Abstract
Adaptation to a novel visuomotor transformation has revealed important principles regarding learning and memory. Computational and behavioral studies have suggested that acquisition and retention of a new visuomotor transformation are distinct processes. However, this dissociation has never been clearly shown. Here, participants made fast reaching movements while unexpectedly a 30-degree visuomotor transformation was introduced. During visuomotor adaptation, subjects received cerebellar, primary motor cortex (M1) or sham anodal transcranial direct current stimulation (tDCS), a noninvasive form of brain stimulation known to increase excitability. We found that cerebellar tDCS caused faster adaptation to the visuomotor transformation, as shown by a rapid reduction of movement errors. These findings were not present with similar modulation of visual cortex excitability. In contrast, tDCS over M1 did not affect adaptation, but resulted in a marked increase in retention of the newly learnt visuomotor transformation. These results show a clear dissociation in the processes of acquisition and retention during adaptive motor learning and demonstrate that the cerebellum and primary motor cortex have distinct functional roles. Furthermore, they show that is possible to enhance cerebellar function using tDCS.
Keywords: adaptation, cerebellum, direct current stimulation, learning, motor cortex
Introduction
The ability of the motor system to adapt to changes in the environment is fundamentally important for the performance of accurate movements (Tseng et al. 2007). Adaptive motor learning often refers to situations where, in order to return to a former level of performance, an error stemming from an altered environment is reduced (Krakauer 2009). Adaptation to external perturbations has been studied through the application of a screen–cursor transformation during reaching or pointing movements (visuomotor adaptation). This form of perturbation causes a systematic directional bias around the hand and can be used to probe adaptive processes (Krakauer 2009). In fact, visuomotor adaptation has revealed important principles that are thought to be generalizable to procedural learning and memory (Krakauer 2009).
Visuomotor adaptation, characterized by a reduction in reaching errors, is believed to be driven by a mismatch between the predicted and actual sensory outcome of a reaching movement (Chen et al. 2006; Diedrichsen et al. 2005; Imamizu et al. 2000; Martin et al. 1996; Maschke et al. 2004; Mazzoni and Krakauer 2006; Tseng et al. 2007; Weiner et al. 1983). Neuropsychological studies have suggested that the successful reduction of errors during adaptation is a cerebellar-dependent process. For example, patients with lesions in the cerebellum are either unable or impaired in their ability to adapt to changes in visuomotor alignment (Weiner et al. 1983; Martin et al. 1996; Rabe et al. 2009). In contrast, other human studies have suggested that after exposure to adaptation paradigms the primary motor cortex (M1) is involved in the retention of the newly learnt visuomotor transformation (Richardson et al. 2006; Hadipour-Niktarash et al. 2007; Hunter et al. 2009). For instance, Hadipour-Niktarash et al. (2007) disrupted M1 with transcranial magnetic stimulation (TMS) and found impaired retention but not acquisition of a novel visuomotor transformation.
These investigations and recent computational models of adaptive motor learning suggest that acquisition and retention are distinct processes with separate neural substrates (Shadmehr and Krakauer 2008; Tanaka et al. 2009). However, this dissociation has not been addressed in a direct manner using the same motor task and intervention. More importantly, no previous brain stimulation study has assessed whether the cerebellum is involved in acquisition, retention or both. Of note, studies in cerebellar patients could not determine deficits in retention because patients were unable to adapt. Finally, prior research investigating the role of specific neural regions during motor adaptation either tested patient populations (Weiner et al. 1983; Martin et al. 1996; Rabe et al. 2009) or healthy individuals with disruptive protocols (i.e., disruption of normal brain function with TMS) (Richardson et al. 2006; Hadipour-Niktarash et al. 2007), leaving the possibility that some of the changes observed, or the lack of them, were due to compensation by other neural regions.
This study sought to double dissociate the roles of the cerebellum and M1 through the application of anodal transcranial direct current stimulation (tDCS), a noninvasive form of electrical stimulation that has been shown to increase M1 (Nitsche and Paulus 2000) and cerebellar (Galea et al. 2009) excitability. We hypothesized that cerebellar tDCS would specifically enhance motor acquisition as reflected by faster error reduction in a visuomotor adaptation paradigm. In contrast, M1 tDCS would augment retention (decrease forgetting), shown by the longer lasting maintenance of errors after the visuomotor transformation is removed (deadaptation).
Materials and Methods
Subject
Seventy-two right-handed healthy individuals with no history of neurological or psychiatric conditions (34 women; mean age 27 ± 6 years, range 19–41 years) participated in the study. None of the participants were taking medications or illicit drugs that can affect the central nervous system. All subjects signed informed consent approved by Johns Hopkins Institutional Review Board and in accordance to the declaration of Helsinki.
At the end of each session, subjects reported their attention, fatigue, and perceived pain of tDCS using a self-scored visual analog scale in which 1 represented poorest attention, maximal fatigue, and pain and 7 represented maximal attention and least fatigue and pain (Stefan et al. 2005).
Experiment 1
Experimental Procedure
Participants (n = 30; 14 women; mean age 25 ± 5 years, range 19–40 years) were seated approximately 60 cm in front of a computer monitor (48 cm width/1280 × 1024 pixel resolution). Subjects were instructed to move a digitizing pen with their right hand over a horizontal digitizing tablet (62 × 46 cm; Intuos4) located at waist height to reach eight different targets projected over a computer screen. The position of the pen was sampled at 75 Hz through a custom Matlab program (The MathWorks), which controlled a circular green cursor (2 mm diameter) on a black screen. Following a well-described protocol (Hadipour-Niktarash et al. 2007; Tseng et al. 2007), participants performed rapid “shooting” reaching movements to 2 mm diameter targets displayed in 1 of 8 positions arrayed radially at 10 cm from a central starting position. In this manner, subjects attempted to move the cursor from a white square (3 mm) centered in the middle of the screen (starting position) through the visible target in a straight line. There was a 1:1 mapping between cursor and hand displacement. The subjects were instructed to not stop at the target but to “shoot” through it. In addition, they were told to make straight movements with no corrections. The reason a “shooting” paradigm was chosen rather than a center-out reaching task is that it reduced the influence of corrective adjustments during movements. At the moment the cursor passed through the invisible boundary circle (invisible circle centered around the starting position with a 10-cm radius trial end), the cursor was hidden, the boundary point (end point) was marked with a yellow square (explicit error signal) and when needed a high- or low-pitched tone informed the subject that their movement (starting position to end point) was either too fast or too slow, respectively (275–375 ms). If the movement was within this time window no audio feedback was given. Importantly, the participants were reminded that spatial accuracy was the main goal of the task. After each trial, subjects moved back to the starting position; however, the cursor indicating their hand position only reappeared when they were within 2 cm. The targets were presented pseudorandomly so that every set of 8 consecutive trials included 1 of each of the target positions. The pseudorandom order was maintained across participants. During 2 blocks, a 30-degree counterclockwise (CCW) screen–cursor (visuomotor) transformation was imposed unexpectedly. To ensure their arm and hand was not visible throughout the study, subjects wore goggles that prevented the view of their upper extremity.
