Abstract
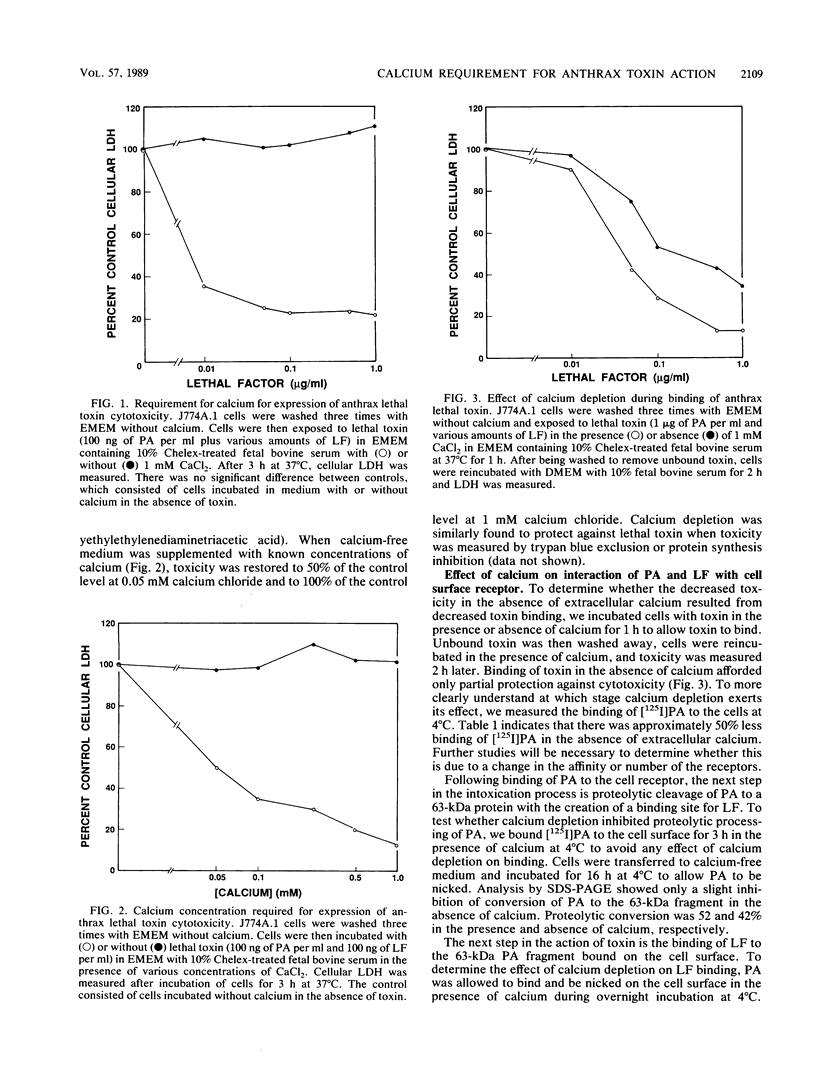
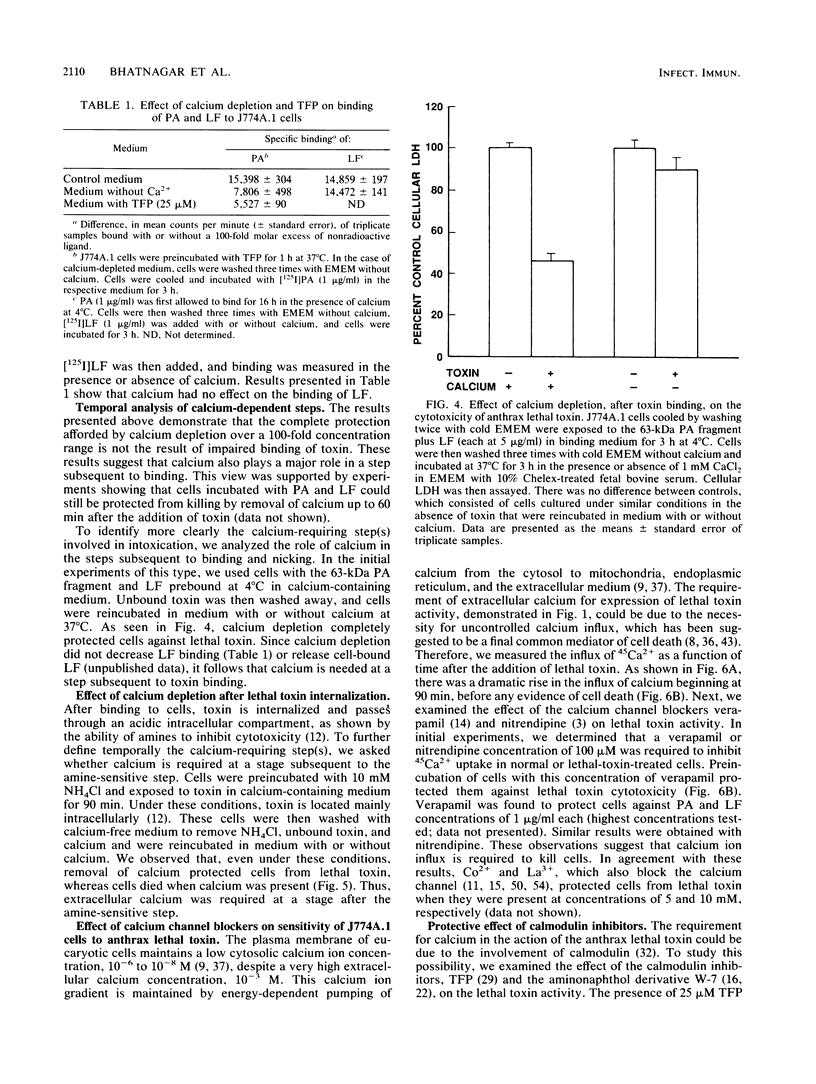
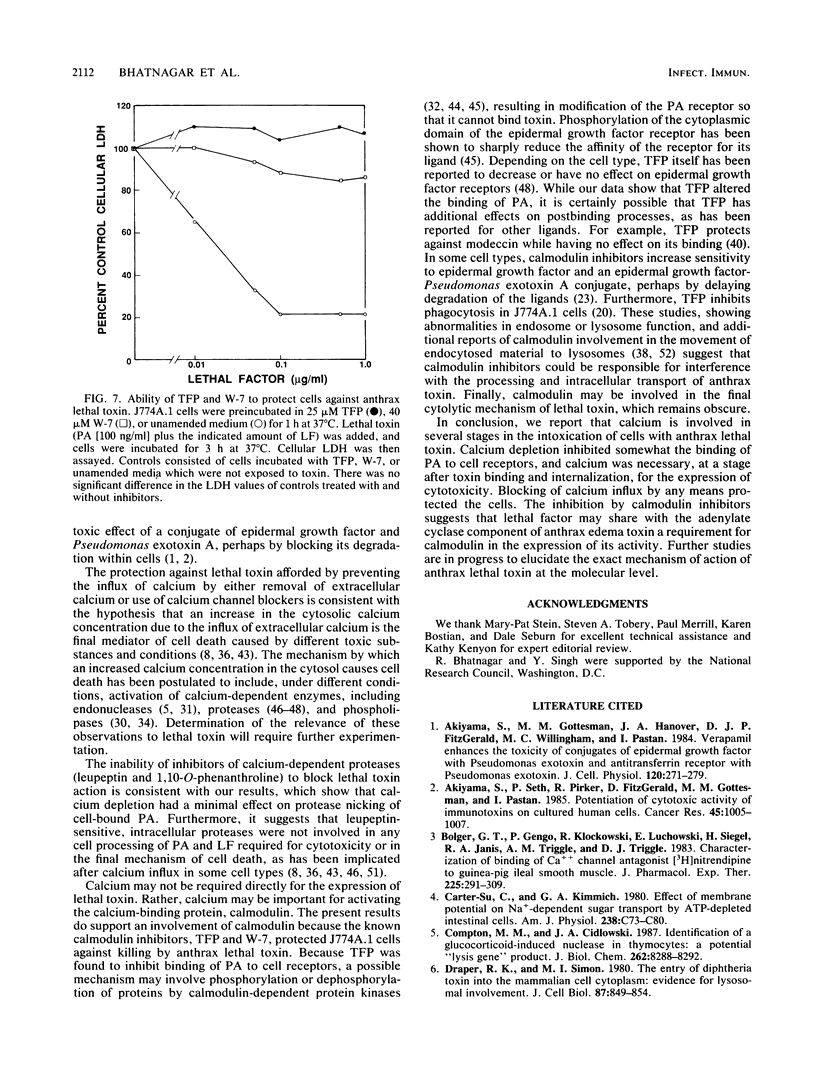
Anthrax lethal toxin, which consists of two separate proteins, protective antigen (Mr, 82,700) and lethal factor (Mr, approximately 83,000), is cytotoxic to the macrophagelike cell line J774A.1. Removal of calcium from the culture medium protected cells against the action of lethal toxin. Calcium depletion during the binding phase of intoxication afforded only partial protection. Further analysis showed that calcium removal caused some inhibition of protective antigen binding but that it had minimal effect on proteolytic conversion of protective antigen to the active 63-kilodalton fragment and that it had no effect on lethal factor binding. Cells to which lethal toxin had bound in the presence of calcium were protected when transferred to calcium-depleted culture medium, indicating a role for calcium at a postbinding stage. When ammonium chloride is present with lethal toxin, toxin accumulates in intracellular vesicles. Calcium-free medium protected these cells upon removal of the amine block, suggesting that calcium is also required at a step after internalization of lethal toxin. Calcium channel blockers inhibited 45Ca2+ uptake and protected cells against cytotoxicity. Calmodulin inhibitors also protected against the action of lethal toxin, suggesting involvement of calmodulin at a step during intoxication. We conclude that calcium is required at several steps in the intoxication of cells by anthrax lethal toxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Gottesman M. M., Hanover J. A., Fitzgerald D. J., Willingham M. C., Pastan I. Verapamil enhances the toxicity of conjugates of epidermal growth factor with Pseudomonas exotoxin and antitransferrin receptor with Pseudomonas exotoxin. J Cell Physiol. 1984 Sep;120(3):271–279. doi: 10.1002/jcp.1041200303. [DOI] [PubMed] [Google Scholar]
- Akiyama S., Seth P., Pirker R., FitzGerald D., Gottesman M. M., Pastan I. Potentiation of cytotoxic activity of immunotoxins on cultured human cells. Cancer Res. 1985 Mar;45(3):1005–1007. [PubMed] [Google Scholar]
