Abstract
Human embryonic stem (hES) cells are capable of differentiation into virtually all cell types and hold tremendous potential as cell sources for regenerative therapies. However, teratoma formation can be the main obstacle for hES cells therapy. In order to understand the biology and physiology of hES cells teratoma formation, we investigated the angiogenic process within teratomas and characterized teratoma cells. In this study, hES cells transduced with double fusion reporter gene that consists of firefly luciferase and enhanced green fluorescent protein (Fluc-eGFP) were injected into hind limbs of SCID mice and performed longitudinal bioluminescence imaging on these animals. To test angiogenic contribution of teratoma from host or hES cells, human and mouse endothelial cells marker CD31 was stained respectively. To further explore the characterization of teratoma derived cells, flow cytometry analysis was carried out and GFP+/SSEA-4+ cells were isolated and subcultured. Then, we re-injected the isolated GFP+/SSEA-4+ teratoma cells into SCID mice and observed by imaging. Our results show that the reporter gene imaging is an ideal technology for monitoring long-term stem cell viability, death, and proliferation. Teratomas contained vasculatures are from hES cells and host. hESCs derived teratomas express a high level of undifferentiated marker SSEA-4 and CD56, and subcultured GFP+/SSEA-4+ cells had similar expression pattern comparing to undifferentiated hES cells, except for a very high level of CD56 and a little lower expression of undifferentiated markers, such as SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81. Moreover, the SSEA-4+ teratoma cells can form teratomas in SCID mice, and this type teratomas grow at a lower rate compared to teratomas derived from hES cells, and are more differentiated.
Keywords: Bioluminescence Imaging, Human Embryonic Stem Cells, Differentiation, Teratoma Formation
INTRODUCTION
Since first shown to propagate as individual lines in vitro in 19981, human embryonic stem (hES) cells have attracted enormous attention for its potential use as raw material for the production of therapeutically useful cell types. However, most preparations of differentiated hES cells are merely enriched for the cell type of interest, and the presence of unknown, confounding, and possible tumor-promoting cell types raises broad alarm about the safety of the clinical application of hES-derived cells2. The ability of hES cells to form noncancerous tumours called teratomas is one of their defining traits. And there also have been many studies showing that hES cells differentiated for extended periods of time are still able to form teratomas2–4. It is also a frightening one, particularly for those who hope to develop therapies from the cells.
To fully understand the mechanism of hES cells therapy, one would like to understand the dynamic process of cells homing, migration, biodistribution, proliferation, and differentiation in the same subject over time. Therefore, the development of noninvasive and sensitive in vivo tracking technologies will play an indispensable role in detailed preclinical studies to optimize the delivery methods and strategies and monitor the mis-behaviors of hES cells after transplantation5. Reporter gene imaging, is useful in assessing kinetic survival status of the implanted cells because the reporter genes can be expressed as long as the cells are alive, the inserted reporter gene(s) can be passed on to daughter cells upon cell division6, 7. Among the different reporter gene imaging techniques, bioluminescence imaging (BLI) is extremely useful because of its high sensitivity, high-throughput screening, and straightforward imaging procedures5.
Angiogenesis is the proliferation of a network of blood vessels that penetrates into cancerous growths, supplying nutrients and oxygen and removing waste products. Antiangiogenic therapy to limit and even reverse the growth of tumors are under investigation and showing promise8. The discovery and characterization of teratoma-derived angiogenesis processes will greatly contribute to our understanding of how teratoma regulates angiogenesis and will provide therapeutic strategy for teratoma formation after hES cells therapy.
In this study, hES cells were stably transduced with a lentiviral vector carrying a novel double-fusion (DF) reporter gene that consists of firefly luciferase (Fluc), enhanced green fluorescence protein (eGFP). Simultaneously, hES cells were transplanted into mice hindlimb. Bioluminescence imaging was used to track the Fluc-labeled hES cells. Moreover, the angiogenesis of teratoma was investigated and the characterization of teratoma derived cells was carried out.
MATERIALS AND METHODS
Maintenance of human embryonic stem cells
Undifferentiated hES cells (H9 line from Wicell, passages 38 to 45) were grown on an inactivated mouse embryonic fibroblast (MEF) feeder layer as previously described7, 9. Briefly, the cell was maintained at an undifferentiated stage on irradiated low-passage MEF feeder layers on 0.1% gelatin-coated plates. The medium was changed daily. The medium consisted of Dulbecco's modified Eagle's medium (DMEM)/F-12, 20% knockout serum replacement, 0.1 mM nonessential amino acids, 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, and 4 ng/ml rhFGF-2 (R&D Systems Inc., Minneapolis). The hES cells were treated by 1 mg/ml collagenase type IV in DMEM/F12 and scraped mechanically on the day of passage. Prior to cell transplantation, hES cells were grown on Matrigel-coated plates in mTeSR1 medium (Stem Cell Technologies, Vancouver, BC, Canada).
