Abstract
Intracerebral haemorrhage is an important public health problem leading to high rates of death and disability in adults. Although the number of hospital admissions for intracerebral haemorrhage has increased worldwide in the past 10 years, mortality has not fallen. Results of clinical trials and observational studies suggest that coordinated primary and specialty care is associated with lower mortality than is typical community practice. Development of treatment goals for critical care, and new sequences of care and specialty practice can improve outcome after intracerebral haemorrhage. Specific treatment approaches include early diagnosis and haemostasis, aggressive management of blood pressure, open surgical and minimally invasive surgical techniques to remove clot, techniques to remove intraventricular blood, and management of intracranial pressure. These approaches improve clinical management of patients with intracerebral haemorrhage and promise to reduce mortality and increase functional survival.
Introduction
Non-traumatic intracerebral haemorrhage results from rupture of blood vessels in the brain. It is a major public health problem1 with an annual incidence of 10–30 per 100 000 population,1,2 accounting for 2 million (10–15%)3 of about 15 million strokes worldwide each year.4 Hospital admissions for intracerebral haemorrhage have in creased by 18% in the past 10 years,5 probably because of increases in the number of elderly people,6 many of whom lack adequate blood-pressure control, and the increasing use of anticoagulants, thrombolytics, and antiplatelet agents. Mexican Americans, Latin Americans, African Americans, Native Americans, Japanese people, and Chinese people have higher incidences than do white Americans.2,7–9 These differences are mostly seen in the incidence of deep intracerebral haemorrhage and are most prominent in young and middle-aged people. Incidence might have decreased in some populations with improved access to medical care and blood-pressure control.8–10
Primary and secondary (anticoagulant-induced) intra-cerebral haemorrhage have similar underlying pathological changes.11 Intracerebral haemorrhage commonly affects cerebral lobes, the basal ganglia, the thalamus, the brain stem (predominantly the pons), and the cerebellum as a result of ruptured vessels affected by hypertension-related degenerative changes or cerebral amyloid angiopathy.1 Most bleeding in hyper tension-related intracerebral haemorrhage is at or near the bifurcation of small penetrating arteries that originate from basilar arteries or the anterior, middle, or posterior cerebral arteries.12 Small artery branches of 50–700 μm in diameter often have multiple sites of rupture; some are associated with layers of platelet and fibrin aggregates. These lesions are characterised by breakage of elastic lamina, atrophy and fragmentation of smooth muscle, dissections, and granular or vesicular cellular degeneration.12,13 Severe atherosclerosis including lipid deposition can affect elderly patients in particular. Fibrinoid necrosis of the subendothelium with subsequent focal dilatations (micro aneurysms) leads to rupture in a small proportion of patients.12
Cerebral amyloid angiopathy is characterised by the deposition of amyloid-β peptide and degenerative changes (microaneurysm formation, concentric splitting, chronic inflammatory infiltrates, and fibrinoid necrosis) in the capillaries, arterioles, and small and medium sized arteries of the cerebral cortex, leptomeninges, and cerebellum.14 Cerebral amyloid angiopathy leads to sporadic intracerebral haemorrhage in elderly people, commonly associated with variations in the gene encoding apolipoprotein E, and a familial syndrome in young patients, typically associated with mutations in the gene encoding amyloid precursor protein.15 White-matter abnormalities (eg, leukoariosis) seem to increase the risk of both sporadic and familial intracerebral haemorrhage, suggesting a shared vascular pathogenesis.16,17
Intracerebral haemorrhage associated with the taking of oral anticoagulants typically affects patients with vasculopathies related to either chronic hypertension or cerebral amyloid angiopathy, which might represent exacerbation of an existing risk of clinical and subclinical disease.16
Pathophysiology
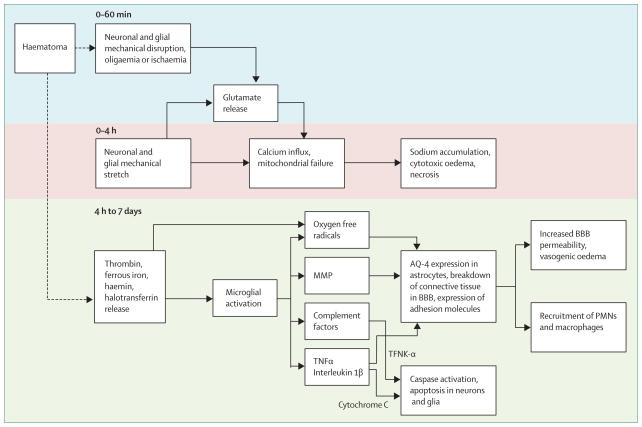
The regions surrounding haematomas are characterised by oedema, apoptosis and necrosis, and inflammatory cells.18 Haematomas induce injury (figure 1) by mechanical disruption of the neurons and glia,1 followed by mechanical deformation causing oligaemia, neuro-transmitter release, mitochondrial dysfunction, and membrane depolarisation.19–21 Dependent on the severity of mitochondrial dysfunction, the results of injury range from temporary metabolic suppression (hibernation phase) to cellular swelling and necrosis. A secondary cascade of injury is started by products of coagulation and haemoglobin breakdown, in particular thrombin, which activate of microglia by 4 h after injury.22–25 Activated microglia26 release products that induce breakdown of the blood–brain barrier, vasogenic oedema, and apoptosis in neurons and glia.27–32
Figure 1. Cascade of neural injury initiated by intracerebral haemorrhage.
The steps in the first 4 h are related to the direct effect of the haematoma, later steps to the products released from the haematoma. BBB=blood–brain barrier. MMP=matrix metallopeptidase. TNF=tumour necrosis factor. PMN=polymorphonuclear cells.
Haemostasis is initiated by local activation of haemostatic pathways and mechanical tamponade.33,34 However, about 73% of patients assessed within 3 h of symptom onset have some degree of haematoma enlargement35 and up to 35% have clinically prominent enlargement35 (figure 2). Most haematoma enlargement occurs within 3 h, although enlargement can occur up to 12 h after onset.36,37 Perihaematomal oedema increases in volume by about 75% in the first 24 h after intracerebral haemorrhage,38 peaks around 5–6 days,39 and lasts up to 14 days.40 Early large oedema volume relative to haematoma volume makes the greatest contribution to outcome.41 However, oedema that is small initially can increase in volume in the first 24 h after haemorrhage.38 An acute hypometabolic and hypoperfusion (hibernation) phase,42,43 with mitochondrial dysfunction44 and metabolic failure,45 has been reported in the region surrounding the haematoma (figure 3). Regional hypoperfusion in clinical46,47 and experimental studies48,49 does not always seem severe enough to induce ischaemia and might be secondary to hypometabolism. In the presence of very high intracranial pressure and low cerebral perfusion pressure, the risk of global ischaemia is high. A variable reperfusion phase lasts from 2 days to 14 days, and a normalisation phase develops after 14 days, with re-establishment of normal cerebral blood flow in all viable regions.
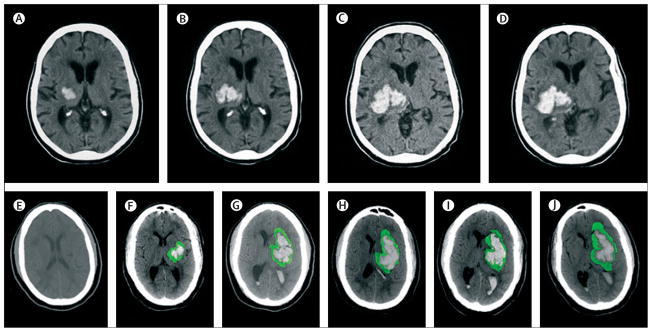
Figure 2. Progression of haemotoma and oedema on CT.
Top: hyperacute expansion of haematoma in a patient with intracerebral haemorrhage on serial CT scans. Small haematoma detected in the basal ganglia and thalamus (A). Expansion of haematoma after 151 min (B). Continued progression of haematoma after another 82 min (C). Stabilisation of haematoma after another 76 min (D). Bottom: progression of haematoma and perihaematomal oedema in a patient with intracerebral haemorrhage on serial CT scans. The first scan (E) was acquired before the intracerebral haemorrhage. Perihaematoma oedema is highlighted in green to facilitate recognition of progression of oedema. At 4 h after symptom onset there is a small haematoma in the basal ganglia (F). Expansion of haematoma with extension into the lateral ventricle and new mass-effect and midline shift at 14 h (G). Worsening hydrocephalus and early perihaematomal oedema at 28 h (H). Continued mass-effect with prominent perihaematomal oedema at 73 h (I). Resolving haematoma with more prominent perihaematomal oedema at 7 days (J).