- Bolger G. T., Gengo P., Klockowski R., Luchowski E., Siegel H., Janis R. A., Triggle A. M., Triggle D. J. Characterization of binding of the Ca++ channel antagonist, [3H]nitrendipine, to guinea-pig ileal smooth muscle. J Pharmacol Exp Ther. 1983 May;225(2):291–309. [PubMed] [Google Scholar]
- Carter-Su C., Kimmich G. A. Effect of membrane potential on Na+-dependent sugar transport by ATP-depleted intestinal cells. Am J Physiol. 1980 Mar;238(3):C73–C80. doi: 10.1152/ajpcell.1980.238.3.C73. [DOI] [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. L. The role of calcium in cell death. Life Sci. 1981 Sep 28;29(13):1289–1295. doi: 10.1016/0024-3205(81)90670-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D., Morris R. E., Saelinger C. B. Essential role of calcium in cellular internalization of Pseudomonas toxin. Infect Immun. 1982 Feb;35(2):715–720. doi: 10.1128/iai.35.2.715-720.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander A. M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J Biol Chem. 1986 Jun 5;261(16):7123–7126. [PubMed] [Google Scholar]
- Guerrero J. R., Martin S. S. Verapamil: full spectrum calcium channel blocking agent: an overview. Med Res Rev. 1984 Jan-Mar;4(1):87–109. doi: 10.1002/med.2610040106. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Naka M., Tanaka T., Hayashi H., Kobayashi R. Calcium-regulated modulator protein interacting agents inhibit smooth muscle calcium-stimulated protein kinase and ATPase. Mol Pharmacol. 1980 Jan;17(1):66–72. [PubMed] [Google Scholar]
- Hirayama T., Kato I. Mode of cytotoxic action of pseudomonal leukocidin on phosphatidylinositol metabolism and activation of lysosomal enzyme in rabbit leukocytes. Infect Immun. 1984 Jan;43(1):21–27. doi: 10.1128/iai.43.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Horiguchi Y., Uemura T., Kozaki S., Sakaguchi G. Effects of Ca2+ and other cations on the action of Clostridium perfringens enterotoxin. Biochim Biophys Acta. 1986 Oct 31;889(1):65–71. doi: 10.1016/0167-4889(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Horwitz S. B., Chia G. H., Harracksingh C., Orlow S., Pifko-Hirst S., Schneck J., Sorbara L., Speaker M., Wilk E. W., Rosen O. M. Trifluoperazine inhibits phagocytosis in a macrophagelike cultured cell line. J Cell Biol. 1981 Dec;91(3 Pt 1):798–802. doi: 10.1083/jcb.91.