Abstract
Purpose
To estimate 4-year incidence and progression of early and advanced age-related macular degeneration (AMD).
Design
Population-based cohort study.
Methods
A comprehensive ophthalmologic examination including stereoscopic fundus photography was performed on adult Latinos at baseline and follow-up. Photographs were graded using a modified Wisconsin Age-Related Maculopathy Grading System. For estimations of incidence and progression of AMD, the Age Related Eye Disease Study Scale was used. Main outcome measures are incidence and progression of early AMD (drusen type, drusen size, and retinal pigmentary abnormalities) and advanced AMD (exudative AMD and geographic atrophy).
Results
4,658/6100 (76%) completed the follow-up examination. The 4-year incidence of early AMD was 7.5% (95%CI:6.6,8.4) and advanced AMD was 0.2% (95%CI:0.1,0.4). Progression of any AMD occurred in 9.3% (95%CI:8.4,10.3) of at-risk participants. Incidence and progression increased with age. Incidence of early AMD in the second eye (10.8%) was higher than incidence in the first eye (6.9%). Baseline presence of soft indistinct large drusen≥250μm in diameter was more likely to predict the 4-year incidence of pigmentary abnormalities, geographic atrophy, and exudative AMD than smaller or hard or soft distinct drusen.
Conclusions
Age-specific incidence and progression of AMD in Latinos are lower than in non-Hispanic whites. While incident early AMD is more often unilateral, the risk of its development in the second is higher than in the first eye. Older persons and those with soft indistinct large drusen had a higher risk of developing advanced AMD compared to those who were younger and did not have soft indistinct large drusen.
Latinos represent the largest of minority populations in the United States. According to the 2004 U.S. Census, 35.6 million people or 12.5% of the nation's residents are Latino.1 This proportion is expected to increase to 20.1% by the year 2030.1 The Latino population has unique demographic, socioeconomic, as well as ocular health characteristics that influence the development of eye disease and its subsequent impact on quality of life.2, 3
Various population-based longitudinal studies of eye disease have been conducted on non-Hispanic whites, including the Beaver Dam Eye Study in Wisconsin, U.S.4 and the Blue Mountains Eye Study in Australia.5 Other studies such as the Barbados Incidence Study of Eye Disease6 have focused on a population of African origin. Although these studies reported data on the incidence and progression of age-related macular degeneration (AMD), given the wide variation in the prevalence and incidence of AMD it would be inaccurate to generalize findings from these studies to persons of other ancestries. As observed in cross-sectional studies, there were differences in the prevalence of both early and advanced AMD between non-Hispanic whites and Latinos.7–9 Findings from the baseline Los Angeles Latino Eye Study (LALES)9 reported that age-specific prevalence of early AMD was lower in Latinos (who have 40% European ancestry and 45% Native American ancestry)10 than the non-Hispanic whites of Beaver Dam, Wisconsin7 but higher than those from Blue Mountains, Australia.8 On the other hand, age-specific prevalence of advanced AMD was marginally lower in Latinos compared to the non-Hispanic white populations of both studies. Most interestingly, the prevalence of large drusen, a well-recognized predictor of incident advanced AMD in non-Hispanic whites,11 was considerably higher in Latinos than in whites. Thus, it is of considerable interest to determine if the incidence and progression of early and advanced AMD in Latinos is comparable to those of non-Hispanic Whites.
In this report we describe the 4-year incidence and progression of early and advanced AMD as well as specific AMD lesions, in a population-based cohort of Latinos.
METHODS
Study Population
The Los Angeles Latino Eye Study (LALES) is a population-based cohort study of eye disease in self-identified Latinos aged 40 years and older living in 6 census tracts in the city of La Puente, Los Angeles County, California. Latinos (Hispanics, Hispanic Americans, and Latino Americans) are individuals who are born into or have descended from a Spanish-speaking community, regardless of race. In the United States they are a heterogeneous group, with the majority of Mexican ancestry (66%). Baseline examination was performed from 2000–2003 with 4-year follow-up examination from 2004–2008. At baseline, 6357 of 7789 eligible participants (82%) completed an in-home questionnaire and a clinical ophthalmic examination. Details of the study design, methods, and baseline data have been reported elsewhere.12 The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and adhered to the recommendations of the Declaration of Helsinki. Written, informed consent was obtained from all participants.
Interview and Examination Procedures
All eligible participants of the baseline LALES examination were invited to return for a home interview and a clinical examination. Similar questionnaire and examination procedures were used for both baseline and follow-up studies. Trained ophthalmologists and technicians performed a comprehensive ocular examination using standardized protocols, which included 30° stereoscopic color fundus photographs of Diabetic Retinopathy Study field one (centered on the optic disc), field two (centered on the macula) and a modified field three (nonstereoscopic, temporal to and including the fovea) on all participants.
Age Related Macular Degeneration (AMD) Grading
A modification of the Wisconsin Age-Related Maculopathy Grading System (WARMGS)13 was used to perform grading of individual age-related macular degeneration (AMD) lesions by masked graders at the Wisconsin Ocular Epidemiology Reading Center. Detailed description of all grading procedures and definitions were previously reported.4, 9 In brief, a lesion-by-lesion evaluation of each fundus photograph taken at the follow-up study was performed to determine maximum drusen size, type, area, and retinal pigmentary abnormalities. Each eye was graded independent of the contralateral eye. Any discrepancies between 2 initial graders were adjudicated by a senior grader using standardized edit rules. All data from the detailed grading were checked for progression or regression of AMD lesions using a custom program. For eyes that had changes in lesion severity by 2 or more steps between the baseline and 4-year follow-up examinations, a longitudinal review was conducted through side-by-side comparison of photographs from both examination periods. Graders were masked to the year the photographs were taken.
Definitions of Age Related Macular Degeneration
Definitions of AMD component lesions which include specific drusen size, drusen types, and retinal pigmentary abnormalities have been described in detail elsewhere.9 In this study, we present incidence and progression for maximum drusen size (<63μm, ≥63μm to <125μm, ≥125μm to <250μm, and ≥250μm), drusen type in increasing severity (hard distinct, soft distinct, and soft indistinct/reticular), increased retinal pigment, retinal pigment epithelial (RPE) depigmentation, and signs of geographic atrophy and exudative AMD.
