Abstract
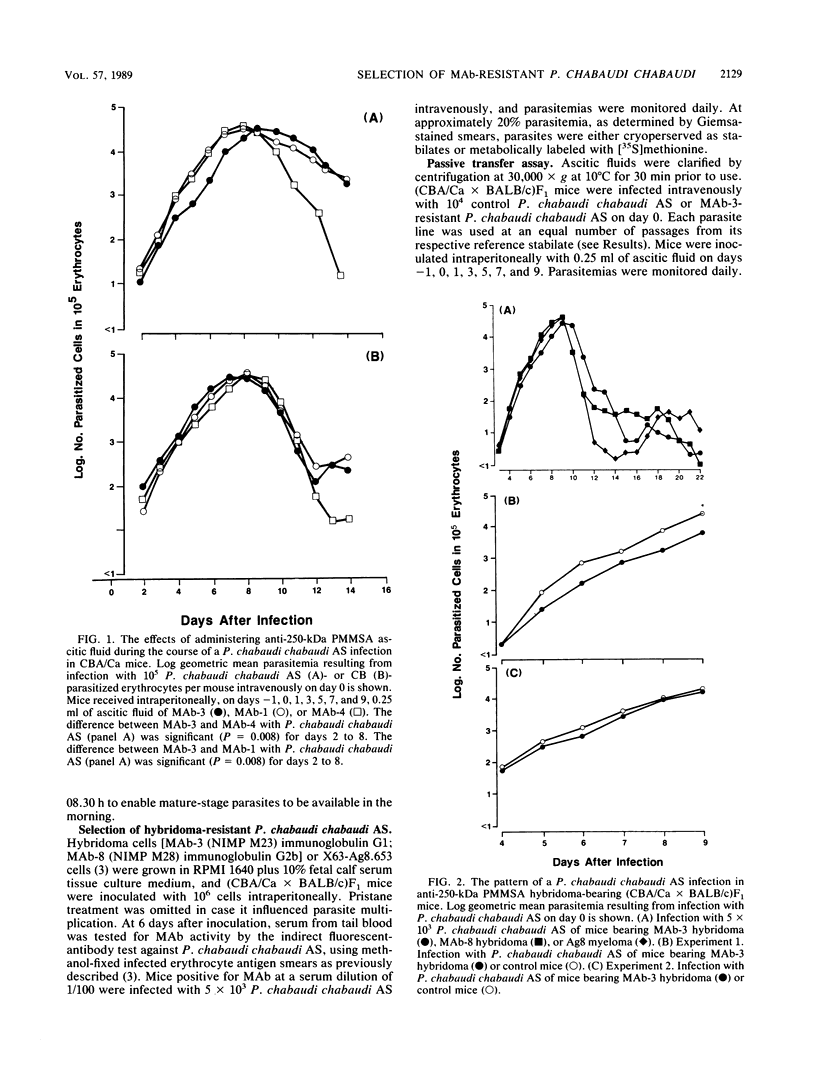
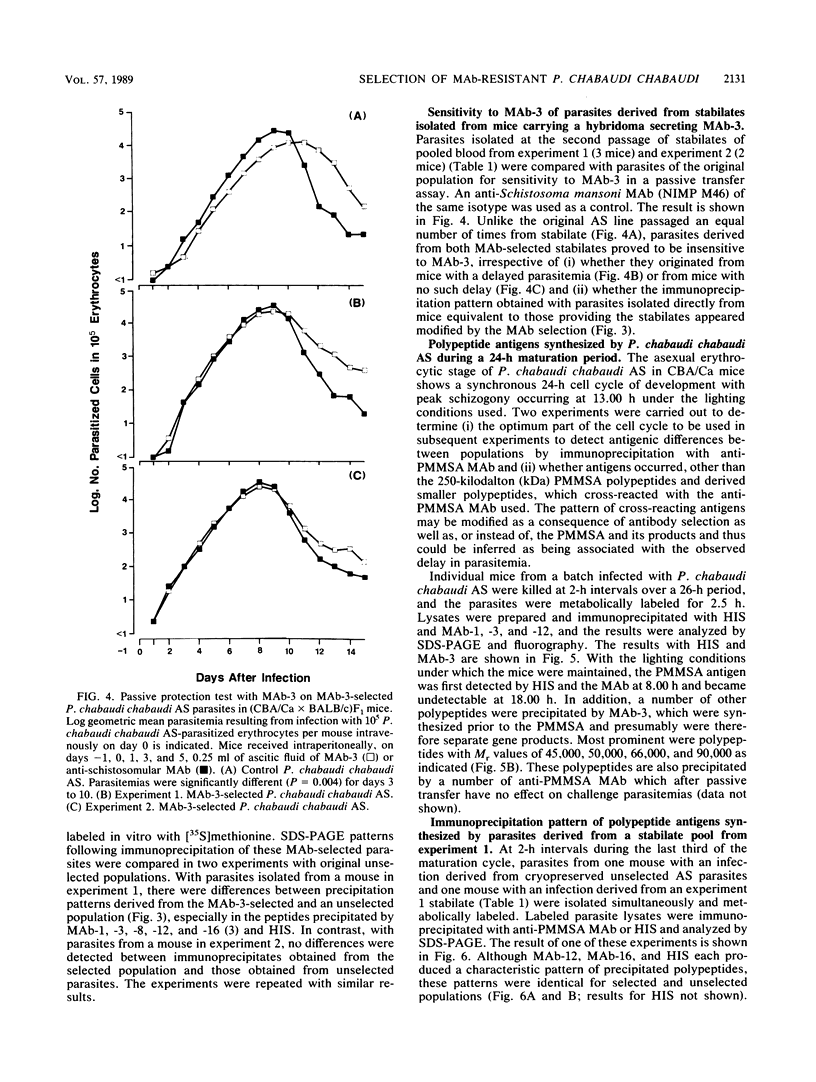
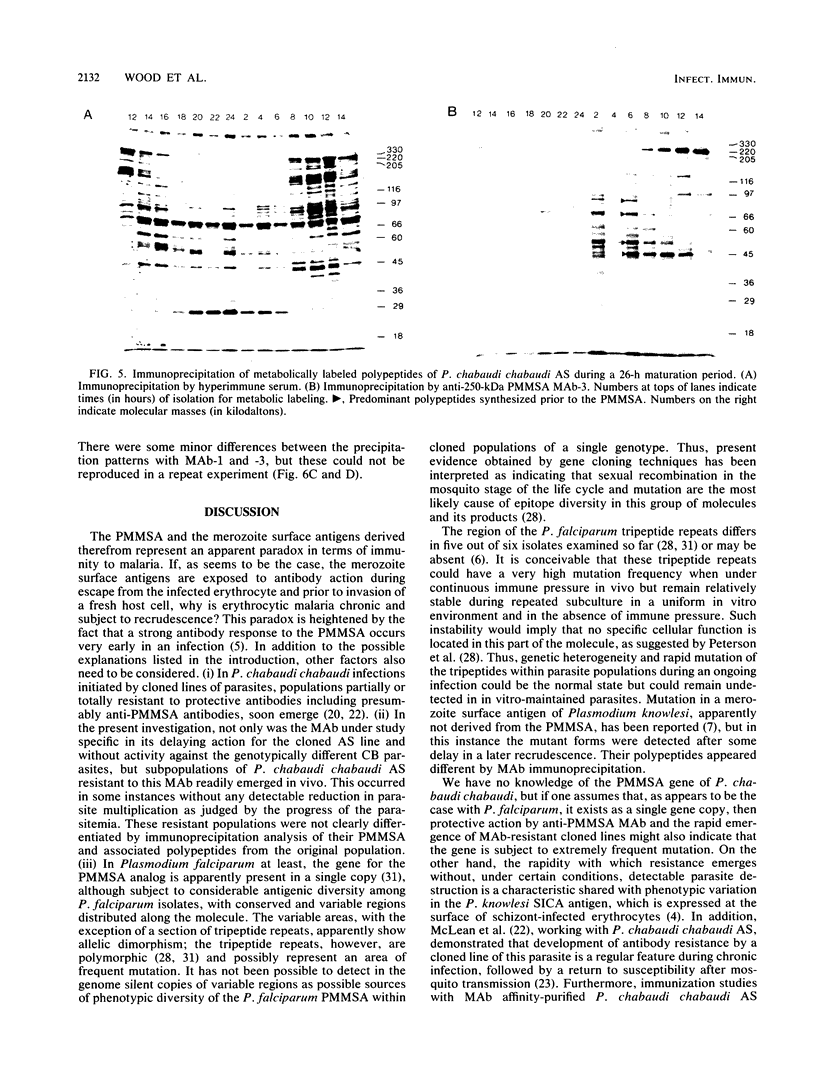
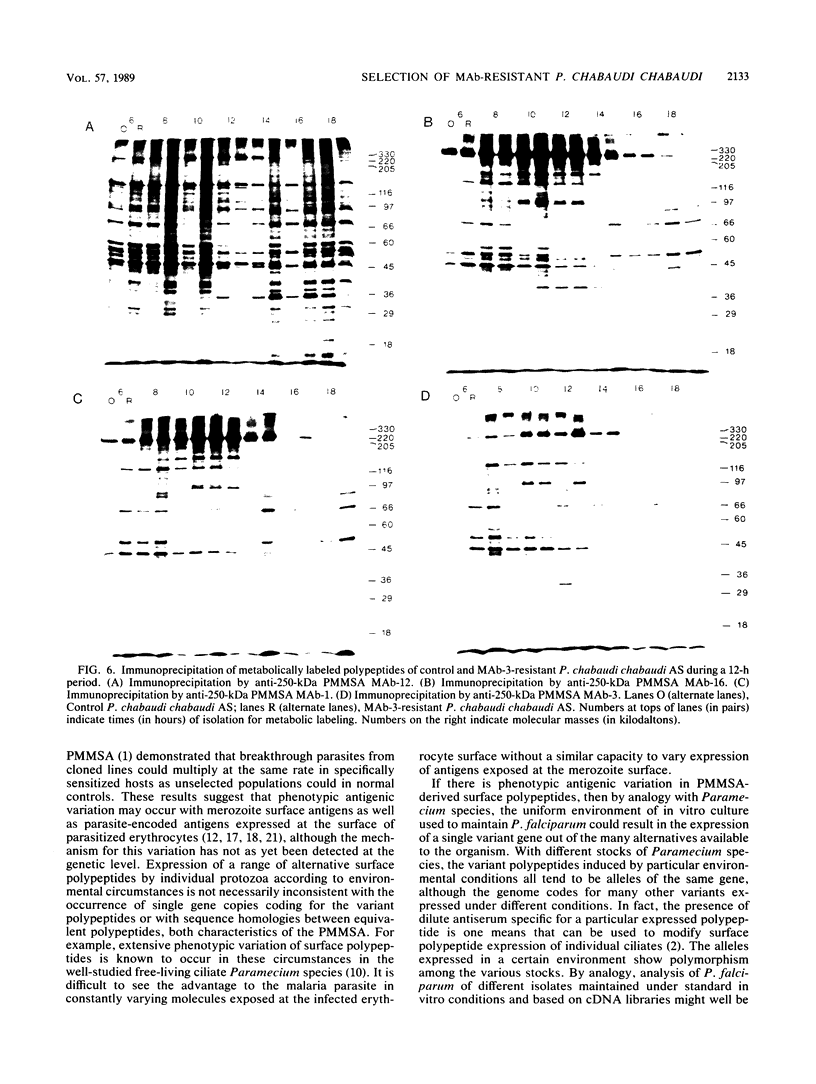
Mice bearing a hybridoma secreting a monoclonal antibody (MAb), MAb-3, which significantly delays the onset of a Plasmodium chabaudi chabaudi AS, but not P. chabaudi chabaudi CB, challenge parasitemia in a passive transfer assay and which is specific for the precursor to the major merozoite surface antigen (PMMSA) of P. chabaudi chabaudi AS, were challenged intravenously with 10(3) P. chabaudi chabaudi AS-parasitized erythrocytes. The resultant parasitemia was very similar to that in normal mice except that initially the parasitemia was sometimes slightly delayed. Parasites derived from cryopreserved stabilates isolated from MAb-3 hybridoma mice with an unmodified parasitemia, or with a delayed parasitemia, were found to have lost their susceptibility to MAb-3 in the passive transfer assay. A number of anti-PMMSA MAb were used to immunoprecipitate lysates of parasite populations isolated directly from hybridoma-bearing mice. In some instances and with certain of the MAb, immunoprecipitation patterns were modified, but other isolates were not detectably different when compared with unselected P. chabaudi chabaudi AS parasites. Using a panel of MAb reacting with the PMMSA of P. chabaudi chabaudi AS, immunoprecipitation patterns of parasites derived from cryopreserved stabilates isolated from hybridoma-bearing mice were determined at 2-h intervals through the appropriate part of the parasite maturation cycle. In these derived populations, resistance to MAb-3 was not associated with a change in the immunoprecipitation reaction with the MAb used. These results are discussed in the context of current knowledge of genotypic and phenotypic antigenic diversity of malaria parasites and other protozoa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates M. D., Newbold C. I., Jarra W., Brown K. N. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi chabaudi in CBA/Ca mice. III. Protective and suppressive responses induced by immunization with purified antigens. Parasite Immunol. 1988 Jan;10(1):1–15. doi: 10.1111/j.1365-3024.1988.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Newbold C. I., Smith C. C., Brown K. N. Monoclonal antibodies that protect in vivo against Plasmodium chabaudi recognize a 250,000-dalton parasite polypeptide. Infect Immun. 1982 Oct;38(1):94–102. doi: 10.1128/iai.38.1.94-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. N. Antibody induced variation in malaria parasites. Nature. 1973 Mar 2;242(5392):49–50. doi: 10.1038/242049a0. [DOI] [PubMed] [Google Scholar]
- Brown K. N., Jarra W., Newbold C. I., Schryer M. Variability in parasite protein antigen structure and protective immunity to malaria. Ann Inst Pasteur Immunol. 1985 Jan-Feb;136C(1):11–23. [PubMed] [Google Scholar]
- Certa U., Rotmann D., Matile H., Reber-Liske R. A naturally occurring gene encoding the major surface antigen precursor p190 of Plasmodium falciparum lacks tripeptide repeats. EMBO J. 1987 Dec 20;6(13):4137–4142. doi: 10.1002/j.1460-2075.1987.tb02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David P. H., Hudson D. E., Hadley T. J., Klotz F. W., Miller L. H. Immunization of monkeys with a 140 kilodalton merozoite surface protein of Plasmodium knowlesi malaria: appearance of alternate forms of this protein. J Immunol. 1985 Jun;134(6):4146–4152. [PubMed] [Google Scholar]