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Davidson H. W., Peshavaria M. Proteolytic processing of chromogranin A in purified insulin granules. Formation of a 20 kDa N-terminal fragment (betagranin) by the concerted action of a Ca2+-dependent endopeptidase and carboxypeptidase H (EC 3.4.17.10). Biochem J. 1987 Jun 1;244(2):457–464. doi: 10.1042/bj2440457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- Kuratomi Y., Akiyama S., Ono M., Shiraishi N., Shimada T., Ohkuma S., Kuwano M. Thioridazine enhances lysosomal accumulation of epidermal growth factor and toxicity of conjugates of epidermal growth factor with Pseudomonas exotoxin. Exp Cell Res. 1986 Feb;162(2):436–448. doi: 10.1016/0014-4827(86)90348-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Production and purification of anthrax toxin. Methods Enzymol. 1988;165:103–116. doi: 10.1016/s0076-6879(88)65019-1. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Malis C. D., Bonventre J. V. Mechanism of calcium potentiation of oxygen free radical injury to renal mitochondria. A model for post-ischemic and toxic mitochondrial damage. J Biol Chem. 1986 Oct 25;261(30):14201–14208. [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. L., Fiera R. A., Matthews N. Involvement of phospholipase A2 activation in tumour cell killing by tumour necrosis factor. Immunology. 1988 May;64(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Salisbury J. L., Condeelis J. S., Satir P. Role of coated vesicles, microfilaments, and calmodulin in receptor-mediated endocytosis by cultured B lymphoblastoid cells. J Cell Biol. 1980 Oct;87(1):132–141. doi: 10.1083/jcb.87.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Brown J. E. Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infect Immun. 1987 Feb;55(2):298–303. doi: 10.1128/iai.55.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. I. Requirement for calcium. J Biol Chem. 1982 Jul 10;257(13):7495–7503. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Interactions between diphtheria toxin entry and anion transport in Vero cells. IV. Evidence that entry of diphtheria toxin is dependent on efficient anion transport. J Biol Chem. 1986 Feb 5;261(4):1570–1575. [PubMed] [Google Scholar]