Various classifications were used to define the presence of early and advanced AMD. In this study we present data based on two systems the WARMGS and the AREDS. The WARMGS13 defined early AMD as the absence of signs of advanced AMD and the presence of 1) soft indistinct or reticular drusen or 2) hard distinct or soft distinct drusen with pigmentary abnormalities (retinal pigment epithelial (RPE) depigmentation or increased retinal pigment). With respect to drusen type, any soft indistinct or reticular drusen is considered early AMD as well as any other type with pigmentary abnormality. With respect to drusen size, any drusen size with a pigmentary abnormality is considered to have early AMD. Any drusen size or type with evidence of geographical atrophy and/or exudative lesion is considered late AMD. Advanced AMD was defined as the presence of either 1) geographic atrophy or 2) exudative AMD. Exudative AMD was defined as presence of any of the following exudative lesions: 1) pigment epithelial detachment or age-related retinal detachment, 2) Subretinal Hemorrhage, 3) Subretinal Scar (subretinal fibrous scar), or 4) laser treatment for exudative ARM. Population-based studies that used this definition include the Beaver Dam Eye Study,4, 7 the Blue Mountains Eye Study,5, 8 as well as the Los Angeles Latino Eye Study for baseline data.9
The Age-Related Eye Disease Study (AREDS) Research Group developed a more detailed severity scale for AMD that allowed for the classification of risk categories and the tracking of AMD development along a 11-step scale.14 Stereoscopic fundus photographs were graded in detail and scored for increasing severity of drusen characteristics (size, type, area), pigmentary abnormalities (increased pigment, RPE depigmentation, geographic atrophy) and retinal abnormalities (RPE detachment, serous or hemorrhagic sensory retinal detachment, subretinal hemorrhage, subretinal fibrous tissue). With this scale, early AMD was defined as steps 4, 5, 6, 7, or 8 while advanced AMD was defined as steps 9, 10, or 11, and steps 1, 2, or 3 signifying presence of questionable lesions or other characteristics not amounting to AMD.
For this report, we present the incidence and progression of early and advanced AMD as defined by the WARMGS and the AREDS severity scale. Progression, regression and disappearance are also reported for individual AMD lesions, following definitions outlined in the WARMGS. All definitions are summarized in Table 1.
Table 1.
Definitions of Incidence, Progression, Regression and Disappearance of Age-related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study
| Incidence |
Progression | Regression | Disappearance |
|||
|---|---|---|---|---|---|---|
| Baseline | Follow-up | Baseline | Follow-up | |||
| Age-Related Eye Disease Study (AREDS) 11-step Severity Scale | ||||||
| Early AMD | ≤ Step 3 | Step 4, 5, 6, 7, or 8 | ≥ 2-step increase | |||
|
| ||||||
| Advanced AMD | ≤ Step 8 | Step 9, 10, or 11 | ||||
|
| ||||||
| Wisconsin Age-Related Maculopathy Grading System Definitions | ||||||
| Early AMD | No early or advanced AMD lesions in both eyes | Either (i) any soft indistinct or reticular drusen, or (ii) retinal pigmentary abnormalities with any type of drusen, in either eye | ≥ 2-step increase from Level 1–3, or ≥ 1-step increase from Level 4 | |||
|
| ||||||
| Advanced AMD | No advanced AMD lesions in both eyes | Exudative AMD or geographic atrophy in either eye | ||||
|
| ||||||
| Drusen size | None or questionable | <63 μm | ≥2 additional involved subfields at follow-up without change in drusen size from baseline | ≥2 fewer involved subfields at follow-up without change in drusen size from baseline | None or questionable | |
| <63 μm | ≥63 to μm <125 μm | <63 μm | None or questionable | |||
| <125 μm | ≥125 μm to <250 μm | ≥63 μm to <125 μm | <63 μm | |||
| <250 μm | ≥250 μm | ≥125 μm to <250 μm | <125 μm | |||
| ≥250 μm | ≥250 μm | <250 μm | ||||
|
| ||||||
| Drusen type | None or HI | HD | ≥2 additional involved subfields at follow-up without change in drusen type from baseline | ≥2 fewer involved subfields at follow-up without change in drusen type from baseline | None or HI | |
| None, HI or HD | SD | HD | None or HI | |||
| None, HI, HD, or | SI | SD | None, HI or HD | |||
| SD | ||||||
| SI | SI | None, HI, HD, or | ||||
| SD | ||||||
|
| ||||||
| Increased retinal pigment | No increased retinal pigment in any subfield | Increased retinal pigment in any subfield | Either (i) ≥2 additional involved subfields at follow-up without change in maximum score from baseline, or (ii) increase in the maximum score at follow-up | Either (i) ≥2 fewer involved subfields at follow-up without change in maximum score from baseline, or (ii) decrease in the maximum score at follow-up | Increased retinal pigment in at least 1 subfield | No increased retinal pigment in all subfields |
|
| ||||||
| RPE depigmentation | No RPE depigmentation in any subfield | Definite RPE depigmentation in any subfield | Either (i) ≥2 additional involved subfields at follow-up without change in maximum score from baseline, or (ii) increase in the maximum score at follow-up | Either (i) ≥2 fewer involved subfields at follow-up without change in maximum score from baseline, or (ii) decrease in the maximum score at follow-up | Definite RPE depigmentation in at least 1 subfield | No RPE depigmentation in all subfields |
|
| ||||||
| Geographic atrophy | No geographic atrophy | Definite geographic atrophy | Increase of ≥2 disc areas, or movement of lesion towards the center | Decrease of ≥2 disc areas, or movement of lesion away from the center | Definite geographic atrophy | No geographic atrophy |
|
| ||||||
| Exudative AMD | No exudative AMD | Exudative AMD | Increase of ≥2 disc areas, or movement of lesion towards the center | Decrease of ≥2 disc areas, or movement of lesion away from the center | Exudative AMD | No exudative AMD |
HI=hard indistinct, HD=hard distinct, SD=soft distinct, SI=soft indistinct, RPE=retinal pigment epithelium
Definitions of Incidence and Progression of Age Related Macular Degeneration
In our study we present the 4-year incidence of early and advanced AMD, and 4-year incidence of specific AMD lesions using 2 mutually exclusive at-risk cohorts of participants. Our first cohort refers to persons who did not have any evidence of AMD at baseline in both eyes, thus being at risk of developing AMD in either or both eyes at follow-up. This definition of incidence in the 1st eye is usually referred to as person-specific incidence in population-based studies of AMD.4–6, 15, 16 Our second cohort refers to persons who had only one eye without evidence of AMD at baseline while the contralateral eye had some evidence of AMD at baseline. Thus in this cohort of participants, the disease-free eye was at risk of developing AMD at follow-up, and any presence of AMD at the follow-up assessment was considered to be incidence in the 2nd eye for that person.