Transcranial Direct Current Stimulation
Anodal tDCS is a form of noninvasive brain stimulation that can increase the excitability of M1 (Nitsche and Paulus 2000) and cerebellum (Galea et al. 2009) with the effects lasting approximately 30 min after the cessation of stimulation (Nitsche et al. 2003; Galea et al. 2009).
Anodal (tDCS) was delivered through 2 sponge electrodes (surface area: 25 cm2) soaked in a saline solution. There were 3 groups (n = 10) each with different electrode placements. For the cerebellar tDCS group (CB), the anodal electrode was centered on the right cerebellar cortex, 3 cm lateral to the inion, and the cathodal electrode was positioned on the right buccinator muscle (Galea et al. 2009). The primary motor cortex tDCS group (M1) had the anodal electrode placed over the left motor “hotspot,” identified by single pulses of TMS delivered at a slightly suprathreshold stimulus intensity to elicit responses on the first dorsal interosseus muscle. TMS was delivered using a 70-mm loop-diameter figure-of-8 coil (Magstim BiStim2; Whitland). The cathodal electrode was placed on the skin overlying the contralateral supraorbital region (Nitsche and Paulus 2000). Anodal stimulation was set at 2 mA (Iyer et al. 2005; Ferrucci et al. 2008; Galea et al. 2009) and delivered using a Phoresor II Auto (Model No.PM850; IOMED). Thus, we applied a current at a density of 0.08 mA/cm2, which is considered to be safe (Iyer et al. 2005) and is far below the threshold for tissue damage (Liebetanz et al. 2009). At the onset of tDCS, the current was increased in a ramp-like fashion over a period of 30 s, a method shown to achieve a good level of blinding (Gandiga et al. 2006). Therefore, the SHAM tDCS group consisted of anodal tDCS applied for a total duration of 30 s over the right cerebellar or left M1 positions chosen at random. Two experimenters were present; 1 delivered tDCS while the other, oblivious to the type of stimulation, ran the experiment. As a result, both the experimenter and subject were blinded as to whether anodal or SHAM tDCS was being applied.
Experimental Protocol
All groups experienced the same experimental protocol consisting of 6 blocks of trials (Fig. 1a). Blocks 1 (Pre1), 2 (Pre2), 4 (Post1), and 6 (Post2) involved 12 repetitions of the 8 targets (96 trials) under veridical conditions (no visual perturbation). The third block (Adapt1) consisted of 25 repetitions (200 trials) where a 30-degree CCW visuomotor transformation was applied to the cursor on the screen. The fifth block (Adapt2) involved the same CCW transformation but only 18 repetitions (144 trials) were performed. This meant acquisition was assessed during Adapt1 and 2 and deadaptation in Post1 and 2. Anodal tDCS was applied during Pre2 and Adapt1 (ca. 15 min; Fig. 1a). During these 2 blocks, the groups differed either in terms of the position of the tDCS electrodes (CB and M1) or the amount of stimulation they received (SHAM).
Figure 1.
Schematic representation of the main experimental setup. (a) Experiment 1: 3 groups (n = 10 each; SHAM, CB, M1) participated in a similar protocol involving 6 blocks. Pre1, Pre2, Post1, and Post2 were all under veridical conditions (no visual perturbation). During Adapt1 and 2, subjects were exposed to a 30-degree CCW screen–cursor transformation. Anodal (CB, and M1) or SHAM tDCS (SHAM) was applied to either the ipsilateral cerebellar cortex (CB) or contralateral motor cortex (M1) during Pre2 and Adapt1 (approximately 15 min; shaded area). The numbers under each block represent the amount of trials, while the numbers in brackets indicate the approximate length of time in minutes for each block. (b) Experiment 2: 3 groups (n = 10 each; SHAM, CB, M1) experienced a similar protocol involving 6 blocks. Pre1 and 2 were under veridical conditions. During Adapt, subjects were exposed to a 30-degree CCW screen–cursor transformation. Anodal or SHAM tDCS was applied during Pre2 and Adapt to M1 or cerebellum. Post1, 2, and 3 all involved trials with no visual feedback. (c) Experiment 3: 2 groups (n = 8 each: CB, OC) experienced a similar first 3 blocks where anodal tDCS was applied during Pre2 and Adapt to either the cerebellum or OC. Before Pre1 (pre) and after Adapt (post), TMS was used to assess phosphene threshold, a measure of visual cortex excitability.
Experiment 2
Experimental Protocol
During deadaptation (Post blocks), at least 2 factors influence the error in reaching movements. With every trial, the participant forgets some of what had previously been acquired, reflecting retention (Smith et al. 2006), and simultaneously learns (acquire) from the movement error (Hadipour-Niktarash et al. 2007). To assess retention alone, in experiment 2, a new group of subjects were exposed to deadaptation trials without visual feedback (Post1, 2, and 3; Fig. 1b). Given that subjects could not observe movement errors, it was possible to assess whether tDCS over the cerebellum or M1 specifically influenced the rate of forgetting (retention) of the previously acquired visuomotor transformation.