- Sasaki T., Lutz F. Action of a cytotoxin from Pseudomonas aeruginosa on human leukemic cell lines. Increase in cell permeability to Ca2+ and Mn2+ and lack of stimulation of inositol lipid turnover. FEBS Lett. 1985 Sep 9;189(1):33–36. doi: 10.1016/0014-5793(85)80836-x. [DOI] [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K. Calcium and ischemic brain damage. Eur Neurol. 1986;25 (Suppl 1):45–56. doi: 10.1159/000116060. [DOI] [PubMed] [Google Scholar]
- Stavenow L., Hultberg B. Release of fibronectin, sulfated glycosaminoglycans and beta-hexosaminidase from injured smooth muscle cells and fibroblasts in culture--possible transferable effects on new cultures. Acta Pathol Microbiol Immunol Scand A. 1985 Sep;93(5):215–222. doi: 10.1111/j.1699-0463.1985.tb03944.x. [DOI] [PubMed] [Google Scholar]
- Sundan A., Sandvig K., Olsnes S. Calmodulin antagonists sensitize cells to pseudomonas toxin. J Cell Physiol. 1984 Apr;119(1):15–22. doi: 10.1002/jcp.1041190104. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Lomedico M. E., Maina D. Role of calcium in the thyrotropin-releasing hormone-stimulated release of prolactin from pituitary cells in culture. Biochem Biophys Res Commun. 1978 Apr 14;81(3):798–806. doi: 10.1016/0006-291x(78)91422-5. [DOI] [PubMed] [Google Scholar]
- Tsokos-Kuhn J. O. Lethal injury by diquat redox cycling in an isolated hepatocyte model. Arch Biochem Biophys. 1988 Sep;265(2):415–424. doi: 10.1016/0003-9861(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Ward M. R., Boyd C. A. Intracellular study of ionic events underlying intestinal membrane transport of oligopeptides. Nature. 1980 Sep 11;287(5778):157–158. doi: 10.1038/287157a0. [DOI] [PubMed] [Google Scholar]
- van Berkel T. J., Nagelkerke J. F., Kruijt J. K. The effect of Ca2+ and the trifluoperazine on the processing of human acetylated low density lipoprotein by non-parenchymal liver cells. FEBS Lett. 1981 Sep 14;132(1):61–66. doi: 10.1016/0014-5793(81)80427-9. [DOI] [PubMed] [Google Scholar]