- Epstein N., Miller L. H., Kaushel D. C., Udeinya I. J., Rener J., Howard R. J., Asofsky R., Aikawa M., Hess R. L. Monoclonal antibodies against a specific surface determinant on malarial (Plasmodium knowlesi) merozoites block erythrocyte invasion. J Immunol. 1981 Jul;127(1):212–217. [PubMed] [Google Scholar]
- Godiska R. Structure and sequence of the H surface protein gene of Paramecium and comparison with related genes. Mol Gen Genet. 1987 Jul;208(3):529–536. doi: 10.1007/BF00328151. [DOI] [PubMed] [Google Scholar]
- Hall R., Hyde J. E., Goman M., Simmons D. L., Hope I. A., Mackay M., Scaife J., Merkli B., Richle R., Stocker J. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. 1984 Sep 27-Oct 3Nature. 311(5984):379–382. doi: 10.1038/311379a0. [DOI] [PubMed] [Google Scholar]
- Handunnetti S. M., Mendis K. N., David P. H. Antigenic variation of cloned Plasmodium fragile in its natural host Macaca sinica. Sequential appearance of successive variant antigenic types. J Exp Med. 1987 May 1;165(5):1269–1283. doi: 10.1084/jem.165.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Protective antigens of rodent and human bloodstage malaria. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):171–177. doi: 10.1098/rstb.1984.0117. [DOI] [PubMed] [Google Scholar]
- Holmberg S., Schulman S., Vanderberg J. P. Role of a serum factor in enhancement of in vitro interactions between Plasmodium berghei sporozoites and hamster peritoneal macrophages. J Parasitol. 1981 Dec;67(6):893–897. [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Barnwell J. W. Immunochemical analysis of surface membrane antigens on erythrocytes infected with non-cloned SICA[+] or cloned SICA[-] Plasmodium knowlesi. Parasitology. 1985 Oct;91(Pt 2):245–261. doi: 10.1017/s0031182000057346. [DOI] [PubMed] [Google Scholar]