Three groups (n = 30; 12 women; mean age 27 ± 6 years, range 19–41 years) were exposed to the same experimental procedures used in experiment one (SHAM, CB, and M1; see “Transcranial Direct Current Stimulation” section for an explanation of the tDCS electrode placement and amount of stimulation). The first three blocks (Pre1 and 2 and Adapt) were identical to experiment 1 with tDCS being applied during Pre2 and Adapt. Blocks 4–6 (Post1, 2, and 3) consisted of 18 repetitions each (144 trials) in which participants made “shooting” reaching movements without visual feedback (Fig. 1b). During these trials, the target was visible, but once the subjects had moved out of the starting position the cursor indicating their hand position was not. In addition, subjects did not receive end point feedback. Participants were instructed that the square (starting position) in the middle of the screen would turn red once they had passed the target. Audio feedback was still given regarding movement time.
Experiment 3
Experimental Protocol
Due to the size and position of tDCS stimulation, it is possible that cerebellar tDCS also stimulated the occipital cortex (OC). As the present task involved a large visual component, it is important to assess whether the results found were a result of cerebellar or OC modulation.
Two additional groups of subjects (n = 16; 10 women: mean age 26 ± 4 years, range 22–38 years) performed the same behavioral task as in experiments 1 and 2 but included only the initial 3 blocks (Pre1 and 2 and Adapt; Fig. 1c). As in the prior experiments, during Pre2 and Adapt blocks, one group received anodal tDCS applied over the cerebellum (CB; see “Transcranial Direct Current Stimulation” section), while the other group was stimulated with anodal tDCS over the OC. Using the 10:20 EEG system, the anodal electrode was positioned over Oz (approximately 3 cm superior to the inion) (Antal et al. 2003) and the cathodal electrode was positioned on the right buccinator muscle. Note that Antal et al. (2003) placed the cathode electrode over Cz; however, we reasoned that this position may modulate M1 excitability and so decided on a cathodal electrode position similar to the cerebellar tDCS group. Importantly, anodal tDCS over this position has been shown to effectively modulate OC excitability (Antal et al. 2003).
In this experiment, to assess the amount of OC stimulation with the 2 electrode configurations, OC excitability was assessed through the measurement of phosphenes. Previous studies have demonstrated that TMS pulses delivered over Oz can elicit light sensations, called stationary phosphenes (Meyer et al. 1991; Marg and Rudiak 1994; Kammer 1999; Antal et al. 2003). The minimum TMS intensity required to elicit phosphenes is defined as the phosphene threshold (PT). Importantly, the PT has been found to be stable within subjects across time and suggested to be an index of visual cortex excitability (Boroojerdi et al. 2000; Stewart et al. 2001; Gothe et al. 2002). Therefore, PT was used to assess OC excitability before Pre1 (pre) and after Adapt (post) in the CB and OC groups (Fig. 1c). To assess PT, the handle of the TMS coil was pointed upward. The subjects sat in a dark room with their eyes open. Single pulses of TMS were applied at 50% of maximum stimulator output (0.2 Hz) to Oz (approximately 3cm superior to the inion). The PT was determined following a standard procedure: starting from the simulation intensity, at which the subject perceived a stable phosphene in the same form and location every time, the intensity was reduced in steps of 5% until phosphenes were no longer perceived. Then, it was increased again in 1% steps until the minimum intensity at which the subject could perceive a phosphene was established. The minimum final values were determined only if the phosphenes were stable sensations, appearing in the same form at the same location in at least 3 cases of the 5 stimulations (Antal et al. 2003). As it was important to obtain stable phosphenes for this experiment, a screening process was used to assess whether participants were able to easily observe them. Four out of the 16 subjects were unable to consistently observe phosphenes and so were removed from the study, leaving 6 subjects in each group.
In this experiment, we hypothesized that a significant decrease in PT would be observed for the OC group relative to the CB group, suggesting a greater change in visual cortex excitability following OC tDCS. Critically, if the changes in behavior found with cerebellar tDCS in the prior experiments were actually due to OC stimulation, we predicted that any improvements observed in the CB group would be larger in the OC group. However, if the behavioral improvements were driven by cerebellar stimulation then greater acquisition improvements would be observed in the CB group.
Data Collection and Analysis (All Experiments)
The 2-D position of the hand was continuously recorded at a rate of 75 Hz using a custom Matlab program (Mathworks). All kinematic data were filtered at 10 Hz with a low-pass Butterworth filter and numerically differentiated to calculate velocity. The onset of each movement was determined as the point at which radial velocity crossed 5% of peak velocity. Performance was quantified in each trial using angular end point error, defined as the angle between the line connecting the starting position to the center of the target and the line connecting the starting position to the end point (Hadipour-Niktarash et al. 2007). Positive values indicated CCW error whereas negative values indicated clockwise (CW) error. Epochs were created by binning 8 consecutive movements. For each block, the initial amount of error (mean error) was determined by averaging over consecutive epochs (Krakauer et al. 2005). For blocks consisting of 96 trials, epochs 2–6 were averaged, whereas for blocks with either 144 or 200 trials, epochs 2–11 were averaged (Krakauer et al. 2005). This form of analysis has been shown to capture the initial rapid rate of learning observed during visuomotor adaptation (Krakauer et al. 2005). Although previous studies have applied exponential models (Krakauer et al. 2000; Ghilardi et al. 2000; Galea and Miall 2006), here we found that individual data varied between a single and double exponential fit and so neither could be reliably fit to all individual subject data. Using mean error as the primary outcome measure, separate repeated-measures analyses of variance (ANOVARM) were used for each experiment to compare factors GROUP (SHAM, CB, and M1) and BLOCK (6). If an interaction was found, separate between-subject 1-way ANOVAs compared GROUP in each BLOCK. Given that these ANOVAs were only performed following a significant initial ANOVARM, further corrections for multiple comparisons were not required. Tukey post hoc tests were performed on all significant results. In addition, identical analysis was performed on (mean) movement duration, reaction time, and maximum velocity. For experiment 3, it was decided to concentrate on the Adapt block as the effect of cerebellar and OC tDCS on acquisition was specifically being assessed. The SHAM group from experiment 2 was used in a between-subject 1-way ANOVA (CB, OC, and SHAM) to compare the groups “mean error” during Adapt. Tukey post hoc tests were performed on significant results. For the TMS assessment of phosphenes, the change in PT following tDCS (post–pre) was computed. An independent t-test compared these values for the OC and CB groups. The threshold for all statistical comparisons was P < 0.05. All data presented represent mean ± standard error of the mean unless otherwise specified.
