and total IgE levels after 2 and 8 weeks of repeated LsAg/PBS injections. (B) Frequency of IL-4 positive basophils in response to LsAg after 2 or 8 weeks of repeated injections. (C) Splenic IL-4, IL-5, and IFNγ production in response to LsAg and (D) anti-CD3/anti-CD28 after eight weeks of repeated exposures to LsAg or PBS. (E) Spleen cell proliferation in response to LsAg or anti-CD3/anti-CD28 after eight weeks of repeated injections. (F) Spontaneous frequency of CD4+CD25+FoxP3+ splenic T cells after eight weeks of repeated exposures. (G) Splenic IL-10 production in response to LsAg and anti-CD3/anti-CD28 from eight week repeatedly injected mice. Timepoints listed are duration of RPEs. Shown are medians. Significant differences were analyzed by Mann-Whitney-U-test (*p<0.05, **p<0.01, ***p<0.001).
The development of cellular tolerance was tested after eight weeks of repeated LsAg/PBS injections by measuring splenocyte proliferation and production of Th1 and Th2 cytokines as well as the frequency of splenic regulatory T cells and splenic IL-10 production in response to LsAg and anti-CD3/anti-CD28.
Sensitized mice that had received repeated LsAg injections exhibited significantly decreased splenocyte production of IL-4, IL-5, and IFNγ in response to LsAg as compared to sensitized mice that had received PBS injections (Fig. 7C). This decrease in antigen-specific cytokine production was not due to a decrease in the capacity of splenocytes from the LsAg exposed group to produce these cytokines, as IL-4, IL-5, and IFNγ production in response to anti-CD3/anti-CD28 were equivalent in the experimental and control group (Fig. 7D).
In addition to exhibiting decreased anitgen-specific cytokine production, spleen cells from repeatedly LsAg-injected mice proliferated significantly less in response to LsAg compared to PBS-injected controls, whereas there was no difference in spleen cell proliferation between both groups in response to anti-CD3/anti-CD28 (Fig. 7E).
Frequencies of spontaneous splenic CD4+CD25+FoxP3+ cells of CD4+ T cells were significantly increased in repeatedly LsAg-injected mice compared to PBS controls (Fig. 7F). However, spleen cells from repeatedly LsAg-injected mice produced significantly less IL-10 in response to LsAg compared to PBS-treated mice (Fig. 7G). There were no differences in IL-10 production in response to anti-CD3/anti-CD28 between both groups.
Overall, these findings demonstrate that an 8 week course of repeated administration of whole worm antigen to LsAg-sensitized BALB/c mice induces significant cellular tolerance as evidenced by upregulation of T-regulatory cells and downregulation of LsAg-specific splenocyte proliferation and cytokine production. Importantly, though, such a course of repeated antigen administration does not suppress IgE responses.
4. Discussion
Chronic helminth infections are among the most prevalent diseases of people in developing countries [2]. By impairing childhood growth and development, limiting the ability to conduct manual labor, and adversely affecting pregnancies these chronic infections impart a tremendous toll on the general health of much of the world’s population.
While there is to date no commercially available vaccine against any helminth pathogen of humans, recently several hookworm vaccine candidates have shown significant promise in animal studies [8, 31–33]. As antigens that induce high titer IgE responses are more protective against primary hookworm infections than those that do not [8, 33], consideration is being given towards developing helminth vaccines that induce parasite-specific IgE responses [11].
To date, the protective efficacy of helminth vaccines in animal studies has only been tested after single challenge infections. In helminth-endemic areas, however, people are continually exposed to helminth infections. Because repeated allergen exposure results in the development of immunological tolerance in allergic patients who have allergen-specific IgE [12], in this study we tested whether L. sigmodontis vaccination remains effective in the face of RPEs.
In the L. sigmodontis filariasis model, vaccination with irradiated larvae induces a type 2 immune response characterized by production of type 2 cytokines such as IL-4 (Fig. 1c) and IL-5 [23]. In this study, we demonstrated that this vaccination protocol also results in production of parasite-specific IgE. Further, we demonstrated that basophils of vaccinated mice increased IL-4 expression in response to LsAg whereas basophils of unvaccinated mice did not. Since basophils primarily become activated through cross-linking of IgE molecules bound to high affinity IgE receptors on their surface, these results suggest the parasite-specific IgE induced by the L. sigmodontis vaccination regimen is functionally active. While the role IgE plays in vaccination-induced protection has not yet been evaluated in the L. sigmodontis model, IgE has been shown to play a protective role after vaccination of mice with larval stages of the human filaria Onchocerca volvulus [10, 34].