Lentiviral transduction of hES cells with double fusion (DF) reporter gene
In order to track transplanted cells in vivo, hES cells were transduced at multiplicity of infection (MOI) of 10 with self-inactivating (SIN) lentiviral vector carrying a human ubiquitin promoter driving firefly luciferase and enhanced green fluorescence protein (Fluc-GFP)7, 9. Stable clones were isolated using FACS for GFP expression. Afterwards, Fluc activity within different cell numbers was confirmed ex vivo using Xenogen IVIS 200 system (Xenogen, Alameda, CA) as described7, 9. To test expression patterns of stem cell markers Oct-4, non-transduced hES cells (control) and hES cells with DF reporter gene (hESC-DF) were stained for Oct-4 (Chemicon, Temecula, CA). Briefly, the undifferentiated hES cell colonies were fixed in 4% paraformaldehyde in PBS for 15 minutes. Nonspecific binding was blocked with 4% normal goat serum for 30 minutes, following which the colonies were stained with antibodies to Oct-4 and incubated with Alexa 594-conjugated rabbit anti-goat secondary antibodies (Invitrogen) for 30 minutes and nuclear counterstained with DAPI. Images were obtained with a Nikon microscopy (Nikon Instruments Inc., Melville, NY).
Transplantation of hES cells into murine hindlimbs
All procedures were performed on 9–11 week old female SCID mice (Laboratory Animal Center of the Academy of Military Medical Science, Beijing, China) (n=10) according to the Nankai University Animal Care and Use Committee guidelines. Mice were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg). Approximately 1x106 undifferentiated hES cells (stably transduced with DF reporter gene) were injected into left hind limbs in 50 μl PBS. 1x106 human umbilical cord mesenchymal stem cells10 stably transduced with DF reporter gene were used as control and injected into right hind limbs.
Flow cytometry sorting (FCS) of teratoma derived cells
Mice were euthanized after cell transplantation at day 28 and teratoma cells were isolated. Briefly, saturated mouse with alcohol, cut the skin and separated teratoma from skeletal muscle using sterilized instruments, then the teratoma was placed in a new plate, washed with PBS, minced tissue with curved dissecting scissors into grain sized pieces. Single cell suspensions of teratomas were obtained by subsequent treatment with 0.56 units/ml of Liberase Blendzyme IV (Roche, Indianapolis) at 37°C for 20–30 min. Cells were passed through a 40-μm cell strainer11. Antibodies used in this study were phycoerythrin (PE) conjugated anti-CD31 (BD Pharmingen), Allophycocyanin (APC) conjugated anti-CD56 (BD), APC conjugated anti-KDR (R&D Systems), APC conjugated anti-CD133 (R&D Systems), APC conjugated anti-mouse IgG/IgM and anti-rat IgM, and rat anti-human SSEA-3 (Chemicon, Temecula, CA), mouse anti-human SSEA-5, TRA-1-60, TRA-1-81 (Chemicon). The stained cells were analyzed using FACS Vantage (Becton-Dickinson, MA). The GFP+/SSEA-4+ cells were isolated and subcultured on Matrigel-coated plates in mTeSR1 medium. The GFP+/SSEA-4+ teratoma cells were subcultured by treating with collagenase type IV and scraped mechanically.
Transplantation of teratoma derived cells into murine hindlimbs
The proliferation activity and multipotency of hES cells derived teratomas has not been determined before. To further investigate in vivo behavior of teratoma derived cells, approximately 1x106 GFP+/SSEA-4+ teratoma cells were injected into left hind limbs of SCID mice (n=10) in 100 μl PBS as mentioned above.
Optical bioluminescence imaging of transplanted cell fate in living mice
Bioluminescence imaging was performed using the Xenogen IVIS 200 system. After intraperitoneal injections of reporter probe D-Luciferin (150 mg luciferin/kg), animals were imaged for 2 seconds to 2 minutes. The same mice were scanned for 4 weeks of hES cells transplantation and 10 weeks of GFP+/SSEA-4+ teratoma cells transplantation. Imaging signals were quantified in units of maximum photons per second per centimeter square per steridian (photons/sec/cm2/sr) as described12.
Immunohistochemical staining for angiogenesis of teratoma
At week 4, the separated teratomas from study were embedded into OCT compound (Miles Scientific). Frozen sections (5 μm thick) were processed for immunostaining. To investigate the angiogenesis of teratoma, rabbit anti-GFP antibody (Invitrogen), mouse anti-human CD31 antibody (BD Pharmingen), and rat anti-mouse CD31 antibody (BD Pharmingen) were used. Alexa Fluor 488, and Alexa Fluor 594-conjugated secondary antibodies were applied appropriately. DAPI was used for nuclearcounterstaining. H&E staining also was carried out to identify the fate of transplanted hES cells. To explore the behavior of transplanted GFP +/SSEA-4+ teratoma cells, the neoplasm were separated from hind limb for H&E staining at day 70.