Results
Experiment 1: Summary
All subjects completed the study without adverse events. In all 3 groups (n = 10), the Pre blocks were characterized by relatively accurate performance with hand trajectories indistinguishable between groups (Fig. 2a). Due to the novel visuomotor transformation, a large error in movement trajectory was initially observed in Adapt1 for all groups (Fig. 2b). Over subsequent trials, all participants adapted, reducing their error values and returning toward baseline performance. As predicted, during adaptation, the CB group showed a smaller amount of error in comparison to the SHAM and M1 groups (Fig. 2c). This faster reduction in angular error is clearly observed when plotting its evolution over time (Fig. 3).
Figure 2.
Single subject data for experiment 1. A sample subject from the SHAM (black), CB (cerebellar anodal tDCS: red), and M1 (M1 anodal tDCS: blue) groups is shown. (a) Under veridical conditions, all groups made similar accurate movement trajectories toward each target (Pre2: epoch 12). (b) When initially exposed to the novel 30-degree CCW screen–cursor transformation (Adapt1: epoch 1), subjects show comparable error in their trajectories. (c) In comparison to the SHAM and M1 participants, the CB participant is able to display a reduced amount of error in their movement trajectories at approximately midpoint of the adaptation block (Adapt1: epoch 8).
Figure 3.
Group data for experiment 1. End point error (degrees) are shown during baseline (Pre1 and 2), adaptation (Adapt1 and 2), and deadaptation (Post1 and 2) for the SHAM (black), CB (red), and M1 (blue) groups (mean ± standard error of the mean [SEM] of 8 trial epochs). Positive values indicate counterclockwise deviation. The shaded area represents blocks in which tDCS was applied (Pre2 and Adapt1). Bar graphs insets indicate “mean end point error” in degrees (±SEM) for SHAM (black), CB (red), and M1 (blue) groups in each block. This was determined for each participant by averaging consecutive epochs (see Materials and Methods). For each block, separate 1-way ANOVAs compared these values across groups. *P < 0.009.
Cerebellar tDCS improves error reduction
ANOVARM comparing mean error across GROUP (SHAM, CB, and M1) and BLOCK (6) revealed no significant difference for GROUP (F2,27 = 1; P = 0.3), but there was a significant main effect of BLOCK (F5,135 = 293; P = 0.0005) and GROUP × BLOCK interaction (F10,135 = 6; P = 0.0005). During Adapt1, there was a significant effect of GROUP (F2,29 = 8; P = 0.002; Fig. 3). Tukey post hoc tests revealed a significant difference between the CB group and both the SHAM (P = 0.003) and M1 (P = 0.01) groups (Fig. 3). This indicates that the CB group experienced the largest reduction of error during adaptation (Fig. 3). In addition, a similar difference between groups was observed for Adapt2 (F2,29 = 9; P = 0.001; Fig. 3), where the CB group showed a greater reduction of error in comparison to either the SHAM (P = 0.009) or M1 groups (P = 0.001; Fig. 3).
The improvement in error reduction could not be explained by differences in baseline performance, in psychological measures, or in other movement kinematics (Table 1). Indeed, although all groups showed a small CW bias at Pre1 and 2, there was no significant effect of GROUP for mean error (F2,29 < 0.3; P > 0.7; Fig. 3). This meant there was no initial performance difference between groups (Pre1). In addition, tDCS applied during reaching movements without a visual perturbation did not affect movement accuracy (Pre2; Fig. 3). The participant's self-reported ratings of attention (5.3 ± 2.7), fatigue (2 ± 1.4), and perceived pain (1.4 ± 0.7) were not significantly different across groups (1-way ANOVA: F2,29 < 0.7; P > 0.5). Finally, other movement kinematic parameters remained constant between groups and across all 6 blocks (Table 1). Separate ANOVARM comparing GROUP (SHAM, CB, and M1) and BLOCK (6) for mean movement duration, reaction time, and maximum velocity showed no significant differences for GROUP (F2,27 < 0.9; P > 0.3), BLOCK (F5,135 < 1; P > 0.2) or GROUP × BLOCK interaction (F10,135 < 0.3; p>0.9; Table1).
Table 1.
Kinematic parameters: in experiments 1 and 2
| Movement duration (ms) |
Reaction time (ms) |
Maximum velocity (cm/s) |
|||||||
| SHAM | CB | M1 | SHAM | CB | M1 | SHAM | CB | M1 | |
| Experiment 1 | |||||||||
| Pre1 | 334 ± 59 | 329 ± 30 | 330 ± 48 | 299 ± 25 | 284 ± 40 | 287 ± 39 | 61 ± 5 | 62 ± 3 | 62 ± 4 |
| Pre2 | 324 ± 23 | 315 ± 19 | 331 ± 23 | 303 ± 20 | 293 ± 35 | 315 ± 42 | 62 ± 3 | 63 ± 4 | 62 ± 2 |
| Adapt1 | 337 ± 25 | 352 ± 27 | 340 ± 23 | 287 ± 33 | 296 ± 21 | 305 ± 32 | 61 ± 1 | 64 ± 4 | 63 ± 4 |
| Post1 | 282 ± 27 | 293 ± 25 | 315 ± 14 | 331 ± 38 | 330 ± 43 | 298 ± 19 | 62 ± 3 | 61 ± 2 | 63 ± 3 |
| Adapt2 | 300 ±21 | 292 ± 28 | 319 ± 24 | 321 ± 29 | 315 ± 31 | 319 ± 27 | 63 ± 2 | 65 ± 2 | 65 ± 5 |
| Post2 | 290 ± 20 | 295 ± 20 | 302 ± 21 | 309 ± 36 | 305 ± 39 | 321 ± 30 | 65 ± 4 | 65 ± 3 | 65 ± 3 |
| ANOVA | G: F = 0.6, P = 0.6 | G: F = 0.7, P = 0.5 | G: F = 0.9, P = 0.3 | ||||||
| B: F = 1, P = 0.2 | B: F = 0.7, P = 0.6 | B: F = 1, P = 0.2 | |||||||
| G × B: F = 0.3, P = 0.9 | G × B: F = 0.2, P = 0.9 | G × B: F = 0.3, P = 0.9 | |||||||
| Experiment 2 | |||||||||
| Pre1 | 345 ± 68 | 339 ± 40 | 342 ± 38 | 300 ± 22 | 281 ± 39 | 291 ± 46 | 63 ± 3 | 62 ± 6 | 63 ± 5 |