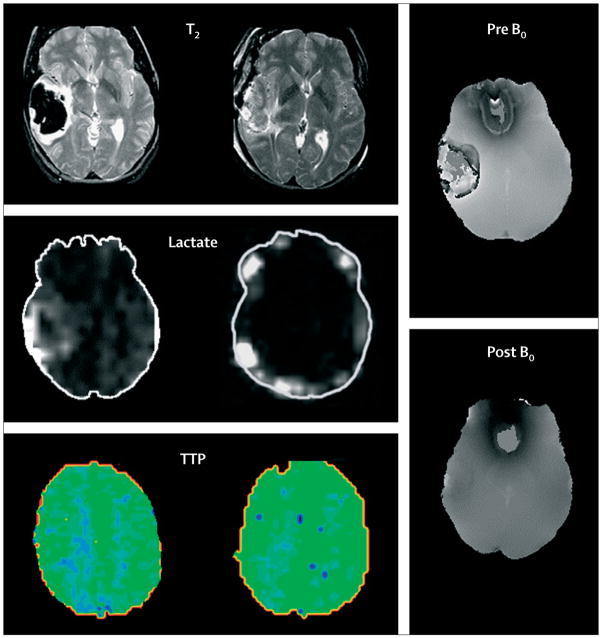
Figure 3. Advanced MRI of lobar intracerebral haemorrhage.
Left: before craniotomy. Middle: after craniotomy for treatment of mass-effect and removal of haematoma. Sequential T2, lactate magnetic resonance spectroscopy, and perfusion studies showed qualitative decreases of perihaematomal oedema and perihaematomal lactate and increased occipital regional perfusion measured as time to peak of bolus injectate (TTP) after removal of clot; TTP is represented by intensity and distribution of green colour. Right: magnetic susceptibility images show paramagnetic influence before surgery and limited susceptibility after removal of the iron-containing blood clot by craniotomy. Figures provided by J Ricardo Carhuapoma (Johns Hopkins Medical Institution, Baltimore, MD, USA).
Diagnosis, clinical features, and outcomes
Although CT scanning is the first-line diagnostic approach, MRI with gradient echo can detect hyperacute intracerebral haemorrhage with equal sensitivity and overall accuracy50,51 and is more accurate for the detection of microhaemorrhages (figure 4). Perihaematomal extravasation of intravenous contrast on CT scan can detect ongoing bleeding.52,53 Cerebral angiography is needed to diagnose secondary causes of intracerebral haemorrhage, such as aneurysms, arteriovenous malformations, dural venous thromboses, and vasculitis1,34,54,55 (figure 5). MRI and magnetic-resonance angiography can also identify secondary causes of intracerebral haemorrhage such as cavernous malformations,55 although their sensitivity is not well established.
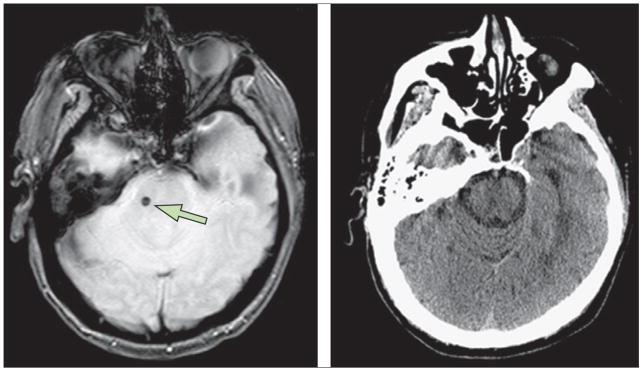
Figure 4. Detection of microhaemorrhages with MRI and CT scans.
Left: asymptomatic pontine microbleed (arrow) in a patient with ischaemic stroke shown as focal hypointensity on gradient echo MRI. Right: microbleed not detected on CT scan. Figures are provided by David S Liebeskind (University of California at Los Angeles, CA, USA).
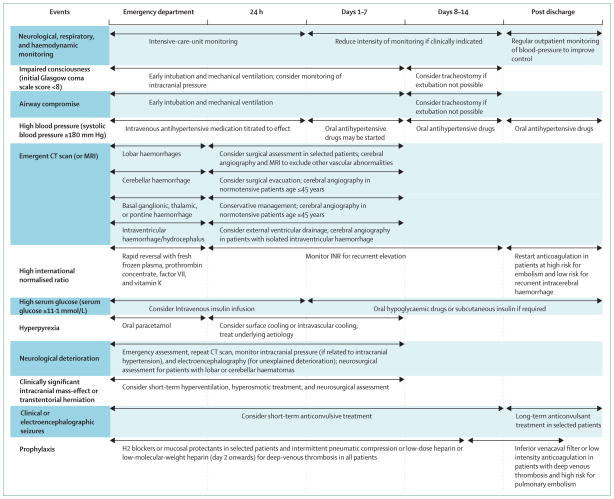
Figure 5.
Management algorithm for patients with intracerebral haemorrhage
Classic presentations, such as rapid-onset focal neurological deficits, decreased consciousness, and signs of brainstem dysfunction, are related to the size and location of haematoma.1 Neurological deterioration is common before56 and during57 hospital admission and is related to early haematoma enlargement or late worsening of oedema.58 Several descriptors of disease severity are predictive of early death, including age, initial score on the Glasgow coma scale (GCS), haematoma volume, ventricular blood volume,59 and haematoma enlargement.35
Mortality at 3 months was 34% in a review of 586 patients with intracerebral haemorrhage from 30 centres.60 In other studies it was 31% at 7 days, 59% at 1 year, 82% at 10 years, and more than 90% at 16 years.61,62 Subsequent risk of other cardiovascular events was 4% for all stroke, 2% for intracerebral haemorrhage, and 1% for ischaemic stroke per patient-year.63 Patients with a lobar haemorrhage had a high rate of recurrence (4% per patient-year). Asymptomatic disease progression is particularly common when microbleeds and white matter abnormalities are taken into account.64 Effects of recurrent bleeding can be changed by antihypertensive treatment;65 whether progressive functional impairments are equally treatable is unknown.66
Management
Overall principles
In a review of 1421 patients with intracerebral haemorrhage, care limitations or withdrawal of life-sustaining interventions was the most common (in 68%) cause of death.67 A state-wide survey in the USA68 showed that the odds of dying in hospital were associated with the frequency of use of do-not-resuscitate orders. In another study, in-hospital mortality was lower in patients treated in an intensive-care neurology unit.69 These studies provide indirect evidence that aggressive medical management and specialist care can improve the overall outcome in patients with intracerebral haemorrhage. In the USA, admissions for haemorrhage to urban teaching hospitals increased from 30% in 1990–91 to 49% in 2000–01.5 Mortality was decreased substantially for patients admitted to urban teaching hospitals but not urban non-teaching hospitals and rural hospitals, suggesting that changing trends in admissions might be beneficial.5 Trials addressing a single severity factor (haemorrhage volume70 or haematoma enlargement71) have been physiologically successful but without clinical benefit. These results emphasise that a single treatment approach might accomplish its physiological goal but be insufficient to produce clinical benefit, thus opening the possibility that well organised, multimodal therapy addressing each of the modifiable factors—haematoma volume, ventricular blood, and haematoma enlargement—might be needed.72
Early assessment and management
Airway support,1 blood-pressure control,73 intracranial pressure treatment,74 and anticoagulation reversal75 are commonly started in emergency departments, which are also the site of many first neurosurgical consultations for patients with intracerebral haemorrhage.1 Observational studies show that about 30% of patients with supra tentorial haemorrhage and almost all patients with brainstem or cerebellar haemorrhage have either decreased consciousness or bulbar muscle dysfunction necessitating intubation.76 Rapid deterioration, clinical evidence of transtentorial herniation, or mass-effect or obstructive hydro cephalus on neuroimaging should mandate an emergent neurosurgical consultation for possible intra ventricular catheter placement or surgical evacuation and concomitant use of hyperventilation and intravenous mannitol77–79 (figure 5). The risk of neurological deterioration and cardiovascular instability is greatest in the first 24 h after symptom onset,80 and frequent assessment of patients’ neurological status and haemodynamic variables in dedicated intensive-care units is needed.