To enable a closer comparison of incidence data between LALES and other population-based studies with similar photography and grading protocols, annual incidences are reported using the WARMGS classification for AMD. Similar age-group stratification was also used to allow uniformity across studies.
For estimations of the 4-year progression of AMD, the AREDS Scale was used. Person-specific progression is reported by concatenating the score given for each eye, therefore defining overall severity using score from the more affected eye. In the 11-step AREDS severity scale, progression was defined as a 2 or more step increase in concatenated score from baseline to the 4-year follow-up examination, and estimated for persons with gradable fundus photographs at both time-points. The cohort at risk for progression was defined as having step 9 or less (no AMD or had early AMD) at baseline.
Data and Statistical Analysis
All clinical and grading data were entered into a central database with internal automated quality control checks. The Statistical Analysis System (version 9.1, SAS Institute Inc, Cary, NC) was used for tabulations and statistical analyses, conducted at the 0.05 significance level. Age at baseline is categorized into 5 groups (40–49 years, 50–59 years, 60–69 years, 70–79 years, and 80+ years) for all analyses. Socio-demographics and clinical characteristics between participants and nonparticipants, and between persons with gradable and ungradable photographs were compared using the Chi-square test for categorical variables and the Student t-test for continuous variables. Associations between age groups and incidence and progression estimates were tested by the Mantel-Hænszel test of trend. Differences in incidence and progression rates for all AMD lesions between right and left eyes were tested using the McNemar's test. The crude overall incidence and progression rates were age-adjusted to the LALES study cohort using direct standardization methods. Results were also annualized to enable comparison across population-based studies. Intergrader and intra-grader agreements were assessed in a random sample of eyes with quadratic weighted K statistics.
RESULTS
Of the 6100 living eligible participants identified, 4658 (76%) participated in the 4-year follow-up study. Mean follow-up period was 4.3±0.03 years. Mean age of participants was 54.7±10.5 years, 60% were females, and 76% were born outside of the U.S. In this study, the country of origin was identified as Mexico in 64%, US in 24%, El Salvador in 5%, Guatemala in 2.5%, and Nicaragua 1%. Other countries were identified in 3.5% of participants. Living eligibles who did not participate at the 4-year examination were mostly males (44% vs. 40%; P<0.01), not married (33% vs. 29%; P=0.01), and did not have health insurance coverage (46% vs. 33%; P<0.001) compared to those who participated.
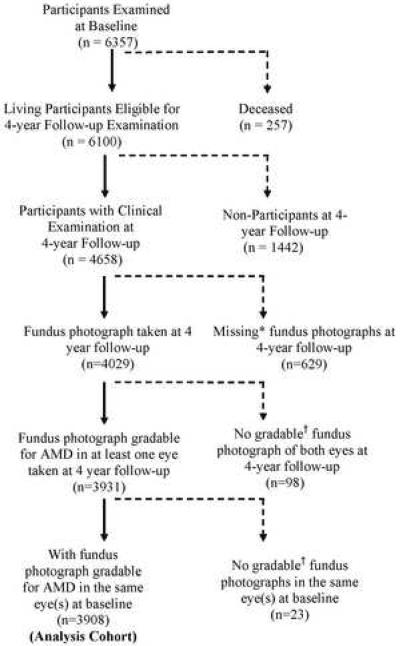
Of those who completed the ophthalmologic examination (n=4658), fundus photographs gradable for AMD lesions in at least 1 eye were available for 4029 participants (Figure). 629 participants did not complete fundus photography due to various reasons. More common reasons included refusal (n=36), poor fixation (n=74), poor dilation (n=109), cataract (n=114), movement (n=253), and poor view (n=51). Sixty-eight of the 98 participants who had completed photography did not produce gradable photographs in both eyes due to presence of other non AMD conditions obscuring AMD lesions (e.g. diabetic macular edema) and 30 had significant media opacities or poor camera focus. Of the 3931 follow-up participants with gradable fundus photo, 3908 had gradable fundus photographs in at least one eye from their baseline examination, thus making this the analysis cohort.
Figure.
Participation Flowchart for Assessing 4-Year Incidence and Progression of Age-Related Macular Degeneration (AMD) in the Los Angeles Latino Eye Study.
*Photographs were not taken due to participant refusal, poor fixation or poor dilation.
†Photographs were not gradable for AMD due to media opacities, poor camera focus, or other non-AMD conditions (e.g. diabetic macular edema) obscuring AMD lesions.
Living eligibles who were not included in this analysis compared to those who were included were less educated (32% vs. 35%; P=0.03), not married (34% vs. 29%; P<0.001), did not have health insurance coverage (41% vs. 33%; P<0.001), had less comorbidities (37% vs. 41%; P=0.001), reported worse visual health status (61% vs. 57%; P=0.01), had a higher proportion with history of eye disease (14% vs. 12% P=0.01), cataract (11% vs. 9%, P=0.001) and diabetic retinopathy (3% vs. 2%, P<0.001). Living eligibles were not included in this analysis due to either missing or non-gradable fundus photographs at baseline or follow-up examinations. The intra-grader agreement for AMD lesions was 100% and the intergrader agreement was 89.7% with a quadratic weighted Kappa of 0.82 (95% confidence interval 0.63, 1.0).
Incidence of Age Related Macular Degeneration
The 4-year incidence of early AMD in at least one eye for persons without signs of drusen or other AMD lesions in both eyes at baseline was 6.9% (95% confidence interval [CI], 6.0–7.8) (Table 2). The incidence was higher in the second eye, with 59 out of 544 persons (10.8%) developing signs of early AMD after 4 years, given the presence of AMD lesions in the contralateral eye at baseline. Similarly, when combined incidence in either eye, 7.5% developed early AMD. Both incidence in the first and second eyes increased significantly with age (test of trend, P<0.001 and P=0.04, respectively).
Table 2.