| Pre2 | 334 ± 34 | 329 ± 18 | 344 ± 34 | 286 ± 38 | 334 ± 18 | 284 ± 36 | 62 ± 4 | 61 ± 3 | 60 ± 4 |
| Adapt1 | 334 ± 59 | 329 ± 30 | 330 ± 48 | 299 ± 25 | 284 ± 40 | 287 ± 39 | 61 ± 5 | 62 ± 3 | 62 ± 4 |
| Post1 | 361 ± 37 | 359 ± 52 | 368 ± 28 | 295 ± 21 | 323 ± 32 | 317 ± 26 | 63 ± 3 | 65 ± 5 | 62 ± 5 |
| Post2 | 353 ± 20 | 360 ± 22 | 366 ± 25 | 313 ± 33 | 298 ± 27 | 301 ± 43 | 64 ± 4 | 62 ± 4 | 63 ± 4 |
| Post3 | 325 ± 25 | 333 ± 20 | 334 ± 19 | 329 ± 26 | 314 ± 34 | 320 ± 24 | 65 ± 5 | 64 ± 4 | 65 ± 4 |
| ANOVA | G: F = 0.2, P = 0.8 | G: F = 0.2, P = 0.8 | G: F = 0.1, P = 0.9 | ||||||
| B: F = 0.9, P = 0.3 | B: F = 0.2, P = 0.9 | B: F = 0.8, P = 0.4 | |||||||
| G × B: F = 0.8, P = 0.6 | G × B: F = 0.9, P = 0.6 | G × B: F = 0.2, P = 0.9 | |||||||
Note: Movement duration (ms), reaction time (ms), and maximum velocity (cm/s) were assessed for the SHAM, CB, and M1 groups in each block. Values depict the mean ± standard error of the mean determined for each subject by averaging over consecutive epochs. For each kinematic parameter, a repeated-measures ANOVA compared GROUP (G; SHAM, CB, M1) and BLOCK (B).
During Adapt2, it is evident that the CB group showed less error in epoch 1 (Fig. 3). Trial-by-trial analysis of this epoch (ANOVARM: GROUP [SHAM, CB, M1] × TRIAL [8]) revealed a significant main effect of GROUP (F2,27 = 8; P = 0.001), TRIAL (F7,189 = 8; P = 0.0005) and GROUP × TRIAL interaction (F14,189 = 2; P = 0.01). Separate 1-way ANOVAs identified no significant main effect of GROUP at trial 1 (SHAM: 25 ± 2 degrees, CB: 28 ± 2, M1: 27 ± 1; F2,29 = 0.7; P = 0.5), but a significant difference was observed by trial 4 (F2,29 = 3; P = 0.05). Tukey post hoc tests revealed that the CB group (16 ± 3degrees) showed less error than either the SHAM (22 ± 2, P = 0.03) or M1 (24 ± 2, P = 0.006) groups. This indicates that at trial 1 of Adapt2 all groups showed a similar amount of error, in contrast on trial 4, the CB group showed a reduction of error that was not observed in either the SHAM or M1 groups.
There was a trend toward a significant GROUP effect for both Post1 (F2,29 = 2.5; P = 0.1) and Post2 (F2,29 = 2.2; P = 0.1; Fig. 3). Surprisingly, this difference was driven by a reduction of error in the CB group and not an increased amount of error in the M1 group (Fig. 3), as previously predicted. However, it is important to recall that during deadaptation (Post blocks), at least 2 factors influence the error in reaching movements: the retention of the previous learned movements and the acquisition of new motor commands resulting from movement errors (Smith et al. 2006; Hadipour-Niktarash et al. 2007).
Initial versus End Movement Error
Reaching “shooting” movements are thought to rely on feedforward control with minimal feedback correction (Hadipour-Niktarash et al. 2007; Tseng et al. 2007). In order to quantify whether there was significant changes in angular error from the start to the end of movement, we calculated angular error at the start of movement. This was taken as the angular difference between a straight line from the start position to the target and start position to the positional marker at 80 ms from the onset of each movement (Sainburg and Wang 2002). For each group, separate ANOVARM compared mean error for the start and end of the movement (factor TIME) across BLOCK. For all comparisons, there was a significant effect for BLOCK (F5,45 > 33; P < 0.005), yet the main effect of TIME (F1,9 < 1; P > 0.7) and TIME × BLOCK interaction (F5,45 < 1; P > 0.2) were both not significant. Importantly, similar results as angular end point error were observed for angular initial mean error in Adapt1 and 2 (F2,29 > 11; P < 0.005). Post hoc analysis revealed a significant difference between the CB group and the other 2 groups (P < 0.006). These results suggest that feedback processes do not play a major role in this task or in the results in general.
Experiment 2: Summary
All subjects completed the experiment without complications. In all 3 groups (n = 10), the Pre blocks were characterized by accurate performance (Fig. 4a). Similarly to experiment 1, the CB group showed a greater reduction of error during adaptation relative to the other 2 groups (Fig. 4b). At the onset of deadaptation (Post1), all groups showed the same amount of initial error (Fig. 4c). However, in subsequent trials within Post1 and later in Post2 and 3, the M1 group remained with marked movement errors indicating increased retention (less forgetting) (Fig. 4d). These differences are clearly observable when plotting epoch data (Fig. 5).
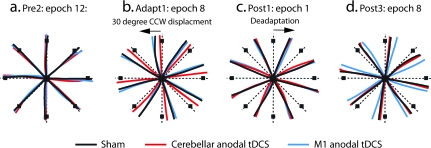
Figure 4.
Single participant data for experiment 2. A sample participant from the SHAM (black), CB (cerebellar anodal tDCS: red), and M1 (M1 anodal tDCS: blue) groups is shown. (a) Under veridical conditions, all groups made similar accurate movement trajectories toward each target (Pre2: epoch 12). (b) Similarly to experiment 1, the CB participant exhibits a reduced amount of error in their movement trajectories by epoch 8, which is not observed in both the SHAM and M1 participants (Adapt1: epoch 8). (c) Initially in the trials with no vision, all participants show a similar amount of error (Post1: epoch 1). (d) By the end of the no-vision blocks, the M1 participant displays a larger amount of movement error relative to the SHAM and CB participants indicative of increased retention (Post3: epoch 8).
