Abstract
This study used functional magnetic resonance imaging to investigate individual differences in the neural underpinnings of sentence comprehension, with a focus on neural adaptability (dynamic configuration of neural networks with changing task demands). Twenty-seven undergraduates, with varying working memory capacities and vocabularies, read sentences that were either syntactically simple or complex under conditions of varying extrinsic working memory demands (sentences alone or preceded by to-be-remembered words or nonwords). All readers showed greater neural adaptability when extrinsic working memory demands were low, suggesting that adaptability is related to resource availability. Higher capacity readers showed greater neural adaptability (greater increase in activation with increasing syntactic complexity) across conditions than did lower capacity readers. Higher capacity readers also showed better maintenance of or increase in synchronization of activation between brain regions as tasks became more demanding. Larger vocabulary was associated with more efficient use of cortical resources (reduced activation in frontal regions) in all conditions but was not associated with greater neural adaptability or synchronization. The distinct characterizations of verbal working memory capacity and vocabulary suggest that dynamic facets of brain function such as adaptability and synchronization may underlie individual differences in more general information processing abilities, whereas neural efficiency may more specifically reflect individual differences in language experience.
Keywords: cortical dynamics, fMRI, individual differences, language, syntax, vocabulary size, working memory
Introduction
The neural underpinnings of reading comprehension are modulated by a complex interaction between reader characteristics and text characteristics. The current study addressed this intersection of reader and text variables by investigating how individuals adapt neurally to changes in the computational demands of a task. Previous research on sentence comprehension has shown that the ability to dynamically configure cortical networks on an “as-needed” basis with changing task demands varies among individuals and is related to comprehension ability (Prat et al. 2007). Here, we explored the mechanism of such neural adaptability by examining individual differences in patterns of activation when healthy adults with varying working memory capacity and vocabulary size read sentences varying in syntactic complexity under different external working memory demands.
Characteristics of Skilled Comprehenders
Verbal Working Memory Capacity
To comprehend sentences, readers must execute multiple component linguistic processes in parallel while storing intermediate representations. Thus, indices of verbal working memory capacity such as the Reading Span Test (Daneman and Carpenter 1980) that measure the ability to simultaneously process sentences and maintain information in memory correlate well with language comprehension abilities under a variety of conditions (for a review, see Daneman and Merikle 1996). The relation between working memory capacity and syntactic processing is particularly well established in the literature. For example, high-capacity individuals are more sensitive to syntactic ambiguities (e.g., MacDonald et al. 1992; Pearlmutter and MacDonald 1995; Long and Prat 2008) and are better able to parse complex syntactic structures (e.g., King and Just 1991; Just and Carpenter 1992) than are low-capacity individuals. A similar enabling relation between working memory capacity and syntactic processing is observed when extrinsic demands on working memory resources are manipulated. For example, adding a secondary working memory task, such as a short list of to-be-remembered words, greatly decreases the ability of all readers to parse sentences (Wanner and Maratsos 1978; King and Just 1991; Gordon et al. 2001, 2002; Fedorenko et al. 2006), especially when the sentence is complex (King and Just 1991; Gordon et al. 2002; Fedorenko et al. 2006) and/or when the reader has a low working memory capacity (King and Just 1991). Thus, several lines of research have converged to highlight the importance of working memory resources for syntactic processing. In the current study, we investigated the nature of such capacity constraints by comparing neural activation patterns during the processing of syntactically simple and complex sentences by individuals with varying resource availability under conditions that placed increasing demands on working memory resources.
Although verbal working memory scores and measures of reading comprehension ability tend to be highly correlated, they do not measure identical constructs (see Daneman and Hannon 2001; Hannon and Daneman 2001; Long et al. 2008). Hannon and Daneman (2001), for example, developed a tool to separately measure 4 components of reading comprehension skill and found that direct measures of reading comprehension skill, such as the Nelson-Denny Reading Test, were highly correlated with each of the 4 components of comprehension ability, whereas reading span scores were most highly correlated with processes based on memory for explicit text information and were more weakly correlated with measures of knowledge access and integration. In another investigation of the factors related to individual differences in memory for discourse, Long et al. (2008) administered a battery of psychometric tests to 149 college undergraduates and used factor analysis to extract 5 factors relevant to reading comprehension abilities. Measures of reading comprehension ability (i.e., Nelson-Denny Reading Test) and print exposure loaded most highly onto a factor related to verbal abilities, which was reliably predictive of later recognition memory for texts, whereas measures of working memory capacity (reading span and operation span) loaded most highly onto a separate working memory factor that did not reliably predict later memory for texts. Thus, verbal working memory scores explain some, but not all, of the variance observed in measures of reading comprehension ability.
Word Knowledge
Reading comprehension, like all complex cognitive tasks, is resource demanding. Hence, the efficiency with which an individual can encode, access, and represent the basic units (words) of a sentence is an important component of comprehension ability. According to the lexical quality hypothesis (e.g., Perfetti 1985, 2007; Perfetti and Hart 2001), variability in the quality of representations of individual words underlies individual differences in reading comprehension abilities. In fact, several studies have shown that individual differences in measures of word knowledge, or the quality of lexical representations, are also related to differences in comprehension ability (e.g., Perfetti 1985, 2007; Bell and Perfetti 1994). These findings are also consistent with the capacity theory of comprehension (Just and Carpenter 1992), which states that the availability of cognitive resources constrains the number and type of linguistic processes that can be executed at any given moment. Thus, a reduction in mental effort necessary for encoding words leads to greater availability of resources for higher level operations (e.g., syntactic processing), and therefore is another factor underlying comprehension ability.
While verbal working memory capacity and word knowledge have been extensively correlated with comprehension ability, the nature and development of these 2 characteristics of readers is quite different. Verbal working memory capacity has been viewed as a general characteristic of fluid information processing ability that peaks in early adolescence (e.g., Gathercole 1999; Gathercole et al. 2004) and then decreases after young adulthood (e.g., Horn and Cattell 1967; Stanovich et al. 1995). Indices of verbal working memory capacity are highly correlated with indices of nonverbal working memory capacity as well as with more general measures of reasoning and fluid intelligence (e.g., Kyllonen and Christal 1990; Engle et al. 1999). Until recently, working memory capacity was believed to be a rather impermeable characteristic of an individual that was unaffected by experience with particular tasks (for recent studies showing improvement of working memory capacity with specialized training, see Jaeggi et al. 2008 and Chein and Morrison 2010). Word knowledge, on the other hand, is acquired through experience with language; it continues to grow with reading experience throughout the life span. Given the distinguishing properties of working memory capacity and word knowledge, it is plausible that the biological bases of these 2 reader characteristics and their influences on comprehension ability are quite different.
The current study explores the neural bases of working memory capacity and word knowledge as they enter into sentence comprehension. We used Reading Span Test scores (Daneman and Carpenter 1980) as indices of working memory capacity and used scores on the vocabulary portion of the Nelson-Denny Reading Test as indices of word knowledge. The Reading Span Test involves reading aloud sets of sentences in progressively increasing block sizes (2–6 consecutive sentences) and recalling the sentences’ final words; it is one of the most widely used indices of verbal working memory capacity. The vocabulary portion of the Nelson-Denny Reading Test consists of 80 multiple choice vocabulary items and has been normed for college readers. In the Long et al. (2008) analysis, scores on this portion of the test loaded most highly onto the verbal ability factor described above and were highly correlated with scores on the comprehension section of the same test (r = 0.69) and with measures of print exposure (r = 0.50). In this experiment, we correlate scores on these 2 behavioral measures with brain-based indices of sentence comprehension under a variety of processing demands to further explore the neural underpinnings of individual differences in comprehension abilities.
Network-Level Characteristics of Skilled Comprehension
Research on individual differences in reading has yielded 3 important facets of brain function that appear to underlie comprehension ability: neural adaptability, neural synchronization, and neural efficiency (e.g., Maxwell et al. 1974; Prat et al. 2007). One goal of the current experiment is to explore the extent to which variability in each of these facets of brain function relates to individual differences in working memory capacity and word knowledge. Another goal is to examine neural adaptability by investigating changes in activation under varying intrinsic (syntactic complexity) and extrinsic (external working memory load) sentence processing demands. Although our research focuses on reading, we propose that the 3 facets of brain function described herein represent more general characterizations of “skilled” information processing in distributed and dynamic cortical networks. Below we summarize existing research on the role of neural adaptability, synchronization, and efficiency in complex cognitive tasks.