Estimated Four-year Incidence of Age-Related Macular Degeneration in the First, Second and Either Eye Utilizing the AREDS Severity Scale, Stratified by Age at Baseline
| Age at baseline (years) | Incidence in 1st Eye |
Incidence in 2nd Eye |
Incidence in Either Eye |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % (CI) | N | n | % (CI) | N | n | % (CI) | |
| Early AMD | |||||||||
| 40–49 | 1316 | 51 | 3.9 (2.9, 5.1) | 162 | 15 | 9.3 (5.3, 14.8) | 1478 | 66 | 4.5 (3.5, 5.6) |
| 50–59 | 1039 | 67 | 6.4 (5.0, 8.1) | 170 | 12 | 7.1 (3.7, 12.0) | 1209 | 79 | 6.5 (5.2, 8.1) |
| 60–69 | 592 | 72 | 12.2 (9.6, 15.1) | 122 | 15 | 12.3 (7.0, 19.5) | 714 | 87 | 12.2 (9.9, 14.8) |
| 70–79 | 199 | 25 | 12.6 (8.3, 18.0) | 80 | 16 | 20.0 (11.9, 30.4) | 279 | 41 | 14.7 (10.8, 19.4) |
| 80+ | 21 | 3 | 14.3 (3.0, 36.3) | 10 | 1 | 10.0 (0.3, 44.5) | 31 | 4 | 12.9 (3.6, 29.8) |
| P < 0.001 | P = 0.04 | P < 0.001 | |||||||
|
| |||||||||
| Overall | 3167 | 218 | 6.9 (6.0, 7.8) | 544 | 59 | 10.8 (8.4, 13.8) | 3711 | 277 | 7.5 (6.6, 8.4) |
|
| |||||||||
| Advanced AMD | |||||||||
| 40–49 | 1446 | 0 | * | 57 | 0 | * | 1503 | 0 | * |
| 50–59 | 1196 | 1 | 0.1 (0.0, 0.5) | 57 | 0 | * | 1253 | 1 | 0.1 (0.0, 0.4) |
| 60–69 | 719 | 1 | 0.1 (0.0, 0.8) | 48 | 0 | * | 767 | 1 | 0.1 (0.0, 0.7) |
| 70–79 | 267 | 4 | 1.5 (0.4, 3.8) | 52 | 0 | * | 319 | 4 | 1.3 (0.3, 3.2) |
| 80+ | 38 | 2 | 5.3 (0.6, 17.7) | 8 | 0 | * | 46 | 2 | 4.3 (0.5, 14.8) |
| P < 0.001 | P < 0.001 | ||||||||
|
| |||||||||
| Overall | 3666 | 8 | 0.2 (0.1, 0.4) | 222 | 0 | * | 3888 | 8 | 0.2 (0.1, 0.4) |
N=number at risk at baseline, n=number of incident cases, CI=confidence interval, P=test of trend, AREDS=Age-Related Eye Disease Study
No incident cases.
Note: AREDS (Age-Related Eye Disease Study) 11-step severity scale: Incidence of early AMD defined as presence of step 4, 5, 6, 7, or 8 characteristics in at least one eye which had characteristics of step 3 or less at baseline. Incidence of advanced AMD defined as presence of step 9, 10, or 11 characteristics in at least one eye which had characteristics of step 8 or less at baseline.
Incidence of advanced AMD remained low in this cohort of Latinos, with only 8 out of 3666 at-risk participants (0.2%) showing signs of exudative AMD, geographic atrophy or photocoagulation for AMD in at least one eye after 4 years of follow-up (Table 2). Advanced AMD was seen only in persons who were 50 years and older at baseline. Incidence estimates increased with age (P<0.001). No incidence of advanced AMD occurred in the second eye.
Drusen Characteristics, Pigmentary Abnormalities, Geographic Atrophy and Exudative Age Related Macular Degeneration
Table 3 shows the 4-year incidence of various AMD lesions stratified by occurrence in the first and second eye. Incidence was reported in over half of participants at risk for drusen <63μm in diameter (61.2% in the first eye, 57.4% in the second eye). Incidence was less frequent for larger sized drusen than those of smaller size. Incidence of soft distinct drusen was higher than soft indistinct drusen (incidence in the first eye, 10.3% versus 2.2%).
Table 3.
Estimated Four-year Incidence of Age-Related Macular Degeneration (AMD) Lesions in the First, Second and Either eye, Stratified by Specific Lesions Present at Baseline
| Incidence in 1st Eye |
Incidence in 2nd Eye |
Incidence in Either Eye |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % (CI) | N | n | % (CI) | N | n | % (CI) | |
| Drusen size (diameter)† | |||||||||
| <63 μm | 152 | 93 | 61.2 (53.0, 69.0) | 787 | 452 | 57.4 (53.9, 60.9) | 939 | 547 | 57.9 (54.7, 61.1) |
| ≥63 μm, <125 μm | 2282 | 420 | 18.4 (16.8, 20.1) | 1058 | 216 | 20.4 (18.0, 23.0) | 3340 | 636 | 19.0 (17.7, 20.4) |
| ≥125 μm, <250 μm | 3152 | 203 | 6.4 (5.6, 7.4) | 584 | 53 | 9.1 (7.0, 11.7) | 3736 | 256 | 6.9 (6.1, 7.7) |
| ≥ 250 μm | 3577 | 57 | 1.6 (1.2, 2.1) | 303 | 14 | 4.6 (2.5, 7.6) | 3880 | 71 | 1.8 (1.4, 2.3) |
| Drusen type | |||||||||
| Soft distinct | 2829 | 290 | 10.3 (9.2, 11.4) | 749 | 87 | 11.6 (9.4, 14.1) | 3578 | 379 | 10.5 (9.5, 11.6) |
| Soft indistinct | 3459 | 77 | 2.2 (1.8, 2.8) | 372 | 26 | 7.0 (4.6, 10.1) | 3831 | 103 | 2.7 (2.2, 3.3) |
| Any pigment abnormality | 3553 | 80 | 2.3 (1.8, 2.8) | 320 | 11 | 3.4 (1.7, 6.1) | 3873 | 91 | 2.3 (1.9, 2.9) |
| Increased retinal pigment | 3145 | 101 | 3.2 (2.6, 3.9) | 525 | 14 | 2.7 (1.5, 4.4) | 3670 | 118 | 3.1 (2.6, 3.7) |
| RPE depigmentation | 3419 | 66 | 1.9 (1.5, 2.4) | 330 | 9 | 2.7 (1.3, 5.1) | 3749 | 75 | 1.9 (1.5, 2.4) |
| Geographic atrophy | 3702 | 5 | 0.1 (0.0, 0.3) | 149 | 0 | * | 3851 | 5 | 0.1 (0.0, 0.3) |
| Pure geographic atrophy | 3882 | 3 | 0.1 (0.0, 0.2) | 24 | 0 | * | 3906 | 3 | 0.1 (0.0, 0.2) |
| Exudative AMD | 3707 | 5 | 0.1 (0.0, 0.3) | 146 | 0 | * | 3853 | 5 | 0.1 (0.0, 0.3) |
N=number at risk at baseline, n=number of incident cases, CI=confidence interval, RP=retinal pigment epithelium.
No incident cases.
Incidence refers to the presence of drusen of a specific size at follow-up when at baseline there was no evidence of drusen of that size in the same eye although drusen of a different size may be present.