- Jarra W., Brown K. N. Protective immunity to malaria: studies with cloned lines of Plasmodium chabaudi and P. berghei in CBA/Ca mice. I. The effectiveness and inter- and intra-species specificity of immunity induced by infection. Parasite Immunol. 1985 Nov;7(6):595–606. doi: 10.1111/j.1365-3024.1985.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Jarra W., Hills L. A., March J. C., Brown K. N. Protective immunity to malaria. Studies with cloned lines of Plasmodium chabaudi chabaudi and P. berghei in CBA/Ca mice. II. The effectiveness and inter- or intra-species specificity of the passive transfer of immunity with serum. Parasite Immunol. 1986 May;8(3):239–254. doi: 10.1111/j.1365-3024.1986.tb01036.x. [DOI] [PubMed] [Google Scholar]
- McLean S. A., Pearson C. D., Phillips R. S. Antigenic variation in Plasmodium chabaudi: analysis of parent and variant populations by cloning. Parasite Immunol. 1986 Sep;8(5):415–424. doi: 10.1111/j.1365-3024.1986.tb00858.x. [DOI] [PubMed] [Google Scholar]
- McLean S. A., Phillips R. S., Pearson C. D., Walliker D. The effect of mosquito transmission of antigenic variants of Plasmodium chabaudi. Parasitology. 1987 Jun;94(Pt 3):443–449. doi: 10.1017/s0031182000055797. [DOI] [PubMed] [Google Scholar]
- Newbold C. I., Boyle D. B., Smith C. C., Brown K. N. Identification of a schizont- and species-specific surface glycoprotein on erythrocytes infected with rodent malarias. Mol Biochem Parasitol. 1982 Jan;5(1):45–54. doi: 10.1016/0166-6851(82)90005-6. [DOI] [PubMed] [Google Scholar]
- Newbold C. I., Boyle D. B., Smith C. C., Brown K. N. Stage specific protein and nucleic acid synthesis during the asexual cycle of the rodent malaria Plasmodium chabaudi. Mol Biochem Parasitol. 1982 Jan;5(1):33–44. doi: 10.1016/0166-6851(82)90004-4. [DOI] [PubMed] [Google Scholar]