Neural Adaptability
Human cognition is characterized by dynamic adaptations to the environment, and therefore a cortical network engaged in performing a skilled task must be able to adapt to changing information processing demands (e.g., Schafer 1982; Garlick 2002). Neuroimaging research provides evidence of such adaptation in terms of the activation of brain areas on an as-needed basis. Although a modal set of areas activates for any given task, additional areas can be recruited to deal with increasing demands. To illustrate, Just et al. (1996) found that as syntactic complexity increased, right hemisphere (RH) homologues of typical left hemisphere (LH) language regions were increasingly activated.
Research on individual differences in sentence comprehension suggests that high-working memory capacity individuals show greater neural adaptability (modulation of activation) in the face of changing task demands (Prat et al. 2007). Specifically, high-capacity readers showed greater increases in activation for sentences with low-frequency nouns versus high-frequency nouns than did low-capacity readers. Therefore, differences in comprehension ability may reflect differences in the adaptability of a neural network in the face of changing demands.
In the current study, we explored various accounts of individual differences in neural adaptability. One account suggests that individual differences in adaptability are a consequence of individual differences in baseline neural efficiency. According to this account, greater adaptability in high-capacity readers arises because individuals with more efficient baseline comprehension processes have more neural resources available for further recruitment when task demands increase. In other words, high-capacity individuals, who utilize a smaller proportion of their resources in performing a baseline task, show greater adaptability (manifested as a greater increase in activation) to increasing demands because they have more resources still available for recruitment.
An alternate possibility is that individual differences in neural adaptability arise from a property of cortical dynamics that is somewhat separable from resource availability and that varies systematically between individuals. Potential facets of brain function that may contribute to individual differences in neural adaptability include differences in structural and functional connectivity between cortical regions, differences in functionality of cognitive control mechanisms in the frontal regions of the brain, and differences in speed of neural processing (including rate of activation and subsequent deactivation). According to this view, systematic differences in one or more of these neural properties in high-capacity individuals result in a tighter coupling between changes in the computational demands of a task and changes in cortical activation (both in amount and topography). These 2 accounts are not mutually exclusive, as it is plausible that both efficient resource utilization and resource allocation are necessary for fluent neural adaptability.
To further explore the mechanism behind these individual differences, the current experiment examined adaptability to differing levels of syntactic complexity (syntactic adaptability) under varying extrinsic working memory demands. If neural adaptability were related to resource availability, then the increased activation for complex sentences over simple sentences would be attenuated as extrinsic working memory demands increased. A similar pattern should be observed as a function of individual differences in working memory capacity, such that smaller increases in activation for complex over simple sentences should be observed in low-capacity individuals. To the extent that individual differences in adaptability arise because of differences in resource availability, it is possible that differential adaptability effects will be most readily observed under conditions where some (lower capacity) individuals have utilized all of their resources (thus having nothing left to recruit), while other (higher capacity) individuals have not (and thus have resources available for recruitment). It is possible, therefore, that individual differences in adaptability will not be observed either in easy conditions, where all individuals have resources available for recruitment, or in extremely hard conditions, where all individuals have utilized all available resources. If, on the other hand, individual differences in adaptability are related to some general facet of brain function separable from resource availability, then individual differences in neural adaptability should be observable in all conditions, irrespective of task difficulty.
Neural Synchronization
In order for a cortical language network to function effectively, the activities of coactivated network centers must be coordinated. Such collaboration between 2 activated regions can be measured in terms of the correlation of the activation time series in one region with the activation time series of another region. The extent to which the activation levels of 2 regions rise and fall in tandem is considered an illustration of the degree to which the 2 regions are collaborating or functionally connected. “Functional connectivity” (Friston 1994) is a measure of the correlation between the activation time series of 2 cortical regions; it does not provide direct evidence that the activity of one region causes activity in another region or that the regions are directly communicating, but it nevertheless provides a useful characterization of brain activity at the network level rather than at the level of the individual region. This level of characterization is particularly appropriate for evaluating the response of an adaptive system to task demands, and it may provide new insight into the nature of individual differences at the network level.
Research on individual differences in sentence comprehension has found that functional connectivity during sentence comprehension is higher in readers with greater working memory capacity (Prat et al. 2007). High-capacity readers showed higher synchronization between Broca’s (left inferior frontal gyrus) and Wernicke’s (left posterior superior temporal gyrus) areas. Additionally, high-capacity readers were better able to maintain or increase the synchronization between brain areas when computational demands (modulated by lexical frequency and syntactic complexity) increased. The current experiment attempted to extend previous findings by investigating changes in cortical synchronization as a function of individual working memory capacity and reading skill under different types of increasing task demands.
Neural Efficiency
Most of the seminal neuroscientific research on individual differences in both reading skill and general intellectual abilities has shown that more-skilled individuals generally accomplish a task more efficiently, using fewer mental resources, than less-skilled individuals (Maxwell et al. 1974; Haier et al. 1988; Reichle et al. 2000; Prat et al. 2007, 2010; Neubauer and Fink 2009). The assumption behind efficiency research is that the amount of “mental resource consumption” that is required to perform a task is reflected by the amount of brain activation observed during the task. Resource consumption can be measured either by the spread of activation (more focal activation patterns are more efficient) or by the intensity of activation in a given area (less activation is more efficient). 4CAPS, a cognitive neuroarchitecture that maps mental resource utilization onto cortical activation (Just and Varma 2007), postulates that the size of the available individual neural resource pool affects the amount of functional magnetic resonance imaging (fMRI)-measured activation observed during a cognitive process. The amount of activation is interpreted as an index of the proportion of the pool that is in use at any given time. Thus, individuals with larger resource pools should show less neural activation during a given sentence comprehension process than individuals with smaller resource pools. This lower amount of brain activation shown during the processing of a given task serves as a measure of neural efficiency. Note that the negative correlation between a psychometrically measured cognitive ability and the amount of activation in a cortical area central to a task is predicted only in groups of healthy, nonbrain damaged individuals who already know how to perform the task and are using the same strategy to do so (for a review, see Neubauer and Fink 2009).
Several recent neuroimaging studies measuring neural efficiency have found that more skilled or proficient individuals have less activation in brain regions that centrally participate in the relevant cognitive processes (e.g., Haier et al. 1988; Reichle et al. 2000; Prat et al. 2007). For example, one study found that in a sentence–picture verification task, participants with higher verbal abilities, as indexed by reading span scores, had lower activation volumes in typical language regions (e.g., Broca’s area) when engaging in verbal strategies. Similarly, individuals with higher visual–spatial skills, as indexed by mental rotation test scores, had lower activation volumes in spatial processing regions (e.g., parietal cortex) when engaging in spatial strategies to solve the task (Reichle et al. 2000). In another fMRI investigation of individual differences in sentence comprehension, Prat et al. (2007) found that compared with low-capacity readers, high-capacity readers showed less activation in bilateral frontal regions across sentences of varying complexity, again suggesting that high-capacity individuals exhibit more efficient use of neural resources. Taken together, these findings provide evidence that skilled readers use a smaller proportion of their resources in performing language comprehension tasks. The current study tested the hypothesis that such differences in neural efficiency underlie individual differences in reading comprehension ability. In addition, the relation between neural efficiency, resource availability, and dynamic network configuration was explored by comparing indices of efficiency, adaptability, and synchronization in readers with varying working memory capacities and word knowledge during sentence comprehension under varying intrinsic and extrinsic demands.
Materials and Methods
Participants
Data were collected from 27 right-handed, native English-speaking participants (15 males and 12 females [gender was included as a nuisance variable in individual differences analyses], aged 18–25 years) who were paid undergraduate volunteers recruited through Carnegie Mellon University. An additional 7 participants were tested but excluded from analysis, either because of head motion greater than 2 mm (4 participants), a structural abnormality (one participant), or poor task performance (greater than 20% error; 2 participants). All participants gave informed consent.
Materials
The materials consisted of 60 sentences that were presented in 3 working memory load conditions: No Load (sentences alone), Low Load (sentences plus words), and High Load (sentences plus nonwords). Half of the sentences were syntactically simple, composed of 2 active-conjoined clauses (e.g., “The kitten licked the puppy and climbed the stairs”), and the other half were syntactically complex, containing an object-relative clause (e.g., “The kitten that the puppy licked climbed the stairs”). All sentences were constructed such that preexisting semantic relations did not more readily pair subjects to verbs or objects (in other words, “kitten” and “puppy” are relatively equally associated with “licked” and “climbed”). Simple and complex sentences were equated for word length and for noun and verb frequency. No subjects, verbs, or objects were repeated across the experiment, in either the sentences or in the word probes.