Figure 5.
Group data for experiment 2. End point error (degrees) are shown during baseline (Pre1 and 2), adaptation (Adapt) and deadaptation with no visual feedback (Post1, 2, and 3) for the SHAM (black), CB (red), and M1 (blue) groups (mean ± standard error of the mean [SEM] of 8 trial epochs). Positive values indicate counterclockwise deviation. The shaded area represents blocks in which tDCS was applied (Pre2 and Adapt). Bar graphs insets indicate mean end point error in degrees (±SEM) for SHAM (black), CB (red), and M1 (blue) in each block. For each block, separate 1-way ANOVAs compared these values across groups. *P < 0.05.
Cerebellar tDCS Improves Error Reduction
ANOVARM comparing mean error across GROUP (SHAM, CB, and M1) and BLOCK (6) showed no significant difference for GROUP (F2,27 = 1.4; P = 0.2). However, there was a significant main effect of BLOCK (F5,135 = 400; P = 0.0005) and GROUP × BLOCK interaction (F10,135 = 5; P = 0.0005). Similarly to experiment 1, separate 1-way ANOVAs comparing GROUP in each block showed a significant effect of GROUP during adaptation (Adapt; F2,29 = 6; P = 0.009; Fig. 5). Tukey post hoc tests revealed a significant difference between the CB (9 ± 1.3degrees) group and the SHAM (P = 0.02) or M1 (p = 0.02) groups (Fig. 5). Again, this indicates that the CB group experienced a greater reduction of error during adaptation.
M1 tDCS Increases Retention
When evaluating the deadaptation blocks (Post1, 2, and 3), separate ANOVAs showed a trend toward a significant GROUP effect for Post1 (F2,29 = 2; P = 0.14; Fig. 5) and a significant effect for Post2 (F2,29 = 3; P = 0.05) and Post3 (F2,29 = 3.7; P = 0.04). For both Post2 and 3, Tukey post hoc tests revealed a significant difference between the M1 group and the SHAM (P < 0.05) and CB (P < 0.05) groups (Fig. 5). Therefore, during deadaptation with no visual feedback, the M1 group showed a persistence of error indicating increased retention of the recently acquired visuomotor transformation, while the CB group now demonstrated similar retention to SHAM.
The results of cerebellar and M1 tDCS on movement accuracy could not be explained by differences in baseline performance, in psychological measures or in other movement kinematics. There was no significant effect of GROUP for Pre1 (F2,29 = 0.7; P = 0.5) or Pre2 for mean error (F2,29 = 1.2; P = 0.3; Fig. 5). In addition, the participant's self-reported ratings of attention (5.7 ± 0.9), fatigue (2.5 ± 1.2), and perceived pain (1.6 ± 0.5) were not significantly different across groups (1-way ANOVA: F2,29 < 2; P > 0.2). Finally, other movement kinematics remained constant between groups and across all 6 blocks (Table 1). Separate ANOVARM comparing GROUP (SHAM, CB, and M1) and BLOCK (6) for mean movement duration, reaction time, and maximum velocity showed no significant differences for GROUP (F2,27 < 0.2; P > 0.8), BLOCK F5,135 < 0.9; P > 0.3) or GROUP × BLOCK interaction (F10,135 < 0.9; P > 0.6; Table 1). Finally, similar results as angular end point mean error were observed for angular initial mean error during Adapt, Post2, and Post3 (F2,29 > 3; P < 0.04). For Adapt, post hoc analysis revealed a significant difference between the CB group and the other 2 groups (P < 0.03). In addition, during Post2 and 3, a significant difference between the M1 group and the other 2 groups (P < 0.05) was observed. Again these results suggest that feedback processes did not play a major role in this task or in the results in general.
Experiment 3: Cerebellar tDCS Results in Faster Error Reduction Relative to OC tDCS
For Adapt, a 1-way ANOVA revealed a significant difference in mean error between groups (F2,21 = 3.6; P = 0.04; Fig. 6a). Tukey post hoc tests found significant differences between the CB and both the OC (P = 0.05) and SHAM (P = 0.02) groups (Fig. 6a). In contrast, there was no difference between the OC and SHAM groups (P = 0.4). This indicates that relative to the OC and SHAM groups, the CB group experienced faster error reduction during adaptation.
Figure 6.
Group data for experiment 3. (a) End point error (degrees) are shown during baseline (Pre1 and 2) and adaptation (Adapt) for the OC (green) and CB (red) groups (mean ± standard error of the mean [SEM] of 8 trial epochs). Positive values indicate counterclockwise deviation. The shaded area represents blocks in which tDCS was applied (Pre2 and Adapt). Bar graph insets indicate mean end point error in degrees (±SEM) during adapt for OC (green), CB (red), and experiment 2 SHAM (black) conditions. For Adapt, an ANOVA compared these values between groups. (b) Changes in visual PTs as measured by TMS (% of stimulator output). These were assessed prior to Pre1 (pre) and after Adapt (post). An independent t-test compared these values (post–pre) between groups. *P < 0.05.
OC tDCS Causes an Increase in Visual Cortex Excitability
An independent t-test revealed a significant difference between OC (−4.8% ± 1.5 stimulator output) and CB (−0.3% ± 1.8; t = 1.8, degrees of freedom = 10, P = 0.04, 1 tailed; Fig. 6b) changes in PT (post–pre), which suggests that the excitability of the visual cortex was enhanced only after OC stimulation, but not with cerebellar tDCS.
Discussion
The current study aimed to dissociate the roles of the cerebellum and M1 during adaptive motor learning to a novel visuomotor transformation. Here, we found that anodal cerebellar tDCS specifically enhanced acquisition, as shown by a faster reduction of movement error, without influencing retention. Conversely, anodal M1 tDCS increased retention but did not affect acquisition.
When individuals are exposed to a novel visuomotor transformation during reaching they initially make a large error. Over subsequent trials, they are able to adapt to the perturbation and gradually reduce the error in their movement. This error reduction process has been interpreted as the acquisition of a novel visuomotor transformation (Miall et al. 1993). If the participant is then reintroduced to a condition where the visual transformation is removed an error in the opposite direction to the perturbation is observed (aftereffect), with this fading over subsequent trials. This “aftereffect” is thought to represent the retention of the acquired visuomotor transformation (Bock et al. 2005; Hadipour-Niktarash et al. 2007).