The 4-year incidence of pigmentary abnormalities and signs of advanced AMD was relatively low in this population. Only 3.2% had increased retinal pigment and 1.9% retinal pigment epithelial (RPE) depigmentation occurring in the first eye (Table 3). Overall only 5 persons developed exudative AMD while 3 developed pure geographic atrophy in at least one eye. Incidence in the second eye was marginally higher than incidence in the first eye for presence of RPE depigmentation (2.7% versus 1.9%, respectively).
Table 4 shows the incidence of various AMD lesions stratified according to baseline drusen type and drusen size for participants who were at-risk for that specific lesion. There was no statistically significant difference in the incidence estimates between right and left eyes, thus results are reported for the right eye only. Presence of soft indistinct drusen at baseline was associated with a higher incidence of RPE depigmentation (13.7%), increased retinal pigment (29.1%), pure geographic atrophy (1.3%) and exudative AMD (1.4%) as compared to other drusen types. Larger drusen (diameter ≥250μm) was also associated with a higher incidence of pigmentary abnormalities (21.6%) and pure geographic atrophy (1.5%) than eyes with smaller drusen present at baseline.
Table 4.
Relation of Drusen Type and Drusen Size at Baseline to the Four-year Incidence of Age-Related Macular Degeneration (AMD) Lesions in the Right Eye
| Four-year Incidence of AMD Lesions |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RPE Depigmentation | Increased Retinal Pigment | Any Pigmentary Abnormalities | Geographic Atrophy | Pure Geographic Atrophy | Exudative Macular Degeneration | |||||||||||||
| N | n | % | N | n | % | N | n | % | N | n | % | N | n | % | N | n | % | |
| Baseline Drusen Type | ||||||||||||||||||
| None or hard indistinct | 549 | 2 | 0.4 | 534 | 3 | 0.6 | 551 | 2 | 0.4 | 559 | 0 | * | 579 | 0 | * | 559 | 0 | * |
| Hard distinct | 2674 | 11 | 0.4 | 2581 | 22 | 0.9 | 2684 | 9 | 0.3 | 2702 | 0 | * | 2726 | 0 | * | 2703 | 0 | |
| Soft distinct | 368 | 16 | 4.4 | 317 | 21 | 6.6 | 380 | 15 | 4.0 | 418 | 0 | * | 426 | 0 | * | 417 | 1 | 0.2 |
| Soft indistinct | 73 | 10 | 13.7 | 55 | 16 | 29.1 | 83 | 16 | 19.3 | 146 | 3 | 2.1 | 149 | 2 | 1.3 | 147 | 2 | 1.4 |
|
| ||||||||||||||||||
| Baseline Drusen Size (maximum diameter in μm) | ||||||||||||||||||
| None or questionable | 549 | 2 | 0.4 | 534 | 3 | 0.6 | 551 | 2 | 0.4 | 559 | 0 | * | 579 | 0 | * | 559 | 0 | * |
| <63 | 2297 | 5 | 0.2 | 2221 | 12 | 0.5 | 2302 | 3 | 0.1 | 2308 | 0 | * | 2328 | 0 | * | 2308 | 0 | * |
| ≥63 to <125 | 573 | 12 | 2.1 | 536 | 24 | 4.5 | 582 | 15 | 2.6 | 626 | 0 | * | 635 | 0 | * | 927 | 1 | 0.2 |
| ≥125 to <250 | 200 | 14 | 7.0 | 162 | 19 | 11.7 | 212 | 11 | 5.2 | 266 | 2 | 0.8 | 271 | 1 | 0.4 | 267 | 2 | 0.8 |
| ≥250 | 45 | 6 | 13.3 | 34 | 4 | 11.8 | 51 | 11 | 21.6 | 66 | 1 | 1.5 | 67 | 1 | 1.5 | 65 | 0 | * |
N=number at risk at baseline, n=number of incident cases, CI=confidence interval, RPE=retinal pigment epithelium.
Note: Data presented as incidence of specific lesion for each category of baseline drusen type. Four-year changes are similar for both right and left eyes.
No incident cases.
In Table 5 the relationship between baseline drusen type and drusen size to the 4-year incidence of hard distinct, soft distinct, and soft indistinct drusen were reported. In increasing severity, presence of hard distinct drusen at baseline predicted the 4-year incidence of soft distinct drusen at 6.6% while presence of soft distinct drusen at baseline predicted the incidence of soft indistinct drusen at 6.5%. Drusen with baseline diameter of 63μm or greater strongly predicted the presence of soft distinct as well as soft indistinct drusen at follow-up, compared to smaller size drusen (<63μm). An increase in drusen size beyond the 63μm threshold did not contribute to a greater incidence of soft indistinct drusen.
Table 5.
Relation of Drusen Type and Drusen Size at Baseline to the Four-year Incidence of Age-Related Macular Degeneration (AMD) Drusen by Type in the Right Eye
| Four-year Incidence of AMD Drusen by Type |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hard Distinct | Soft Distinct | Soft Indistinct | |||||||
| N | n | % (CI) | N | n | % (CI) | N | n | % (CI) | |
| Baseline Drusen Type | |||||||||
| None or hard indistinct | 523 | 322 | 61.6 (57.4, 65.7) | 552 | 29 | 5.3 (3.4, 7.11) | 554 | 2 | 0.4 (0.2, 3.2) |
| Hard distinct | * | 2664 | 177 | 6.6 (5.7 7.6) | 2691 | 27 | 1.0 (0.6, 1.4) | ||
| Soft distinct | * | * | 414 | 27 | 6.5 (4.1, 8.9) | ||||
| Soft indistinct | * | * | * | ||||||
|
| |||||||||
| Baseline Drusen Size (maximum diameter in μm) | |||||||||
| None or questionable | 523 | 322 | 61.6 (51.8, 65.2) | 552 | 29 | 5.3 (3.4, 7.1) | 554 | 2 | 0.4 (0.1, 0.9) |
| <63 | * | 2287 | 102 | 4.5 (3.6, 5.3) | 2300 | 13 | 0.6 (0.3, 0.9) | ||
| ≥63 to <125 | * | 377 | 75 | 20.0 (15.9, 23.9) | 599 | 31 | 5.2 (3.4, 7.0) | ||
| ≥125 to <250 | * | * | 179 | 8 | 4.5 (1.4, 7.5) | ||||
| ≥250 | * | * | 27 | 2 | 7.4 (2.5, 17.3) | ||||
N=number at risk at baseline, n=number of incident cases, CI=confidence interval.
Note: Data presented as incidence of specific drusen type for each category of baseline drusen type or baseline drusen size. Four-year changes are similar for both right and left eyes. Four-year changes are similar for both right and left eyes.