The materials also included a set of memory items composed of 60 concrete, highly imageable nouns (e.g., pickle, jacket, cow) taken from the online MRC Psycholinguistics Database and 60 pronounceable nonwords (e.g., trokey, saft, ponget), constructed by changing 1 or 2 phonemes of real words. The memory items were grouped into 20 sets of 3 words and 20 sets of 3 nonwords. Each set was composed of 2 one-syllable items and one 2-syllable item. In the word condition, memory items were not highly semantically associated with each other or with the words in the sentence they preceded.
Sixty true/false memory probes tested recognition of either the memory items or the sentences. In the No Load condition, where sentences were presented alone, the memory probes tested recognition of information presented in the sentences. In the Low and High Load conditions, where the sentences were preceded by memory items, half of the memory probes tested recognition of information presented in the sentence and the other half tested recognition of the memory items. This design resulted in 40 sentence probes, 10 word probes, and 10 nonword probes. Half of the sentence probes tested recognition of information presented in the first clause of the sentence and the other half of tested recognition of information presented in the second half of the sentence. False sentence probes were constructed by creating a mismatch between the actors and actions mentioned in the sentence rather than introducing new information (e.g., “The pianist that the composer admired had many fans could be followed by the false comprehension probe “The pianist admired the composer” or “The composer had many fans”). False memory item probes included 2 of the 3 original items (words or nonwords) with the third item replaced by a foil of similar length. The foil replaced the first, second, or third item with approximately equal frequency. In both the Low and High Load conditions, participants did not know whether comprehension probes would investigate memory for sentences or for memory items and were instructed to pay equal attention to both sources of information.
Procedure
All participants underwent behavioral testing and fMRI practice sessions 1 or 2 days prior to their scan. First, the Reading Span Test (Daneman and Carpenter 1980), the Nelson-Denny Reading Test (Riverside Publishing Company), and the Edinburgh Handedness Inventory (Oldfield 1971) were administered, in that order. Second, each participant practiced the paradigm with sample stimuli inside a mock scanner.
Explicit instructions were given to minimize the likelihood that participants would engage in different strategies during the fMRI scanning paradigm. In the No Load condition, participants were instructed to read each sentence for comprehension. In the Low Load condition, participants were instructed to read the 3 concrete nouns and form a mental picture that included the 3 items. They were then instructed to hold the image in mind while reading the subsequent sentence for comprehension. In the High Load condition, participants were instructed to read the 3 nonwords and mentally rehearse them while reading the sentences that followed for comprehension.
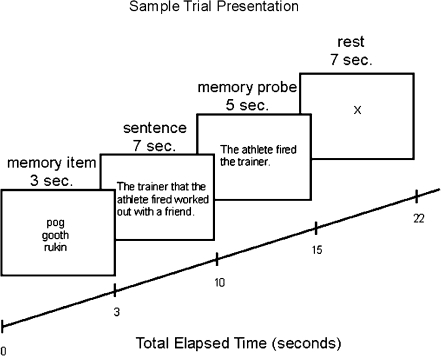
In the Low and High Load conditions, each trial began with a list of 3 memory items, presented simultaneously in a vertical column in the center of the screen for 3 s. Immediately after the memory items disappeared, a sentence was presented for 7 s (with words arrayed normally from left to right). In the No Load condition, each trial began with a sentence presented for 7 s. In all conditions, the sentences were followed by a memory probe presented for 5 s. Participants were instructed to press a mouse button corresponding to “true” or “false” based on the information presented in the comprehension probe as quickly and as accurately as possible. Each trial concluded with a 7-s rest, where an X appeared on the center of the screen. A schematic diagram for timing in the High Load condition is presented in Figure 1.
Figure 1.
Schematic depiction of the presentation of a High Load trial.
The 3 working memory conditions were presented in blocks. All participants received the No Load condition first. The order of Low and High Load conditions varied across participants, such that half received the High Load condition first and the other half received the Low Load condition first. Within blocks, sentence type (simple and complex) and memory probe type (sentence or memory item) were presented in random order. Four baseline periods occurred at the beginning, end, and between blocks. During these baseline periods, participants viewed an X on the center of the screen for 30 s and were instructed to relax and clear their mind. Participants viewed the stimulus materials (subtending a visual angle of approximately 30°) through a mirror that reflected a projection screen behind the participant’s head.
fMRI Data Acquisition
Data were collected using a Siemens Allegra 3.0 T scanner at the Brain Imaging Research Center jointly operated by Carnegie Mellon University and the University of Pittsburgh. The study was performed with a gradient echo planar pulse sequence with time repetition = 1000 ms, time echo = 30 ms, and a 60° flip angle. Seventeen oblique-axial slices were imaged, and each slice was 5-mm thick with a gap of 1 mm between slices. The acquisition matrix was 64 × 64 with 3.125 × 3.125 × 5 mm voxels.
Data Analysis Methods
Behavioral Analyses
Response times for correct trials and error rates to probes were analyzed separately for memory items and sentence comprehension probes. This resulted in 2 separate analyses of variance (ANOVAs): a 2 (Syntactic Complexity) × 3 (Working Memory Load) ANOVA for sentence probes and a 2 (Syntactic Complexity) × 2 (Working Memory Load) ANOVA for memory items (as there were no memory items in the No Load condition), with syntactic complexity and working memory load as within-participant variables. Nelson-Denny Vocabulary and reading span scores were used as between-participant covariates in all analyses. Participants’ Nelson-Denny Vocabulary percentiles ranged from 43 to 99, with a mean of 84 and standard deviation of 15.69. Reading span scores ranged from 2.0 to 5.0, with a mean of 3.2 and standard deviation of 0.88. Nelson-Denny and span scores were not correlated (r27 = −0.15, P = 0.47) (While both vocabulary size and working memory capacity are highly correlated with reading comprehension ability, they measure distinct reader characteristics and are not necessarily correlated with one another. In a larger analysis of 84 individuals, we also found no correlation between vocabulary size and working memory capacity [Prat et al. 2010].). Scatterplots depicting the distribution of Nelson-Denny Vocabulary scores as a function of Reading Span is depicted in supplementary Figure 1. All effects were tested at a significance level of P < 0.05.
fMRI Data Analyses
The data were analyzed using SPM2 (Wellcome Department of Imaging Neuroscience, www.fil.ion.ucl.ac.uk/spm) to examine the distribution of activation during sentence comprehension as a function of working memory load and individual working memory capacity. Images were corrected for slice acquisition timing, motion-corrected, normalized to the Montreal Neurological Institute (MNI) template, resampled to 2 × 2 × 2 mm voxels, and smoothed with an 8-mm Gaussian kernel to decrease spatial noise. Statistical analyses were performed on individual and group data using the general linear model (Independent regressors were created for sentences, response probes, and memory items. Memory items were not separated in time from sentences, but as participants were instructed to rehearse the items in mind, there was no feasible way to completely separate the hemodynamic response to memory items and sentence probes.) as implemented in SPM2 (Friston et al. 1995). Only results falling within gray matter were reported. For individual participants, a fixed-effects model that incorporated a high-pass filter with a cutoff of 378 s and an AR(1) correction for serial autocorrelation was used to estimate parameters. Syntactic adaptability was calculated in each of the 3 working memory load conditions as the contrast of the parameter estimates for syntactically complex—syntactically simple sentences. False discovery rate corrections (Genovese et al. 2002) were applied to the group analyses with a corrected height threshold of P < 0.05 and an extent threshold of 12 voxels, roughly corresponding to 2 voxels in native space. Individual differences in syntactic adaptability and neural efficiency were assessed on a voxel-wise basis using random-effects multiple regression models in which Nelson-Denny vocabulary percentile and reading span scores were entered simultaneously as regressors of interest, with gender included as a nuisance variable, and the contrast of parameter estimates for the syntactic complexity effect (all object-relative sentences minus all active sentences) and for efficiency (all sentences minus fixation) were the dependent variables. An uncorrected height threshold of P < 0.001 and an extent of 12 voxels were used for individual differences analyses.