Acute haemostatic treatment
Activated recombinant factor VII (fVIIa) promotes haemostasis at sites of vascular injury and limits haematoma enlargement after intracerebral haemorrhage. A randomised, double-blind, placebo-controlled phase II trial81 treated 399 patients within 3 h of onset with placebo or 40 μm/kg, 80 μm/kg, or 160 μg/kg of fVIIa. Overall, the mean increase in haematoma volume was 29% in the placebo group, compared with 11–16% in the groups given fVIIa. Mortality at 90 days was 29% for patients who received placebo and 18% for those who received fVIIa. The phase III fVIIa for Acute Hemorrhagic Stroke Treatment (FAST) trial71 assessed the efficacy of fVIIa in patients with intracerebral haemorrhage who presented within 3 h of symptom onset. Of 821 patients, 263 received placebo, 265 received 20 μg/kg, and 293 received 80 μg/kg of fVIIa. The ability of fVIIa to limit expansion was similar to the initial trial for both the 20 μg/kg and 80 μg/kg doses. However, at 3 months, 24% given placebo had died or had disability compared with 26% and 29% of patients given 20 μg/kg and 80 μg/kg of fVIIa, respectively; mortality was not different between the groups. The rate of arterial thrombosis was higher in patients treated with 80 μg/kg of fVIIa (10%) than in those treated with placebo (5%) or 20 μg/kg of fVIIa (6%). Thus, this pivotal trial of fVIIa did not confirm better functional outcomes despite producing a significant reduction in rate of haematoma expansion. The absence of major benefit for fVIIa, despite its ability to stabilise bleeding, suggests that additional treatments, such as surgical evacuation after stabilisation might be needed to change the natural history of intracerebral haemorrhage. The FAST trial subgroup analysis82 suggested potential benefit for patients younger than 70 years, with baseline haematoma volume less than 60 mL, baseline intraventricular haemorrhage volume less than 5 mL, and time from onset less than or equal to 2·5 h.
Management of mass-effects causing intracranial hypertension
Mass-effects resulting from haematomas, oedematous tissue surrounding haematomas, and obstructive hydrocephalus with subsequent herniation are a major cause of death in the first few days after intracerebral haemorrhage. Monitoring of intracranial pressure might identify the risk of neurological deterioration83 in patients with impaired consciousness.55 Intensive care leading to controlled cerebral perfusion pressure of 50–70 mm Hg might improve outcome.83
Two randomised trials showed no benefit on regional cerebral blood flow, neurological improvement, mortality, and functional outcomes from regular use of intravenous mannitol boluses.84–87 Therefore, only short-term use of mannitol in patients with intra-cerebral haemorrhage under special circumstances, such as transtentorial herniation or acute neurological deterioration associated with high intracranial pressure or mass-effect, should be considered. A single-centre observational study suggested that aggressive, timely reversal of transtentorial herniation through the use of hyperventilation and osmotic drugs improved the long-term outcome.74
The American Stroke Association (ASA) Stroke Council88 recognises the absence of definitive clinical trial evidence in this specialty but recommends monitoring of intracranial pressure in patients treated with osmotic diuretics, cerebrospinal fluid drainage via ventricular catheter, neuromuscular blockade, and hyperventilation. The European Stroke Initiative (EUSI) guidelines54 recommend monitoring of intracranial pressure for patients who need mechanical ventilation and recommend treatment in patients who have neurological deterioration related to increasing cerebral oedema on neuroimaging or high intracranial pressure. Both guidelines recommend selective use of mannitol, hypertonic saline, and short-term hyperventilation to maintain cerebral per fusion pressure greater than 70 mm Hg.
Management of blood pressure
The acute hypertensive response in intracerebral haemorrhage is characterised by its high prevalence, self-limiting nature, and prognostic significance.89 In an analysis of 45 330 patients with intracerebral haemorrhage, 75% had systolic blood pressure greater than 140 mm Hg and 20% greater than 180 mm Hg at presentation.90 The high blood pressure might be secondary to uncontrolled chronic hypertension, with disruption of central autonomic pathways by intra-cerebral haemorrhage.89 High blood pressure is associated with haematoma enlargement and poor outcome;37 however, an exact cause and effect relation is not proven.34 The 1999 ASA guidelines55 are based on expert opinion and recommend lowering of blood pressure to keep mean arterial pressure at less than 130 mm Hg in patients with a history of hypertension. Patients with intracerebral haemorrhage treated with intravenous infusion of calcium channel blockers consistent with 1999 ASA guidelines within 24 h of symptom onset tolerated treatment well and had low rates of neurological deterioration and haematoma expansion.36 Comparisons suggest that intravenous-bolus-based regimens produce more variable blood-pressure control than do infusion-based regimens of antihypertensive treatment.73
The current ASA Stroke Council88 guidelines recommend “until ongoing clinical trials of blood pressure intervention for intracerebral haemorrhage are completed, physicians must manage blood pressure on the basis of the present incomplete evidence…” by maintaining systolic blood pressure less than 180 mm Hg in the acute period with short half-life intravenous anti-hypertensive drugs. Both guidelines consider more aggressive systolic blood-pressure lowering in the absence of clinical signs of high intracranial pressure88 or chronic hypertension.54 Recent data suggest a greater therapeutic benefit with more aggressive lowering of blood pressure.91 In one observational study, haematomas enlarged in 9% of patients with systolic blood pressure maintained below 150 mm Hg and in 30% of those with systolic blood pressure maintained at less than 160 mm Hg or a higher threshold.91 The Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial92 and the Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage (INTERACT) trial reported that aggressive reduction of blood pressure to less than 140 mm Hg probably decreases the rate of substantial haematoma enlargement93 without increasing adverse events.94 In subgroup analyses from INTERACT,93 patients recruited within 3 h and those with an initial systolic blood pressure of 181 mm Hg or more seemed to have the greatest benefit with aggressive lowering of blood pressure. No difference in rates of death and disability at 3 months were seen between patients treated with aggressive and conservative lowering of blood pressure in ATACH or INTERACT studies, although the analyses were limited by small sample sizes. Because the effect on clinical outcome has not been fully assessed, the more conservative targets set in the ASA Stroke Council88 and the EUSI guidelines54 should be followed. Great caution is advised about lowering blood pressure too aggressively without concomitant management of cerebral perfusion pressure.
Management of intraventricular haemorrhage and hydrocephalus
Two clinical trials70,81 confirmed that intraventricular haemorrhage and hydrocephalus are independent predictors of poor outcome in spontaneous intracerebral haemorrhage.95 Impaired flow of cerebrospinal fluid and direct mass-effects of ventricular blood lead to obstructive hydrocephalus. External drainage of cerebrospinal fluid through ventricular catheters reduces intracranial pressure,96 but clots in the catheter and infections prevent sustained beneficial effects on hydrocephalus and neurological status in many patients.79,97 Shortening the length of external ventricular drainage with early ventriculoperitoneal shunt placement98 or lumbar drainage for communicating hydrocephalus99 might lower the rate of infections. Substitution of lumbar drainage for external ventricular drainage in patients with communicating hydrocephalus might also lessen the need to change temporary ventricular catheters and to use ventriculoperitoneal shunts.99
Intraventricular haemorrhage is a dynamic process that follows intracerebral haemorrhage. In a recent study of fVIIa, 45% of 374 patients with intracerebral haemorrhage had intraventricular haemorrhage by 24 h after presentation.100 Growth of the intraventricular haemorrhages occurred in 17% of placebo-treated patients and 10% of those given fVIIa. Risk factors for growth included a baseline mean arterial pressure of more than 120 mm Hg, large baseline volume of intracerebral haemorrhage, presence of intraventricular haemorrhage at baseline, shorter time from symptom onset to first CT scan, and lack of treatment with fVIIa. Presence of intraventricular haemorrhage at any time and growth of this haemorrhage increased the likelihood of death or severe disability by 90 days.
To facilitate early and effective clearance of blood in the ventricles, recent efforts have focused on intraventricular use of thrombolytic drugs in patients who have intra ventricular haemorrhage in association with spontaneous intracerebral haemorrhage.77,78,101 In a randomised, double-blind, controlled trial,79 intra-ventricular thrombolytics given every 12 h led to faster resolution of intraventricular haemorrhage than did treatment with ventricular drainage alone. Two systematic reviews of clinical studies102,103 found a 30–50% reduction in mortality associated with thrombolytic treatment for intraventricular haemorrhage. Clinical trials have not clearly shown improved neurological outcome in survivors of intraventricular haemorrhage. The Clot Lysis: Evaluating Accelerated Resolution of IVH (CLEAR-IVH) trial is investigating this issue.104
Observational studies showed encouraging results for endoscopic removal of intraventricular haemorrhage.105–107 In one study, 24 of 25 patients with intra-ventricular haemorrhage and obstructive hydrocephalus had resolution of hydrocephalus after endoscopic evacuation.107 In a single-centre, non-randomised comparison study,105 endoscopic removal of intraventricular haemorrhage resulted in a higher rate of good recovery at 2 months than did external ventricular drainage alone.