Statistical Analysis
ANOVA and repeated measures ANOVA with post-hoc testing as well as the two-tailed Student's t-test were used. Differences were considered significant at P-values of <0.05. Unless otherwise specified, data are expressed as mean ± standard deviation.
Results
Generation of hES cells with double fusion (DF) reporter gene
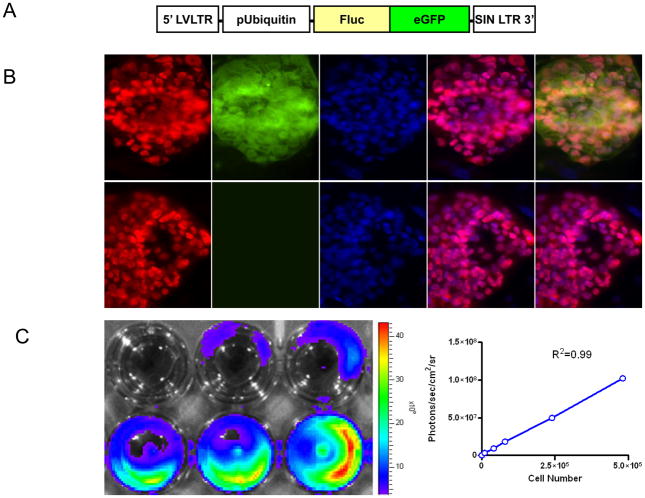
To develop an imaging platform for tracking hES cells in mouse, we used a DF reporter gene consisting of Fluc-eGFP. The double fusion reporter gene was cloned into a self-inactivating lentiviral vector downstream from the constitutive ubiquitin promoter (Fig. 1A). Both control nontransduced hES cells and stably transduced hES cells showed similar expression patterns of stem cell markers Oct-4 on immunostaining, suggesting the side effects by reporter gene on maintaining stem cell state are negligible (Fig. 1B). Upon culturing onto 24-well plates, we also observed a strong correlation (γ2=0.99) between Fluc activity and cell numbers ex vivo using the Xenogen IVIS system (Fig. 1C). Moreover, there is no difference between nontransduced cells and transduced hES cells with respect to the cell proliferation and cell viability (data not shown). In general, these data are consistent with a previous study in which hES cells with double fusion reporter gene have no difference with nontransduced hES cells on cell survival, proliferation, and differentiation7.
Figure 1. Stable lentiviral transduction of hES cells with the double fusion reporter genes.
(A) Schema of the double fusion reporter gene containing fusion of Fluc-GFP. The double fusion reporter gene was cloned into a self-inactivating lentiviral vector downstream from the constitutive ubiquitin promoter. (B) Control nontransduced hES cells and transduced hES cells showed similar expression pattern of Oct-4 under fluorescence microscopy. DAPI staining is used as a nuclear marker. Scale bar=20μm. (C) Ex vivo imaging analysis of stably transduced hES cells show increasing bioluminescence signals with cell numbers of hES cells (r2=0.99).
Longitudinal reporter gene imaging of hES cell survival in living animals
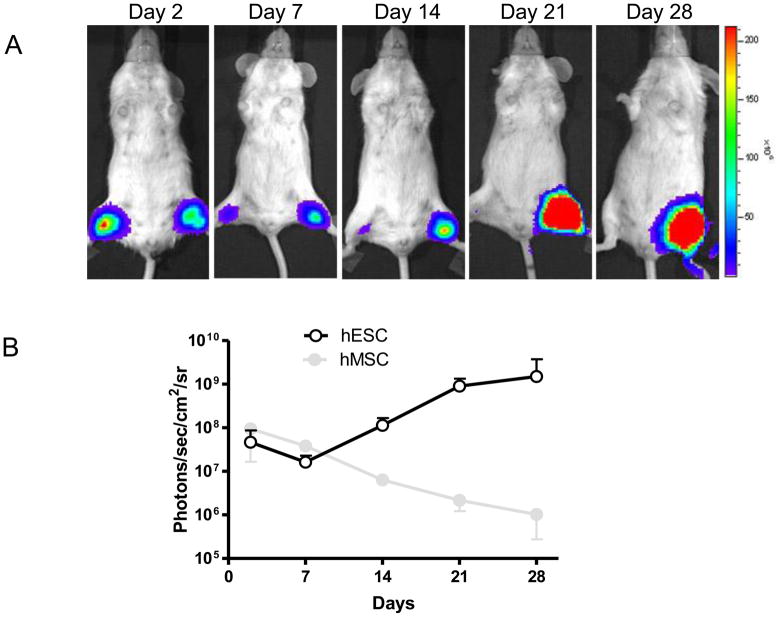
To analyze the ability of reporter gene imaging to assess stem cell fate, we injected approximately 1x106 hES cells stably transduced with DF reporter gene into left hind limbs in 50μl PBS and 1x106 human umbilical cord mesenchymal stem cells (MSC) stably transduced with DF reporter gene into right hind limbs as control. After injection, we performed longitudinal bioluminescence imaging on these animals for 4 weeks (Fig. 2A). In both hind limbs, bioluminescence signals were robust 2 days after injection. In the MSC group, bioluminescence signals progressively decreased from day 2 to day 28, indicating acute MSC death. Different from the survival pattern of MSC group, the bioluminescence signals of the hESC group first experienced a sharp drop from day 2 to day 7, which was followed by a dramatic increase in the following 3 weeks. At the end of the forth week, the signals of hESC were approximately 10-fold higher than those of day 2 (Fig. 2B). This special pattern of hESC death followed by cell proliferation can be seen in most animals analyzed.