Connectivity Analyses
Twelve functionally defined and 2 anatomically defined regions of interest (ROIs) were used for the connectivity analyses. Seven ROIs were selected from a group of spherical ROIs previously defined to encompass all the major regions of activation across a series of discourse comprehension experiments. The selected ROIs were those germane to sentence processing and working memory tasks and included bilateral inferior frontal gyri (including Broca’s area and its RH homologue), bilateral superior posterior temporal gyri (including Wernicke’s area and its RH homologue), bilateral parietal regions, and a medial frontal region. Because this task relied more heavily on working memory and control centers than simpler discourse comprehension paradigms, an additional 5 functional ROIs were defined to encompass regions of activation for which we did not have previously defined ROIs, including bilateral superior frontal ROIs, LH striatum (caudate and putamen), RH caudate, and precuneus (extending into both hemispheres). We used 2 anatomically defined ROIs (as outlined in Tzourio-Mazoyer et al. 2002) to capture activation in the hippocampi because these regions are not captured well by spheres. This resulting set of 14 ROIs was then grouped into 4 functional networks: a LH language network (consisting of Broca’s and Wernicke’s areas), a RH language network (consisting of the RH homologues of Broca’s and Wernicke’s areas), a control network (consisting of bilateral superior frontal, parietal, striatum, and medial frontal ROIs), and a memory network (consisting of bilateral hippocampal ROIs and the medial precuneus ROI). Network labels, centroid MNI coordinates, and corresponding Brodmann’s areas (where applicable) for the 14 ROIs are listed in Table 1.
Table 1.
Locations of 14 ROIs used in network connectivity analyses
| Network | Cortical region | Brodmann’s area | Radius | Centroid MNI coordinates |
||
| x | y | z | ||||
| LH language | LH inferior frontal (Broca’s area) | 45 | 14 | −48 | 18 | 18 |
| LH language | LH posterior temporal (Wernicke’s area) | 40 | 14 | −52 | −54 | 18 |
| RH language | RH inferior frontal (Broca’s homologue) | 46 | 14 | 48 | 22 | 26 |
| RH language | RH posterior temporal (Wernicke’s homologue) | 22 | 14 | 48 | −50 | 6 |
| Control | Medial frontal | 8 | 14 | −2 | 20 | 50 |
| Control | LH superior frontal | 6 | 12 | −14 | 6 | 60 |
| Control | RH superior frontal | 6 | 12 | 36 | −12 | 52 |
| Control | LH parietal | 40 | 14 | −42 | −42 | 42 |
| Control | RH parietal | 40 | 14 | 40 | −40 | 42 |
| Control | LH striatum (caudate and putamen) | 12 | −20 | 22 | 0 | |
| Control | RH caudate and cingulum | 25 | 12 | 8 | 12 | 14 |
| Memory | Precuneus | 7 | 14 | −2 | −60 | 46 |
| Memory | LH hippocampus–anatomical | |||||
| Memory | RH hippocampus–anatomical | |||||
The activation time course that served as the basis of the functional connectivity analysis was extracted for each participant, averaged over only the voxels within each of the functional ROIs that were activated above a threshold of P = 0.001 (uncorrected) in any of the conditions. The input data were the normalized and smoothed images that had been low-pass filtered and had the linear trend removed. The functional connectivity was computed separately for each participant as a correlation between the activation time courses (averaged over all of the activated voxels) in a pair of ROIs. A participant was excluded from analysis if the number of voxels activated in either of the ROIs constituting the pair was less than 12. Network-level connectivity analysis was conducted such that the average functional connectivity of each pair of ROIs within a functional network or between 2 functional networks was computed for each participant. Fisher’s r to z transformation was applied to the correlation coefficients for each participant prior to averaging. Separate 2 (Syntactic Complexity) × 3 (Working Memory Load) ANOVAs were computed within and between each of the 4 functional networks, with Nelson-Denny and reading span scores as covariates. Syntactic complexity and working memory load were within-subjects variables. All effects were tested at a significance level of P < 0.05.
Results
Behavioral Results
Accuracy in the responses to the recognition probes across conditions was relatively high (M = 89%; range = 82–98%), as participants were trained prior to participating in the fMRI experiment, and the sample included only participants with an overall accuracy greater than 80%. These high accuracies assured that the activation data reflected complete and accurate processing of the task. All participants made more errors on nonword memory items than word items, resulting in a main effect of the type of working memory load (F1,24 = 7.91; Mean Square Error (MSE) = 266.5). This effect was greater in lower vocabulary individuals, for whom the nonword items generated a greater increase in errors than for the higher vocabulary individuals. This resulted in a reliable vocabulary by working memory load interaction (F1,24 = 7.645; MSE = 266.5). There were no reliable effects in the response times to memory item probes, nor in the response times or accuracies to sentence probes. Mean error rates and response times to probes as a function of syntactic complexity and working memory load are shown in Table 2.
Table 2.
Response times (RTs) and error rates (standard deviation [SD] in parenthesis) to comprehension probes as a function of syntactic complexity and working memory load
| No Load |
Low Load |
High Load |
||||
| Sentence alone |
Sentence + word |
Sentence + nonword |
||||
| RT (SD) | Error (SD) | RT (SD) | Error (SD) | RT (SD) | Error (SD) | |
| Active conjoined | ||||||
| Sentence | 2119 (78) | 8.2 (1.9) | 2389 (96) | 11.9 (2.9) | 2391 (94) | 7.4 (2.6) |
| Memory item | — | — | 1833 (89) | 8.1 (2.5) | 2154 (98) | 14.8 (3.5) |
| Object relative | ||||||
| Sentence | 2144 (99) | 5.2 (1.8) | 2525 (106) | 11.9 (2.7) | 2492 (106) | 11.9 (3.1) |
| Memory item | — | — | 1959 (89) | 8.1 (2.4) | 2099 (100) | 21.1 (5.3) |
Distribution of Activation
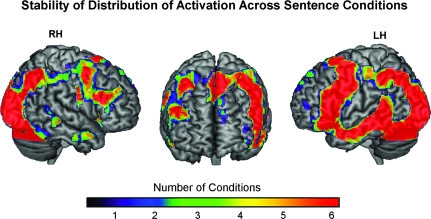
Each of the 6 sentence comprehension conditions resulted in activation of broadly distributed networks including the dominant LH language regions and their RH homologues, as well as frontal, parietal, occipital, and subcortical regions. Comprehension of these sentences was computationally demanding, as preexisting semantic relationships did not facilitate pairings of agents and actions and resulted in rather extensive activation clusters. While the level of activation in given regions changed significantly across conditions and individuals, the set of activated areas was similar across conditions. This large overlap in distribution of activation across the 6 conditions is depicted in Figure 2, with red indicating voxels that were active in every condition and blue indicating voxels that were active in only one of the 6 conditions.
Figure 2.
Activation map showing the large amount of overlap in voxels activated across the 6 sentence comprehension conditions. Red regions indicate voxels that were consistently activated in each condition, whereas blue regions show voxels that were only active in one condition.
Variation in Syntactic Adaptability
Individuals with higher working memory capacities showed better syntactic adaptability (recruitment of additional resources with increasing syntactic complexity) than did individuals with lower working memory capacities. This resulted in a positive correlation between working memory capacity and the contrast of complex–simple sentence activation across extrinsic demand conditions, in a network of control and memory regions (including prefrontal cortex, striatum, hippocampus, and precuneus). These results are consistent with our previous findings indicating that high-capacity individuals showed greater neural adaptability to lexical frequency manipulations (also in prefrontal cortex and striatum) than did low-capacity readers (Prat et al. 2007). There was also one small cluster in the right paracentral lobule that showed a positive correlation between vocabulary size and syntactic adaptability. MNI coordinates, Brodmann’s areas, and peak T values of clusters of activation where syntactic adaptability was positively correlated with working memory span and vocabulary size are listed in Table 3.
Table 3.
Positive correlations between working memory span, vocabulary size, and syntactic adaptability
| Cortical Region | Peak BA | Cluster size | Peak T value | MNI coordinates |
||
| x | y | z | ||||
| Positive correlation between working memory capacity and syntactic adaptability | ||||||
| LH superior frontal | 6 | 16 | 3.78 | −16 | 6 | 58 |
| RH superior frontal/precentral | 6 | 12 | 3.85 | 36 | −12 | 56 |
| LH caudate/putamen | 70 | 4.69 | −20 | 20 | −2 | |
| RH caudate | 26 | 4.21 | 18 | 18 | 8 | |
| RH caudate/cingulum | 202 | 5.35 | 16 | 14 | 24 | |
| LH hippocampus | 28 | 4.02 | −34 | −36 | −4 | |
| LH precuneus | 7 | 16 | 3.76 | −10 | −68 | 50 |
| RH insula | 13 | 194 | 4.88 | 16 | −36 | 24 |
| RH paracentral | 5 | 14 | 4.12 | 18 | −40 | 52 |
| Positive correlation between vocabulary size and syntactic adaptability | ||||||
| RH paracentral | 4 | 28 | 4.38 | 2 | −38 | 62 |
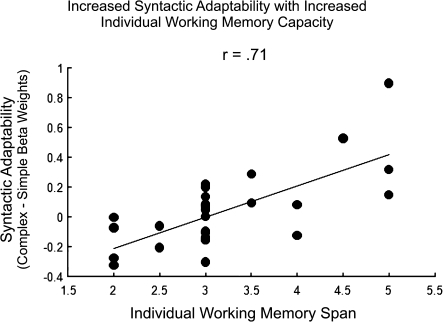
To quantify the relation between working memory capacity and syntactic adaptability across conditions, beta weights (for the contrast of all complex minus all simple sentence activation) were extracted for each voxel over the regions in which there were reliable correlations between working memory capacity and the activation difference between complex and simple sentences. These regions were 9 spherical ROIs with a 6-mm diameter (corresponding to 1 voxel in native space) whose centroids were the peaks of the clusters obtained in the whole-brain correlation between working memory capacity and complex–simple activation differences, as listed in Table 3. Average beta weights were calculated within each region and then averaged across the 9 regions for each individual. The resulting indices of syntactic adaptability across the network of areas were highly correlated with individual differences in working memory capacity (r27 = 0.71). A scatterplot depicting this relation of increasing neural adaptability with increasing working memory capacity (averaged across the 6 ROIs) is shown in Figure 3.