We chose to conduct RPEs for two and eight weeks with injections every other day or three times a week, respectively, as desensitization protocols of patients with allergen-specific IgE differ in their duration from a few days with several treatments per day in accelerated immunotherapy to 3–6 months with bi- or weekly treatments [35]. Also, animal studies of allergy desensitization have used from 5–6 treatments on consecutive days to 3 weekly injections for four weeks [36–38]. For the majority of our studies, RPEs were performed with irradiated L3 larvae, which are attenuated but still capable of migrating through the subcutaneous tissues without developing to adult worms. Utilization of irradiated L3s for RPEs thus allowed for comparison of worm recovery between RPE and control groups of mice after challenge with equal numbers of viable infectious L3 larvae.
Repeated L3 injections over eight, but not two weeks, resulted in a tendency towards increased parasite-specific IgE levels in vaccinated mice compared to vaccination controls. Increases in specific IgE have been reported in SIT, and are typically followed by gradual decreases over months or years of treatment. Although the decrease of serum IgE is not correlated with clinical improvement after SIT [13, 28, 39], early desensitization of mast cells and basophils occurs [12]. However, in our study basophils were not desensitized after RPEs, as they showed no decreased expression of IL-4 in response to LsAg compared to vaccination controls.
Furthermore, neither two nor eight weeks of RPEs in vaccinated mice decreased concentrations of Th2 cytokines or increased Th1 cytokine levels in spleen cell cultures stimulated with LsAg. In contrast, eight weeks of RPEs resulted in slightly increased amounts of IL-4, IL-5, and IFNγ. This is in contrary to the expected effects of SIT, in which an allergen-specific T-cell shift from Th2 to Th1 occurs that is characterized by decreases in IL-4+ and IL-5+ T-cells and increases in IFNγ+ T-cells [12, 13, 40].
Another immunological manifestation of SIT is the induction of peripheral T-cell tolerance that occurs within days. This peripheral T-cell tolerance is believed to be due to increased IL-10 production from antigen-specific and CD4+CD25+ T-cells which suppress proliferation and cytokine production in response to allergen [12, 13, 29, 30]. In our study, though, RPEs failed to increase splenic IL-10 production or to increase the frequency of splenic CD4+CD25+FoxP3+ regulatory T-cells in vaccinated mice. Additionally, neither proliferation of spleen nor lymph node cells to LsAg was decreased in vaccinated mice that received RPEs compared to vaccination controls.
Most importantly, this study clearly showed that RPEs did not reduce the protective response induced by a Type 2 immune-inducing vaccine. Indeed, mice given RPEs had lower parasite burdens at study endpoint compared to mice given vaccination alone. The inability of RPEs to reduce the protective efficacy of helminth vaccination was not due to the use of irradiated larvae for parasite exposures, as repeated administration with infectious, non-irradiated L3 larvae also resulted in improved vaccine efficacy at study endpoint. To our knowledge, this is the first study to demonstrate that a vaccine which induces a type 2 immune response and pathogen-specific IgE maintains protective efficacy even after repeated exposures to the pathogen.
Interestingly, the results of this study suggest that repeated low dose parasite exposures might actually boost vaccine-induced protective mechanisms by inducing or maintaining a stronger Th2 immune response. Immunologic studies on vaccinated mice that were repeatedly parasite exposed demonstrated increased production of Th2 cytokines, increased total and antigen-specific IgE levels, increased basophil activation, and increased cellular proliferation to antigen in the axillary and brachial lymph nodes compared to vaccinated controls that did not receive RPEs. If such results can be confirmed in other models of helminth vaccination, then it is possible that vaccine regimens which induce type 2 immune responses might be more effective in endemic settings than initially predicted by experiments that utilize just one infectious challenge.