Figure 2. Reporter gene imaging of hES cell fate after transplantation.
(A) A representative animal injected with 1x106 MSC (right hind limb) shows significant bioluminescence activity at day 2, which decreases progressively over the following 4 weeks. In contrast, undifferentiated hES cells (left hind limb) show the lowest bioluminescence signals at day 7, which increases dramatically during week 2 and week 4. (B) Detailed quantitative analysis of signals from all animals transplanted with hES cells versus MSC. Signal activity is expressed as photons/sec/cm2/sr.
Postmortem histology and immunohistochemistry of teratoma
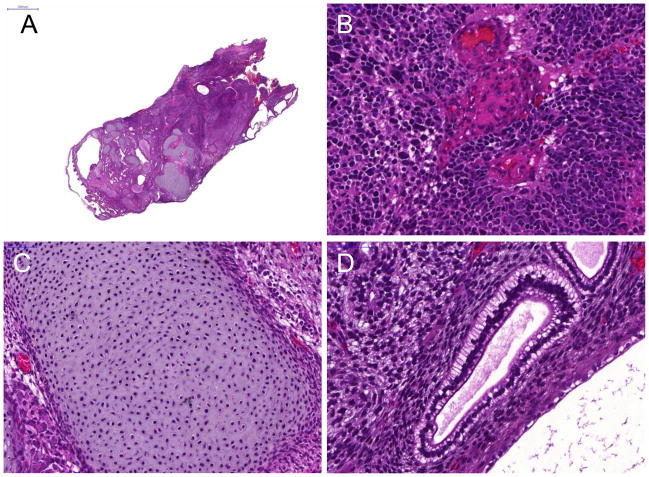
All animals were sacrificed 4 weeks after cell transplantation. Histologic analysis of the harvested hES-cell tumors showed teratoma formation (Fig. 3A). And H&E staining of teratoma formation after hES cell implantation identifies various differentiated cells representative of the three embryonic germ layers, such as neural epithelium representative of the ectoderm (Fig. 3B), osteoid formation representative of the mesoderm (Fig. 3C), and gut epithelium for endoderm (Fig. 3D), as well as immature components.
Figure 3. Histologic analysis of double labeled hES cells.
H&E staining of teratoma formation (A) after hES cell implantation identifies various cell types, which contains tissues of all three embryonic germ layers, such as (B) neural epithelium (ectoderm), (C) osteoid formation (mesoderm), and (D) gut epithelium (endoderm). Scale bar=2000μm (A) and 20μm (B, C, D).
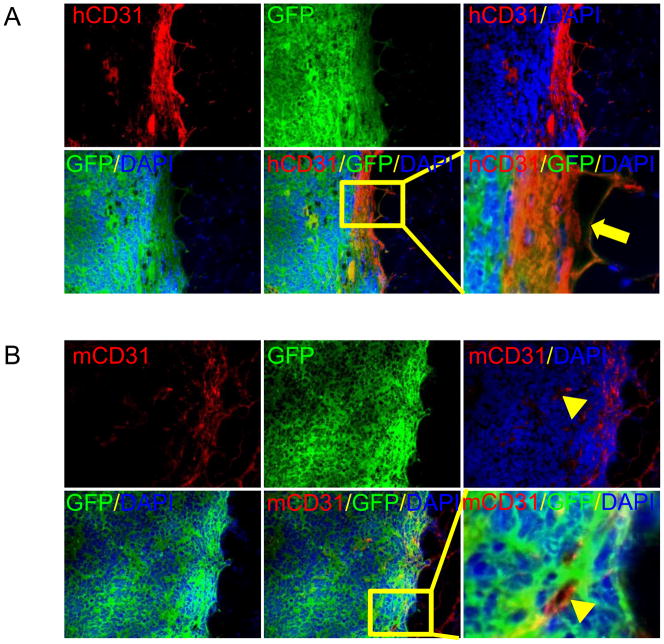
To determine whether the teratomas generated from hESCs in SCID mice can be used as a practical platform to study human neovasculogenesis, we should first investigate the origin of the vascular structure observed in the teratomas. First, we immunostained for GFP and human CD31 on teratomas derived from transplanted double fusion hES cells. In this way, we found cells coexpressing GFP and human CD31, indicating endothelial differentiation of the transplated GFP positive hES cells in vivo (Fig. 4A). Next, we immnostained for GFP and mouse CD31. We observed some cells inside the teratoma formations expressing mouse CD31 but not GFP, indicating that host vasculatures expand into teratomas and support the nutriention supply of teratomas (Fig. 4B).