Not applicable. Participants can only be at-risk for incidence of drusen type of greater severity given a less severe drusen type or drusen size at baseline. For instance, participants with baseline drusen size ≥63 μm to <125 μm may develop incident soft distinct or soft indistinct drusen, but not incidence hard distinct drusen which is of lesser severity than their baseline drusen of that particular size.
Progression, Regression, and Disappearance of Age Related Macular Degeneration Lesions
The overall 4-year progression of any AMD in either eye was 9.3% (95% CI, 8.4–10.3), which correspond to a 2-step or more increase in severity along the AREDS severity scale (Table 6). Increasing age was associated with higher rates of progression (test of trend, P<0.0001), ranging from 6.2% in persons aged 40–49 years at baseline to 21.7% in persons over 80 years of age.
Table 6.
Estimated Four-year Progression of Age-Related Macular Degeneration (AMD) Defined by the AREDS Severity Scales, Stratified by Age at Baseline
| Age at baseline (years) | Four-year Progression |
||
|---|---|---|---|
| AREDS 11-step severity scale | |||
| N | n | % (CI) | |
| 40–49 | 1503 | 93 | 6.2 (5.0, 7.5) |
| 50–59 | 1253 | 99 | 7.9 (6.5, 9.5) |
| 60–69 | 767 | 102 | 13.3 (11.0, 15.9) |
| 70–79 | 319 | 58 | 18.2 (14.1, 22.9) |
| 80+ | 46 | 10 | 21.7 (10.9, 36.4) |
| P < 0.0001 | |||
|
| |||
| Overall | 3888 | 362 | 9.3 (8.4, 10.3) |
N=number at risk at baseline, n=number of progression cases, CI=confidence interval, P=test of trend, AREDS=Age-Related Eye Disease Study.
Note: The Age-Related Eye Disease Study (AREDS) AMD severity scale defines progression as a ≥2-step increase in severity from baseline to follow-up in either eye.
For participants with drusen <63μm in size, 17.1% had a change of ≥2 additional involved subfields at follow-up without a change in drusen size (Table 7). The increase in additional involved subfields was not substantial for drusen sized ≥63μm to <250μm, but was slightly higher for drusen sized ≥250μm (6.1%). Regression, which corresponded to a decrease in ≥2 involved subfields, was similar across different categories of drusen size. In terms of actual reduction in drusen size from baseline to follow-up (also defined as disappearance), there was greater proportion of change for drusen of larger size (50.0% changed from ≥250μm to <250μm, compared to 14.1% changed from <63μm to absence of drusen).
Table 7.
Estimated Four-Year Progression, Regression, and Disappearance of Age-Related Macular Degeneration (AMD) Lesions in the Right Eye
| AMD Lesion at Baseline | Progression |
Regression |
Disappearance |
||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % (CI) | N | n | % (CI) | N | n | % (CI) | |
| Drusen size (maximum diameter)† | |||||||||
| <63 μm | 2113 | 361 | 17.1 (15.5, 18.8) | 1362 | 395 | 29.0 (26.6, 31.5) | 2300 | 324 | 14.1 (12.7, 15.6) |
| ≥63 μm, <125 μm | 618 | 21 | 3.4 (2.1, 5.1) | 77 | 19 | 24.7 (15.6, 35.8) | 619 | 177 | 28.6 (25.1, 32.3) |
| ≥125 μm, <250 μm | 260 | 6 | 2.3 (0.9, 5.0) | 25 | 6 | 24.0 (9.4, 45.1) | 261 | 92 | 35.2 (29.5, 41.4) |
| ≥250 μm | 66 | 4 | 6.1 (1.7, 14.8) | 7 | 2 | 28.6 (3.7, 71.0) | 66 | 33 | 50.0 (37.4, 62.6) |
| Drusen type‡ | |||||||||
| Soft distinct | 414 | 10 | 2.4 (1.2, 4.4) | 48 | 4 | 8.3 (2.3, 20.0) | 414 | 106 | 25.6 (21.5, 30.1) |
| Soft indistinct | 138 | 21 | 15.2 (9.7, 22.3) | 29 | 8 | 27.6 (12.7, 47.2) | 141 | 19 | 13.5 (8.3, 20.2) |
| Increased retinal pigment§ | 97 | 26 | 26.8 (18.3, 36.8) | 18 | 9 | 50.0 (26.0, 74.0) | 97 | 18 | 18.6 (11.4, 27.7) |
| RPE depigmentation§ | 42 | 14 | 33.3 (19.6, 49.5) | 12 | 5 | 41.7 (15.2, 72.3) | 42 | 11 | 26.2 (13.9, 42.0) |
| Geographic atrophy∥ | 3 | 1 | 33.3 (0.8, 90.6) | 2 | 0 | * | 3 | 1 | 33.3 (0.8, 90.6) |
| Exudative AMD∥ | 2 | 0 | * | 2 | 1 | 50.0 (1.3, 98.7) | 3 | 1 | 33.3 (0.8, 90.6) |
N=number at risk at baseline, n=number of progression cases, CI=confidence interval, RPE=retinal pigment epithelium.
No progression or regression cases.
Progression defined as ≥2 additional involved subfields at follow-up but without change in drusen size from baseline. Regression defined as ≥2 fewer involved subfields at follow-up also without change in drusen size. Disappearance for each category of drusen size defined as reduction in drusen size at follow-up [i.e. change, from baseline to follow-up: (i) <63 μm to 0 μm; (ii) 63–125 μm to <63 μm; (iii) 125–250 μm to <125 μm; or (iv) ≥250 μm to <250 μm].
Progression defined as ≥2 additional involved subfields at follow-up but without change in drusen type. Regression defined as ≥2 fewer involved subfields at follow-up but without change in drusen type. Disappearance defined as change in drusen type at follow-up [i.e. change, from baseline to follow-up: (i) soft distinct to none, hard indistinct, or hard distinct; or (ii) soft indistinct to none, hard indistinct, hard distinct, or soft distinct].
Progression defined as either (i) ≥2 additional involved subfields at follow-up but without change in maximum score from baseline, or (ii) an increase in the maximum score at follow-up. Regression defined as either (i) ≥2 fewer involved subfields at follow-up but without change in maximum score from baseline, or (ii) a decrease in the maximum score at follow-up. Disappearance defined as absence of the specific lesion in all subfields at follow-up when the same lesion was definitely present in at least 1 subfield at baseline.