Although there is a body of evidence suggesting distinct roles for the cerebellum and M1 during adaptive motor learning, this dissociation had never been directly tested. Experiments 1 and 2 demonstrated that anodal cerebellar tDCS led to faster reduction of error relative to SHAM or M1 stimulation in all blocks requiring rapid correction of movement errors. Given that deadaptation (post) is thought to assess retention (Hadipour-Niktarash et al. 2007), it is possible that the observable but nonsignificant reduction in error in the cerebellar group during deadaptation in experiment 1 may indicate that cerebellar tDCS also led to faster forgetting. However, during deadaptation, at least 2 factors influence the amount of error in reaching movements. With every trial, the participant forgets some of what had previously been acquired, reflecting retention (Smith et al. 2006), and simultaneously learns new motor commands from the movement errors (Hadipour-Niktarash et al. 2007). Thus, it is likely that deadaptation in experiment 1 was simultaneously assessing retention and the acquisition of new motor commands. In order to eliminate this confound and specifically assess retention, experiment 2 involved deadaptation with no vision. Since participants were not exposed to visual movement errors the main factor influencing performance had to be retention or the rate of forgetting. Under these conditions, the cerebellar group now performed similarly to the SHAM group. More importantly, M1 tDCS resulted in a longer lasting maintenance of error, representing enhanced retention or slower forgetting. These results provide clear evidence that during visuomotor adaptation, tDCS over the cerebellum increases a participant's ability to learn from movement error but has little effect on the subsequent retention of the learnt information. In contrast, M1 tDCS does not influence the ability to learn from movement error but augments retention. As there were no differences between the groups during baseline performance, the changes associated with tDCS were learning specific rather than secondary to changes in simple performance. In addition, these changes were only observed in movement error and not in other kinematic parameters such as reaction time, maximum velocity, or movement duration. However, this is not surprising as the visuomotor perturbation specifically caused a large spatial error and therefore was the only movement kinematic which required a process of adaptive error reduction.
Recent modeling studies have attempted to explain the neural network involved during adaptive motor learning (Shadmehr and Krakauer 2008; Tanaka et al. 2009). It has been suggested that the cerebellum forms a forward model predicting the sensory consequences of motor commands based on the current estimation of the state (Miall et al. 1993; Wolpert and Miall 1996; Shadmehr and Krakauer 2008). The output of the forward model could be sent to the parietal cortex where a prediction error is generated by comparing the predicted and actual sensory feedback (Shadmehr and Krakauer 2008). Any discrepancy between the expected and actual sensory consequences of the action will then result in an update of the forward model, that is, the prediction error will be fed back to the cerebellum in order to modify the forward model. Within this framework, it is thought that the inhibitory output of Purkinje cells is partially modulated by climbing fiber inputs transmitting the sensory prediction error signals presumably originating in the parietal cortex (Miall et al. 1993; Wolpert and Miall 1996; Shadmehr and Krakauer 2008). As it is thought that cerebellar tDCS may modulate the output of Purkinje cells (Galea et al. 2009), it is possible that tDCS changed these cells response to the input of the climbing fibers perhaps by affecting secondary events such as long-term depression. In other words, anodal tDCS may have increased the Purkinje cells response to error.
A recent study by Fritsch et al. (2010) has shown that the cellular mechanisms underlying anodal direct current stimulation include an enhanced secretion of the neurotrophin brain-derived neurotrophic factor (BDNF) and increased activation of the high-affinity tyrosine kinase receptors (TrkB). In M1, when these processes are combined with synaptic activity, they lead to long-term potentiation and their presence is critical for the beneficial effects of tDCS on plasticity and motor skill learning (Fritsch et al. 2010). Likely, the same mechanisms can explain the increase in retention found when subjects performed the current visuomotor adaptation paradigm whilst receiving anodal tDCS over M1. Interestingly, in the cerebellum, TrkB promotes activity-dependent inhibitory synaptogenesis of Purkinje cells and modulates inhibitory synaptic function (for review, see Drake-Bauman 2006). Although direct evidence to date is lacking, it is possible that the changes observed with anodal direct current stimulation on TrkB secretion in the motor cortex may also occur in the cerebellum. This could provide a molecular explanation as to the increased inhibitory cerebellar output (Galea et al. 2009) and enhanced visuomotor acquisition observed following cerebellar anodal tDCS.
Due to the size of the tDCS electrodes (25cm2), it is important to consider as an alternative explanation that the site of cerebellar stimulation influenced the excitability of the brainstem and OC, rather than solely the cerebellum. First, it is unlikely that brainstem changes occurred as our previous study assessed 3 different measures of brainstem excitability and found tDCS placed over the cerebellum as done in this study (3 cm lateral to the inion) elicited no excitability changes (Galea et al. 2009). Second, experiment 3 of the current study indicates that increasing OC excitability does not result in the behavioral changes observed with cerebellar tDCS. If OC activity were the driving factor behind the improved error reduction during cerebellar tDCS, then moving the tDCS electrode onto an area where previous studies have shown tDCS increases OC excitability (Antal et al. 2003; Lang et al. 2007) should have enhanced adaptation. However, anodal tDCS placed over Oz (3 cm superior to the inion) increased OC excitability but resulted in similar acquisition to SHAM tDCS. Of note, OC tDCS was placed 3 cm superior to the inion, an area thought to represent the primary visual cortex (V1; Antal et al. 2003, Lang et al. 2007). However, one could argue that during cerebellar tDCS, stimulation of the visual movement–sensitive area (V5) could result in faster visuomotor adaptation. When stimulating V5, the tDCS electrode has previously been positioned 4 cm superior and 7 cm lateral to the inion (Antal et al. 2004). As we did not observe any changes in V1 excitability following cerebellar tDCS (Experiment 3: TMS phosphene threshold), we believe it is highly unlikely that the excitability of a more distant area of the OC would have been modulated. Although a role of more distant OC areas cannot be completely ruled out, the results from experiment 3 indicate that cerebellar modulation was the driving factor behind faster acquisition. In sum, these results provide a reason to explore cerebellar tDCS in terms of its cellular basis, clinical applications, and how useful it might be in understanding human cerebellar function.