Surgical evacuation
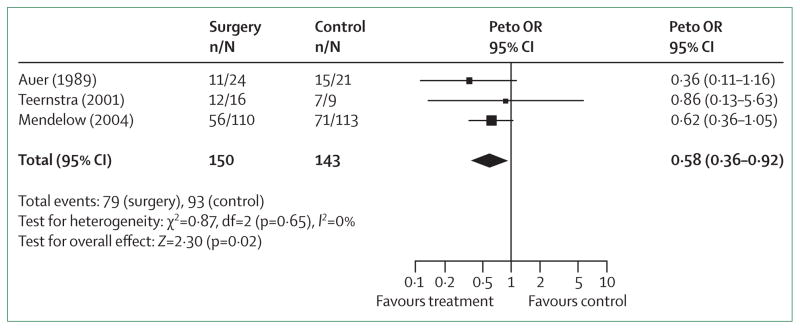
Surgical evacuation may prevent expansion, decrease mass-effects, block the release of neuropathic products from haematomas, and thus prevent initiation of pathological processes. The Surgical Trial in Intracerebral Haemorrhage (STICH) trial70 compared early surgery (median time of 20 h from presentation to surgery) with medical treatment. 1033 patients were randomly assigned to early surgery or initial conservative treatment. At 6 months, early surgery had no benefit compared with initial conservative treatment: 24% versus 26% had good recovery or moderate disability after treatment.70 The benefits of surgery via open craniotomy can be outweighed by neural damage incurred and recurrence of bleeding, especially in deep lesions. In a subgroup analysis of the STICH trial, surgical treatment of lobar haematomas and haematomas within 1 cm of the cortical surface were most likely to benefit70,108–110 (figure 6). The STICH II trial has started, and will prospectively test for benefits of surgery in lobar intracerebral haemorrhage when clots extend to within 1 cm of the cortical surface but remain intraparenchymal without spread to the ventricular system.108 Another potential indication for surgery is acute neurological worsening. One report111 suggested that emergent surgical evacuation could result in functional independence in a quarter of patients if they had not lost upper brainstem reflexes and did not show extensor posturing. Another prospective ran domised study112 suggested that the benefit of early surgery is limited to patients presenting with initial Glasgow coma scale scores of 8 or more or intra cerebral haemorrhage volumes of 80 mL or less.
Figure 6. Odds ratio for death or disability in patients with lobar intracerebral haemorrhage treated surgically or conservatively.
Boxes are Peto’s odds ratio (OR), lines are 95% CI. Adapted with permission from Lippincott Williams and Wilkins.108
To limit neural damage and the risk of recurrent bleeding associated with open craniotomy, studies are now focusing on less invasive stereotactic and endoscopic evacuation with the use of thrombolytic drugs.113 A randomised trial114 showed that stereotactic evacuation of putaminal haematoma was associated with lower mortality and better recovery to functional independence in patients with mildly reduced consciousness. Another trial109 randomly assigned 36 patients to stereotactic aspiration after liquefaction with urokinase and 35 to conservative management. Surgery showed a greater haematoma reduction (18 mL compared with 7 mL with conservative management), but no clinical improvement. The ongoing Minimally Invasive Surgery plus Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation (MISTIE)108 trial is designed to find the best dose of thrombolytics capable of removing 80% of intracerebral haemorrhage volume by use of stereotactic aspiration followed by catheter-based removal irrigation of intra cerebral haemorrhage with thromboytics.
The ASA Stroke Council88 and EUSI guidelines54 do not recommend routine evacuation of supratentorial haemorrhage by standard craniotomy within 96 h of ictus. Both guidelines recommend surgery for patients presenting with lobar haemorrhage within 1 cm of the surface, particularly for those with good neurological status who are deteriorating clinically. Guidelines acknowledge that operative removal within 12 h, particularly with minimally-invasive methods, has the most evidence for beneficial effect and could be considered for deep haemorrhages in the presence of mass-effect.54 However, guidelines note that very early craniotomy might be associated with an increased risk of recurrent bleeding.115
Posterior fossa surgery
Timely decompression in cerebellar haematomas can lower morbidity and mortality related to compression of the brainstem. In an analysis of the data from a national stroke registry,116 patients treated surgically had significantly greater improvement in neurological scores than did those treated medically, independent of age and initial severity of deficits. In most institutions, evidence of neurological deterioration is an indication for surgical evacuation;117 although surgical intervention before neurological deterioration might be more beneficial if there is severe fourth ventricular compression.118 The best functional results are seen with early craniotomy in patients with a cerebellar haemorrhage who had an initial Glasgow coma scale score of less than 14 or large haemorrhages (≥40 mL).1 Endoscopic removal of cerebellar haemorrhage119 can also effectively remove the haematoma with lower procedure time and a shorter period of cerebrospinal fluid drainage than with craniectomy.
The ASA Stroke Council88 and EUSI guidelines54 recommend urgent surgery for patients with cerebellar haemorrhages with a relatively good neurological status or haematoma larger than 3 cm who are deteriorating clinically, or who have brainstem compression or hydrocephalus from ventricular obstruction. Cerebellar haemorrhage is commonly complicated by obstructive hydrocephalus120 with delayed but rapidly rising intracranial pressure, which can be treated successfully with external ventricular drainage.121 The consequences of longlasting intracranial hypertension with delayed drainage should be avoided by careful monitoring of intracranial pressure and neurological status and use of serial CT scans.
Neuroprotective and seizure treatment
NXY-059, a free-radical-trapping neuroprotectant,122 was investigated in a randomised trial of 607 patients with intracerebral haemorrhage within 6 h of symptom onset.123 Although the use of NXY-059 was associated with slightly less haematoma growth than use of placebo (mean change of 4·5 mL vs 6·7 mL), on comparison of baseline scans to those 72 h after treatment onset, the drug had no effect on mortality at 3 months, disability, or neurological deficit scores.
8% of patient with intracerebral haemorrhage have clinical seizures124 within 1 month of symptom onset, associated with lobar location or haematoma enlargement. However, continuous electro encephalographic monitoring in an observational study125 showed that 28% of patients with intracerebral haemorrhage had (predominantly subclinical) seizures within the first 72 h of admission. Seizures were associated with neurological worsening, an increase in midline shift, and poorer outcomes. In another study of 45 patients with intracerebral haemorrhage,126 sub clinical seizures and non-convulsive status epilepticus were detected in 13% and 9% of the patients, respectively. Therefore, a low threshold for obtaining electroencephalographic studies and use of anticonvulsants in patients with intracerebral haemorrhage might be advisable. On the basis of risk reduction reported in observational studies,124 a 30-day course of prophylactic anticonvulsants is recommended in patients with lobar haemorrhage or those who develop seizures.54,88 Patients who have a seizure more than 2 weeks after intracerebral haemorrhage onset are at greater risk of recurrent seizures than those who do not and might need long-term prophylactic treatment with anticonvulsants.
Management of medical complications
About 30% of patients with intracerebral haemorrhage have gastric haemorrhages. Prophylactic H2 blockers or drugs that can protect the mucosa lower the numbers of such events.127 In a randomised trial,127 gastric haemorrhages occurred in 23%, 11%, and 14% of patients treated with placebo, ranitidine, and sucralfate, respectively; in-hospital mortality was 28%, 11%, and 25%.