Figure 3.
Scatterplot depicting the relation between average syntactic adaptability (mean complex—simple peak beta weights) and individual working memory capacity.
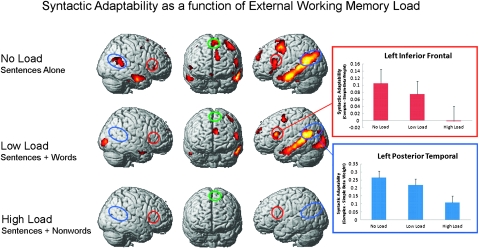
Syntactic adaptability decreased in all participants as the extrinsic working memory demands of the task increased. In the No Load condition, where sentences were presented alone, syntactically complex sentence comprehension resulted in greater activation than syntactically simple sentences throughout the LH language regions (including Broca’s and Wernicke’s areas), as well as in the RH homologues of these regions and in left middle and superior frontal gyri. In the Low Load condition (sentences preceded by to-be-remembered words), syntactically complex sentences again resulted in more activation in the LH language regions (and in the medial frontal region) than simple sentences but with a smaller effect size than in the No Load condition. In the High Load condition (sentences preceded by to-be-remembered nonwords), there were no reliable differences in activation evoked by syntactically simple versus complex sentences (It is possible that the greater adaptability observed in the No Load condition vs. the Low and High Load conditions arose, in part, because of improved modeling of the hemodynamic response for sentences when they were not preceded by memory items. If this is true, however, it does not explain why the largest reduction in adaptability was observed between the Low and High Load conditions, which both constituted complex trials with memory items preceding the sentences.). Activation maps depicting the syntactic adaptability effect (activation in syntactically complex minus syntactically simple sentences) as a function of external working memory load (increasing down the 3 rows) are shown in Figure 4. Also shown in Figure 4 are bar graphs depicting the average beta weights across individuals for the contrast of complex minus simple sentences in the 3 working memory conditions in left inferior frontal (Broca’s) and posterior temporal (Wernicke’s) ROIs. MNI coordinates, Brodmann’s areas, and peak T values of the regions of increased activation to syntactically complex sentences are listed in Table 4.
Figure 4.
Activation maps show regions of increased activation for complex versus simple syntax in each of the 3 working memory load conditions. Inferior frontal regions (including Broca’s area and its RH homologue) are circled in red, superior posterior temporal regions (including Wernicke’s area and its RH homologue) are circled in blue, and the medial frontal region is circled in green. The bar graphs depict mean signal change (syntactically complex—simple sentence beta weights) averaged across LH inferior frontal (red) and posterior temporal (blue) ROIs in each of the 3 working memory load conditions (error bars show standard error of the mean).
Table 4.
Significant activation differences between complex and simple sentences as a function of working memory load
| Cortical region | Brodmann’s areas | Cluster size | Peak T value | MNI coordinates |
||
| x | y | z | ||||
| Sentences alone: complex > simple | ||||||
| LH inferior frontal | 44, 45 | 491 | 4.49 | −44 | 16 | 14 |
| LH insula, inferior frontal | 47 | 280 | 4.87 | −26 | 30 | −2 |
| LH middle frontal/precentral | 6 | 1247 | 6.54 | −40 | 4 | 48 |
| LH superior frontal, bilateral SMA | 6 | 564 | 6.62 | −6 | 10 | 64 |
| LH superior/medial frontal | 10 | 289 | 4.84 | −12 | 58 | 24 |
| Bilateral medial orbital frontal | 11 | 29 | 3.96 | 6 | 50 | −16 |
| LH middle/superior temporal, precuneus | 21, 22, 39 | 12, 767 | 9.42 | −58 | −10 | −20 |
| RH inferior frontal, insula, putamen | 13 | 383 | 4.49 | 36 | 26 | 12 |
| RH inferior frontal | 45, 47 | 43 | 3.29 | 54 | 30 | 0 |
| RH middle frontal/precentral | 6 | 29 | 2.97 | 34 | −2 | 44 |
| RH anterior middle/inferior temporal | 20, 21 | 634 | 6.19 | 50 | −10 | −26 |
| RH middle/superior temporal, angular | 21, 37, 39 | 572 | 4.21 | 50 | −46 | 10 |
| RH fusiform | 36, 37 | 209 | 4.31 | 26 | −32 | −26 |
| LH parahippocampus/hippocampus | 28 | 23 | 3.00 | −12 | −12 | −18 |
| RH hippocampus, amygdala | 28 | 26 | 3.13 | 22 | −6 | −14 |
| RH caudate nucleus | 28 | 3.06 | 8 | 12 | 14 | |
| RH thalamus, pallidum | 34 | 363 | 3.83 | 8 | −2 | −8 |
| LH rolandic operculum, Heschl’s | 13 | 20 | 3.09 | −30 | −32 | 14 |
| LH cerebellum | 15 | 3.04 | −10 | −60 | −28 | |
| Sentences + words: complex > simple | ||||||
| LH inferior frontal | 44, 45 | 334 | 6.00 | −56 | 22 | 16 |
| LH middle frontal/precentral | 6 | 201 | 4.34 | −34 | 0 | 64 |
| LH superior/medial frontal | 10 | 200 | 4.23 | −8 | 58 | 24 |
| LH middle/superior temporal, angular | 21, 22, 39 | 3303 | 7.74 | −54 | −40 | 0 |
| LH precuneus | 7 | 496 | 4.95 | −10 | −52 | 40 |
| LH middle occipital | 19 | 73 | 3.75 | −28 | −84 | 24 |
| LH occipital/fusiform | 17, 18, 19, 37 | 1380 | 5.97 | −12 | −86 | 0 |
| RH anterior middle temporal | 21 | 57 | 4.59 | 56 | 2 | −28 |
| RH occipital/fusiform | 17, 18 | 358 | 5.03 | 40 | −90 | 0 |
| RH occipital | 17 | 114 | 3.7 | 8 | −82 | 6 |
| Sentences + words: simple > complex | ||||||
| LH middle frontal | 10, 46 | 177 | 5.62 | −42 | 54 | 4 |
| RH inferior parietal | 40 | 141 | 5.3 | 48 | −54 | 46 |
| RH middle/orbital frontal | 10, 11, 46 | 281 | 5.52 | 40 | 46 | −8 |
In the Low Load condition only, activation in bilateral inferior occipital regions increased for complex versus simple sentences. This increase may have occurred because readers were instructed to generate images from the to-be-remembered words and hold them in mind during the sentences; the comprehension of complex sentences may have increased the difficulty of concurrently maintaining the images. There was only one case, in the Low Load condition, in which there was more activation (in bilateral middle frontal and RH inferior parietal regions) for simple than complex sentences. This finding may be attributable to a greater availability of resources for maintenance of the generated mental image of the to-be-remembered words during comprehension of the simple sentences. These regions are described in Table 4.
Each of the regions that showed reduced adaptability with decreased individual working memory capacity, with the exception of the caudate nucleus, also showed decreased adaptability with increased external working memory demands. Taken together, these results indicate that the ability to recruit additional resources in the face of increasing syntactic processing demands decreases as resource availability decreases.