Finally, our studies also demonstrated that repeated infections of the intrinsically resistant C57BL/6 strain of mice with infectious L3 stage larvae did not alter the susceptibility these mice have for L. sigmodontis infection. These results are consistent with prior studies that evaluated the effects of repeated parasite infections in the absence of initial vaccination in other animal models of helminth infections. Repeated infections with the filarial nematode Acanthocheilonema viteae in jirds was shown to reduce the recovery rate of worms compared to jirds given a single challenge of larvae [41] and repeated infections with Brugia pahangi in cats resulted in many of them becoming amicrofilaraemic and free of any adult worms [42, 43]. It is important to note, however, that some studies have shown evidence of tolerance with frequent worm exposures. For example, repeated exposures with the nematode Trichuris muris in resistant C57BL/6 mice caused an increase in worm numbers and prolongation of worm survival [44].
It is unclear why RPEs did not induce immunological changes akin to specific immunotherapy. The cause does not seem to be due to the frequency or duration of the RPEs administered since repeated injections of parasite antigen given at the same frequency and for the same duration as the 8 week course of RPEs clearly resulted in the development of cellular tolerance to LsAg in LsAg-sensitized mice. Specifically, repeated LsAg administration to LsAg-sensitized mice caused decreased LsAg-specific cytokine production, decreased antigen-specific spleen cell proliferation, and an increase of CD4+CD25+FoxP3+ regulatory T cells. In contrast, mice vaccinated against L. sigmodontis developed none of these immunologic changes after RPEs. The issue may be one of antigen concentration, since the concentration of antigens released by larvae may have been substantially different than the amount of LsAg injected into mice. Alternatively, RPEs may not cause immune tolerance due to the tissue sites where they localize. Irradiated and live L3 larvae migrate to the pleural cavity, whereas soluble LsAg regularly injected into the peritoneum likely enters quickly into the circulation. Regardless of the reason, though, it is clear from these studies that RPEs do not cause immune tolerance in mice given a type-2 inducing helminth vaccine.
It is important to note that neither RPEs nor repeated antigen administration given three times a week for 8 weeks caused decreased parasite-specific IgE responses. As such, our study does not preclude the possibility that longer periods or greater frequencies of repeated parasite exposures may suppress IgE responses and perhaps vaccine efficacy.
In summary, this study demonstrates that a helminth vaccine which induces type 2 immune responses remains effective even in the face of multiple parasite exposures for up to 8 weeks. Frequent exposures to parasites after vaccination resulted in no substantial alteration in Th2 skewing and no increases in immunoregulatory mechanisms. Most importantly, protective efficacy was maintained despite repeated parasite exposures. These results suggest that if a safe and effective type 2 immune-inducing vaccine can be made against helminth infections, it may be able to maintain protective efficacy even in settings of frequent parasite exposures.
Acknowledgments
We thank Ellen Mueller and David Larson for their assistance with the infections. Additionally, we thank Karen Wolcott and Kateryna Lund at the USU Biomedical Instrumentation Center for their valuable assistance with flow cytometry as well as Michael Woolbert from USU Environmental Health & Occupational Safety for the performed irradiations.