In the first 2 weeks, deep-venous thrombosis can be detected by ultrasonography in 40% of patients.128 Patients with severe neurological deficits and high d-dimer concentrations are at highest risk.128 The rate of clinical deep-venous thrombosis was 4% and pulmonary embolism 1% within 3 months, in a combined analysis of placebo-treated patients in fVIIa trials.129 A randomised study130 showed that intermittent pneumatic compression decreased the occurrence of asymptomatic deep-venous thromboembolism compared with elastic stockings alone and should be used in all patients. The seventh American College of Chest Physicians panel recommends that a low-dose regimen of sub cutaneous heparin or low-molecular-weight heparin can be started on the second day after onset of intracerebral haemorrhage in neurologically stable patients.131 A small study showed a low incidence of pulmonary embolism without an incremental rate of new intracerebral haemorrhage if low-dose heparin was started on the second day after onset (compared with later intervals).132 Once a deep-venous thromboembolism develops, treatment should be given to patients at high risk of pulmonary embolism. Inferior vena-cava filters or a 5–10-day course of full-dose low-molecular-weight heparin followed by 3 months of lower-dose low-molecular-weight heparin are possible alternatives to warfarin.133
10% of intensively treated patients with intracerebral haemorrhage need tracheostomies, and early use might reduce the risk of aspiration and long-term mechanical ventilation.134 Recent guidelines have placed emphasis on control of hyperthermia and hyperglycaemia with antipyretic medication and possibly insulin infusion in the acute period of intracerebral haemorrhage.54,88
Intracerebral haemorrhage related to use of oral anticoagulants
A population based study135 reported that intracerebral haemorrhage associated with oral anticoagulant use comprised 5% of all intracerebral haemorrhages in 1988, 9% in 1993–94, and 17% in 1999, with the observed increase presumably due to increasing prevalence of atrial fibrillation and higher rates of warfarin use.11 Although most cases associated with oral anticoagulant use occur when international normalised ratios are within the therapeutic range, higher ratios increase the risk.136 Advancing age and cerebral amyloid angiopathy are also important contributory factors to intracerebral haemorrhage associated with oral anticoagulant use.11,137 In a multicentre study, a progressive neurological deterioration during the first 24–48 h was seen in almost half of patients with intracerebral haemorrhage associated with oral anticoagulant use and a high mortality (64%) by 6 months.138 The high mortality in these patients was mediated by a high rate of early and delayed haematoma enlargement139 which was commonly associated with persistently high international normalised ratio after admission.140,141
Rapid reversal of systemic anticoagulation with a combination of intravenous vitamin K, prothrombin complex concentrates, or fresh frozen plasma and fVIIa is recommended preferably within 2 h of onset.11,142,143 Prothrombin complex concentrates or fVIIa can achieve rapid reversal although the international normalised ratio might increase in subsequent hours owing to the short half-lives of these drugs requiring follow-up monitoring. In a single-centre review,141 haematomas enlarged in 19% of patients given prothrombin complex concentrates, 33% given fresh frozen plasma, and 50% given vitamin K. An early reversal of international normalised ratio (within 2 h) was achieved in 84% with prothrombin complex concentrates, 39% with fresh frozen plasma, and 0% with vitamin K. International normalised ratio reversal to less than 1·4 within 2 h was associated with low rates of haematoma enlargement. A retrospective study144 compared the outcomes of neurosurgical patients with intracranial haemorrhage treated with fresh frozen plasma and fVIIa and those managed with fresh frozen plasma alone. International normalised ratios returned to normal over a mean period of 7 h in those given fVIIa and 47 h in those who were not. More patients treated with fVIIa had good functional outcome than did those who received only fresh frozen plasma. Rapid reversal of international normalised ratios also enables urgent surgical evacuations in patients who are deteriorating neurologically with intracerebral haemorrhage related to oral anticoagulant use. One study145 reported a high rate (65%) of favourable outcomes in patients with prominent midline shift (with or without uncal herniation) who had emergent surgical evacuation after reversal.
The clinical issue regarding reinstitution of anticoagulation is controversial. Two studies concluded that antithrombotic drugs should be avoided where possible in patients with acute intracerebral haemorrhage.146,147 A subgroup at high risk of thromboembolic stroke and low risk of recurrence might benefit from long-term anticoagulation or aspirin. Both the ASA Stroke Council88 and the EUSI guidelines54 recommend that warfarin can be started again in patients at a very high risk of thromboembolism at 7–14 days after onset of the original intracerebral haemorrhage.148,149
Future directions
Clinical evidence suggests the importance of three management tasks in intracerebral haemorrhage: stopping the bleeding,81 removing the clot,70 and controlling cerebral perfusion pressure.92 The precision needed to achieve these goals and the degree of benefit attributable to each clinical goal would be precisely defined when the results of trials in progress become available. An NIH workshop150 identified the importance of animal models of intracerebral haemorrhage and of human pathology studies. Use of real-time, high-field MRI with three-dimensional imaging and high-resolution tissue probes is another priority. Trials of acute blood-pressure treatment and coagulopathy reversal are also medical priorities. And trials of minimally invasive surgical techniques including mechanical and pharmacological adjuncts are surgical priorities. The STICH II trial should determine the benefit of craniotomy for lobar haemorrhage. A better understanding of methodological challenges, including establishment of research networks and multispecialty approaches, is also needed.150 New information created in each of these areas should add substantially to our knowledge about the effcacy of treatment for intracerebral haemorrhage.
Search strategy and selection criteria.
We based our review on personal knowledge of the subject supplemented by data derived from multicentre randomised trials, and selected non-randomised or observational clinical studies. The information was identified with multiple searches on Medline from 2002 to the present by cross referencing the following keywords: “cerebral haemorrhage”, “intracerebral hemorrhage”, “neuroimaging”, “clinical studies”, “randomised trials”, “cytotoxicity”, “oedema”, “haemostatic treatment”, “factor VII”, “acute hypertension”, “surgery”, “endoscopic evacuation”, “stereotactic surgery”, “intraventricular catheter”, “hydrocephalus”, and “oral anticoagulants”. Other pertinent articles were identified through review of bibliography from selected articles. We also reviewed abstracts from pertinent scientific meetings.
Acknowledgments
AIQ has received funding from National Institutes of Health RO-1-NS44976-01A2 (medication provided by ESP Pharma), American Heart Association Established Investigator Award 0840053N, and Minnesota Medical Foundation (Minneapolis, MN, USA). ADM is the director of the Newcastle Neurosurgery Foundation, and has received honoraria for attending Advisory Committee Meetings for Codman and for Novo Nordisk. DFH receives funding through the US Food and Drug Administration orphan-drugs programme grant 5RO1-FD 001693, National Institute of Neurological Disorders and Stroke (NINDS) planning grant, 1R34-NS056638, MISITIE: NINDS, 1R01-NS 046309, Jeffrey and Harriet Legum professorship, Genentech, sponsored research agreement; he also has disavowed interest in this patent (Johns Hopkins University use patent application # 10/509,694) and has received an honorarium from Novo Nordisk.
Footnotes
Contributors
All authors contributed equally to the preparation of this Seminar.
Contributor Information
Adnan I Qureshi, Zeenat Qureshi Stroke Research Center, Department of Neurology and Neurosurgery, University of Minnesota, MN, Minnesota, USA.
A David Mendelow, Department of Neurosurgery, University of Newcastle, Newcastle, UK.
Daniel F Hanley, Division of Brain Injury Outcomes, Johns Hopkins Medical Institutions, Baltimore, MD, USA.
References
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–60. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- 2.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and hispanics. Neurology. 2005;65:518–22. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 3.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. Stroke. 1997;28:491–99. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Organization. [accessed Nov 21, 2007];International cardiovascular disease statistics: cardiovascular disease (CVD) http://www.americanheart.org/downloadable/heart/1140811583642InternationalCVD.pdf.