Network Synchronization
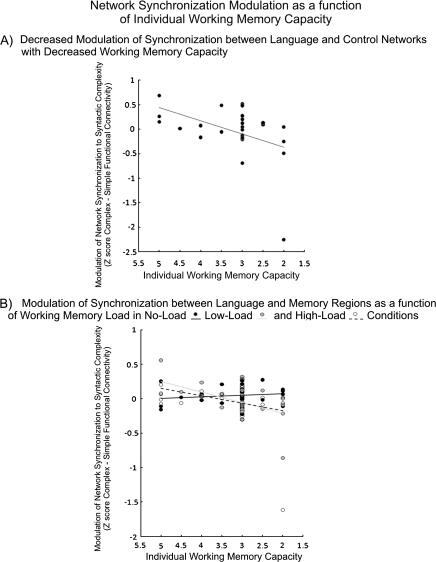
Network functional connectivity analyses showed that individuals with higher working memory capacities were better able to maintain or increase cortical synchronization with increasing syntactic complexity than were individuals with lower working memory capacities. This was reflected by a reliable interaction in the synchronization measure within executive control regions between Syntactic Complexity and Working Memory Capacity. The same 2 variables had the same interactive effect on the synchronization between executive control regions and LH language regions. These findings suggest that differences in working memory capacity may be driven by the ability of executive control areas of higher capacity individuals to maintain or increase synchronization with relevant cortical processing centers as task demands increase. This was especially true in the Low and High Load conditions, resulting in reliable Syntactic Complexity × Working Memory Capacity × Working Memory Load interactions between LH language and memory regions, between RH language and memory regions, between LH and RH language regions, and within RH language regions. These results suggest that synchronization between LH language regions and their RH homologues and memory regions is particularly important when language processing occurs under high demands and that high-capacity readers are better able to maintain such synchronization than are lower capacity readers. Between LH language and memory regions, the mean synchronization increased with syntactic complexity only in the No Load condition, resulting in a Syntactic Complexity × Working Memory Load interaction. The increase in synchronization at the group level only in the No Load condition may have occurred because only the high-capacity readers had the ability to increase synchronization during Low and High Load conditions (as indicated by the Syntactic Complexity × Working Memory Capacity × Working Memory Load interaction described above). Scatterplots depicting (A) changes in synchronization between language and control networks for increased syntactic complexity as a function of working memory capacity and (B) between language and memory regions as a function of both working memory demand and working memory capacity are shown in Figure 5. ANOVA and follow-up statistics for network analyses are summarized in Table 5. Individual differences in vocabulary were not related to variability in network synchronization.
Figure 5.
Scatterplots depicting modulation of synchronization (functional connectivity in syntactically complex–simple sentences) between (A) the language and control networks across conditions, a function of individual working memory capacity, and (B) between the language and memory networks as a function of individual working memory capacity in the No Load, Low Load, and High Load conditions.
Table 5.
ANOVA statistics for network connectivity analyses
| Networks | df | F | MSE | Follow-up analyses |
| Syntactic complexity × capacity | Correlation: WM capacity and (complex–simple) | |||
| Control (within) | 1, 24 | 4.28 | 0.036 | r = 0.44** |
| Control-LH language | 1, 24 | 4.39 | 0.037 | r = 0.44** |
| Load × syntactic complexity | Complex–simple in 3 WM Load Conditions | |||
| LH language–memory | 2, 23 | 3.45 | 0.022 | t = 1.67* (no), t = −0.58 (low), t = −0.65 (high) |
| Load × syntactic complexity × capacity | Correlation: WM and (complex–simple) by Load | |||
| LH language–memory | 2, 23 | 7.17 | 0.022 | r = −0.14 (no), r = 0.55** (low), r = 0.27 (high) |
| LH-RH language | 2, 23 | 3.81 | 0.022 | r = −0.10 (no), r = 0.50** (low), r = 0.35* (high) |
| RH language–memory | 2, 23 | 4.04 | 0.021 | r = −0.16 (no), r = 0.48** (low), r = 0.34* (high) |
| RH language (within) | 2, 23 | 4.14 | 0.03 | r = 0.06 (no), r = 0.53** (low), r = 0.33* (high) |
Note: Probabilities for follow-up: *P < 0.10, **P < 0.05. df, degrees of freedom.
Neural Efficiency
Higher vocabulary individuals showed more efficient use of (i.e., less activation in) frontal cortical regions during sentence comprehension across conditions than did lower vocabulary individuals. The resulting negative correlations between Nelson-Denny Vocabulary scores and neural activation were observed in bilateral inferior and middle frontal gyri and in the superior medial frontal region. No positive correlations between vocabulary size and activation were observed.
The relation between working memory capacity and neural efficiency was not as straightforward as the relation between increased vocabulary and increased neural efficiency. Both positive correlations (in left orbital and right superior frontal regions) and negative correlations (in the right parahippocampal region) were found. MNI coordinates, Brodmann’s areas, and peak T values for clusters of activation that reliably correlated with vocabulary and working memory capacity are listed in Table 6.
Table 6.
Correlations between reading skill, working memory capacity, and activation across sentence types
| Cortical region | Peak, BA | Cluster size | Peak T value | MNI coordinates |
||
| x | y | z | ||||
| Negative correlation between reading skill and sentence activation | ||||||
| LH middle/inferior frontal | 9 | 85 | 4.50 | −28 | 44 | 10 |
| RH inferior frontal | 46 | 42 | 4.10 | 52 | 22 | 28 |
| LH inferior frontal | 47 | 18 | 4.5 | −58 | 20 | −2 |
| RH middle frontal | 10 | 12 | 4.09 | 32 | 44 | 4 |
| Superior/medial frontal, SMA | 6 | 45 | 4.47 | −4 | 6 | 66 |
| Negative correlation between working memory capacity and sentence activation | ||||||
| RH parahippocampal | 36 | 19 | 4.03 | 14 | −2 | 20 |
| Positive correlation between working memory capacity and sentence activation | ||||||
| LH inferior orbital frontal | 11 | 32 | 4.58 | −20 | 26 | −12 |
| RH superior frontal | 6 | 14 | 3.94 | 20 | 0 | 70 |
Discussion
The Neural Correlates of Reading Experience and Dynamic Information Processing
To our knowledge, this experiment represents the first attempt to independently characterize the neural underpinnings of a specific index of word knowledge (vocabulary) and a general measure of cognitive capacity (verbal working memory) during sentence comprehension. The results suggest that individual differences in word knowledge are primarily related to the total amount of resources utilized, whereas individual differences in working memory capacity are primarily related to dynamic facets of brain function. The separable characteristics of word knowledge and working memory capacity described here help to shed light on the complexities of the neural foundations of comprehension abilities.
Readers with better word knowledge showed greater neural efficiency (less activation) across task conditions than did readers with lower vocabulary scores in the current experiment. These results are consistent with Maxwell’s seminal investigation of reading skill and efficiency (Maxwell et al. 1974) as well as with a more recent investigation of individual differences in inferential processes (Prat et al. forthcoming). We propose that this increased efficiency is related to increased reading experience in the high-vocabulary individuals. The correlation between increased print exposure and increased vocabulary has been well established in the literature (Stanovich and West 1989; Stanovich 1993; Stanovich et al. 1995; Acheson et al. 2008; Long et al. 2008). Thus, the task of encoding and comprehending words and sentences is more practiced by and should be less demanding of processing activity for experienced readers. This reduction in processing is indexed by reduced neural activation. Neuroimaging studies provide direct evidence of such increased neural efficiency with training. For example, practice with mirror reading paradigms results in reduced activation in the right superior parietal regions associated with the mental transformations required for mirror reading (Poldrack et al. 1998; Kassubek et al. 2001; Poldrack and Gabrieli 2001; Ilg et al. 2008), whereas practice with Tetris, a visuospatially demanding video game, results in decreased activation primarily in frontal regions (Haier et al. 2009). It is plausible, therefore, that the observed reduction of activation in the frontal regions of skilled readers results from increased reading experience.
The relation between individual working memory capacity and neural efficiency is less clear. Rypma and Prabhakaran (2009) found that the relation between working memory capacity and efficiency varied across brain regions and a function of the nature of the task and hypothesized that increased activation in frontal regions during complex tasks may result in better binding and faster and more efficient retrieval at a later stage of processing. Our results may reflect this to some extent, as increased working memory capacity was associated with both increased activation in prefrontal control regions and with decreased activation in parahippocampal memory regions. Our previous investigation of individual differences in sentence comprehension found increased neural efficiency as a function of increased individual working memory capacity (Prat et al. 2007). A possible explanation for the difference between these and our previous results is that our previous results did not account for differences in reading experience. Prat et al. (2007) compared extreme working memory capacity groups, increasing the likelihood that high-capacity readers also differed in reading experience from low-capacity readers. Thus, individual differences in reading experience may explain the efficiency differences observed in our previous study. The current study investigated individuals across a broad range of working memory span scores. In these participants, working memory capacity was not correlated with vocabulary size (r27 = −0.15, P = 0.47). The lack of a correlation between working memory capacity and vocabulary in this sample allows us to separately consider the influence of the 2 factors. In the current study, vocabulary size was a better and more consistent predictor of neural efficiency than was working memory capacity. These results suggest that increased neural efficiency in frontal regions with increased vocabulary size may result from less-effortful encoding of materials associated with increased reading experience.