Figure 4. Demonstration of angiogenesis in teratoma.
(A) Immunofluorescence staining for GFP and human CD31 at week 4 after transplantation of hES cells. GFP indicates cells come from hES cells and human CD31 suggests endothelial differentiation of hES cells in vivo. The transplanted GFP positive hES cells were found endothelial differentiation and integrating into host vasculatures. Scale bar=100μm. (B) Immnostaining of GFP and mouse CD31 for teratoma formation of transplanted double fusion hES cells, and mouse CD31 for microvasculature of teratoma coming from mouse itseslf, and GFP for hES cells. Nuclei were stained with DAPI (blue). The mouse CD31 positive cells were detected in teratoma, which implied that host vasculatures expand into teratoma and support the nutriention supply of teratoma. Scale bar=100μm.
Subculture and characterization of teratoma derived cells
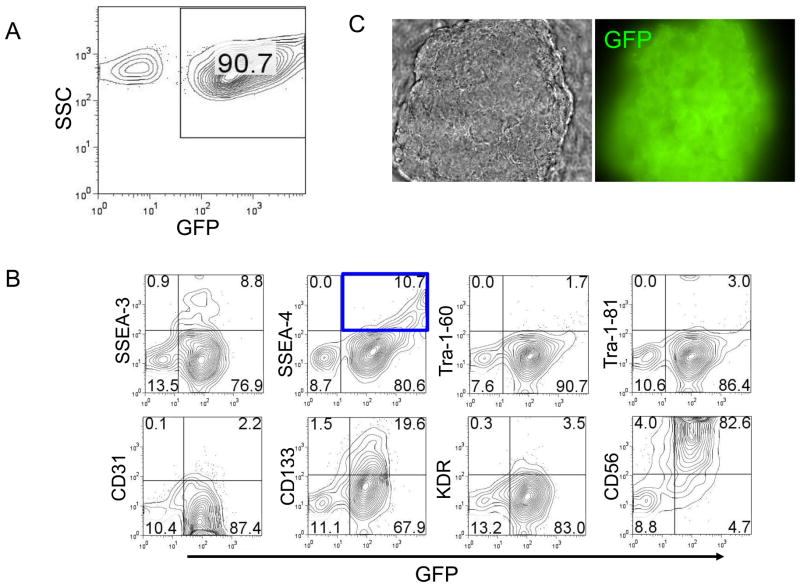
At day 28 after cell transplantation, we euthanized the mice and obtained single cell suspensions of teratomas. Flow cytometric analysis showed that more than 90 percent of the teratoma cells expressed GFP, indicating that those cells came from hES cells (Fig. 5A).
Figure 5. In vitro characterization of teratoma cells.
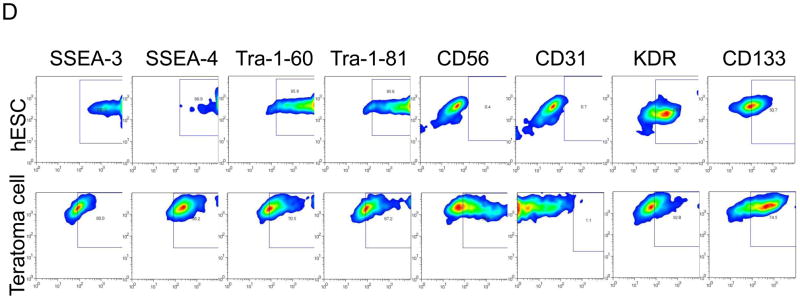
(A) Flow cytometric analysis showed most teratoma cells were GFP positive, which indicates those cells come from hES cells. (B) Flow cytometric analysis revealed that teratoma express a panel of differentiated and undifferentiated markers. Especially, undifferentiated marker SSEA-4 and NK cell marker CD56 expressed at high level. (C) In vitro subculture GFP+/SSEA-4+ teratoma cells. The morphology was like undifferentiated hES cells and GFP positive. (D) Characterization of subcultured GFP+/SSEA-4+ cells isolated from teratoma. hES cells were used as positive control. The results indicate subcultured GFP+/SSEA-4+ cells had similar expression pattern comparing to undifferentiated hES cells, but had very high level CD56 and a little lower expression of undifferentiated markers, such as SSEA-3, SSEA-4, TRA-1–60, and TRA-1–81. Single representative sample of triplicates are shown here.