Progression defined as either (i) increase ≥2 disc areas, or (ii) movement of lesion towards the center. Regression defined as either (i) decrease of ≥2 disc areas, or (ii) movement of lesion away from the center. Disappearance defined as absence of the specific lesion in all subfields at follow-up when the same lesion was definitely present in at least 1 subfield at baseline.
DISCUSSION
The Los Angeles Latino Eye Study (LALES) is the first longitudinal study to provide data on the incidence and progression of AMD and its associated lesions in a large, well-defined cohort of adult Latinos. The use of standardized protocols at both baseline and follow-up, particularly the identical grading procedure carried out by the same graders, ensured data compatibility between the two time points.
The LALES also used the AREDS classification scheme in describing AMD incidence and progression. This scheme defines risk categories and grades for the severity of AMD characteristics along an ordinal scale. Using this scale, AMD progression of 2 or more steps was found in 9% of persons over 4 years in our population. Using this severity scale, we found that 7.5% and 0.2% of the overall LALES population had a 4-year incidence of early and advanced AMD, respectively.
The use of near identical methods and photography grading procedures between the LALES, the Beaver Dam Eye Study (BDES),4 and the Blue Mountains Eye Study (BMES)5 allows for general comparison of data across populations although the numbers of incidence cases in LALES was low. Annualized results show that the incidence of early AMD in Latinos was lower than observed in non-Hispanic whites of the other 2 studies (annual incidence, 0.8% in LALES, 1.6% in BDES, 1.5% in BMES) (Table 8). A similar trend was also observed for the incidence of advanced AMD (annual incidence, 0.05% in LALES, 0.18% in BDES, 0.16% in BMES). Given that the LALES study cohort was generally younger than the BDES and BMES populations, we stratified the annual incidence rates by age groups consistent with the 2 studies, and removed from our analysis 18 persons aged 40–42 at-risk for early AMD and another 192 persons at-risk for advanced AMD of the same age range. We found that the annual incidences for both early and advanced AMD at each age strata remained lower in LALES than in BDES or BMES (Table 8). We also age standardized the overall rates of the three studies to the LALES population. The age standardized rates for early AMD were 0.95, 1.73 and 1.41 for LALES, BDES and BMES, respectively; still consistent with the unadjusted rates. Similarly the rates for late AMD were suggestive of a lower trend for LALES (age standardized rates: LALES 0.11; BDES 0.18: BMES: 0.15). This suggests possible variations in AMD disease development along the different ethnicities. Further examination of known genetic and environmental risk factors may explain these differences. For example the homozygous CFH Tyr402His polymorphism is present in only 3% of our LALES population compared to 9%–21% of non-Hispanic Whites.17
Table 8.
Estimated Annual Incidence of Early and Advanced Age-Related Macular Degeneration (AMD) Defined by the Wisconsin AMD Definitions in Population-Based Studies
| Age at baseline (years) | Annual Incidence by Population-Based Studies | |||||
|---|---|---|---|---|---|---|
| LALES | BDES | BMES | ||||
| N | % (CI) | N | % (CI) | N | % (CI) | |
| Earlv AMD | ||||||
| 43–54 | 1728 | 0.48 (0.15, 0.80) | 1135 | 0.78 (0.27, 1.29) | 332 | 0.54 (0.00, 1.32) |
| 55–64 | 873 | 0.55 (0.06, 1.04) | 855 | 0.94 (0.30, 1.59) | 838 | 0.93 (0.28, 1.58) |
| 65–74 | 473 | 1.65 (0.50, 2.80) | 660 | 3.22 (1.90, 4.57) | 759 | 2.40 (1.31, 3.49) |
| ≥ 75 | 112 | 2.45 (0.00, 5.31) | 184 | 4.56 (1.50, 7.57) | 269 | 3.94 (1.62, 6.26) |
|
| ||||||
| Overall | 3186 | 0.75 (0.45, 1.05) | 2834 | 1.64 (1.20, 2.11) | 2198 | 1.50 (0.99, 2.01) |
|
| ||||||
| Advanced AMD | ||||||
| 43–54 | 1817 | 0.03 (0.00, 0.10) | 1254 | * | 334 | * |
| 55–64 | 985 | * | 1033 | 0.06 (0.00, 0.21) | 847 | 0.02 (0.00, 0.13) |
| 65–74 | 533 | 0.15 (0.00, 0.48) | 901 | 0.26 (0.00, 0.59) | 810 | 0.20 (0.00, 0.50) |
| ≥ 75 | 150 | 0.68 (0.00, 1.99) | 314 | 1.08 (0.00, 2.22) | 322 | 0.99 (0.00, 2.08) |
|
| ||||||
| Overall | 3485 | 0.05 (0.00, 0.12) | 3502 | 0.18 (0.00, 0.32) | 2313 | 0.16 (0.00, 0.32) |
LALES=Los Angeles Latino Eye Study, BDES=Beaver Dam Eye Study, BMES=Blue Mountains Eye Study.
N=number at risk at baseline, stratified by age-groups different from usual LALES format to allow uniformity across studies. For early AMD, 18 persons aged 40–42 were excluded from the LALES at-risk group. For advanced AMD, 192 persons were excluded. Incidence data presented as percent (95% confidence interval). Annual incidence estimated from 4-year incidence for LALES, and 5-year incidence for BDES and BMES.
No incidence cases.
Note: The Wisconsin AMD definitions: Incidence of early AMD defined as absence of AMD lesions in both eyes at baseline and presence of either soft indistinct or reticular drusen or the co-presence of any drusen and retinal pigmentary abnormalities in either or both eyes at follow-up. Incidence of advanced AMD defined as absence of AMD lesions in both eyes at baseline and presence of exudative AMD or geographic atrophy in either or both eyes at follow-up. If definitions other than the Wisconsin system are used, incidence rates may differ.
Comparisons with other population-based studies are difficult because different methodologies were employed for the assessment of AMD. Descriptively, the Barbados Eye Studies reported a 4-year incidence of 5.2% for early AMD in their predominantly Afro-Caribbean population,6 which appeared to be lower than the rate found in our Latino population. This parallels the prevalence data of both studies where early as well as advanced AMD were less prevalent in the Barbados cohort than in LALES.9, 18 Elsewhere, the Melbourne Visual Impairment Project reported a 5-year incidence of 5.4% for early AMD when definitions were standardized to that used in BMES.16 Two other studies in Europe also provided estimates of AMD incidence and progression in non-Hispanic White populations. In the Copenhagen City Eye Study, Buch et. al.15 found similar incidence rate of early ARM when compared to BDES (a U.S. study), and slighter higher rate of late AMD possibly due to their older population (60–80 years of age). Whereas in the Rotterdam Study, Klaver et al, found similar incidence estimates of late AMD, but not early ARM when compared to BDES, possibly due to the short follow-up duration.15, 19
In this LALES study we have detailed the interrelation of AMD lesions over time. Soft indistinct drusen and large drusen (≥250μm) stood out as the strongest predictors of incident pigmentary abnormalities. Although the presence of soft indistinct drusen and large drusen at baseline also predicted the incidence of geographic atrophy and exudative AMD, this relationship is relatively weak due to the low number of cases from this Latino population. Other studies have previously shown that these type and size of drusen increase the risk of advanced AMD.4, 5, 20 However, we cannot conclude that this is the case for the Latino population due to the low incidence of advanced AMD in the current 4-year cohort.