- 5.Qureshi AI, Suri MFK, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–84. doi: 10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- 6.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 7.Morgenstern LB, Spears WD. A triethnic comparison of intracerebral hemorrhage mortality in Texas. Ann Neurol. 1997;42:919–23. doi: 10.1002/ana.410420614. [DOI] [PubMed] [Google Scholar]
- 8.Kubo M, Kiyohara Y, Kato I, et al. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke. 2003;34:2349–54. doi: 10.1161/01.STR.0000090348.52943.A2. [DOI] [PubMed] [Google Scholar]
- 9.Jiang B, Wang WZ, Chen H, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke. 2006;37:63–68. doi: 10.1161/01.STR.0000194955.34820.78. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Coull AJ, Giles MF, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study) Lancet. 2004;363:1925–33. doi: 10.1016/S0140-6736(04)16405-2. [DOI] [PubMed] [Google Scholar]
- 11.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–62. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- 12.Takebayashi S, Kaneko M. Electron microscopic studies of ruptured arteries in hypertensive intracerebral hemorrhage. Stroke. 1983;14:28–36. doi: 10.1161/01.str.14.1.28. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani T, Kojima H, Miki Y. Arterial dissections of penetrating cerebral arteries causing hypertension-induced cerebral hemorrhage. J Neurosurg. 2000;93:859–62. doi: 10.3171/jns.2000.93.5.0859. [DOI] [PubMed] [Google Scholar]
- 14.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–51. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 15.Rost NS, Greenberg SM, Rosand J. The genetic architecture of intracerebral hemorrhage. Stroke. 2008;39:2166–73. doi: 10.1161/STROKEAHA.107.501650. [DOI] [PubMed] [Google Scholar]
- 16.Hart RG. What causes intracerebral hemorrhage during warfarin therapy? Neurology. 2000;55:907–08. doi: 10.1212/wnl.55.7.907. [DOI] [PubMed] [Google Scholar]
- 17.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–12. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi AI, Suri MF, Ostrow PT, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–47. [PubMed] [Google Scholar]
- 19.Qureshi AI, Ali Z, Suri MF, et al. Extracellular glutamate and other amino acids in experimental intracerebral hemorrhage: an in vivo microdialysis study. Crit Care Med. 2003;31:1482–89. doi: 10.1097/01.CCM.0000063047.63862.99. [DOI] [PubMed] [Google Scholar]
- 20.Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J Neurotrauma. 2004;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- 21.Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol. 2000;59:641–51. doi: 10.1093/jnen/59.8.641. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Xi G, Park JW, Hua Y, Hoff JT, Keep RF. Holo-transferrin and thrombin can interact to cause brain damage. Stroke. 2005;36:348–52. doi: 10.1161/01.STR.0000153044.60858.1b. [DOI] [PubMed] [Google Scholar]
- 23.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Keep RF, Hua Y, Nagao S, Hoff JT, Xi G. Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochir Suppl. 2006;96:194–48. doi: 10.1007/3-211-30714-1_42. [DOI] [PubMed] [Google Scholar]
- 25.Wagner KR, Packard BA, Hall CL, et al. Protein oxidation and heme oxygenase-1 induction in porcine white matter following intracerebral infusions of whole blood or plasma. Dev Neurosci. 2002;24:154–60. doi: 10.1159/000065703. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Tsirka SE. Tuftsin fragment 1–3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke. 2005;36:613–48. doi: 10.1161/01.STR.0000155729.12931.8f. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Sabin J, Delgado P, Abilleira S, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–22. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 28.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 29.Hua Y, Wu J, Keep RF, Nakamura T, Hoff JT, Xi G. Tumor necrosis factor-alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542–50. doi: 10.1227/01.NEU.0000197333.55473.AD. [DOI] [PubMed] [Google Scholar]
- 30.Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48:875–82. doi: 10.1097/00006123-200104000-00037. [DOI] [PubMed] [Google Scholar]
- 31.Matz PG, Lewen A, Chan PH. Neuronal, but not microglial, accumulation of extravasated serum proteins after intracerebral hemolysate exposure is accompanied by cytochrome c release and DNA fragmentation. J Cereb Blood Flow Metab. 2001;21:921–28. doi: 10.1097/00004647-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Yang S, Nakamura T, Hua Y, et al. The role of complement C3 in intracerebral hemorrhage-induced brain injury. J Cereb Blood Flow Metab. 2006;26:1490–95. doi: 10.1038/sj.jcbfm.9600305. [DOI] [PubMed] [Google Scholar]
- 33.Fujii Y, Takeuchi S, Harada A, Abe H, Sasaki O, Tanaka R. Hemostatic activation in spontaneous intracerebral hemorrhage. Stroke. 2001;32:883–90. doi: 10.1161/01.str.32.4.883. [DOI] [PubMed] [Google Scholar]
- 34.Broderick JP, Diringer MN, Hill MD, et al. Determinants of intracerebral hemorrhage growth: an exploratory analysis. Stroke. 2007;38:1072–75. doi: 10.1161/01.STR.0000258078.35316.30. [DOI] [PubMed] [Google Scholar]
- 35.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 36.Qureshi AI, Harris-Lane P, Kirmani JF, et al. Treatment of acute hypertension in patients with intracerebral hemorrhage using American Heart Association guidelines. Crit Care Med. 2006;34:1975–80. doi: 10.1097/01.CCM.0000220763.85974.E8. [DOI] [PubMed] [Google Scholar]
- 37.Kazui SMK, Sawada T, Yamaguchi T. Predisposing factors to enlargement of spontaneous intracerebral hematoma. Stroke. 1997;28:2370–75. doi: 10.1161/01.str.28.12.2370. [DOI] [PubMed] [Google Scholar]
- 38.Gebel JM, Jr, Jauch EC, Brott TG, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2631–35. doi: 10.1161/01.str.0000035284.12699.84. [DOI] [PubMed] [Google Scholar]
- 39.Inaji M, Tomita H, Tone O, Tamaki M, Suzuki R, Ohno K. Chronological changes of perihematomal edema of human intracerebral hematoma. Acta Neurochir Suppl. 2003;86:445–48. doi: 10.1007/978-3-7091-0651-8_91. [DOI] [PubMed] [Google Scholar]
- 40.Butcher KS, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal edema in primary intracerebral hemorrhage is plasma derived. Stroke. 2004;35:1879–85. doi: 10.1161/01.STR.0000131807.54742.1a. [DOI] [PubMed] [Google Scholar]
- 41.Gebel JM, Jr, Jauch EC, Brott TG, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–41. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 42.Qureshi AI, Hanel RA, Kirmani JF, Yahia AM, Hopkins LN. Cerebral blood flow changes associated with intracerebral hemorrhage. Neurosurg Clin N Am. 2002;13:355–70. doi: 10.1016/s1042-3680(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 43.Siddique MS, Fernandes HM, Wooldridge TD, Fenwick JD, Slomka P, Mendelow AD. Reversible ischemia around intracerebral hemorrhage: a single-photon emission computerized tomography study. J Neurosurg. 2002;96:736–41. doi: 10.3171/jns.2002.96.4.0736. [DOI] [PubMed] [Google Scholar]
- 44.Kim-Han JS, Kopp SJ, Dugan LL, Diringer MN. Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke. 2006;37:2457–62. doi: 10.1161/01.STR.0000240674.99945.4e. [DOI] [PubMed] [Google Scholar]
- 45.Carhuapoma JR, Wang PY, Beauchamp NJ, Keyl PM, Hanley DF, Barker PB. Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke. 2000;31:726–32. doi: 10.1161/01.str.31.3.726. [DOI] [PubMed] [Google Scholar]
- 46.Zazulia AR, Diringer MN, Videen TO, et al. Hypoperfusion without ischemia surrounding acute intracerebral hemorrhage. J Cereb Blood Flow Metab. 2001;21:804–10. doi: 10.1097/00004647-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Schellinger PD, Fiebach JB, Hoffmann K, et al. Stroke MRI in intracerebral hemorrhage: is there a perihemorrhagic penumbra? Stroke. 2003;34:1674–79. doi: 10.1161/01.STR.0000076010.10696.55. [DOI] [PubMed] [Google Scholar]
- 48.Orakcioglu B, Fiebach JB, Steiner T, et al. Evolution of early perihemorrhagic changes—ischemia vs edema: an MRI study in rats. Exp Neurol. 2005;193:369–76. doi: 10.1016/j.expneurol.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Qureshi AI, Wilson DA, Hanley DF, Traystman RJ. No evidence for an ischemic penumbra in massive experimental intracerebral hemorrhage. Neurology. 1999;52:266–72. doi: 10.1212/wnl.52.2.266. [DOI] [PubMed] [Google Scholar]
- 50.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004;35:502–06. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 51.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 52.Becker KJ, Baxter AB, Bybee HM, Tirschwell DL, Abouelsaad T, Cohen WA. Extravasation of radiographic contrast is an independent predictor of death in primary intracerebral hemorrhage. Stroke. 1999;30:2025–32. doi: 10.1161/01.str.30.10.2025. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–94. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 54.Steiner T, Katse M, Forsting M, et al. Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. Cerebrovasc Dis. 2006;22:294–316. doi: 10.1159/000094831. [DOI] [PubMed] [Google Scholar]