Flow cytometric analysis revealed that teratomas expressed a panel of differentiated (CD31, CD133, KDR, and CD56) and undifferentiated markers (SSEA-3, SSEA-4, Tra-1–60, Tra-1–81). Especially, CD56 (82.6%) and undifferentiated marker SSEA-4 (10.7%) were expressed at high level (Fig. 5B). Then we isolated the GFP+/SSEA-4+ cells and subcultured this cell population on Matrigel-coated plates in mTeSR1 medium. The morphology of in vitro subcultured GFP+/SSEA-4+ teratoma cells was like undifferentiated hES cells and GFP positive (Fig. 5C). Flow cytometric analysis showed that subcultured GFP+/SSEA-4+ cells had similar expression pattern comparing to undifferentiated hES cells, but had very high level CD56 and a little lower expression of undifferentiated markers, such as SSEA-3, SSEA-4, TRA-1–60, and TRA-1–81 (Fig. 5D).
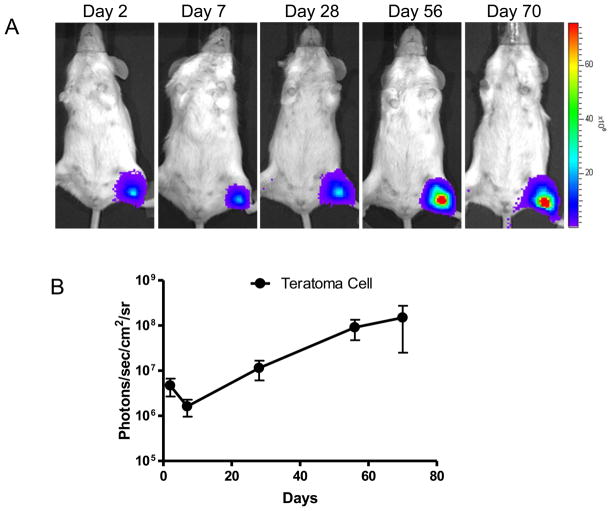
We next injected approximately 1x106 GFP+/SSEA-4+ teratoma cells into left hind limbs of SCID mice and performed longitudinal bioluminescence imaging on these animals for 10 weeks. Similar to the animals injected with hES cells we mentioned above, the special pattern of cell death followed by cell proliferation was also observed in mice injected with GFP+/SSEA-4+ teratoma cells. But the latter population proliferated with a relatively lower rate. A representative animal injected with GFP+/SSEA-4+ teratoma cells showed significant bioluminescence activity at day 2, which decreased in first 7 days, then increased progressively over the following 10 weeks. At the end of the tenth week, the signals of teratoma cells were approximately 10-fold higher than those of day 2 (Fig. 6).
Figure 6. Reporter gene imaging of GFP+/SSEA-4+ teratoma cells fate after transplantation.
(A) A representative animal injected with 1x106 GFP+/SSEA-4+ teratoma cells (left hind limb) shows significant bioluminescence activity at day 2, which decreases in first 7 days, then increases progressively over the following 10 weeks. (B) Detailed quantitative analysis of signals from all animals transplanted with GFP+/SSEA-4+ teratoma cells. Signal activity is expressed as photons/sec/cm2/sr.
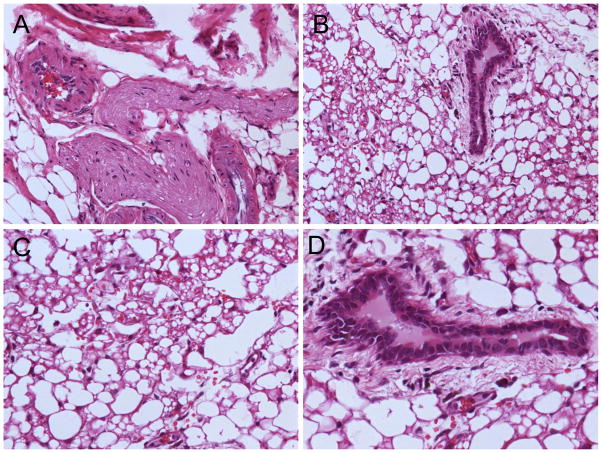
Upon injection into SCID mice, GFP+/SSEA-4+ teratoma cells form teratoma again in vivo. The postmortem histological analysis showed the teratoma formed by GFP+/SSEA-4+ teratoma cells also contained tissues of all three embryonic germ layers, such as neural epithelium representative of ectoderm (Fig. 7A), adipose tissue representative of mesoderm (Fig. 7C), and gut epithelium for endoderm (Fig. 7D). But compared to hES cells derived teratoma, the latter one contained less immature components.