This work was supported by grant number R073MX from the Uniformed Services University of the Health Sciences, Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–21. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 3.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 4.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 5.Babayan SA, Attout T, Harris A, Taylor MD, Le Goff L, Vuong PN, et al. Vaccination against filarial nematodes with irradiated larvae provides long-term protection against the third larval stage but not against subsequent life cycle stages. Int J Parasitol. 2006;36:903–14. doi: 10.1016/j.ijpara.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara RT, Loukas A, Mendez S, Williamson AL, Bueno LL, Wang Y, et al. Vaccination with irradiated Ancylostoma caninum third stage larvae induces a Th2 protective response in dogs. Vaccine. 2006;24:501–9. doi: 10.1016/j.vaccine.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Wu H, Chen S, Hu L, Xie Z, Qiu Y, et al. Association between IgE antibody against soluble egg antigen and resistance to reinfection with Schistosoma japonicum. Trans R Soc Trop Med Hyg. 1997;91:606–8. doi: 10.1016/s0035-9203(97)90047-x. [DOI] [PubMed] [Google Scholar]
- 8.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. Faseb J. 2005;19:1743–5. doi: 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- 9.Kooyman FN, Schallig HD, Van Leeuwen MA, MacKellar A, Huntley JF, Cornelissen AW, et al. Protection in lambs vaccinated with Haemonchus contortus antigens is age related, and correlates with IgE rather than IgG1 antibody. Parasite Immunol. 2000;22:13–20. doi: 10.1046/j.1365-3024.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 10.Abraham D, Leon O, Schnyder-Candrian S, Wang CC, Galioto AM, Kerepesi LA, et al. Immunoglobulin E and eosinophil-dependent protective immunity to larval Onchocerca volvulus in mice immunized with irradiated larvae. Infect Immun. 2004;72:810–7. doi: 10.1128/IAI.72.2.810-817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotez PJ, Zhan B, Bethony JM, Loukas A, Williamson A, Goud GN, et al. Progress in the development of a recombinant vaccine for human hookworm disease: the Human Hookworm Vaccine Initiative. Int J Parasitol. 2003;33:1245–58. doi: 10.1016/s0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- 12.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–91. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Jutel M, Akdis M, Blaser K, Akdis CA. Mechanisms of allergen specific immunotherapy--T-cell tolerance and more. Allergy. 2006;61:796–807. doi: 10.1111/j.1398-9995.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Al-Qaoud KM, Ungeheuer MN, Paehle K, Vuong PN, Bain O, et al. IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Med Microbiol Immunol. 2000;189:67–74. doi: 10.1007/pl00008258. [DOI] [PubMed] [Google Scholar]
- 15.Martin C, Le Goff L, Ungeheuer MN, Vuong PN, Bain O. Drastic reduction of a filarial infection in eosinophilic interleukin-5 transgenic mice. Infect Immun. 2000;68:3651–6. doi: 10.1128/iai.68.6.3651-3656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA, et al. Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun. 2006;74:5236–43. doi: 10.1128/IAI.00329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkmann L, Bain O, Saeftel M, Specht S, Fischer K, Brombacher F, et al. Murine filariasis: interleukin 4 and interleukin 5 lead to containment of different worm developmental stages. Med Microbiol Immunol. 2003;192:23–31. doi: 10.1007/s00430-002-0155-9. [DOI] [PubMed] [Google Scholar]
- 18.Le Goff L, Lamb TJ, Graham AL, Harcus Y, Allen JE. IL-4 is required to prevent filarial nematode development in resistant but not susceptible strains of mice. Int J Parasitol. 2002;32:1277–84. doi: 10.1016/s0020-7519(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 19.Folkard SG, Hogarth PJ, Taylor MJ, Bianco AE. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology. 1996;112:323–9. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007;179:4626–34. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 21.Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005;174:4924–33. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann W, Petit G, Schulz-Key H, Taylor D, Bain O, Le Goff L. Litomosoides sigmodontis in mice: reappraisal of an old model for filarial research. Parasitol Today. 2000;16:387–9. doi: 10.1016/s0169-4758(00)01738-5. [DOI] [PubMed] [Google Scholar]
- 23.Le Goff L, Martin C, Oswald IP, Vuong PN, Petit G, Ungeheuer MN, et al. Parasitology and immunology of mice vaccinated with irradiated Litomosoides sigmodontis larvae. Parasitology. 2000;120:271–80. doi: 10.1017/s0031182099005533. [DOI] [PubMed] [Google Scholar]