- Newbold C. I. Intraerythrocytic development and antigenicity of asexual malaria parasites. Mol Biochem Parasitol. 1984 Apr;11:1–22. doi: 10.1016/0166-6851(84)90051-3. [DOI] [PubMed] [Google Scholar]
- Newbold C. I., Schryer M., Boyle D. B., McBride J. S., McLean A., Wilson R. J., Brown K. N. A possible molecular basis for strain specific immunity to malaria. Mol Biochem Parasitol. 1984 Apr;11:337–347. doi: 10.1016/0166-6851(84)90077-x. [DOI] [PubMed] [Google Scholar]
- Peterson M. G., Coppel R. L., McIntyre P., Langford C. J., Woodrow G., Brown G. V., Anders R. F., Kemp D. J. Variation in the precursor to the major merozoite surface antigens of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):291–301. doi: 10.1016/0166-6851(88)90049-7. [DOI] [PubMed] [Google Scholar]
- Saul A. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 1987 Jan;9(1):1–9. doi: 10.1111/j.1365-3024.1987.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Shaw J. M., Feagin J. E., Stuart K., Simpson L. Editing of kinetoplastid mitochondrial mRNAs by uridine addition and deletion generates conserved amino acid sequences and AUG initiation codons. Cell. 1988 May 6;53(3):401–411. doi: 10.1016/0092-8674(88)90160-2. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Mackay M., Goman M., Scaife J. G. Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. J Mol Biol. 1987 May 20;195(2):273–287. doi: 10.1016/0022-2836(87)90649-8. [DOI] [PubMed] [Google Scholar]
- Uy R., Wold F. Posttranslational covalent modification of proteins. Science. 1977 Dec 2;198(4320):890–896. doi: 10.1126/science.337487. [DOI] [PubMed] [Google Scholar]