Figure 7. H&E staining of teratoma formation.
Upon injection into SCID mice, GFP+/SSEA-4+ teratoma cells form teratoma again in vivo, which contains tissues of all three embryonic germ layers, such as (A) neural epithelium (ectoderm), (B) mesoderm and endoderm, (C) adipose tissue (mesoderm), and (D) gut epithelium (endoderm). Scale bar=20μm (A, C, D) and 40μm (B).
Discussion
In this study, hES cells were stably transduced with double fusion reporter gene (Fluc-eGFP), enabling us to track cells by both noninvasive imaging and invasive histopathology. Upon bioluminescence imaging and histology, we demonstrated that the engraftment of hES cells show dramatically increasing signal and lead to teratoma formation confirmed by histology. Moreover, the angiogenesis of teratoma was investigated and CD31 staining revealed that teratoma vasculatures are from both differentiated hES cells and host. Furthermore, we report here first that those cells exhibit multipotency, retaining their differentiation ability in vivo confirmed by their differentiation into representatives of three germ layers, but their proliferation activity is not very high comparing to authentic hES cells.
For stem cell research to make the next quantum leap, it is imperative to understand the dynamic processes of hES cells homing, migration, biodistribution, proliferation, and differentiation in the same subject over time. In this study, we were able to determine the kinetics of cell survival over time within the same individual. We transduced hES cells with a double fusion reporter gene consisting of Fluc-eGFP to track the cells in vivo. We observed no apparent side effects by the reporter gene on maintaining stem cell state, cell viability and cell proliferation. And a close relationship was found between hES cell number and luciferase activity, which ensures that noninvasive imaging of Fluc-eGFP-labeled hES cells provides a sensitive and simple means to observe the presence of engrafted hES cells. And in this way it is practicable to monitor the kinetics of hES cells before palpable teratomas are formed which typically takes several weeks.
hES cells are remarkable for their unlimited self-renewal and pluripotency capacityand an obstacle to their therapeutic use is teratomas formation. Tumor growth, including teratomas, needs need blood vessels and antiangiogenic therapy to limit and even reverse the growth of tumors are showing promise8. By immunostaining, we observed vasculatures formation from hES cells confirmed by GFP and human CD31 double positive in teratomas, indicating endothelial differentiation of the transplanted GFP positive hES cells in vivo. And we also found some cells expressing mouse CD31 but not GFP inside the teratoma formation, showing that host vasculatures expanded into teratoma and support the nutriention supply of teratoma. When discussing the clinical application of hESC-derived cells, teratoma formation is a significant concern. Enlightened by the vascular targeted therapies in oncology, we consider that agents targeting endothelial cells differentiated from hES cells or preventing host vasculatures from expanding into teratomas may be a practicable choice in preventing and treating teratomas formed by undifferentiated hES cells.
Formation of three somatic germ layers within the teratoma is taken as the best indicator of the pluripotency of hES cell lines13, 14. A further understanding of this process should aid in the development of safer hES cells therapies and may help elucidate teratomas formation. Our FACS data revealed that CD56 expressed robustly in teratoma derived cells. CD56, a 200–220 kDa cell surface glycoprotein, identified as an isoform of the neural adhesion molecules (NCAM), has been found frequently expressed on the surface of neurons, glia, skeletal muscle and natural killer cells, as well as in several lympho-hematopoietic neoplasms15–19. CD56 was intensely expressed in the mesenchyme20 and is a marker of early neuro-ectodermal differentiation 21, which has been used to identify neural differentiation of hESCs by flow-cytometric analysis and sorting 15, 22. We suggest that the expression of CD56 upon hES cell transplantation in vivo may indicate the teratomas formation with a large degree of embryonic neural derivatives.
With regard to histopathology, teratomas are characterized as either mature and benign or as immature and malignant14. Though containing well-defined somatic structures in hES derived teratomas, undifferentiated tissue may be noted and should be addressed and evaluated for future hES cells therapy. Our FACS data revealed that teratomas express undifferentiated markers SSEA-3, SSEA-4 about 10%, as well as Tra-1–60, Tra-1–81. Moreover, in vivo transplantation indicated that those cells exhibit multipotency, but their proliferation activity is not very high comparing to hES cells by bioluminescence imaging. H&E staining showed nuclear enlargement, increased nuclear-to-cytoplasmic ratio in hES cells derived teratomas, where as more differentiated in teratomas derived cells, which indicates that teratomas are benign, multidifferentiated tissue and contain immature cells, but not malignant. Of note, the teratomas derived cells were capable of forming teratoma-like masses and proliferated at low level in vivo, an indication of incomplete potency when compared to hES cells.