- 55.Broderick JP, Adams HP, Jr, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–15. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 56.Moon J-S, Janjua N, Ahmed S, et al. Prehospital neurologic deterioration in patients with intracerebral hemorrhage. Crit Care Med. 2008;36:172–75. doi: 10.1097/01.CCM.0000297876.62464.6B. [DOI] [PubMed] [Google Scholar]
- 57.Leira R, Davalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–67. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 58.Mayer SA, Sacco RL, Shi T, Mohr JP. Neurologic deterioration in noncomatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379–84. doi: 10.1212/wnl.44.8.1379. [DOI] [PubMed] [Google Scholar]
- 59.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–97. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 60.Weimar C, Weber C, Wagner M, et al. Management patterns and health care use after intracerebral hemorrhage. a cost-of-illness study from a societal perspective in Germany. Cerebrovasc Dis. 2003;15:29–36. doi: 10.1159/000067119. [DOI] [PubMed] [Google Scholar]
- 61.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66:1182–86. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]
- 62.Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry. 2005;76:1534–38. doi: 10.1136/jnnp.2004.055145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56:773–77. doi: 10.1212/wnl.56.6.773. [DOI] [PubMed] [Google Scholar]
- 64.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 66.Hachinski V. Vascular behavioral and cognitive disorders. Stroke. 2003;34:2775. doi: 10.1161/01.STR.0000107480.16433.4C. [DOI] [PubMed] [Google Scholar]
- 67.Zurasky JA, Aiyagari V, Zazulia AR, Shackelford A, Diringer MN. Early mortality following spontaneous intracerebral hemorrhage. Neurology. 2005;64:725–27. doi: 10.1212/01.WNL.0000152045.56837.58. [DOI] [PubMed] [Google Scholar]
- 68.Hemphill JC, 3rd, Newman J, Zhao S, Johnston SC. Hospital usage of early do-not-resuscitate orders and outcome after intracerebral hemorrhage. Stroke. 2004;35:1130–34. doi: 10.1161/01.STR.0000125858.71051.ca. [DOI] [PubMed] [Google Scholar]
- 69.Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–40. doi: 10.1097/00003246-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 70.Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 71.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 72.Tuhrim S. Intracerebral hemorrhage—improving outcome by reducing volume? N Engl J Med. 2008;358:2174–76. doi: 10.1056/NEJMe0801856. [DOI] [PubMed] [Google Scholar]
- 73.Qureshi AI, Mohammad YM, Yahia AM, et al. A prospective multicenter study to evaluate the feasibility and safety of aggressive antihypertensive treatment in patients with acute intracerebral hemorrhage. J Intensive Care Med. 2005;20:34–42. doi: 10.1177/0885066604271619. [DOI] [PubMed] [Google Scholar]
- 74.Qureshi AI, Geocadin RG, Suarez JI, Ulatowski JA. Long-term outcome after medical reversal of transtentorial herniation in patients with supratentorial mass lesions. Crit Care Med. 2000;28:1556–64. doi: 10.1097/00003246-200005000-00049. [DOI] [PubMed] [Google Scholar]
- 75.Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–55. doi: 10.1161/01.STR.0000195047.21562.23. [DOI] [PubMed] [Google Scholar]
- 76.Gujjar AR, Deibert E, Manno EM, Duff S, Diringer MN. Mechanical ventilation for ischemic stroke and intracerebral hemorrhage: indications, timing, and outcome. Neurology. 1998;51:447–51. doi: 10.1212/wnl.51.2.447. [DOI] [PubMed] [Google Scholar]
- 77.Naff NJ, Tuhrim S. Intraventricular hemorrhage in adults: complications and treatment. New Horizons. 1997;5:359–63. [PubMed] [Google Scholar]
- 78.Naff NJ, Carhuapoma JR, Williams MA, et al. Treatment of intraventricular hemorrhage with urokinase: effects on 30-day survival. Stroke. 2000;31:841–47. doi: 10.1161/01.str.31.4.841. [DOI] [PubMed] [Google Scholar]
- 79.Naff NJ, Hanley DF, Keyl PM, et al. Intraventricular thrombolysis speeds blood clot resolution: results of a pilot, prospective, randomized, double-blind, controlled trial. Neurosurgery. 2004;54:577–83. doi: 10.1227/01.neu.0000108422.10842.60. [DOI] [PubMed] [Google Scholar]
- 80.Qureshi AI, Safdar K, Weil J, et al. Predictors of early deterioration and mortality in black Americans with spontaneous intracerebral hemorrhage. Stroke. 1995;26:1764–67. doi: 10.1161/01.str.26.10.1764. [DOI] [PubMed] [Google Scholar]
- 81.Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777–85. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- 82.Mayer SA, Davis SM, Begtrup K, et al. Subgroup analysis in the FAST trial: a subset of intracerebral hemorrhage patients that benefit from recombinant activated factor VII. Stroke. 2008;39:528. doi: 10.1161/STROKEAHA.108.524470. [DOI] [PubMed] [Google Scholar]
- 83.Fernandes HM, Siddique S, Banister K, et al. Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl. 2000;76:463–66. doi: 10.1007/978-3-7091-6346-7_96. [DOI] [PubMed] [Google Scholar]
- 84.Misra UK, Kalita J, Ranjan P, Mandal SK. Mannitol in intracerebral hemorrhage: a randomized controlled study. J Neurol Sci. 2005;234:41–45. doi: 10.1016/j.jns.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 85.Kalita J, Misra UK, Ranjan P, Pradhan PK, Das BK. Effect of mannitol on regional cerebral blood flow in patients with intracerebral hemorrhage. J Neurol Sci. 2004;224:19–22. doi: 10.1016/j.jns.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 86.Sansing LH, Kaznatcheeva EA, Perkins CJ, Komaroff E, Gutman FB, Newman GC. Edema after intracerebral hemorrhage: correlations with coagulation parameters and treatment. J Neurosurg. 2003;98:985–92. doi: 10.3171/jns.2003.98.5.0985. [DOI] [PubMed] [Google Scholar]
- 87.Dziedzic T, Szczudlik A, Klimkowicz A, Rog TM, Slowik A. Is mannitol safe for patients with intracerebral hemorrhages? Renal considerations. Clin Neurol Neurosurg. 2003;105:87–89. doi: 10.1016/s0303-8467(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 88.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 89.Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118:176–87. doi: 10.1161/CIRCULATIONAHA.107.723874. [DOI] [PubMed] [Google Scholar]
- 90.Qureshi AI, Ezzeddine MA, Nasar A, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med. 2007;25:32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage: relationship between elevated blood pressure and hematoma enlargement. Stroke. 2004;35:1364–67. doi: 10.1161/01.STR.0000128795.38283.4b. [DOI] [PubMed] [Google Scholar]
- 92.Qureshi AI. Antihypertensive treatment of acute cerebral hemorrhage (ATACH): rationale and design. Neurocrit Care. 2007;6:56–66. doi: 10.1385/ncc:6:1:56. [DOI] [PubMed] [Google Scholar]
- 93.Anderson CS, Huang Y, Wang G, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a pilot randomised trial. Lancet Neurol. 2008;7:391–99. doi: 10.1016/S1474-4422(08)70069-3. [DOI] [PubMed] [Google Scholar]
- 94.Qureshi AI. Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) trial: International Stroke Conference; New Orleans, LA. Feb 20–22, 2008. [Google Scholar]
- 95.Bhattathiri PS, Gregson B, Prasad KS, Mendelow AD. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl. 2006;96:65–68. doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- 96.Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Surgery for patients with severe supratentorial intracerebral hemorrhage. Neurocrit Care. 2006;5:15–20. doi: 10.1385/NCC:5:1:15. [DOI] [PubMed] [Google Scholar]
- 97.Hanley DF. Intraventricular hemorrhage and ICH outcomes: severity factor and treatment target. [accessed March 12, 2009];Stroke. 2009 doi: 10.1161/STROKEAHA.108.535419. published online Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yilmazlar S, Abas F, Korfali E. Comparison of ventricular drainage in poor grade patients after intracranial hemorrhage. Neurol Res. 2005;27:653–56. doi: 10.1179/016164105X35657. [DOI] [PubMed] [Google Scholar]
- 99.Huttner HB, Nagel S, Tognoni E, et al. Intracerebral hemorrhage with severe ventricular involvement: lumbar drainage for communicating hydrocephalus. Stroke. 2007;38:183–87. doi: 10.1161/01.STR.0000251795.02560.62. [DOI] [PubMed] [Google Scholar]
- 100.Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery. 2006;59:767–73. doi: 10.1227/01.NEU.0000232837.34992.32. [DOI] [PubMed] [Google Scholar]
- 101.Huttner HB, Tognoni E, Bardutzky J, et al. Influence of intraventricular fibrinolytic therapy with rt-PA on the long-term outcome of treated patients with spontaneous basal ganglia hemorrhage: a case-control study. Eur J Neurol. 2008;15:342–49. doi: 10.1111/j.1468-1331.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 102.Andrews CO, Engelhard HH. Fibrinolytic therapy in intraventricular hemorrhage. Ann Pharmacother. 2001;35:1435–48. doi: 10.1345/aph.10383. [DOI] [PubMed] [Google Scholar]
- 103.Nieuwkamp DJ, de Gans K, Rinkel GJ, Algra A. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol. 2000;247:117–21. doi: 10.1007/pl00007792. [DOI] [PubMed] [Google Scholar]
- 104.Nyquist P, Hanley DF. The use of intraventricular thrombolytics in intraventricular hemorrhage. J Neurol Sci. 2007;261:84–88. doi: 10.1016/j.jns.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Z, Li X, Liu Y, Shao Y, Xu S, Yang Y. Application of neuroendoscopy in the treatment of intraventricular hemorrhage. Cerebrovasc Dis. 2007;24:91–96. doi: 10.1159/000103122. [DOI] [PubMed] [Google Scholar]