When documenting the incidence of eye disease, it would be useful to quantify occurrence in the subject's first eye as well as second eye, which we have done in this report. The respective incidence estimates would refer to two separate at-risk cohorts of participants, where the former was free of disease in both eyes at baseline while the latter had previously reported presence of unilateral disease. In the case for AMD, a study by Gudnadottir et al. (2005)21 reported that 82% of individuals with unilateral exudative AMD developed the same condition in the second eye within 4 years, resulting in bilateral advanced AMD. A systematic review by Wong et al (2008)22 reported a 26.8% involvement in the contralateral eye by 4 years in 4362 treatment-naive neovascular AMD patients or eyes in 53 study groups. In addition, persons with bilateral severe AMD had reported significantly lower vision-related quality of life than persons with AMD of varying severity.23 These findings highlight the importance of capturing incidence in the second eye, which has not been reported in other population-based longitudinal studies. Although generally perceived to be a relatively symmetrical condition, the differential incidence of AMD in the first and second eye support the use of a concatenated score like the WARMGS or the AREDS simplified severity scheme.24
In conclusion, this study presents the 4-year incidence and progression of AMD in Latinos, with findings that were different from previous studies of non-Hispanic whites. While these data show low incidence and progression of AMD in the Latino population, it is not yet understood whether this is due to protective genetic and lifestyle factors relative to whites.
ACKNOWLEDGEMENTS
Support: National Institutes of Health Grants: NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
Other Acknowledgements: The Los Angeles Latino Eye Study Group, University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH; Stanley P. Azen, PhD; Roberta McKean-Cowdin, PhD; Sylvia H. Paz, MS; Mina Torres, MS; Jaime Barrera; Farzana Choudhury, MBBS, MPH; Lupe Cisneros, COA; Jessica Chung, MPH; Elizabeth Corona; Carolina Cuestas, OD; Jeanne Dzekov; Athena W.P. Foong; Carlos Lastra, MD; Mei-Ying Lai, MS; George Martinez; Corina Shtir, MS; Ronald E. Smith, MD; LaVina Tetrow; Ying Wang, MS; Joanne Wu, MPH.
Battelle Survey Research Center, St. Louis, MO: Lisa John, MSW; Karen Tucker, MA. Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI: Ronald Klein, MD, MPH; Stacy E. Meuer; Michael D. Knutson, MA; Michael Neider.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
REFERENCES
- 1.U.S. Interim Projections by Age, Sex, Race, and Hispanic Origin: U.S. Census Bureau, Population Division, Population Projections Branch. 2004. [Google Scholar]
- 2.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP. Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143:1013–23. doi: 10.1016/j.ajo.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma R, Wu J, Chong K, Azen SP, Hays RD. Impact of severity and bilaterality of visual impairment on health-related quality of life. Ophthalmology. 2006;113:1846–53. doi: 10.1016/j.ophtha.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Jensen SC, Meuer SM. The five-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1997;104:7–21. doi: 10.1016/s0161-6420(97)30368-6. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109:1092–7. doi: 10.1016/s0161-6420(02)01055-2. [DOI] [PubMed] [Google Scholar]
- 6.Leske MC, Wu SY, Hyman L, Hennis A, Nemesure B, Schachat AP. Four-year incidence of macular changes in the Barbados Eye Studies. Ophthalmology. 2004;111:706–11. doi: 10.1016/j.ophtha.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 9.Varma R, Fraser-Bell S, Tan S, Klein R, Azen SP. Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111:1288–97. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Shtir CJ, Marjoram P, Azen S, et al. Variation in genetic admixture and population structure among Latinos: the Los Angeles Latino eye study (LALES) BMC Genet. 2009;10:71. doi: 10.1186/1471-2156-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130:658–63. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 12.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–31. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 14.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buch H, Nielsen NV, Vinding T, Jensen GB, Prause JU, la Cour M. 14-year incidence, progression, and visual morbidity of age-related maculopathy: the Copenhagen City Eye Study. Ophthalmology. 2005;112:787–98. doi: 10.1016/j.ophtha.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Mukesh BN, Dimitrov PN, Leikin S, et al. Five-year incidence of age-related maculopathy: the Visual Impairment Project. Ophthalmology. 2004;111:1176–82. doi: 10.1016/j.ophtha.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Tedeschi-Blok N, Buckley J, Varma R, Triche TJ, Hinton DR. Population-based study of early age-related macular degeneration: role of the complement factor H Y402H polymorphism in bilateral but not unilateral disease. Ophthalmology. 2007;114:99–103. doi: 10.1016/j.ophtha.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 18.Schachat AP, Hyman L, Leske MC, Connell AM, Wu SY. Features of age-related macular degeneration in a black population. The Barbados Eye Study Group. Arch Ophthalmol. 1995;113:728–35. doi: 10.1001/archopht.1995.01100060054032. [DOI] [PubMed] [Google Scholar]
- 19.Klaver CC, Assink JJ, van Leeuwen R, et al. Incidence and progression rates of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2001;42:2237–41. [PubMed] [Google Scholar]
- 20.van Leeuwen R, Klaver CC, Vingerling JR, Hofman A, de Jong PT. The risk and natural course of age-related maculopathy: follow-up at 6 1/2 years in the Rotterdam study. Arch Ophthalmol. 2003;121:519–26. doi: 10.1001/archopht.121.4.519. [DOI] [PubMed] [Google Scholar]
- 21.Gudnadottir GS, Magnusson KP, Stefansson E, Jonasson F, Helgadottir G, Sigurdsson H. The time pattern of bilateral exudative age-related macular degeneration. Acta Ophthalmol Scand. 2005;83:333–6. doi: 10.1111/j.1600-0420.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 22.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Cahill MT, Banks AD, Stinnett SS, Toth CA. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology. 2005;112:152–8. doi: 10.1016/j.ophtha.2004.06.036. [DOI] [PubMed] [Google Scholar]
- 24.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]