In summary, our study demonstrates that bioluminescence imaging can be used to monitor misbehavior of hES cells noninvasively in living animal. Teratomas formed by hES cells in SCID mice contained vasculatures of both hES cells and host origin and targeting endothelial cells differentiated from hES cells or preventing host vasculatures from expanding into teratomas may be a practicable choice in preventing and treating teratomas. FACS analysis revealed that hES cells derived teratomas express a high level of CD56, and CD56 would be a good indicator for teratoma formation of hES cells in vivo. Moreover, teratoma tissues are composed with undifferentiated cell population, which expressing SSEA-3, SSEA-4, Tra-1–60 and Tra-1–81 and this population cells are more differentiated and with less proliferation capacity comparing to hES cells and they would be difficult to form teratoma like masses in vivo.
Acknowledgments
This project was supported by grants from National Development Plan of High Technology 863 (2007AA021010) and 973 (2007CB914804) to RX.
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009 doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Xu XQ, Zweigerdt R, Soo SY, Ngoh ZX, Tham SC, Wang ST, Graichen R, Davidson B, Colman A, Sun W. Highly enriched cardiomyocytes from human embryonic stem cells. Cytotherapy. 2008;10(4):376–389. doi: 10.1080/14653240802105307. [DOI] [PubMed] [Google Scholar]
- 4.Phillips BW, Hentze H, Rust WL, Chen QP, Chipperfield H, Tan EK, Abraham S, Sadasivam A, Soong PL, Wang ST, Lim R, Sun W, Colman A, Dunn NR. Directed differentiation of human embryonic stem cells into the pancreatic endocrine lineage. Stem Cells and Development. 2007;16(4):561–578. doi: 10.1089/scd.2007.0029. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Han Z, Wu JC. Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases. J Cell Biochem. 2009;106(2):194–199. doi: 10.1002/jcb.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116(11 Suppl):I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, Yang PC, Wu JC. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem cells (Dayton, Ohio) 2008;26(4):864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, Wu JC. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. Plos One. 2009;4(12):e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao QJ, Ren HY, Li XY, Chen Z, Zhang XY, Gong W, Liu YJ, Pang TX, Han ZC. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy. 2009;11(4):414–426. doi: 10.1080/14653240902849754. [DOI] [PubMed] [Google Scholar]
- 11.Xu CH, He JQ, Kamp TJ, Police S, Hao XM, O'Sullivan C, Carpenter MK, Lebkowski J, Gold JD. Human embryonic stem cell-derived cardiomyocytes can be maintained in defined medium without serum. Stem Cells and Development. 2006;15(6):931–941. doi: 10.1089/scd.2006.15.931. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53(14):1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nat Biotechnol. 2009;27(3):281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 14.Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma Formation Assays with Human Embryonic Stem Cells: A Rationale for One Type of Human-Animal Chimera. Cell stem cell. 2007;1(3):253–258. doi: 10.1016/j.stem.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Pruszak J, Sonntag KC, Aung MH, Sanchez-Pernaute R, Isacson O. Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations. Stem cells (Dayton, Ohio) 2007;25(9):2257–2268. doi: 10.1634/stemcells.2006-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, Mariotti C, Birtolo S, Tozzi M, Lauria F. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia. 2001;15(8):1161–1164. doi: 10.1038/sj.leu.2402174. [DOI] [PubMed] [Google Scholar]
- 17.Kontogianni K, Nicholson AG, Butcher D, Sheppard MN. CD56: a useful tool for the diagnosis of small cell lung carcinomas on biopsies with extensive crush artefact. Journal of Clinical Pathology. 2005;58(9):978–980. doi: 10.1136/jcp.2004.023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohishi Y, Kaku T, Oya M, Kobayashi H, Wake N, Tsuneyoshi M. CD56 expression in ovarian granulosa cell tumors, and its diagnostic utility and pitfalls. Gynecol Oncol. 2007;107(1):30–38. doi: 10.1016/j.ygyno.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Kurokawa M, Nabeshima K, Akiyama Y, Maeda S, Nishida T, Nakayama F, Amano M, Ogata K, Setoyama M. CD56: a useful marker for diagnosing Merkel cell carcinoma. J Dermatol Sci. 2003;31(3):219–224. doi: 10.1016/s0923-1811(03)00029-x. [DOI] [PubMed] [Google Scholar]
- 20.Jin L, Hemperly JJ, Lloyd RV. Expression of neural cell adhesion molecule in normal and neoplastic human neuroendocrine tissues. Am J Pathol. 1991;138(4):961–969. [PMC free article] [PubMed] [Google Scholar]
- 21.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19(12):1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- 22.Sundberg M, Jansson L, Ketolainen J, Pihlajamaki H, Suuronen R, Skottman H, Inzunza J, Hovatta O, Narkilahti S. CD marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel CD marker for exclusion of pluripotent stem cells. Stem Cell Research. 2009;2(2):113–124. doi: 10.1016/j.scr.2008.08.001. [DOI] [PubMed] [Google Scholar]