- 106.Longatti PL, Martinuzzi A, Fiorindi A, Maistrello L, Carteri A. Neuroendoscopic management of intraventricular hemorrhage. Stroke. 2004;35:e35–38. doi: 10.1161/01.STR.0000113736.73632.F6. [DOI] [PubMed] [Google Scholar]
- 107.Yadav YR, Mukerji G, Shenoy R, Basoor A, Jain G, Nelson A. Endoscopic management of hypertensive intraventricular haemorrhage with obstructive hydrocephalus. BMC Neurol. 2007;7:1. doi: 10.1186/1471-2377-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendelow AD, Unterberg A. Surgical treatment of intracerebral haemorrhage. Curr Opin Crit Care. 2007;13:169–74. doi: 10.1097/MCC.0b013e3280a9e5c2. [DOI] [PubMed] [Google Scholar]
- 109.Teernstra OP, Evers SM, Lodder J, Leffers P, Franke CL, Blaauw G. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA) Stroke. 2003;34:968–74. doi: 10.1161/01.STR.0000063367.52044.40. [DOI] [PubMed] [Google Scholar]
- 110.Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530–35. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 111.Rabinstein AA, Atkinson JL, Wijdicks EFM. Emergency craniotomy in patients worsening due to expanded cerebral hematoma: to what purpose? Neurology. 2002;58:1367–72. doi: 10.1212/wnl.58.9.1367. [DOI] [PubMed] [Google Scholar]
- 112.Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early surgical treatment vs conservative management for spontaneous supratentorial intracerebral hematomas: a prospective randomized study. Surg Neurol. 2006;66:492–501. doi: 10.1016/j.surneu.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 113.Broderick JP. The STICH trial: what does it tell us and where do we go from here? Stroke. 2005;36:1619–20. doi: 10.1161/01.STR.0000170714.43167.34. [DOI] [PubMed] [Google Scholar]
- 114.Hattori N, Katayama Y, Maya Y, Gatherer A. Impact of stereotactic hematoma evacuation on activities of daily living during the chronic period following spontaneous putaminal hemorrhage: a randomized study. J Neurosurg. 2004;101:417–20. doi: 10.3171/jns.2004.101.3.0417. [DOI] [PubMed] [Google Scholar]
- 115.Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56:1294–99. doi: 10.1212/wnl.56.10.1294. [DOI] [PubMed] [Google Scholar]
- 116.Wang CX, Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs Aging. 2007;24:537–46. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 117.Lyden PD, Shuaib A, Lees KR, et al. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT Trial. Stroke. 2007;38:2262–69. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- 118.Morioka J, Fujii M, Kato S, et al. Surgery for spontaneous intracerebral hemorrhage has greater remedial value than conservative therapy. Surg Neurol. 2006;65:67–72. doi: 10.1016/j.surneu.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 119.Wijdicks EF, St Louis EK, Atkinson JD, Li H. Clinician’s biases toward surgery in cerebellar hematomas: an analysis of decision-making in 94 patients. Cerebrovasc Dis. 2000;10:93–96. doi: 10.1159/000016036. [DOI] [PubMed] [Google Scholar]
- 120.Kirollos RW, Tyagi AK, Ross SA, van Hille PT, Marks PV. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurgery. 2001;49:1378–86. doi: 10.1097/00006123-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 121.Yamamoto T, Nakao Y, Mori K, Maeda M. Endoscopic hematoma evacuation for hypertensive cerebellar hemorrhage. Minim Invasive Neurosurg. 2006;49:173–78. doi: 10.1055/s-2006-944242. [DOI] [PubMed] [Google Scholar]
- 122.St Louis EK, Wijdicks EF, Li H. Predicting neurologic deterioration in patients with cerebellar hematomas. Neurology. 1998;51:1364–69. doi: 10.1212/wnl.51.5.1364. [DOI] [PubMed] [Google Scholar]
- 123.Sumer MM, Acikgoz B, Akpinar G. External ventricular drainage for acute obstructive hydrocephalus developing following spontaneous intracerebral haemorrhages. Neurol Sci. 2002;23:29–33. doi: 10.1007/s100720200020. [DOI] [PubMed] [Google Scholar]
- 124.Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175–80. doi: 10.1046/j.1528-1157.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 125.Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–46. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 126.Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–48. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- 127.Misra UK, Kalita J, Pandey S, Mandal SK, Srivastava M. A randomized placebo controlled trial of ranitidine versus sucralfate in patients with spontaneous intracerebral hemorrhage for prevention of gastric hemorrhage. J Neurol Sci. 2005;239:5–10. doi: 10.1016/j.jns.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 128.Ogata T, Yasaka M, Wakugawa Y, Inoue T, Ibayashi S, Okada Y. Deep venous thrombosis after acute intracerebral hemorrhage. J Neurol Sci. 2008;272:83–86. doi: 10.1016/j.jns.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 129.Christensen MC, Dawson J, Vincent C. Risk of thromboembolic complications after intracerebral hemorrhage according to ethnicity. Adv Ther. 2008;25:831–41. doi: 10.1007/s12325-008-0092-0. [DOI] [PubMed] [Google Scholar]
- 130.Lacut K, Bressollette L, Le Gal G, et al. Prevention of venous thrombosis in patients with acute intracerebral hemorrhage. Neurology. 2005;65:865–69. doi: 10.1212/01.wnl.0000176073.80532.a2. [DOI] [PubMed] [Google Scholar]
- 131.Albers GW, Amarenco P, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126 (3 suppl):483–512S. doi: 10.1378/chest.126.3_suppl.483S. [DOI] [PubMed] [Google Scholar]
- 132.Boeer A, Voth E, Henze T, Prange HW. Early heparin therapy in patients with spontaneous intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 1991;54:466–67. doi: 10.1136/jnnp.54.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kelly J, Hunt BJ, Lewis RR, Rudd A. Anticoagulation or inferior vena cava filter placement for patients with primary intracerebral hemorrhage developing venous thromboembolism? Stroke. 2003;34:2999–3005. doi: 10.1161/01.STR.0000102561.86835.17. [DOI] [PubMed] [Google Scholar]
- 134.Huttner HB, Kohrmann M, Berger C, Georgiadis D, Schwab S. Predictive factors for tracheostomy in neurocritical care patients with spontaneous supratentorial hemorrhage. Cerebrovasc Dis. 2006;21:159–65. doi: 10.1159/000090527. [DOI] [PubMed] [Google Scholar]
- 135.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–21. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]
- 136.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;48:23–28. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 137.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55:947–51. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 138.Sjoblom L, Hardemark HG, Lindgren A, et al. Management and prognostic features of intracerebral hemorrhage during anticoagulant therapy: a Swedish multicenter study. Stroke. 2001;32:2567–74. doi: 10.1161/hs1101.098523. [DOI] [PubMed] [Google Scholar]
- 139.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–64. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 140.Yasaka M, Minematsu K, Naritomi H, Sakata T, Yamaguchi T. Predisposing factors for enlargement of intracerebral hemorrhage in patients treated with warfarin. Thromb Haemost. 2003;89:278–83. [PubMed] [Google Scholar]
- 141.Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- 142.Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–55. doi: 10.1161/01.STR.0000195047.21562.23. [DOI] [PubMed] [Google Scholar]
- 143.Brody DL, Aiyagari V, Shackleford AM, Diringer MN. Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care. 2005;2:263–67. doi: 10.1385/NCC:2:3:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roitberg B, Emechebe-Kennedy O, Amin-Hanjani S, Mucksavage J, Tesoro E. Human recombinant factor VII for emergency reversal of coagulopathy in neurosurgical patients: a retrospective comparative study. Neurosurgery. 2005;57:832–36. doi: 10.1227/01.neu.0000180816.80626.c2. [DOI] [PubMed] [Google Scholar]
- 145.Rabinstein AA, Wijdicks EFM. Determinants of outcome in anticoagulation-associated cerebral hematoma requiring emergency evacuation. Arch Neurol. 2007;64:203–06. doi: 10.1001/archneur.64.2.noc60131. [DOI] [PubMed] [Google Scholar]
- 146.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34:1710–16. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 147.Keir SL, Wardlaw JM, Sandercock PA, Chen Z. Antithrombotic therapy in patients with any form of intracranial haemorrhage: a systematic review of the available controlled studies. Cerebrovasc Dis. 2002;14:197–206. doi: 10.1159/000065661. [DOI] [PubMed] [Google Scholar]
- 148.Butler AC, Tait RC. Restarting anticoagulation in prosthetic heart valve patients after intracranial haemorrhage: a 2-year follow-up. Br J Haematol. 1998;103:1064–66. doi: 10.1046/j.1365-2141.1998.01078.x. [DOI] [PubMed] [Google Scholar]
- 149.Phan TG, Koh M, Wijdicks EF. Safety of discontinuation of anticoagulation in patients with intracranial hemorrhage at high thromboembolic risk. Arch Neurol. 2000;57:1710–13. doi: 10.1001/archneur.57.12.1710. [DOI] [PubMed] [Google Scholar]
- 150.Anonymous. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:e23–41. doi: 10.1161/01.STR.0000155685.77775.4c. [DOI] [PubMed] [Google Scholar]