The results from M1 tDCS are in accordance with previous reports suggesting a role of this area in the retention of new motor memories (Muellbacher et al. 2002; Richardson et al. 2006; Hadipour-Niktarash et al. 2007; Galea and Celnik 2009; Hunter et al. 2009; Reis et al. 2009). For instance, disruptive TMS of M1 can interfere with the retention process without significantly influencing acquisition (Richardson et al. 2006; Hadipour-Niktarash et al. 2007). Additionally, it has been shown that M1 anodal tDCS can increase the retention of a motor skill between days but not the rate of acquisition during performance (Reis et al. 2009) and also enhance the retention of a motor memory resulting from simple thumb movements (Galea and Celnik 2009). At first glance, it may be surprising that M1 tDCS did not influence deadaptation during experiment 1 considering it measured both forgetting and learning. A plausible explanation is that the rate of deadaptation within the task was heavily weighted toward error-dependent learning, rather than retention. If M1 tDCS is specifically manipulating retention, then any changes may have been masked by the unaffected error-dependent learning.
Finally, the current results are in agreement with a recent modeling study that suggests that M1 may store the new visuomotor mappings resulting from adaptation (Tanaka et al. 2009). The authors suggest that in order to reduce the prediction error, the synaptic weights between the parietal cortex and motor cortical areas are modified. These changes, which would require plasticity to occur in M1, are reflected in the increased activity of neurons in motor areas whose preferred direction in hand space matches the required visual trajectory (Tanaka et al. 2009). The previously mentioned cellular results from Fritsch et al. (2010) support the view that anodal tDCS combined with a learning paradigm may increase the plasticity of M1 and so lead to a greater retention of the new visuomotor transformation.
In experiment 1, cerebellar tDCS seemed to result in changes after the cessation of stimulation (adapt 2). This could be a result of either tDCS continuing to modulate cerebellar excitability after the cessation of tDCS (Galea et al. 2009) or due to improved initial adaptation (Huang and Shadmehr 2009). Although not relevant to the conclusions of this study, this issue may be important to understand when using cerebellar tDCS in the context of rehabilitation. For example, does tDCS need to be applied simultaneously with motor practice or can the aftereffect of tDCS also be useful to augment acquisition?
In conclusion, anodal tDCS was used to clearly dissociate the processes of acquisition and retention during adaptive motor learning and show that the cerebellum and primary motor cortex have distinct functional roles. Specifically anodal cerebellar tDCS enhanced acquisition, as shown by a faster reduction in movement errors. In contrast, anodal M1 tDCS did not influence acquisition but led to increased retention of the new visuomotor transformation. Furthermore, the results indicate that it is possible to augment cerebellar function using tDCS.
Funding
National Institute of Child, Health and Development (NICHD), National Institutes of Health (R01 HD053793 and R21 HD060169), and the Brain Science Institute of Johns Hopkins University; Fonds Spéciaux de Recherche from Université catholique de Louvain (Belgium) and the Fondation pour la Vocation (Belgium) (to J.J.O.).
Acknowledgments
J.J.O. is a fellow of the Belgian American Educational Foundation. Conflict of Interest: None declared.
References
- Antal A, Kincses TZ, Nitsche MA, Paulus W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia. 2003;41(13):1802–1807. doi: 10.1016/s0028-3932(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kruse W, Kincses TZ, Hoffmann KP, Paulus W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. 2004;16(4):521–527. doi: 10.1162/089892904323057263. [DOI] [PubMed] [Google Scholar]
- Bock O, Worringham C, Thomas M. Concurrent adaptations of left and right arms to opposite visual distortions. Exp Brain Res. 2005;162:513–519. doi: 10.1007/s00221-005-2222-0. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Prager A, Muellbacher W, Cohen LG. Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology. 2000;54:1529–1531. doi: 10.1212/wnl.54.7.1529. [DOI] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex. 2006;16:1462–1473. doi: 10.1093/cercor/bhj087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake-Baumann R. Activity-dependent modulation of inhibition in Purkinje cells by TrkB ligands. Cerebellum. 2006;5:220–226. doi: 10.1080/14734220600621344. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–1697. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Celnik P. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Miall RC. Concurrent adaptation to opposing visual displacements during an alternating movement. Exp Brain Res. 2006;175:676–688. doi: 10.1007/s00221-006-0585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, Antonini A, Eidelberg D. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Gothe J, Brandt SA, Irlbacher K, Roricht S, Sabel BA, Meyer BU. Changes in visual cortex excitability in blind subjects as demonstrated by transcranial magnetic stimulation. Brain. 2002;125:479–490. doi: 10.1093/brain/awf045. [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 2007;27:13413–13419. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol. 2009;102:931–940. doi: 10.1152/jn.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol. 2009;587:2949–2961. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64(5):872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Kammer T. Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia. 1999;37:191–198. doi: 10.1016/s0028-3932(98)00093-1. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 2009;629:405–421. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Chadaide Z, Boros K, Nitsche MA, Rothwell JC, Paulus W, Antal A. Bidirectional modulation of primary visual cortex excitability: a combined tDCS and rTMS study. Optom Vis Sci. 2007;48:5782–5787. doi: 10.1167/iovs.07-0706. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Koch R, Mayenfels S, König F, Paulus W, Nitsche MA. Safety limits of cathodal transcranial direct current stimulation in rats. Clin Neurophysiol. 2009;120:1161–1167. doi: 10.1016/j.clinph.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Marg E, Rudiak D. Phosphenes induced by magnetic stimulation over the occipital brain: description and probable site of stimulation. Optom Vis Sci. 1994;71:301–311. doi: 10.1097/00006324-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–238. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BU, Diehl R, Steinmetz H, Britton TC, Benecke R. Magnetic stimuli applied over motor and visual cortex: influence of coil position and field polarity on motor responses, phosphenes, and eye movements. Electroencephalogr Clin Neurophysiol. 1991;(Suppl. 43):121–134. [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol. 2009;101:1961–1971. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Overduin SA, Valero-Cabre A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res. 2002;145:437–447. doi: 10.1007/s00221-002-1140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sejnowski TJ, Krakauer JW. Adaptation to visuomotor rotation through interaction between posterior parietal and motor cortical areas. J Neurophysiol. 2009;102:2921–2932. doi: 10.1152/jn.90834.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]