- 24.Le Goff L, Marechal P, Petit G, Taylor DW, Hoffmann W, Bain O. Early reduction of the challenge recovery rate following immunization with irradiated infective larvae in a filaria mouse system. Trop Med Int Health. 1997;2:1170–4. doi: 10.1046/j.1365-3156.1997.d01-218.x. [DOI] [PubMed] [Google Scholar]
- 25.Hubner MP, Torrero MN, McCall JW, Mitre E. Litomosoides sigmodontis: a simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus) Exp Parasitol. 2009;123:95–8. doi: 10.1016/j.exppara.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubner MP, Stocker JT, Mitre E. Inhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cells. Immunology. 2009;127:512–22. doi: 10.1111/j.1365-2567.2008.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy. 2009;39:361–9. doi: 10.1111/j.1365-2222.2008.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleich GJ, Zimmermann EM, Henderson LL, Yunginger JW. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–71. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 29.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis JN, Till SJ, Durham SR. Induction of IL-10+CD4+CD25+ T cells by grass pollen immunotherapy. J Allergy Clin Immunol. 2003;111:1255–61. doi: 10.1067/mai.2003.1570. [DOI] [PubMed] [Google Scholar]
- 31.Hotez PJ, Ashcom J, Bin Z, Bethony J, Williamson A, Hawdon JM, et al. Effect of vaccinations with recombinant fusion proteins on Ancylostoma caninum habitat selection in the canine intestine. J Parasitol. 2002;88:684–90. doi: 10.1645/0022-3395(2002)088[0684:EOVWRF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6:733–41. doi: 10.1016/S1473-3099(06)70630-2. [DOI] [PubMed] [Google Scholar]
- 33.Fujiwara RT, Zhan B, Mendez S, Loukas A, Bueno LL, Wang Y, et al. Reduction of worm fecundity and canine host blood loss mediates protection against hookworm infection elicited by vaccination with recombinant Ac-16. Clin Vaccine Immunol. 2007;14:281–7. doi: 10.1128/CVI.00404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange AM, Yutanawiboonchai W, Scott P, Abraham D. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–11. [PubMed] [Google Scholar]
- 35.Cox L. Accelerated immunotherapy schedules: review of efficacy and safety. Ann Allergy Asthma Immunol. 2006;97:126–37. doi: 10.1016/S1081-1206(10)60003-8. quiz 37–40, 202. [DOI] [PubMed] [Google Scholar]
- 36.Bauer L, Bohle B, Jahn-Schmid B, Wiedermann U, Daser A, Renz H, et al. Modulation of the allergic immune response in BALB/c mice by subcutaneous injection of high doses of the dominant T cell epitope from the major birch pollen allergen Bet v 1. Clin Exp Immunol. 1997;107:536–41. doi: 10.1046/j.1365-2249.1997.d01-953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Garnier C, Astori M, Kettner A, Dufour N, Heusser C, Corradin G, et al. Allergen-derived long peptide immunotherapy down-regulates specific IgE response and protects from anaphylaxis. Eur J Immunol. 2000;30:1638–45. doi: 10.1002/1521-4141(200006)30:6<1638::AID-IMMU1638>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 38.Yasue M, Yokota T, Fukada M, Takai T, Suko M, Okudaira H, et al. Hyposensitization to allergic reaction in rDer f 2-sensitized mice by the intranasal administration of a mutant of rDer f 2, C8/119S. Clin Exp Immunol. 1998;113:1–9. doi: 10.1046/j.1365-2249.1998.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Ree R, Van Leeuwen WA, Dieges PH, Van Wijk RG, De Jong N, Brewczyski PZ, et al. Measurement of IgE antibodies against purified grass pollen allergens (Lol p 1, 2, 3 and 5) during immunotherapy. Clin Exp Allergy. 1997;27:68–74. doi: 10.1046/j.1365-2222.1997.d01-416.x. [DOI] [PubMed] [Google Scholar]
- 40.Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Muller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-gamma secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995;154:4187–94. [PubMed] [Google Scholar]
- 41.Barthold E, Wenk P. Dose-dependent recovery of adult Acanthocheilonema viteae (Nematoda: Filarioidea) after single and trickle inoculations in jirds. Parasitol Res. 1992;78:229–34. doi: 10.1007/BF00931731. [DOI] [PubMed] [Google Scholar]
- 42.Denham DA, McGreevy PB, Suswillo RR, Rogers R. The resistance to re-infection of cats repeatedly inoculated with infective larvae of Brugia pahangi. Parasitology. 1983;86:11–8. doi: 10.1017/s0031182000057127. [DOI] [PubMed] [Google Scholar]
- 43.Denham DA, Ponnudurai T, Nelson GS, Rogers R, Guy F. Studies with Brugia pahangi. II. The effect of repeated infection on parasite levels in cats. Int J Parasitol. 1972;2:401–7. doi: 10.1016/0020-7519(72)90084-7. [DOI] [PubMed] [Google Scholar]
- 44.Bancroft AJ, Else KJ, Humphreys NE, Grencis RK. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int J Parasitol. 2001;31:1627–37. doi: 10.1016/s0020-7519(01)00281-8. [DOI] [PubMed] [Google Scholar]