Working memory capacity, on the other hand, was primarily related to dynamic facets of network function. Higher capacity readers showed a better ability to recruit additional resources while maintaining or increasing synchronization between regions than did lower capacity readers when the syntactic complexity of tasks increased under a variety of extrinsic working memory demands. Our previous research similarly found that high-capacity readers showed greater recruitment of resources with increasing lexical processing demands (decreased lexical frequency) and better maintenance or increase of synchronization with increasing lexical and syntactic processing demands (Prat et al. 2007). Taken together, these studies suggest that individual differences in the ability to dynamically configure and coordinate neural resources with changes in task demands are important for reading comprehension ability and are related to differences in working memory capacity.
It is likely that the observed individual differences in dynamic network configuration and synchronization are not specific to language comprehension tasks but may reflect more general fluid information processing abilities. The demands imposed by complex cognitive tasks are dynamic in nature, changing both qualitatively and quantitatively from moment to moment. It follows that a neural system that can effectively adapt by recruiting resources on an as-needed basis will perform better than one that is less flexible, particularly when the task is more challenging. As discussed previously, psychometric indices of verbal working memory capacity correlate very highly with indices of reasoning and fluid information processing ability (e.g., Kyllonen and Christal 1990; Engle et al. 1999), and neuroanatomical investigations have shown considerable overlap in the cortical regions correlated with individual differences in working memory capacity and intelligence (Colom et al. 2007). Early electrophysiological experiments showed such increasing neural adaptability with increased IQ (e.g., Shucard and Horne 1973; Schafer 1982). Garlick’s (2002) model of information processing in parallel distributed networks highlighted the importance of neural adaptability for intelligent behavior. In addition, the parietofrontal integration theory of intelligence (Jung and Haier 2007) highlights the importance of synchronization of function between frontal and posterior regions for intelligent behavior. Our results are consistent with this theory and extend it by showing that modulation of synchronization between frontal and parietal regions (which were included in our “control” network) increased with increasing working memory capacity. In summary, our finding that high-capacity readers are able to dynamically recruit and synchronize neural networks with changing task demands is likely a reflection of their improved general information processing abilities. One prediction that can be derived from these characterizations of our results is that the experientially dependent neural efficiency results should be observed only in learned verbal tasks, whereas the relation between working memory capacity and neural adaptability and synchronization should apply to novel tasks and a broad range of domains. We view this as an avenue for future research.
Neural Adaptability and Resource Availability
These results extend our previous findings showing that adaptability or dynamic recruitment of neural resources in the face of increasing task demands is one of the defining characteristics of mental abilities (Garlick 2002; Newman et al. 2005; Prat et al. 2007). One of the goals of this study was to investigate the mechanisms underlying individual differences in neural adaptability. One possible account of the phenomenon is that individual differences in adaptability arise because more efficient processes in baseline conditions (requiring a smaller proportion of the resources) result in greater availability of resources for recruitment when demands increase. Another account is that individual differences in adaptability arise because of systematic variability between individuals in cortical dynamics. These findings provide support for both accounts.
The first account predicts that neural adaptability will decrease as resource availability decreases. The current study found that all individuals showed decreased adaptability to syntactic demands when the extrinsic working memory task was added (decreasing resource availability) and no adaptability to syntactic demands in the High Load condition (which presumably exhausted resource availability). In addition, we replicated our previous findings by showing that low-capacity individuals (who presumably have fewer resources available) showed poorer adaptability to syntactic demands across working memory loads. Taken together, these results provide evidence that neural adaptability is related to resource availability and that individual differences in adaptability are therefore related to individual differences in resource availability.
The individual differences in neural adaptability observed cannot, however, be fully explained by resource availability. First, factors that predicted neural efficiency were different from those that predicted neural adaptability. For example, these results showed that individuals with higher reading skills exhibited more efficient use of frontal cortical resources than did individuals with lower reading skills. Based on the resource availability account, this should also generate an environment for greater syntactic adaptability in frontal regions for highly skilled readers due to a greater availability of remaining resources. However, increased adaptability with increased reading skill was observed in only one small cluster of activation in the RH paracentral region. Similarly, high-capacity individuals showed reliably greater syntactic adaptability in prefrontal cortex and in the striatum, although no reliable differences in neural efficiency were detected in these regions. Additionally, we did not find any reliable differences in the correlations between individual working memory capacity and adaptability as working memory load increased. In fact, the relation between adaptability and working memory capacity was essentially the same in the High Load and No Load conditions. Thus, although resource availability is clearly a necessary condition for neural recruitment, our data suggest some other facet of brain function that underlies cortical dynamics may also systematically differ between individuals. Below we describe how the cofunctioning of the prefrontal cortex and the striatum may contribute to one such mechanism.
Neural Adaptability and Cortical Information Transfer
In 2 investigations of individual differences in sentence comprehension, we have shown that higher working memory capacity individuals show greater modulation of the amount of activation in the prefrontal cortex and the striatum in the face of changing linguistic demands (e.g., syntactic complexity and lexical frequency) than do lower working memory capacity individuals. These regions have been extensively reported as activating in neuroimaging investigations of working memory (e.g., Braver et al. 1997; Rypma et al. 1999; Lewis et al. 2004). With respect to language comprehension processes, researchers have claimed that the striatum (specifically the caudate nuclei) is involved in the recruitment of controlled processes (Friederici 2006), explaining why activation is found during linguistic tasks, especially complex ones (e.g., Crinion et al. 2006; Mason and Just 2007; Ketteler et al. 2008).
Of particular interest are a class of theories that propose that the striatum serves a general role in routing information throughout the cortex (Gurney et al. 2001; O'Reilly and Frank 2006; Stocco et al. 2010). Although these theories differ in their proposed mechanisms, the consensus among them is that the striatum functions like a gate, receiving input from all the cortical regions, working in collaboration with the prefrontal cortex (which receives most of the outputs from the striatum) to select which signals should pass between cortical regions, limiting interference and ensuring that selected representations will transfer to appropriate cortical centers.
In this study, we found that high-capacity readers showed greater recruitment of both striatum and prefrontal regions with increased syntactic complexity. We also showed greater increases in synchronization within control regions and between control and language regions with increased syntactic complexity. These results suggest that improved prefrontal–striatal communication may be a mechanism modulating the neural adaptability that underpins cognitive abilities in high-working memory capacity individuals.
In light of current theories of the role of the striatum in information transfer, it is possible that adaptability results such as those observed in prefrontal and caudate regions in this experiment may be related to individual differences in the ease with which relevant information can be dynamically transferred to cortical processing centers and that this ability underpins individual differences in general mental abilities that require maintenance and manipulation of information, such as working memory capacity and fluid intelligence.
Summary
In summary, understanding how neural systems adapt in the face of changing task demands is an important, yet often overlooked, determinant of individual differences in performance in cognitive tasks. Such dynamic network configuration and synchronization properties are related to individual differences in verbal working memory capacity, and we propose that they reflect a more general fluidity in information processing capabilities. In addition, more specific indices of verbal abilities or experience on a given task are reflected by the total amount of neural resources used to complete the task. Applying these definitions, we may be able to determine how individual ability (or inability) to perform a task arises from an interaction of general information processing abilities and experience with a particular task or class of tasks. In addition, these findings suggest that neural adaptability is related to, but somewhat separable from, resource availability.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Institute of Mental Health (grant MH029617); National Institute on Deafness and Communication Disorders (grant DC009634).
Supplementary Material
Acknowledgments
We would like to thank Jennifer Moore, Andrea Stocco, and the members of the Center for Cognitive Brain Imaging reading group for their helpful comments on this manuscript. Conflict of Interest: None declared.
References
- Acheson DJ, Wells JB, MacDonald MC. New and updated tests of print exposure and reading abilities in college students. Behav Res Methods. 2008;40:278–289. doi: 10.3758/brm.40.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell LC, Perfetti CA. Reading skill: some adult comparisons. J Educ Psychol. 1994;86:244–255. [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Chein JM, Morrison AB. Expanding the mind's workspace: training and transfer effects with a complex working memory span task. Psychon Bull Rev. 2010;17:193–199. doi: 10.3758/PBR.17.2.193. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ. General intelligence and memory span: evidence for a common neuroanatomic framework. Cogn Neuropsychol. 2007;24:867–878. doi: 10.1080/02643290701781557. [DOI] [PubMed] [Google Scholar]
- Crinion J, Turner R, Grogan A, Hanakawa T, Noppeney U, Devlin JT, Price CJ. Language control in the bilingual brain. Science. 2006;312:1537–1540. doi: 10.1126/science.1127761. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter PA. Individual differences in working memory and reading. J Ver Lear Behav. 1980;19:450–466. [Google Scholar]
- Daneman M, Hannon B. Using working memory theory to investigate the construct validity of multiple-choice reading comprehension tests such as the SAT. J Exp Psychol Gen. 2001;130:208–223. doi: 10.1037//0096-3445.130.2.208. [DOI] [PubMed] [Google Scholar]
- Daneman M, Merikle PM. Working memory and language comprehension: a meta-analysis. Psychon Bull Rev. 1996;3:422–433. doi: 10.3758/BF03214546. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway RA. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Fedorenko E, Gibson E, Rohde D. The nature of working memory capacity in sentence comprehension: evidence against domain-specific working memory resources. J Mem Lang. 2006;54:541–553. [Google Scholar]
- Friederici AD. What's in control of language? Nat Neurosci. 2006;9:991–992. doi: 10.1038/nn0806-991. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Garlick D. Understanding the nature of the general factor of intelligence: The role of individual differences in neural plasticity as an explanatory mechanism. Psychol Rev. 2002;109:116–136. doi: 10.1037/0033-295x.109.1.116. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Cognitive approaches to the development of short-term memory. Trends Cogn Sci. 1999;3:410–418. doi: 10.1016/s1364-6613(99)01388-1. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. Dev Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gordon PC, Hendrick R, Johnson M. Memory interference during language processing. J Exp Psychol Learn Mem Cogn. 2001;27:1411–1423. doi: 10.1037//0278-7393.27.6.1411. [DOI] [PubMed] [Google Scholar]
- Gordon PC, Hendrick R, Levine WH. Memory-load interference in syntactic processing. Psychol Sci. 2002;13:425–430. doi: 10.1111/1467-9280.00475. [DOI] [PubMed] [Google Scholar]
- Gurney K, Prescott TJ, Redgrave P. A computational model of action selection in the basal ganglia. I. A new functional anatomy. Biol Cybern. 2001;84:401–410. doi: 10.1007/PL00007984. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Sherif K, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2:1–7. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Siegel BV, Nuechterlein KH, Hazlett E, Wu JC, Paek J, Browning H, Buchsbaum MS. Cortical glucose metabolic-rate correlates of abstract reasoning and attention studied with Positron Emission Tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- Hannon B, Daneman M. A new tool for measuring and understanding individual differences in the component processes of reading comprehension. Br J Educ Psychol. 2001;93:103–128. [Google Scholar]
- Horn JL, Cattell RB. Age differences in fluid and crystallized intelligence. Acta Psychol. 1967;26:107–129. doi: 10.1016/0001-6918(67)90011-x. [DOI] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The parieto-frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007;30:135–187. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: individual differences in working memory. Psychol Rev. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Just MA, Varma S. The organization of thinking: what functional brain imaging reveals about the neuroarchitecture of complex cognition. Cogn Affect and Behav Neurosci. 2007;7:153–191. doi: 10.3758/cabn.7.3.153. [DOI] [PubMed] [Google Scholar]
- Kassubek J, Schmidtke K, Kimmig H, Lucking CH, Greenlee MW. Changes in cortical activation during mirror reading before and after training: an fMRI study of procedural learning. Brain Res Cogn Brain Res. 2001;10:207–217. doi: 10.1016/s0926-6410(00)00037-9. [DOI] [PubMed] [Google Scholar]
- Ketteler D, Kastrau F, Vohn R, Huber W. The subcortical role of language processing: high level linguistic features such as ambiguity-resolution and the human brain; an fMRI study. Neuroimage. 2008;39:2002–2009. doi: 10.1016/j.neuroimage.2007.10.023. [DOI] [PubMed] [Google Scholar]
- King J, Just MA. Individual differences in syntactic processing: the role of working memory. J Mem Lang. 1991;30:580–602. [Google Scholar]
- Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci. 2004;19:755–760. doi: 10.1111/j.1460-9568.2004.03108.x. [DOI] [PubMed] [Google Scholar]
- Long DL, Prat C, Johns C, Morris P, Jonathan E. The importance of knowledge in vivid text memory: an individual-differences investigation of recollection and familiarity. Psychon Bull Rev. 2008;15:604–609. doi: 10.3758/PBR.15.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DL, Prat CS. Individual differences in syntactic ambiguity resolution: readers vary in their use of plausibility information. Mem Cognit. 2008;36:375–391. doi: 10.3758/mc.36.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC, Just MA, Carpenter PA. Working memory constraints on the processing of syntactic ambiguity. Cogn Psychol. 1992;24:56–98. doi: 10.1016/0010-0285(92)90003-k. [DOI] [PubMed] [Google Scholar]
- Mason RA, Just MA. Lexical ambiguity in sentence comprehension. Brain Res. 2007;1146:115–127. doi: 10.1016/j.brainres.2007.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell AE, Fenwick PB, Fenton GW, Dollimore J. Reading ability and brain function: a simple statistical model. Psychol Med. 1974;4:274–280. doi: 10.1017/s0033291700042963. [DOI] [PubMed] [Google Scholar]
- Neubauer AJ, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Newman SD, Klatzky RL, Lederman SJ, Just MA. Imagining material versus geometric properties of objects: an fMRI study. Brain Res Cogn Brain Res. 2005;23:235–246. doi: 10.1016/j.cogbrainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Pearlmutter NJ, MacDonald MC. Individual differences and probabilistic constraints in syntactic ambiguity resolution. J Mem Lang. 1995;34:521–542. [Google Scholar]
- Perfetti CA. Reading ability. New York: Oxford University Press; 1985. [Google Scholar]
- Perfetti CA. Reading ability: lexical quality to comprehension. Sci Stud Read. 2007;11:357–383. [Google Scholar]
- Perfetti CA, Hart L. The lexical bases of comprehension skill. 2001. In: Gorfien D, editor. On the consequences of meaning selection 67–86. Washington (DC): American Psychological Association pp. 67–86; 2001. [Google Scholar]
- Poldrack RA, Desmond JE, Glover GH, Gabrieli JDE. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb Cortex. 1998;8:1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Prat CS, Keller TA, Just MA. Individual differences in sentence comprehension: a functional magnetic resonance imaging investigation of syntactic and lexical processing demands. J Cogn Neurosci. 2007;19:1950–1963. doi: 10.1162/jocn.2007.19.12.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat CS, Mason RA, Just MA. Right hemisphere contributions to reading: a multi-experiment individual differences investigation. 2010 Poster, Human Brain Mapp, Barcelona, Spain. [Google Scholar]
- Prat CS, Mason RA, Just MA. Forthcoming. Individual differences in the neural basis of Causal Inferencing. Brain Lang. doi: 10.1016/j.bandl.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle ED, Carpenter PA, Just MA. The neural bases of strategy and skill in sentence-picture verification. Cogn Psychol. 2000;40:261–295. doi: 10.1006/cogp.2000.0733. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: the mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37:207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Schafer EWP. Neural adaptability: a biological determinant of behavioral intelligence. Int J Neurosci. 1982;17:183–191. doi: 10.3109/00207458208985922. [DOI] [PubMed] [Google Scholar]
- Shucard DWM, Horn JL. Evoked potential amplitude change related to intelligence and arousal. Psychophys. 1973;10:445–452. doi: 10.1111/j.1469-8986.1973.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Stanovich KE. Does reading make you smarter? Literacy and the development of verbal intelligence. In: Reese HW, editor. Advances in child development and behavior. Vol. 24. San Diego (CA): Academic Press; 1993. pp. 133–180. [DOI] [PubMed] [Google Scholar]
- Stanovich KE, West RF. Exposure to print and orthographic processing. Read Res Q. 1989;24:402–433. [Google Scholar]
- Stanovich KE, West RF, Harrison MR. Knowledge growth and maintenance across the life span: the role of print exposure. Dev Psychol. 1995;31:811–826. [Google Scholar]
- Stocco A, Lebiere C, Anderson JR. Conditional routing of information to the cortex: a model of the basal ganglia's role in cognitive coordination. Psychol Rev. 2010;117:541–574. doi: 10.1037/a0019077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wanner E, Maratsos M. An ATN approach to comprehension. In: Halle M, Bresnan J, Miller G, editors. Linguistic theory and psychological reality. Cambridge (MA): MIT Press; 1978. pp. 119–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.